Abstract
Recurrent hypoglycemia (RH), the most common side-effect of intensive insulin therapy for diabetes, is well established to diminish counterregulatory responses to further hypoglycemia. However, despite significant patient concern, the impact of RH on cognitive and neural function remains controversial. Here we review both the data from human studies and recent animal studies regarding the impact of RH on cognitive, metabolic, and neural processes. Overall, RH appears to causes brain adaptations which may enhance cognitive performance and fuel supply when euglycemic but which pose significant threats during future hypoglycemic episodes.
Our brain runs on glucose: in contrast to cells elsewhere, which will generally take their fuel where they can find it, neurons and glia both rely on a constant supply of glucose from blood for their metabolic support (with some of that glucose being metabolised by astrocytes into lactate which is then exported for subsequent use by neurons). Unsurprisingly, acute interruption of this supply by systemic hypoglycemia produces marked cognitive impairment and leads eventually to coma and death. The consequences of acute hypoglycemia, including effects on cognitive and neural functions, are relatively well-understood. In addition and unsurprisingly, repeated severe hypoglycemia causes both significant neuronal death and cognitive impairment [1, 2]. However, in recent years the impact on the brain and on cognitive functions of repeated, moderate interruptions of glucose supply, which cause little or no loss of neurons in hippocampus or cortex [3, 4], has become of increasing interest. This interest is primarily the result of increased hypoglycemic incidence as a side-effect of advances in therapy for diabetes, but might also be of wider interest because of a potential relationship with the emerging practice of caloric restriction as an aid to long-term physical and mental health. The present review will examine data from both animal and human studies at a variety of levels of analysis. The primary conclusion drawn will be that the long-term consequences of moderate repeated hypoglycemia can be significant, but in general appear to be potentially beneficial at most times. There is, however, significant acerbation of the impact of further episodes of hypoglycemia on cognitive function, so that this risk should be recognised and borne in mind by those receiving or initiating protocols likely to produce such hypoglycemia.
The most common cause of hypoglycemia in modern Western society is the use of exogenous insulin as a therapeutic agent for treatment of diabetes. Hypoglycemia has historically been primarily seen in individuals with type I diabetes mellitus (T1DM), but increasingly is also being experienced by patients with type 2 diabetes (T2DM) as a result of more aggressive therapy. Over the past two decades, the gold standard for treatment of T1DM has been intensive insulin therapy, aimed at aggressively preventing hyperglycemia and associated neuropathies. While successful, this treatment approach has also resulted in a marked increase in frequency of hypoglycemia subsequent to insulin administration [5]. The long term consequences of such recurrent moderate hypoglycemia (RH) for brain and cognitive functions remain controversial not least because of the difficulty in human studies of accurately parsing the effects of RH from such confounds as duration of diabetes, age of onset, hyperglycemic neuropathies, and so on. Notwithstanding the uncertainty with regard to the neural and cognitive impact of RH, hypoglycemia has become the most feared side-effect of insulin therapy [6], with widespread concern for e.g. the possibility of neural damage because of interrupted fuel supply.
There are several good reviews of the diminution of counter-regulatory hormonal responses following RH, with the attendant impairment to systemic glucose homeostasis in the face of further hypoglycemic episodes (e.g. [7–9]). The systemic and glucose-sensing changes in response to RH, and mechanisms of hypoglycemia detection and counterregulation, are outside the scope of this mini-review which will focus solely on brain, and primarily cognitive, effects of RH.
1. Cognitive studies
1.1. Human studies
There have been several attempts to determine the neural and cognitive impact of RH, but results have been mixed. The human literature contains reports of RH producing enhanced, impaired, or unaffected subsequent cognitive function [6, 10–23]. The lack of uniformity is likely due in large part to difficulty in controlling and determining the glycemic history of diabetic patients and to the confounds unavoidably introduced by individual variations in hypoglycemic history, exposure to hyperglycemia, cerebrovascular and neuropathic conditions, and chronic illness. Any impairment to brain function might be expected to be reflected especially in reduced performance on hippocampally-mediated tasks: the hippocampus, in addition to being the primary site involved in declarative, spatial, and several other forms of memory, is very sensitive to variations in fuel supply at times of cognitive demand [24–26]. Hence, it is to be expected that perturbations in, for instance, glucose supply would have an especially marked impact on hippocampal function. The fact that RH is not consistently reported to impair performance on memory tasks even in diabetic patients (where impairment might be contributed to by e.g. diabetic neuropathies and/or vascular impairments) might suggest that RH does not in fact lead to any marked cognitive impairment. Perhaps the single most convincing finding that RH does not appear to produce long-term cognitive deficits comes from follow-up studies on the large-scale Diabetes Control and Complications Trial (DCCT), where no association was found between hypoglycemia and cognitive function even over many years [27] and which came to the blunt conclusion that “repeated episodes of hypoglycemia were not related to cognitive decrement.” The recently-published EDIC follow-up study essentially confirmed this conclusion and further suggested that moderate RH does not impair subsequent cognitive performance [28].
