Abstract
Neural, vascular and structural variables contributing to the BOLD signal response variability were investigated in younger and older humans. Twelve younger healthy human subjects (6M and 6F; mean age: 24 years; range: 19–27 years) and twelve older healthy subjects (5M and 7F; mean age: 58 years; range: 55–71 years) with no history of head trauma and neurological disease were scanned. FMRI measurements using the BOLD contrast were made when participants performed a motor, cognitive or a breath hold task. Activation volume and the BOLD response amplitude were estimated for the younger and older at both group and subject levels. Mean activation volume was reduced by 45, 40 and 38% in the elderly group during the motor, cognitive and breath hold tasks respectively compared to the younger. Reduction in activation volume was substantially higher compared to the reduction in the gray matter volume of 14% in the older compared to the younger. A significantly larger variability in the inter-subject BOLD signal change occurred during the motor task, compared to the cognitive task. BH-induced BOLD signal change between subjects was significantly less-variable in the motor task-activated areas in the younger compared to older whereas such a difference between age groups was not observed during the cognitive task. Hemodynamic scaling using the BH signal substantially reduced the BOLD signal variability during the motor task compared to the cognitive task. The results indicate that the origin of the BOLD signal variability between subjects was predominantly vascular during the motor task while being principally a consequence of neural variability during the cognitive task. Thus, in addition to gray matter differences, the type of task performed can have different vascular variability weighting that can influence age-related differences in brain functional response.
Keywords: fMRI, breath hold, BOLD, CBF, hypercapnia, variability, motor, cognitive, neural, vascular
Introduction
Functional Magnetic Resonance Imaging (fMRI), using Blood oxygen level dependent (BOLD) contrast, has been used to infer differences or patterns in neural activity both within participants and between participant groups. However, BOLD fMRI is an indirect measure of neural activity. FMRI signal changes result from changes in proportions of oxygenated to deoxygenated blood in the capillary beds that feed neural systems. Therefore, subject-specific cerebrovascular and baseline physiological factors, in addition to neural activity, affect BOLD fMRI. Thus drawing inferences of altered neural activity due to aging and disease can present several challenges due to these factors [1]. Despite growing evidence of the effects of these factors on group differences in BOLD results (e.g.,), most studies still assume equivalence of these factors between the groups under study.
Normal aging is associated with changes in cerebrovascular function, neuronal structure and cellular metabolism [2]. As a person ages, dramatic changes occur in neural and cerebrovascular structures leading to differences in task-induced fMRI-BOLD responses. A decreased fMRI response in the elderly has been observed by earlier studies using visual, motor and cognitive tasks [1,3,4]. While age-related differences in fMRI response have been well documented, it is difficult to infer if these differences stem from age-related neural plasticity that alters the mechanisms by which brain structures implement cognition or age-related cerebrovascular changes that alter the mechanisms by which blood-flow supports neural activity [5]. This is because aging-induced changes in vascular reactions to neural activity can also alter task-induced fMRI response [6,7].
Tests of age-related changes in the hemodynamics that accompany neural activity have been based on the suspicion that known age-related cerebrovascular changes may alter relationships between neural activity and blood-flow [8,9], making assumptions of hemodynamic equivalence untenable. These studies have shown contrasting age-related hemodynamic changes in the motor cortex. One study used a simple-sequential-grasping-response to demonstrate age-related slowing of hemodynamic rise-time [10]. Another study used periodic button-press and found age-related signal-to-noise ratio reduction and reduced suprathreshold activation [11]. In contrast to these results, other studies using finger-tapping have shown age-related reductions in signal intensity [12] while others have shown age-related increases in signal intensity [13]. Studies investigating other primary sensory regions have observed age-related decreases in MRI signal. Ross and colleagues [3], recorded fMRI signal from young and old adults’ V1 during periodic photic stimulation and observed reduced signal amplitude in that region. Reicker and colleagues [14] compared younger and older groups performing a motor task with an increasing functional demand on the motor system. They found age-related activation increases within the motor system not related to the functional demand and concluded that it does not necessarily reflect compensation for neurobiological age changes. In contrast, Heuninckx and colleagues [15] observed age-equivalent activity when younger and older subjects performed a simple motor task but age-related increases in activity when subjects performed a complex motor task. Three conclusions may be drawn from the above studies. First, all of these studies show age-related hemodynamic differences. Second, results from these studies have not been consistent with respect to extent and direction of HRF age differences. Third, several task factors, including the stimuli employed, the response required, and the extent of cognitive demand, have varied across these studies. More conclusive results would necessitate studies that (1) take the subject-specific cerebrovascular and baseline physiological factors into account and (2) are not based on the assumption of between-group hemodynamic equivalence, but instead take account of the origin and functional characteristics of the observed signal [16,17]. Indeed, any study of age-related changes in brain function requires consideration of structural changes, task design and the activation responses normalized to vascular reactivity to obtain valid quantitative estimates of age related changes in neural activity [18].
Examples of how task and age can reflect the extent of reduction in vascular variability is evident from earlier studies of hemodynamic scaling (hypercapnic calibration or hypercapnic normalization) of functional responses. In younger subjects performing a working memory task, hypercapnic calibration using a BH task led to a 25% reduction in the fractional inter-subject BOLD variability [19] when compared to an 85% reduction in a mixed group of young and old subjects performing a motor task [20].
The present study focused on understanding the brain structural, neural and cerebrovascular influences on BOLD fMRI data collected on young and elderly participants engaged in motor and cognitive tasks. To address issues with regards to vascular variability with age that may alter spatial responses to tasks, subjects were separated into younger (18–30 years) and older (55–75 years) groups. Subjects performed motor, cognitive and breath hold (BH) tasks in the same scanning session. Hemodynamic scaling of functional activation was performed using the BH response to eliminate vascular variability in the motor and cognitive task-responses. The results indicated that BOLD signal amplitude and spatial variability in task-induced fMRI response is predominantly vascular during motor task performance and mostly neural in origin during the cognitive task performance.
METHODS
Subjects
Twelve younger healthy subjects (6M and 6F; mean age: 24 years; range: 19–27 years) and twelve older healthy subjects (5M and 7F; mean age: 58 years; range: 55–71 years) with no history of head trauma and neurological disease were scanned. The Institutional Review Board of the University of Texas at Dallas approved all experimental procedures. Written informed consent was obtained from all subjects who were paid on an hourly basis during the study.
Experimental Paradigm
During the FMRI session, participants completed four tasks to distinguish FMRI signal changes due to neural activity versus vascular reactivity: (1) rest, (2) periodic breath-hold, (3) periodic bimanual finger-tapping and (4) Digit Symbol Verification Task [16]. Participants completed these tasks in six separate scanning runs within the FMRI session.