One limitation to the human literature is that there has been little investigation of whether acute and chronic glycemic states might interact to affect brain function. An exception is a recent study which compared T1DM patients with and without impaired counter-regulatory responses and showed that patients with impaired hypoglycemia awareness (suggesting a history of more frequent and/or severe hypoglycemia) might have slightly less impairment of cognitive performance on relatively simple tasks during further hypoglycemia [29]. Several studies (e.g. [30]) have attempted to make some retrospective evaluation of reported cognitive function during episodes of self-reported hypoglycemia. Such studies suggest that impairments seen, as a result of acute hypoglycemia, in e.g. driving ability (a complex task, requiring spatial processing and judgement, and which imposes significant metabolic demands of its own [31]) may correlate with the severity of previous hypoglycemic history. These data hence suggest an interaction between previous history and glycemic state at the time of cognitive challenge in modulating the cognitive impact of RH. Task difficulty is an important variable to consider when examining the literature: it has been suggested [32] that preservation of simple cognitive functions following RH at the expense, where necessary, of complex processing, may be an adaptive response. This suggestion has some support in the literature [33, 34] but has been relatively little studied. The fact that deficits are found most commonly in cognitively-demanding tasks, and possibly in tasks mediated by the hippocampus, is highly consistent with the extensive literature on enhancement of memory following provision of additional exogenous glucose (reviewed in [35]): enhancement is seen only when the memory task used is challenging for the subjects being tested, and is most reliably seen on tasks mediated by the hippocampus.
Beyond purely cognitive ability, a second aspect of psychological function commonly reported as being affected (generally, impaired) by RH is control of affective functions, as measured by changes in mood and anxiety [36–38]; these functions might be expected to be mediated by the amygdala and/or frontal cortex, rather than by the hippocampus, but have received less attention than memory and task performance. By their nature, such measures are also more difficult to evaluate in animal studies, and this area would benefit from more comprehensive and long-term studies.
Problems in interpreting the human literature include not only disease state effects but also the fact that several of the hormonal parameters most dramatically affected by RH - in particular, plasma glucocorticoids and epinephrine, which rise rapidly in response to hypoglycemia in control subjects but whose rise is blunted following RH [39] - are themselves cognitive enhancers [40]. More problematically, this is also true of the insulin used to induce hypoglycemia, which (especially in studies using hyperinsulinemic clamps to maintain plasma glucose at a target threshold) is given in very high doses; insulin has recently been shown to enhance cognitive function when given e.g. intranasally [41, 42] or centrally [43, 44] and is known to cross the blood-brain barrier. Adding further to the complexity of interpretation is the fact that several recent studies have shown that insulin may (i) directly modulate hormonal responses to hypoglycemia [45], (ii) directly affect brain glucose-sensing [46], and (iii) directly modulate neuronal firing and plasticity [46, 47]. Timing of studies may also be an issue, especially for repeated-measures designs, as hypoglycemia even several hours after an event may impair optimal consolidation of memories for that event [48].
Overall, designing well-controlled studies of the impact of RH on cognitive performance in human patients remains a challenge, and attributing any cognitive effects seen to RH as the causal factor is difficult, especially in the context of pre-existing diabetes. In sum, though, the literature suggests that RH may have little or no deleterious effect on future cognitive performance when euglycemic, or on simple tasks, but may perhaps lead to impairment at times of further metabolic challenge and/or complex task demand.
1.2. Animal studies
Two recent animal studies from our lab were the first to investigate the cognitive effects of RH in an animal system, using a well-controlled model of RH to examine both spatial working memory performance and hippocampal function and examining whether cognitive function was affected by an interaction between RH and acute glycemic state at the time of testing. The results showed a clear interaction: RH enhanced spatial memory in rats when the testing was done at euglycemia but impaired performance during a further hypoglycemic episode [32, 49]. This pattern of results was consistent whether the RH was a short-term, fairly intense protocol or a long-term paradigm using once-weekly induction of hypoglycemia to mimic the reported experience of human T1DM patients [5]; in this latter case, RH acted to attenuate age-related memory impairments (tested at euglycemia) for at least 12 months, well past the point at which control animals were unable to perform above chance levels. Reassuringly, the data from animal studies are very consistent with the overall pattern of data seen in humans, both at the cognitive level and (discussed below) with regard to e.g. metabolic changes. Data from recently-diabetic and non-diabetic animals were essentially identical [32], suggesting that animal studies in non-diabetic animals may be effective at allowing determination of the effects of RH without confound from any chronic disease-state variables. Nevertheless, several of the confounds and complexities discussed above with regard to human studies are also seen in the animal literature: for instance, many animal studies use hyperinsulinemic hypoglycemic clamps and hence must consider the impact of highly hyperphysiological insulin levels, and attenuation of hormonal responses to hypoglycemia following RH is seen in animal models as in humans.
Additional data recently obtained in our laboratory have extended the examination of cognitive changes following RH to examine performance on tests of anxiety (elevated plus-maze and open-field testing) and mental flexibility (a ‘set-shifting’ task analogous to the human Wisconsin card-sorting task [50]). Interestingly, the impact of RH varies markedly by paradigm; for instance, anxiety is increased only by the interaction of RH and acute hypoglycemia (neither increases anxiety alone), and mental flexibility is impaired following RH but only when euglycemic (unpublished data). Ongoing work is attempting to determine the underlying basis for these differences between tasks and the brain regions subserving them; however, it is important to note that reports of effects of RH on psychological measures in humans (e.g. on anxiety and judgement) can at least to some extent be reproduced in a rodent model.
Although there are few if any further studies of RH and cognitive function in animal models, our findings are also consistent with the growing body of work in various animal models regarding the cognitive benefits of chronic caloric restriction, including data from primates [51]; recently, similar results have been obtained in human studies also [52]. The general pattern of such findings is one of beneficial adaptation, albeit in the context of non-diabetic subjects who are hence unlikely to experience acute hypoglycemia.
2. Metabolic and neural studies
2.1. Fuel transport and supply
Antecedent hypoglycemia has been shown by several groups to increase blood-brain barrier (BBB) glucose transport, and glucose transporter expression is increased at both the BBB and neuronal surface [53–55], although in humans the question of whether glucose uptake is in fact increased has been somewhat controversial [56–59]. Using in vivo microdialysis in our rat studies, we showed (i) that even short-term, moderate RH markedly increased the elevation in hippocampal interstitial fluid glucose following i.p. glucose administration, and (ii) that at euglycemia RH prevented task-associated drainage of glucose from the hippocampus during spatial memory testing [32]. We have previously shown that at times of high cognitive load, hippocampal glucose demand exceeds supply, and administration of exogenous glucose enhances performance by correcting this imbalance [24]; animals with prior RH, tested at euglycemia, closely resembled control animals who had received exogenous glucose, in both task performance and hippocampal glucose levels [32]. The pattern of results strongly supports the suggestion from human studies that RH leads to higher capacity for transport of glucose to the brain, and measurements of BBB and neuronal glucose transporters in rats following RH show upregulation of glucose transporters (GluT1 and 3), also consistent with this finding (unpublished data).