Rest
For the rest run, participants were instructed to rest with their eyes closed for 3 minutes.
Periodic Breath hold (BH)
Subjects performed end-inspirational BH inhaling a normal volume of air, which they would perform in a normal breathing cycle [17, 21]. For the periodic breath-hold task, participants completed three breath-hold trials lasting 20 seconds each, separated by 40 seconds of normal respiration, and 40 seconds of normal respiration preceded the first and followed the last trial. A white circle remained centered on the screen during the normal respiration periods, and to signal the breath-hold periods, the circle changed color to cyan and began flashing at 0.5 Hz. Subjects completed two practice trials prior to entering the scanner. Before the practice trials, subjects were instructed on the style of the BH and the visual cue they would observe to perform the BH task. Participants were instructed to take a normal breath when the changed colors began flashing and to hold their breath until the circle stopped flashing. They were instructed to avoid a “deep belly-breath,” which would be equivalent to a valsalva maneuver. During the practice sessions, the subjects were timed for their ability to hold their breath for the duration of 20 seconds, which was comfortably performed by all subjects. Additional practice sessions were given to subjects who needed more trials to learn the BH paradigm. One of the experimenters observed the performance of the subjects during the practice sessions and advised minor corrective measures necessary to perfect the task.
Periodic Bilateral fingertapping (FTAP)
For the periodic bimanual finger-tapping task, participants completed four finger-tapping trials lasting 20 seconds each, each separated by 20 seconds of rest, and 20 seconds of rest preceded the first and followed the last trial. A white circle remained centered on the screen during the rest periods, and to signal the finger-tapping periods, the circle changed color to cyan and began flashing at 0.5 Hz. Participants were instructed to sequentially touch each finger to its respective thumb making one touch and release, as best they could, in synchrony with the flashing circle. All subjects were able to perform this task with relative ease. Thus this task was behaviorally normalized between the young and old groups.
Digit symbol verification task
The Digit-Symbol Verification Task was adapted from the task reported by Rypma et al., [16], which was modeled after the Digit-symbol Substitution Task (DSST) from the Wechsler Adult Intelligence Scale [22]. Participants completed 156 trials across three scanning runs (52 trials per run). On each trial, a digit-symbol key and a digit-symbol probe appeared simultaneously (Figure 1) for 4 s. Participants were to indicate as quickly and accurately as possible whether the probe pair matched one of the pairs in the key (right thumb response button = yes; left = no). On half of the trials, the probe pair matched a digit-symbol pair in the key. Participants were to respond within the 4 s that the stimuli were presented on the screen. The DSST trials for each run were randomly intermixed (jittered) with twenty-three 4 s rest periods.
Figure 1.
Shows the schematic of the DSST task. On each trial, a digit-symbol key and a digit-symbol probe appeared simultaneously for 4 seconds jittered with 4 second periods of rest.
MRI acquisition
MR Imaging was performed on a 3T Philips scanner. The imaging system was equipped with a fixed asymmetric head gradient coil and a quadrature transmit/receive birdcage radio-frequency coil. Subjects were positioned in a supine position on the gantry with head in a midline location in the coil. Foam padding and a pillow were used to minimize head motion. High-resolution T1 weighted anatomical images were obtained from all subjects. Gradient echo-EPI images were subsequently obtained during rest, BH, FTAP and the DSST task. 32 slices were obtained in the axial plane covering the entire brain. Imaging parameters were: FOV of 22 cm, matrix size of 64×64, TR/TE = 2000/30 msec and slice thickness of 4mm. 110 EPI images were obtained during each of rest, BH, DSST and FTAP tasks. Imaging parameters were kept the same for all four runs.
Data Analysis
All fMRI data sets were preprocessed using AFNI [23]. The EPI images were corrected for motion using a rigid-body volume registration algorithm available in AFNI. We used motion correction parameters to calculate the total amount of motion in six directions of rotation and translation throughout each run. The maximal displacement (D) was computed after considering motion in all six directions to obtain a single D value for each volume [24]. A maximal displacement of >2mm was considered as the criteria to reject data sets for further analysis. Analysis was done only on voxels that represented brain tissue. All data sets were detrended to correct for quadratic trends. Resting state data from one young subject (due to corruption) and data in one elderly subject with the exception of the DSST task (due to excess motion) was not considered for further analysis.
To determine activated areas during each task, a gamma-variate function was convolved with the task reference function and cross correlated with the BOLD signal on a voxel-wise basis. During BH, the reference function was appropriately shifted to take into account the large hemodynamic delay during the BH response [21]. As each task had different time series lengths, different correlation threshold values were used to obtain similar Bonferroni corrected P-values. Activation maps were determined using a correlation coefficient threshold of 0.30 for the BH task, 0.20 for the DSST task and 0.35 for the FTAP task (corresponding to a Bonferroni corrected p<0.01; [25]). The average of the activation from the three DSST runs was considered as the subject activation for the cognitive task. To minimize false positives, a minimum cluster size of 20 voxels was considered for generating the activation maps during all tasks. Group activation maps were determined by converting each subject’s functional map to standard stereotaxic space based on the Talariach and Tournoux atlas [26] using a linear transformation. The correlation coefficients ‘r’ from each individual subject’s functional maps were z-transformed (z=0.5*log[(1+r)/(1−r)]) by considering the arctanh of ‘r’ on a voxel-wise basis. The z-transformed map from each subject was averaged. The averaged z-maps were transformed by considering the tanh of the z-values to obtain the average correlation coefficient map for each group [27]. The number of suprathreshold voxels in each subject was estimated to determine the activation volume on a subject-wise basis.
Gray matter volumes were determined for both the younger and older subjects using the respective high-resolution anatomical T1 images. The skull was stripped from the anatomical images using the AFNI program 3dSkullStrip prior to any segmentation. An automated gray matter segmentation was carried out on every subject using FAST (a hidden Markov random field model and an associated Expectation-Maximization algorithm) available in FSL [28]. The gray matter segmented image was visually compared to the high resolution T1 anatomical image of each of the subjects.
Hemodynamic amplitude scaling was accomplished by dividing the BOLD signal response amplitude during the motor and cognitive task with the BH-induced BOLD response amplitude in the corresponding voxels [19, 20, 29–31].
Significant differences between group means were established using the unpaired student’s t-test and the equality of variances between groups was tested using the Bartlett’s test. P<0.05 was required for significance.