The interaction of acute and chronic glycemic states on cognitive performance in our animal studies was accompanied by a similar interaction with regard to brain glucose levels: in contrast to the enhanced glucose supply at euglycemia, animals with prior RH showed a more rapid decline in brain glucose in response to induction of acute hypoglycemia. Recent work in this area used magnetic resonance spectroscopy and spin-labelled fuels to measure brain glucose usage, following RH, under both euglycemic and hypoglycemic conditions, and reassuringly confirmed the pattern of results seen with microdialysis: increased brain glucose metabolism at euglycemia but impaired metabolism during further hypoglycemia [60]. As yet it is unclear whether the alterations seen in hippocampal metabolism extend to other brain regions or whether perhaps, as with cognitive function, the impact of RH on metabolism may vary by brain region
[Note that potential confounds, such as the use of insulin to induce hypoglycemia, must be considered in the context of metabolism also. In humans, even very low levels of insulin administration can affect brain glucose usage, for example [61]. Insulin produces systemic hypoglycemia by increasing glucose uptake into tissues via the insulin-sensitive glucose transporter GluT4; that transporter is also present in the brain (along with the recently-discovered isoform GluTX, which may also be regulated by insulin [62]); moreover, insulin has been shown to directly modulate the brain’s ability to sense glucose levels via actions at the ATP-sensitive potassium channel [46, 63].]
In addition to supply from the circulation, neurons and glia can draw on reserves of glucose stored as glycogen in astrocytes. Initial studies of brain glycogen levels following RH suggested that there might be a ‘supercompensation’ of glycogen storage, which might then act as a buffer against further episodes of interrupted glucose supply [64]. Improvements in technique, allowing for measurements to be taken from awake (rather than anesthetised) animals, however, have recently shown that no such effect occurs [65].
A further potential adaptation which might act to preserve the brain’s energy supply would be to derive energy not solely from glucose but rather to also metabolise e.g. ketones derived from fatty acids. Such an adaptation is well-known to occur in the brain of animals or humans placed on a ketogenic diet [66], and monocarboxylates have been shown in vitro to support synaptic function in the absence of glucose [67]; Page and colleagues have recently shown that medium-chain fatty acids are capable of attenuating cognitive impairment during hypoglycemia [68]. Preliminary findings in our lab suggest that in our animal model of RH, monocarboxylate transporter expression is increased at both neuronal surface and (possibly) BBB following RH, suggesting a role for use of fuels beyond glucose in adapting to RH.
A major concern of diabetic patients is that repeated episodes of hypoglycemia may result in neuronal loss because of impaired fuel supply. However, while fairly severe acute hypoglycemia has been shown to produce neuronal damage and death (primarily in cortex following moderate hypoglycemia [69] with hippocampal damage also occurring following extremely severe, hypoglycemia [2]), there has been no demonstration of any negative impact of RH on such cell loss, and moderate RH does not appear to cause any cell loss [70]. Monocarboxylate provision may again be helpful in avoiding neuronal loss following hypoglycemia [71].
2.2. Synaptic and electrophysiological effects
There has been relatively little work done in humans on the impact of RH beyond the cognitive and metabolic levels. One study in humans used event-related potentials in an attempt to evaluate automatic versus effortful processing, and found that RH reduced signals associated with complex processes during further hypoglycemia, while sparing those associated with more automatic functions [38]. Consistent with this, we found in our rat studies that several measures of hippocampal synaptic function (measured in vitro post-mortem) were enhanced, following RH, when slices were maintained at euglycemia, but that synaptic function was markedly impaired under conditions of further hypoglycemia [49]; other labs have also found, in rats, that synaptic function is impaired, under reduced glucose conditions, in brain slices from diabetic brains compared to those from control brains [72]. These data are remarkably consistent with the cognitive and metabolic results discussed above. However, work in this area has been limited, and the underlying mechanisms behind these observations of altered synaptic function remain to be determined.
Similarly, there have been few or no RH studies focussed on e.g. neurotransmitter release, neuronal morphology, spine density, plasticity, or other mechanisms and measures of cognitive and neural function. There is much scope and need for further work in this area.
3. Summary
In contrast to the concerns of many diabetic patients, there is little or no evidence for a long-term deleterious impact of recurrent moderate hypoglycemia on brain function. Indeed, the literature suggests that if anything, the brain responds to RH by increasing support for cognitive functions, and in particular by enhancing fuel supply, resulting in improved cognitive performance which may extend across large portions of the lifespan. The major caveat to that rosy picture is that there is a clear interaction between hypoglycemic history and acute glycemic state in modulation of cognitive and neural function: animals or humans with a history of RH have a variety of alterations which contribute to impaired ability to meet the challenges posed by a further hypoglycemic event. This is important clinically, as the primary time of concern is when a diabetic patient with a history of RH is about to experience a further episode of hypoglycemia and may be considering e.g. driving. Moreover, the fact that RH impairs both the patient’s ability to detect further episodes of hypoglycemia and counterregulatory responses to such hypoglycemia means that RH remains a significant clinical and therapeutic issue. Increased understanding of the mechanisms involved in the brain’s response to RH, however, may allow for introduction of preventative therapies such as the use of medium-chain fatty acids as a nutritional supplement. For non-diabetic individuals considering adopting a regimen of reduced caloric intake, who are at low risk for future hypoglycemia, the data are supportive of potential benefits to cognitive function and brain fuel supply.