RESULTS
Subject motion
We estimated the rotation and translation motion parameters during all tasks in every subject. The mean motion estimates are shown in Table 1. Most subjects had a maximal displacement (D) within 2 mm except one older subject whose data was not considered for further analysis. Motion in the older subjects was significantly larger than the younger subjects during the BH and cognitive task (Table 1). Several older subjects exhibited larger motion during the rest and motor task, however no significant group difference in motion was observed during the rest and motor task.
Table 1.
Mean motion in all directions and the maximal displacement (D) during the four functional tasks in the younger and older subject groups. Data shown are mean±SD of 12 subjects in each group.
| subjects | task | motion | ||||||
|---|---|---|---|---|---|---|---|---|
| roll (deg) | pitch (deg) | yaw (deg) | dS (mm) | dL (mm) | dP (mm) | D (mm) | ||
| young | rest | 0.05±0.05 | 0.18±0.14 | 0.06±0.05 | 0.07±0.08 | 0.03±0.02 | 0.23±0.22 | 1.19±0.43* |
| motor | 0.05±0.05 | 0.13±0.13 | 0.05±0.04 | 0.07±0.07 | 0.03±0.03 | 0.03±0.03 | 0.78± 0.36** | |
| cognitive | 0.06±0.07 | 0.09±0.10 | 0.06±0.06 | 0.11±0.11 | 0.04±0.04 | 0.05±0.05 | 0.75± 0.43§ | |
| BH | 0.04±0.05 | 0.12±0.12 | 0.07±0.07 | 0.09±0.10 | 0.04±0.04 | 0.06±0.07 | 0.78± 0.34§§ | |
| old | rest | 0.10±0.08 | 0.11±0.11 | 0.10±0.08 | 0.15±0.12 | 0.06±0.06 | 0.30±0.15 | 1.44± 0.90 |
| motor | 0.09±0.06 | 0.14±0.12 | 0.09±0.06 | 0.17±0.16 | 0.07±0.06 | 0.08±0.08 | 1.21± 0.93 | |
| cognitive | 0.13±0.17 | 0.17±0.13 | 0.15±0.15 | 0.19±0.17 | 0.10±0.13 | 0.07±0.07 | 1.37± 0.67 | |
| BH | 0.09±0.09 | 0.11±0.09 | 0.09±0.07 | 0.14±0.11 | 0.07±0.07 | 0.11±0.08 | 1.06± 0.39 | |
Not significantly different from older subjects
P<0.20;
P<0.08, student’s t-test
Significantly different from older subjects
P<0.04;
P<0.01, student’s t-test
Group activation volume
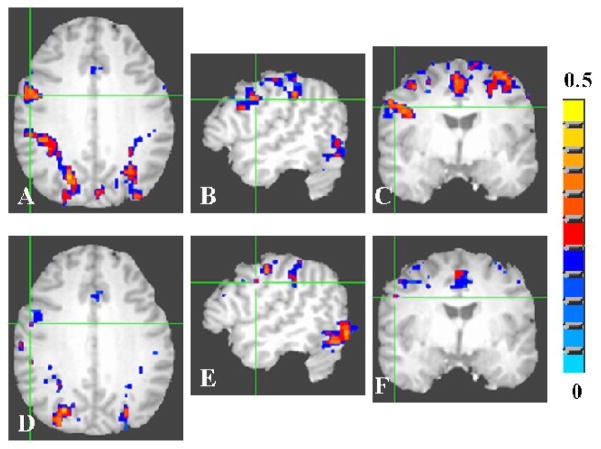
Group activation volume was estimated after averaging the individual activation maps across the younger and older group of subjects. Group activation volume was significantly smaller in the older subjects when compared to younger during all tasks. Figures 2A–C shows the group activation during the motor task for the young. Figures 2D–F show these data for the older group. The group activation volume during the motor task in the younger and older group was 29 and 16 cm3 respectively. Activation volume during the motor task in aged subjects was about 45% lesser than the activation volume observed in younger subjects.
Figure 2.
Average activation map during the motor (FTAP) task for the young (A-C) and old (D–F) groups. A voxel-wise cross-correlation of the BOLD signal time course was performed with the boxcar reference function representing the motor task for each subject. The correlation coefficient maps obtained from each subject were averaged to obtain the average map for the young and old groups. The group activation map was determined using a threshold of 0.35 for the correlation coefficient (Bonferroni corrected p<0.003).
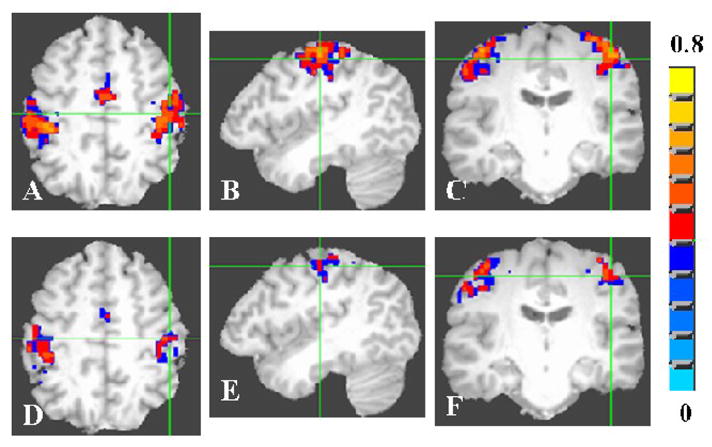
The DSST activated several brain regions including the Brodmann’s areas 7, 9, 18, 19, 24, 31, 40, 44 and 46 in both younger and older subjects. Figures 3A–C shows the activation during the DSST in Brodmann’s Area-9 in the young. Figures 3D–F shows these data for the older group. Figures 4A–C shows the activation during the DSST in Brodmann’s area-18 in the young. Figures 4D–F shows these data for the older group. The older subject group showed reduced areas of activation when compared to young in all areas activated by the DSST. The group activation volume during the DSST in the younger and older group was 156 and 94 cm3 respectively indicating a 40% less activation volume in the older subjects compared to the younger.
Figure 3.
Average activation map during the cognitive (DSST) task for the young (A–C) and old (D–F) groups in Broadman area 9. A voxel-wise cross-correlation of the BOLD signal time course was performed with a cannonical HRF convolved with the reference function representing the DSST task for each subject. The correlation coefficient maps obtained from each subject were averaged to obtain the average map for the young and old groups. The group activation map was determined using a threshold of 0.20 for the correlation coefficient (Bonferroni corrected p<0.01).
Figure 4.