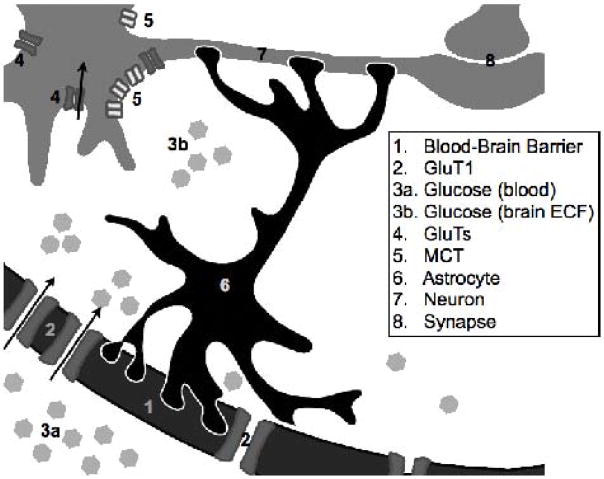
Figure 1.
This schematic illustrates some of the sites at which known alterations in hippocampal fuel supply and function occur in response to recurrent hypoglycemia. In particular, glucose transporter (2, 4) expression increases at both the blood-brain barrier (1) and neuronal cell-surface; the dominant GluT forms will be GluT1 at the BBB, and GluTs 3 and perhaps 4 on neurons. This upregulation of transport capacity means that glucose (3a, 3b) metabolism increases, following RH, at euglycemia; the reason for decreased glucose metabolism when acutely hypoglycemic subsequent to RH remains to be determined.. Monocarboxylate transporter (MCT, 5) expression may also increase, permitting increased use of lactate exported from astrocytes. Finally, RH has been shown to alter several aspects of neural transmission across synapses (8), with the impact of RH depending on glycemic state.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. Journal of Clinical Investigation. 2007;117:910–8. doi: 10.1172/JCI30077. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bree A, Puente E, Daphna-Iken D, Fisher S. Diabetes increases brain damage caused by severe hypoglycemia. American Journal of Physiology, endocrinology and metabolism. 2009;297:E194–201. doi: 10.1152/ajpendo.91041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tkacs NC, Dunn-Meynell A, Levin BE. Presumed Apoptosis and Reduced Arcuate Nucleus Neuropeptide Y and Pro-Opiomelanocortin mRNA in Non-Coma Hypoglycemia. Diabetes. 2000;49:820–826. doi: 10.2337/diabetes.49.5.820. [DOI] [PubMed] [Google Scholar]
- 4.Tkacs NC, Pan Y, Raghupathi R, Dunn-Meynell AA, Levin BE. Cortical Fluoro-Jade staining and blunted adrenomedullary response to hypoglycemia after noncoma hypoglycemia in rats. J Cereb Blood Flow Metab. 2005;25:1645–1655. doi: 10.1038/sj.jcbfm.9600152. [DOI] [PubMed] [Google Scholar]
- 5.Diabetes Care and Complications Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in diabetes mellitus. New England Journal of Medicine. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 6.Amiel S. Cognitive function testing in studies of acute hypoglycaemia: rights and wrongs? Diabetologia. 1998;41:713–719. doi: 10.1007/s001250050973. [DOI] [PubMed] [Google Scholar]
- 7.Cryer P. Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes. 2005;54:3592–3601. doi: 10.2337/diabetes.54.12.3592. [DOI] [PubMed] [Google Scholar]
- 8.Dagogo-Jack S. Hypoglycemia in type 1 diabetes mellitus: pathophysiology and prevention. Trends in Endocrinology. 2004;3:94–103. doi: 10.2165/00024677-200403020-00004. [DOI] [PubMed] [Google Scholar]
- 9.Diedrich L, Sandoval D, Davis S. Hypoglycemia associated autonomic failure. Clinical Autonomic Research. 2002;12:358–65. doi: 10.1007/s10286-002-0035-9. [DOI] [PubMed] [Google Scholar]
- 10.Amiel S, Pottinger R, Archibald H, Chusney G, Cunnah D, Prior P, et al. Effect of antecedent glucose control on cerebral function during hypoglycemia. Diabetes Care. 1991;14:109–118. doi: 10.2337/diacare.14.2.109. [DOI] [PubMed] [Google Scholar]