Average activation map during the cognitive (DSST) task for the young (A–C) and old (D–F) groups in Broadman area 18. A voxel-wise cross-correlation of the BOLD signal time course was performed with a cannonical HRF convolved with the reference function representing the DSST task for each subject. The correlation coefficient maps obtained from each subject were averaged to obtain the average map for the young and old groups. The group activation map was determined using a threshold of 0.20 for the correlation coefficient (Bonferroni corrected p<0.01).
As cerebrovascular reactivity can influence fMRI-BOLD signal changes, we measured the cerebrovascular reactivity in the younger and older subject groups using the BH task. The BH task tests cerebrovascular reactivity and can be used in lieu of CO2 [21, 32, 33]. BH induced a global brain response in both younger and older subjects (Figure 5). The group activation volume during BH in the younger and older group was 395 and 243 cm3 respectively. Activation volume during the BH task in aged subjects was about 38% less than the activation volume observed in younger subjects.
Figure 5.
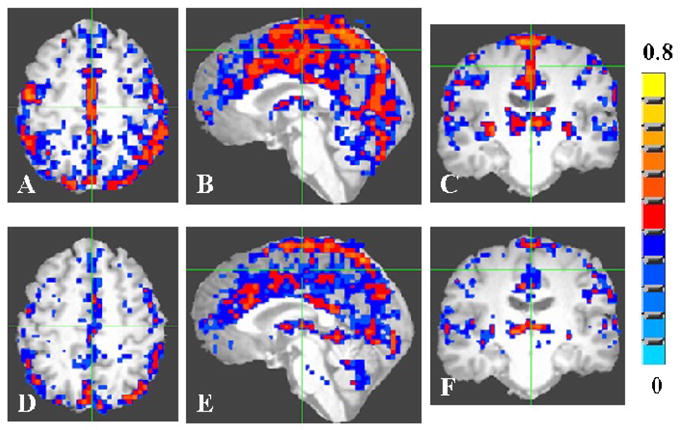
Average activation map during the breath hold (BH) task for the young (A–C) and old (D–F) groups. A voxel-wise cross-correlation of the BOLD signal time course was performed with the boxcar reference function representing the BH task for each subject. The correlation coefficient maps obtained from each subject were averaged to obtain the average map for the young and old groups. The group activation map was determined using a threshold of 0.30 for the correlation coefficient (Bonferroni corrected p<0.005).
Brain volume appeared to be more pronounced in the gray matter compared to white matter areas. To investigate possible gray-matter age differences, we segmented the gray matter from each individual brain and estimated the volume. Figure 6A shows the gray matter volume as a function of participants’ age. Gray matter volume in the younger and older groups were 643± 44 cm3 and 553±45 cm3 respectively, a 14% reduction in the older subject group when compared to the younger. A significant group difference in the gray matter volume was observed between the younger and older groups (P<6×10−5; student’s t-test). As shown above, the age related decline in fMRI-BOLD activation volume during all tasks was substantial (around 40%) compared to the 14% decrease in the mean gray matter volume for the older subject group. As there is a possibility that age related reduction in brain volume can contribute to the reduction in the fMRI-BOLD activation in the elderly we investigated the relationship between gray matter volume and BOLD activation volume during all the tasks. The gray matter volume was correlated with BOLD activation volume during the motor, DSST and BH tasks for all subjects (Fig 6B–D). A correlation of 0.1 to 0.4 was obtained for all the tasks indicating that there was a task-activation volume dependence on the gray matter volume. Thus a 14% gray matter volume decrease as indicated above in the older subjects could have significantly contributed to the reduction in the fMRI-BOLD activation volume.
Figure 6.
A. Gray matter volume as a function of the participant’s age. The two horizontal lines indicate the mean gray matter volume for the younger and older groups respectively. Correlation of gray matter volume and BOLD activation volume over all subjects during, B. motor task, C. DSST and D. BH task.
Subject-level activation volume and its variability
Because the gray matter volume significantly differed between the younger and older groups, all task-induced responses were normalized to the mean gray matter volume of the whole population scanned. The subject level activation volume for all the three tasks is shown in Table 2. No significant difference was observed in the mean activation volume between the younger and older groups during all tasks (Table 2). Inter-subject variability in the activation volume was assessed through the coefficient of variation (CV=ratio of standard deviation and mean). Inter-subject variability in the activation volume during all tasks was significantly higher in the older subject group during the motor task compared to the younger (Table 2). However, no significant variability was observed between the younger and older group during the cognitive and BH tasks (Table 2).
Table 2.
Activation volume (in cm3) in response to the motor (FTAP), cognitive (DSST) and hypercapnia (BH) tasks in young and old subjects. Values from each subject were normalized to the average gray matter volume of the whole population scanned.
| Subject | Young | old | ||||||
|---|---|---|---|---|---|---|---|---|
| NC | FTAP | DSST | BH | NC | FTAP | DSST | BH | |
| 1 | 0.86 | 53.57 | 50.93 | 337.36 | 1.10 | ---- | ---- | ---- |
| 2 | 0.86 | 35.69 | 33.47 | 587.31 | 0.99 | 52.60 | 12.87 | 654.45 |
| 3 | 0.93 | 41.77 | 44.74 | 646.64 | 1.18 | 17.88 | 32.33 | 307.13 |
| 4 | 0.91 | 159.52 | 46.31 | 603.30 | 1.06 | 34.42 | 41.00 | 325.41 |
| 5 | 0.93 | 52.41 | 55.29 | 331.32 | 1.09 | 39.87 | 48.96 | 603.47 |
| 6 | 0.94 | 72.88 | 83.13 | 624.25 | 1.28 | 301.38 | 27.83 | 354.65 |
| 7 | 1.03 | 61.53 | 35.80 | 556.53 | 1.13 | 54.83 | 25.38 | 501.96 |
| 8 | 0.90 | 37.53 | 46.19 | 182.98 | 1.15 | 132.63 | 48.94 | 207.94 |
| 9 | 0.86 | 40.17 | 33.59 | 228.83 | 0.99 | 168.53 | 39.03 | 431.77 |
| 10 | 1.08 | 80.49 | 30.98 | 360.14 | 1.10 | 52.85 | 55.09 | 431.98 |
| 11 | 0.94 | 79.24 | 77.17 | 422.80 | 0.99 | 28.46 | 88.51 | 318.92 |
| 12 | 0.96 | 44.70 | 66.20 | 220.36 | 0.99 | 133.06 | 45.99 | 437.70 |
| Mean | 0.95 | 63.29 | 50.32 | 425.15 | 1.07 | 92.41 | 42.36 | 415.94 |
| SD | 0.06 | 34.23 | 17.29 | 171.59 | 0.09 | 81.50 | 18.77 | 126.82 |
| CV | 0.06 | 0.54* | 0.34** | 0.40§ | 0.08 | 0.88 | 0.44 | 0.30 |
NC- normalization constant
Significantly different compared to older,
P<0.03; Bartlett’s test of equality of variance.