- 11.Pramming S, Thorsteinsson B, Theilgaard A, Pinner E, Binder C. Cognitive function during hypoglycaemia in type I diabetes mellitus. British Medical Journal. 1986;292:647–650. doi: 10.1136/bmj.292.6521.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones T, Borg W, Borg M, Boulware S, McCarthy G, Silver D, et al. Resistance to neuroglycopenia; an adaptive response during intensive insulin treatment of diabetes. Journal of Endocrinology and Metabolism. 1997;82:1713–1718. doi: 10.1210/jcem.82.6.3993. [DOI] [PubMed] [Google Scholar]
- 13.Fruehwald-Schultes B, Born J, Kern W, Peters A, Fehm H. Adaptation of cognitive function to hypoglycemia in healthy men. Diabetes Care. 2000;23:1059–1066. doi: 10.2337/diacare.23.8.1059. [DOI] [PubMed] [Google Scholar]
- 14.Hershey T, Bhargava N, Sadler M, White NH, Craft S. Conventional versus intensive diabetes therapy in children with type 1 diabetes: effects on memory and motor speed. Diabetes Care. 1999;22:1318–1324. doi: 10.2337/diacare.22.8.1318. [DOI] [PubMed] [Google Scholar]
- 15.Hershey T, Craft S, Bhargava N, White NH. Memory and insulin dependent diabetes mellitus (IDDM): effects of childhood onset and severe hypoglycemia. Journal of the International Neuropsychological Association. 1997;3:509–520. [PubMed] [Google Scholar]
- 16.Dagogo-Jack SE, Cryer PE. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus: recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. Journal of Clinical Investigation. 1993;91:821–828. doi: 10.1172/JCI116302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hvidberg A, Fanelli C, Hershey T, Terkamp C, Craft S, Cryer P. Impact of recent antecedent hypoglycemia on hypoglycemic cognitive dysfunction in non-diabetic humans. Diabetes. 1996;45:1030–1036. doi: 10.2337/diab.45.8.1030. [DOI] [PubMed] [Google Scholar]
- 18.Langan SJ, Deary IJ, Hepburn DA, Frier B. Cumulative cognitive impairment following recurrent severe hypoglycaemia in adult patients with insulin-treated diabetes mellitus. Diabetologia. 1991;34:337–344. doi: 10.1007/BF00405006. [DOI] [PubMed] [Google Scholar]
- 19.Strachan MWJ, Ewing FME, Deary IJ, Frier B. Recovery of cognitive function and mood after severe hypoglycemia in adults with insulin-treated diabetes. Diabetes Care. 2000;23:305–12. doi: 10.2337/diacare.23.3.305. [DOI] [PubMed] [Google Scholar]
- 20.Lincoln N, Faleiro R, Kelly C, Kirk B, Jeffcoate W. Effect of long-term glycemic control on cognitive function. Diabetes Care. 1996;19:656–658. doi: 10.2337/diacare.19.6.656. [DOI] [PubMed] [Google Scholar]
- 21.Deary IJ, Crawford J, Hepburn DA, Langan SJ, Blackmore L, Frier B. Severe hypoglycemia and intelligence in adult patients with insulin-treated diabetes. Diabetes. 1993;42:341–344. doi: 10.2337/diab.42.2.341. [DOI] [PubMed] [Google Scholar]
- 22.Sherwin RS. Bringing Light to the Dark Side of Insulin: A Journey Across the Blood-Brain Barrier. Diabetes. 2008;57:2259–2268. doi: 10.2337/db08-9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strachan MWJ, Deary IJ, Ewing FME, Frier BM. Recovery of cognitive function and mood after severe hypoglycemia in adults with insulin-treated diabetes. Diabetes Care. 2000;23:305–312. doi: 10.2337/diacare.23.3.305. [DOI] [PubMed] [Google Scholar]
- 24.McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:2881–5. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNay EC, Gold PE. Age-related differences in hippocampal extracellular fluid glucose concentration during behavioral testing and following systemic glucose administration. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2001;56:B66–71. doi: 10.1093/gerona/56.2.b66. [DOI] [PubMed] [Google Scholar]