No significant difference compared to older,
P< 0.6 and
P< 0.3; Bartlett’s test of equality of variance.
Group level BOLD signal response amplitude
Figures 7A–C shows the average BOLD response amplitude during all tasks in the younger subjects. Figures 7D–F shows these data for the older subjects. The average percent change in BOLD signal during all tasks was computed from the respective group activation volumes. During the motor task, a higher mean BOLD signal change of 1.84% was observed compared to 1.68% in the older group. During the cognitive task, the mean BOLD signal change was comparable with a change of 1.54% in the younger and 1.51% in the older group. During the BH task, the mean BOLD signal change was 2.4% in the younger and 2.7% in the older subject group.
Figure 7.
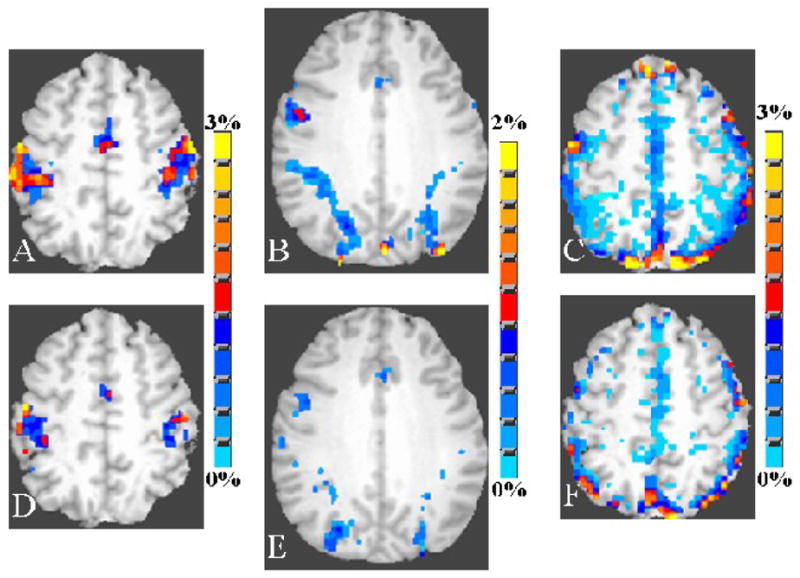
Average BOLD signal response amplitude from the pre-stimulus baseline (in percent) during all tasks for young (A–C) and old (D–F) groups. Activation during the motor task is shown in panel A and D, cognitive task in panel B and E and the breath hold task in panel C and F. The percent BOLD signal change was estimated on a voxel-wise basis for each subject and subsequently averaged over all subjects in the younger and older group. The correlation coefficient threshold for activation was 0.35 for the FTAP task, 0.20 for the DSST task and 0.30 for the BH task.
Subject level BOLD signal response amplitude and its variability
We analyzed the BOLD signal response amplitude on a subject-wise basis by spatially averaging the BOLD signal change in the active voxels from each subject during all tasks. The average BOLD signal amplitude from the motor and cognitive task-activated areas in the young was not significantly different from the old (Table 3). These results were similar to those obtained using the HRF amplitude in the motor areas [11] and visual areas [4].
Table 3.
Average BOLD response amplitude (%) from activated voxels during the motor (FTAP), cognitive (DSST) and hypercapnia (BH) task in the younger and older subjects.
| Subject | Young | old | ||||
|---|---|---|---|---|---|---|
| FTAP | DSST | BH | FTAP | DSST | BH | |
| 1 | 3.27 | 3.56 | 4.78 | ---- | ---- | ---- |
| 2 | 2.62 | 3.78 | 5.26 | 3.00 | 5.91 | 4.95 |
| 3 | 2.58 | 4.96 | 7.14 | 1.64 | 2.79 | 6.18 |
| 4 | 3.6 | 3.12 | 5.55 | 2.36 | 2.86 | 3.91 |
| 5 | 2.47 | 3.16 | 4.15 | 2.94 | 5.12 | 6.05 |
| 6 | 3.39 | 4.48 | 9.20 | 5.94 | 4.12 | 5.64 |
| 7 | 2.35 | 3.12 | 4.16 | 8.91 | 2.90 | 6.84 |
| 8 | 2.94 | 3.75 | 4.79 | 3.28 | 3.83 | 3.89 |
| 9 | 3.15 | 3.42 | 5.51 | 3.68 | 3.77 | 7.45 |
| 10 | 1.83 | 6.81 | 4.91 | 2.04 | 3.22 | 6.05 |
| 11 | 2.71 | 4.00 | 4.17 | 2.71 | 4.44 | 7.37 |
| 12 | 2.69 | 3.12 | 4.70 | 3.70 | 4.17 | 4.62 |
| Mean | 2.80 | 3.94 | 5.36 | 3.65 | 3.92 | 5.72 |
| SD | 0.49 | 1.10 | 1.46 | 2.07 | 0.99 | 1.25 |
| CV | 0.18* | 0.28** | 0.27§ | 0.57 | 0.25 | 0.22 |
Significantly different compared to older,
P<0.0001,
Not significant compared to older
P< 0.73 and
P< 0.6
Bartlett’s test of equality of variance.
During the motor task, the coefficient of variation (CV) in the young subject group was 0.18, which increased to 0.57 in the older subject group. However, during the cognitive task, the CV in the younger subject group was 0.28, which was comparable to 0.25 in the older subject group (Table 3). Thus the older subjects responded with significantly higher BOLD amplitude variability among themselves during the motor task, which was not apparent during the cognitive task. Considering the whole population of younger and older subjects, the inter-subject BOLD amplitude variability during the motor task was 0.47 when compared to 0.26 during the cognitive task. The younger subjects showed significantly different BOLD amplitude variability during the motor task (CV=0.18) when compared to the whole population (CV=0.47). A significant difference in variability compared to the whole population was not apparent in the younger subject group when they performed the cognitive task.
Vascular sensitivity is known to play a significant role in the task-induced BOLD signal change [17]. The extent of vascular sensitivity in the BOLD signal response can vary depending on the tissue in a particular voxel [29, 33]. To verify the extent of vascular variability in the task induced responses, we estimated the BH-induced BOLD signal change in the younger and older subjects from the task-activated regions of interest (ROIs). Table 4 shows the BH-induced signal change from all subjects from the regions activated by the motor and the cognitive tasks. The older subjects exhibited a larger vascular variability in the motor task regions when compared to the younger. However the increase in variability was not significant between the younger and older groups during the BH task (Table 4).
Table 4.
Breath hold (BH)-induced BOLD signal amplitude change in the younger and older subjects in brain regions active during the motor (FTAP) or cognitive (DSST) task. Values are the voxel average from the activated regions.
| Subject | BH-induced BOLD signal change (%) | |||
|---|---|---|---|---|
| young | old | |||
| FTAP | DSST | FTAP | DSST | |
| 1 | 3.73 | 2.94 | n.a | n.a |
| 2 | 3.71 | 4.48 | 2.59 | 4.86 |
| 3 | 4.74 | 4.63 | 2.44 | 2.81 |
| 4 | 5.57 | 5.49 | 1.80 | 1.96 |
| 5 | 2.43 | 2.08 | 2.22 | 5.84 |
| 6 | 3.52 | 4.08 | 3.7 | 2.52 |
| 7 | 3.50 | 4.43 | 6.32 | 4.91 |
| 8 | 1.84 | 2.73 | 3.17 | 2.78 |
| 9 | 2.70 | 2.63 | 4.19 | 4.14 |
| 10 | 2.65 | 4.25 | 2.74 | 4.45 |
| 11 | 4.01 | 4.79 | 2.06 | 3.06 |
| 12 | 2.06 | 2.19 | 5.62 | 4.52 |
| Mean | 3.37 | 3.72 | 3.35 | 3.80 |
| SD | 1.10 | 1.13 | 1.48 | 1.23 |
| CV | 0.32* | 0.30** | 0.44 | 0.32 |
No significant difference compared to older
P< 0.3 and
P< 0.8;
Bartlett’s test of equality of variance.
Inter-subject spatial variability in BOLD activation
We estimated the spatial overlap of activation for all subjects in the young and old groups. Binary activation maps were obtained for each subject in the younger and older groups and subsequently averaged. Figure 8 shows the spatial extent of activation during the motor, cognitive and the BH task. All active voxels, irrespective of the color, in Figures 8A1 and B1, are the union of active voxels during the motor task from all subjects in the younger and older groups respectively. Overlap of motor activation from at least 2, and as many as 11 subjects were linearly color coded and the maps from at least 2 up to 5 subjects as a threshold are shown in figures 8A2–A5 and 8B2–B5 for the younger and older subjects respectively. Figures 8C and D and 8E and F show these data for the cognitive and BH tasks respectively. As evident from Figures 8A1 and B1, the task-induced motor response was more spatially distributed in the older as compared to younger subjects. In other words, the older subject group displayed a relatively larger spatial variation in the motor task-induced response when compared to the young. This larger spatial variability in the motor task-induced activity in older subjects resulted in a faster rate of decline in the spatial overlap of activity for higher subject thresholds in the older group (Figures 8B1–B7 and G) as compared to the younger group (Figures 8A1–A7 and G). During the cognitive task, the spatial variability in the older subjects was also relatively higher when compared to young. This was similar to that observed during the motor task. In other words, the progressive decline in the spatial overlap of activation with increasing subject threshold occurred at a slower rate in the younger (Figures 8C1–C5 and H) when compared to the older subjects (Figures 8D1–D5 and H). A similar result was observed during the BH task as depicted in Figures 8E1–E5 and I indicating the BH response in young subjects and Figures 8F1–F5 and I indicating the BH response in old subjects. The rate of decline in the BH induced response in a larger number of subjects was higher in the older group when compared to the younger.
Figure 8.
Activation maps during the motor task (FTAP) from a single axial slice covering the motor cortex in young A1–A7, and old B1–B7. Activation maps during the cognitive task (DSST) from BA9 in young C1–C5, and old D1–D5. Breath hold induced activation in a single axial slice covering the motor cortex in young E1–E5, and old F1–F5. Binary activation maps were created for every subject and subsequently averaged over each group for the specific task. Color in each voxel represents common spatial activation in any one, up to eleven subjects in each group. Plot of the of the activation area common from one to eleven subjects during the motor G, cognitive H, and Breath hold I, in the young and old. Activation area is plotted in logarithmic scale.
BOLD response amplitude variability and the effect of hemodynamic scaling using BH
Neural and vascular variability contributes to the total variability in the BOLD signal response. In order to decipher the contribution of vascular components to the variation in the BOLD signal change observed during task activation, we hemodynamically scaled the task-induced response in each subject with the respective BOLD signal change during BH from the same subject. Hemodynamic scaling reduced the mean BOLD signal change during the motor and cognitive tasks in addition to significantly reducing the inter-subject variation in the younger and older subject groups. After hemodynamic scaling with BH, a significantly larger reduction in the variation in the motor task-induced BOLD signal change was observed in the older subject group where the inter-subject coefficient of variation (CV) reduced from 0.57 to 0.22 (P<1×10−6; Bartlett’s test). Such a large reduction in inter-subject variation was not evident in the young subject group where the CV reduced moderately from 0.18 to 0.15 ((P<0.002; Bartlett’s test) after hemodynamic scaling with BH. During the cognitive task, the inter-subject variation in the BOLD signal change reduced moderately from 0.31 to 0.28 (P<1×10−6; Bartlett’s test) in the old subject group and from 0.28 to 0.25 (P<1×10−6; Bartlett’s test) in the younger subject group. This indicates that vascular sensitivity variation during the motor task was more prominent in the elderly when compared to the young.
DISCUSSION
Our results indicated that age related BOLD signal differences may be predominantly attributed to neural and vascular variables after age-related differences in gray-matter volume are accounted for. Age-equivalent motor task performance was observed. Motor task-induced activation was minimal in the group maps (Figure 2), and not significantly different as observed from the subject-wise mean activation volumes (Table 2), due to a larger inter-subject variability in the older subjects. The mean BH-induced BOLD signal response amplitude within the areas activated by the motor task (an indicator of vascular sensitivity), though not significantly different, was highly variable in the older subject group when compared to the younger (Table 4). Hence motor task-induced activation was significantly affected by vascular variables. Accordingly, we observed, significant reductions in the group variability after hemodynamic scaling with the BH task.
DSST results indicated that older subjects had reduced group activation volume (Figures 3 and 4) that was not significantly different when compared to young (Table 2). However, the mean BH-induced BOLD signal response amplitude and its variability within the areas activated by the DSST (an indicator of vascular sensitivity) were not significantly different between the younger and older groups (Table 4). Hence the DSST-induced activation was not significantly affected by vascular variables. This result is consistent with the relatively smaller reduction of vascular variability after hemodynamic scaling with the BH task [19]. As the vascular sensitivity determined by the BH induced response from the regions activated by the motor task and DSST was not significantly different in our study, it is likely that the larger vascular variability to the motor task induced BOLD response stems from the design of the motor task (block design) as opposed to the DSST (event related). Additionally, in a recent study vascular variability has been shown to play an insignificant role in the age-related differences in the BOLD signal response [31]. This study, however, used an event related task design in which subjects performed a visuomotor saccade task.
Activation clusters could be reduced in older samples due to differences in baseline BOLD variance affecting statistical significance [4,11]. We analyzed the temporal standard deviation (SD) using the motion corrected resting-state scan on a voxel-wise basis. SD was estimated from the motor task activated voxels in each subject. The SD was 7.26±1.1 for the younger group and 8.34±1.97 for the older group respectively. Average baseline noise in the older group was larger but not significantly different when compared to the average baseline noise from the younger group. While the baseline noise can be larger in some subjects within the older group making an impact on the subject’s statistical maps, it cannot significantly affect the group results. Thus the substantially smaller group activation and mean BOLD signal change in the elderly during the DSST and motor task may stem from larger subject-wise spatial variation in the task-induced responses (Figure 8) as the subject-wise voxel average analysis failed to show any significant difference in the area of activation and mean BOLD signal change during all tasks between the two groups (Table 2 and 3).
We hypothesize that, during normal aging, subtle functional reorganization may occur to account for regional changes in cerebrovascular function. Experimental evidence including these data with diminished gray matter volume in the elderly support the above hypothesis [34,35]. Structural imaging studies have shown considerable anatomical variability between the brains of younger and older adults and within a random sample of older adults [34,35] and previous functional imaging studies have also indicated an age-related increase in functional activity [36]. Some researchers have attributed these age differences to strategic cognitive compensation [13,15,37], our results suggest that age-related brain atrophy may result more in spatial shifts in the functional cell assemblies that support cognitive activity than in shifts in cognitive strategy. We observed a faster rate of decline in the activation volume with increasing subject threshold in the older group even during the non-neural BH task (Figure 8). This larger spatial variation in cerebral reactivity in the older subjects may represent structural and functional alterations in the vascular properties with aging. Thus cognitive activity in the elderly may be optimal or suboptimal depending on the extent of vascular alterations. Age-related brain plasticity may occur with neural function following neighboring regions that optimally support the task related neural activity. The results during the motor task and the DSST indicate this possibility as both tasks led to a faster decline in activation volume in the older group. Such an age-related plasticity may optimally or sub-optimally support cognitive operations. This migration from optimal to suboptimal functional regions could result in an overall reduction in neural efficiency, and consequent reductions in processing speed [38]. Indeed, behavioral and neuroimaging studies that have explicitly tested hypotheses of age-related strategic changes in DSST and other cognitive tasks have produced null results [39,40].
Vascular sensitivity varied considerably between subjects and more in the older group than the younger in areas activated by the motor task. However vascular sensitivity variation in the elderly may not have a significant role in the inter-subject BOLD variation during the cognitive task indicating a predominantly neural variability. This is supported by results in young subjects, where the inter-subject variability during the motor task was CV=0.18, (Table 3) while the same set of subjects performing the cognitive task (during the same session) had a relatively larger inter-subject variability of CV=0.28, (Table 3). However, the inter-subject vascular variability, as determined by the BH measurements, was similar in the activated clusters during the motor or cognitive tasks (Table 4). In the absence of any vascular sensitivity differences, this result indicates that relatively larger neural variability contributes to the inter-subject variability in the BOLD signal change during the DSST in the younger compared to the older sample. Furthermore, in the older sample, DSST-induced inter-subject variability (CV=0.25; Table 3) was not significantly different to that observed in the younger sample (CV=0.28; Table 3) while exhibiting a similar extent of inter-subject vascular variability as determined from the BH measurements (CV= 0.32; Table 4). The above results strongly suggest a neural source for the DSST-induced variability in the BOLD signal change in both young and old subjects.
In conclusion, a significant gray matter loss in older participants accounted for almost one third of the reduction in activation volume during all tasks. A larger inter-subject spatial variability in the activation led to decreased group-activation during the motor, cognitive and BH tasks in the older participants. Hemodynamic scaling using the BH task indicated a vascular contribution to the BOLD signal amplitude variability during the motor task while neural variability contributed significantly to the BOLD signal amplitude variability during the cognitive task in the younger and older subject groups. As vascular and neural contribution to the BOLD response can vary depending on the type of task, age-related differences to neural activation-induced functional response should be appropriately weighted. Tasks that have a large vascular variability weighting could lead to larger BOLD signal variability in older subjects thus complicating efforts to accurately determine the neural basis of age-related differences in task performance.
Acknowledgments
This study was supported by NIH grants NS049176-01A2 (BB) and AG029523-02 (BR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nature Reviews Neuroscience. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- 2.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 3.Ross MH, Yurgelun-Todd DA, Renshaw PF, Maas LC, Mendelson JH, Mello NK, Cohen BM, Levin JM. Age-related reduction in functional MRI response to photic stimulation. Neurology. 1997;48:173–176. doi: 10.1212/wnl.48.1.173. [DOI] [PubMed] [Google Scholar]
- 4.Huettel SA, Singerman JD, McCarthy G. The effects of aging upon the hemodynamic response measured by functional MRI. Neuroimage. 2001;13:161–75. doi: 10.1006/nimg.2000.0675. [DOI] [PubMed] [Google Scholar]
- 5.Buckner RL, Snyder AZ, Sanders AL, Raichle ME, Morris JC. Functional brain imaging of young, nondemented, and demented older adults. J Cogn Neurosci. 2000;2:24–34. doi: 10.1162/089892900564046. [DOI] [PubMed] [Google Scholar]
- 6.Riecker A, Grodd W, Klose U, Schulz JB, Gröschel K, Erb M, Ackermann H, Kastrup A. Relation between regional functional MRI activation and vascular reactivity to carbon dioxide during normal aging. J Cereb Blood Flow Metab. 2003;23:565–73. doi: 10.1097/01.WCB.0000056063.25434.04. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto M, Meyer JS, Sakai F, Yamaguchi F. Aging and cerebral vasodilator responses to hypercarbia: responses in normal aging and in persons with risk factors for stroke. Arch Neurol. 1980;37:489–496. doi: 10.1001/archneur.1980.00500570037005. [DOI] [PubMed] [Google Scholar]
- 8.Fang HCH. Observation of aging characteristics of cerebral blood vessels: Macroscopic and microscopic features. In: Terry RD, Gershon S, editors. Neurobiology of aging. NewYork: Raven; 1976. [Google Scholar]
- 9.Kalaria RN. Cerebral vessels in ageing and alzheimer’s disease. Pharmacology & Therapeutics. 1996;72:193–214. doi: 10.1016/s0163-7258(96)00116-7. [DOI] [PubMed] [Google Scholar]
- 10.Taoka T, Iwasaki S, Uchida H, Fukusumi A, Nakagawa H, Kichikawa K, Takayama K, Yoshioka T, Takewa M, Ohishi H. Age correlation of the time lag in signal change on EPI-fMRI. Journal of Computer Assisted Tomography. 1998;22:514–517. doi: 10.1097/00004728-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 11.D’Esposito M, Zarahn E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the bold hemodynamic response. Neuroimage. 1999;10:6–14. doi: 10.1006/nimg.1999.0444. [DOI] [PubMed] [Google Scholar]
- 12.Hesselmann V, Weber OZ, Wedekind C, Krings T, Schulte O, Kugel H, Krug B, Klug N, Lackner K. J Age related signal decrease in functional magnetic resonance imaging during motor stimulation in humans. Neuroscience Letters. 2001;308:141–144. doi: 10.1016/s0304-3940(01)01920-6. [DOI] [PubMed] [Google Scholar]
- 13.Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer-Lindenberg A, Goldberg TE, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci Lett. 2002;392:32–7. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Riecker A, Gröschel K, Ackermann H, Steinbrink C, Witte O, Kastrup A. Functional significance of age-related differences in motor activation patterns. Neuroimage. 2006;32:1345–54. doi: 10.1016/j.neuroimage.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Heuninckx S, Wenderoth N, Swinnen SP. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci. 2008;28:91–99. doi: 10.1523/JNEUROSCI.3300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rypma B, Berger JS, Prabhakaran V, Bly BM, Kimberg DY, Biswal BB, D’Esposito M. Neural correlates of cognitive efficiency. Neuroimage. 2006;33:969–79. doi: 10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 17.Biswal BB, Kannurpatti SS, Rypma B. Magnetic Resonance. Hemodynamic scaling of fMRI-BOLD signal: validation of low-frequency spectral amplitude as a scalability factor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samanez-Larkin GR, D’Esposito M. Group comparisons: imaging the aging brain. Soc Cogn Affect Neurosci. 2008;3:290–297. doi: 10.1093/scan/nsn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomason ME, Foland LC, Glover GH. Calibration of BOLD fMRI using breath holding reduces group variance during a cognitive task. Hum Brain Mapp. Hum Brain Mapp. 2007;28:59–68. doi: 10.1002/hbm.20241. [DOI] [PMC free article] [PubMed] [Google Scholar]; Imaging. 2007;25:1358–1369. doi: 10.1016/j.mri.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kannurpatti SS, Biswal BB. Detection and scaling of task-induced fMRI-BOLD response using resting state fluctuations. Neuroimage. 2008;40:1567–1574. doi: 10.1016/j.neuroimage.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kannurpatti SS, Biswal BB, Hudetz AG. Differential fMRI-BOLD signal response to apnea in humans and anesthetized rats. Magn Reson Med. 2002;47:864–870. doi: 10.1002/mrm.10131. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Revised. New York: The Psychological Corp; 1981. [Google Scholar]
- 23.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 24.Jiang A, Kennedy DN, Baker JR, Weisskoff RM, Tootell RBH, Woods RP, Benson RR, Kwong KK, Brady TJ, Rosen BR, Belliveau JW. Motion detection and correction in functional MR imaging. Human Brain Mapp. 1995;3:224–235. [Google Scholar]
- 25.Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for the time-course data sets in fMRI of the human brain. Mag Reson Med. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- 26.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Theime Medical; New York: 1988. [Google Scholar]
- 27.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Trans on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 29.Bandettini PA, Wong EC. A hypercapnia-based normalization method for improved spatial localization of human brain activation with fMRI. NMR Biomed. 1997;10:197–203. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<197::aid-nbm466>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 30.Cohen ER, Rostrup E, Sidaros K, Lund TE, Paulson OB, Ugurbil K, Kim SG. Hypercapnic normalization of BOLD fMRI: comparison across field strengths and pulse sequences. Neuroimage. 2004;23:613–624. doi: 10.1016/j.neuroimage.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Handwerker DA, Gazzaley A, Inglis BA, D’Esposito M. Reducing vascular variability of fMRI data across aging populations using a breathholding task. Hum Brain Mapp. 2007;28:846–59. doi: 10.1002/hbm.20307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kastrup A, Kruger G, Glover GH, Mosley EM. Assessment of cerebral oxidative metabolism with breath-holding and fMRI. Mag Reson Med. 1999;42:608–611. doi: 10.1002/(sici)1522-2594(199909)42:3<608::aid-mrm26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 33.Li QT, Kastrup A, Takahashi A, Glover GH, Mosley EM. Functional MRI of human brain during breath-holding by BOLD and FAIR techniques. Neuroimage. 1999;9:243–249. doi: 10.1006/nimg.1998.0399. [DOI] [PubMed] [Google Scholar]
- 34.Raz N. Aging of the brain and its impact on cognitive performance: integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. Handbook of aging and cognition II. Mahwah, New Jersey: Erlbaum; 2000. pp. 1–90. [Google Scholar]
- 35.Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral Cortex. 2005;15:1676–89. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 36.Rypma B, Prabhakaran V, Desmond JE, Gabrieli JDE. Age differences in prefrontal cortical activity in working memory. Psychology and Aging. 2001;16:371–384. doi: 10.1037//0882-7974.16.3.371. [DOI] [PubMed] [Google Scholar]
- 37.Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 38.Rypma B. A neural efficiency hypothesis of age-related changes in human working memory performance. In: Osaka N, Logie R, D’Esposito M, editors. The cognitive neuroscience of working memory. Oxford University Press; Oxford, UK: 2007. [Google Scholar]
- 39.Salthouse TA. Independence of age-related influences on cognitive abilities across the life span. Dev Psychol. 1998;34:851–64. doi: 10.1037//0012-1649.34.5.851. [DOI] [PubMed] [Google Scholar]
- 40.Rypma B, Berger JS, Genova HM, Rebbechi D, D’Esposito M. Dissociating age-related changes in cognitive strategy and neural efficiency using event-related fMRI. Cortex. 2005;41:582–94. doi: 10.1016/s0010-9452(08)70198-9. [DOI] [PubMed] [Google Scholar]