- 26.Rex A, Bert B, Fink H, Voigt J-P. Stimulus-dependent changes of extracellular glucose in the rat hippocampus determined by in vivo microdialysis. Physiology & Behavior. 2009;98:467–473. doi: 10.1016/j.physbeh.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Austin E, Deary I. Effects of Repeated Hypoglycemia on Cognitive Function: A psychometrically validated reanalysis of the Diabetes Control and Complications Trial data. Diabetes Care. 1999;22:1273–1277. doi: 10.2337/diacare.22.8.1273. [DOI] [PubMed] [Google Scholar]
- 28.Group TDCaCTEoDIaCDESR. Long-Term Effect of Diabetes and Its Treatment on Cognitive Function. New England Journal of Medicine. 2007;356:1842–1852. doi: 10.1056/NEJMoa066397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zammitt N, Warren R, Deary I, Frier B. Delayed Recovery of Cognitive Function Following Hypoglycemia in Adults With Type 1 Diabetes. Effect of Impaired Awareness of Hypoglycemia Diabetes. 2008;57:732–736. doi: 10.2337/db07-0695. [DOI] [PubMed] [Google Scholar]
- 30.Cox D, Penberthy J, Zrebiec J, Weinger K, Aikens J, Stetson B, DeGroot M, Trief P, Chaechinger H, Hermanns N, Gonder-Frederick L, Clarke W. Diabetes and Driving Mishaps: Frequency and correlations from a multinational survey. Diabetes Care. 2003;26:2329–2334. doi: 10.2337/diacare.26.8.2329. [DOI] [PubMed] [Google Scholar]
- 31.Cox D, Gonder-Frederick L, Kovatchev B, Clarke W. The metabolic demands of driving for drivers with type 1 diabetes mellitus. Diabetes/Metabolism Research and Reviews. 2002;18:381–385. doi: 10.1002/dmrr.306. [DOI] [PubMed] [Google Scholar]
- 32.McNay EC, Sherwin RS. Effect of recurrent hypoglycemia on spatial cognition and cognitive metabolism in normal and diabetic rats. Diabetes. 2004;53:418–25. doi: 10.2337/diabetes.53.2.418. [DOI] [PubMed] [Google Scholar]
- 33.Jacob R, Dziura J, Blumberg M, Morgen J, Sherwin R. Protective adaption during acute hypoglycemia. Diabetes. 1999;48:141–145. doi: 10.2337/diabetes.48.1.141. [DOI] [PubMed] [Google Scholar]
- 34.Jacob RJ, Dziura J, Blumberg M, Morgen JP, Sherwin RS. Effects of recurrent hypoglycemia on brainstem function in diabetic BB rats: protective adaptation during acute hypoglycemia. Diabetes. 1999;48:141–5. doi: 10.2337/diabetes.48.1.141. [DOI] [PubMed] [Google Scholar]
- 35.McNay EC, Gold PE. Food for thought: fluctuations in brain extracellular glucose provide insight into the mechanisms of memory modulation. Cognitive and Behavioural Neuroscience Reviews. 2002;1:264–280. doi: 10.1177/1534582302238337. [DOI] [PubMed] [Google Scholar]
- 36.Strachan M, Deary I, Ewing F, Frier B. Recovery of Cognitive Function and Mood After Severe Hypoglycemia in Adults With Insulin-Treated. Diabetes Diabetes Care. 2000;23:305–312. doi: 10.2337/diacare.23.3.305. [DOI] [PubMed] [Google Scholar]
- 37.Cryer P, Davis S, Shamoon H. Hypoglycemia in Diabetes. Diabetes Care. 2003;26:1902–1912. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- 38.Schultes B, Kern W, Oltmanns K, Peters A, Gais S, Fehm H, Born J. Differential adaptation of neurocognitive brain functions to recurrent hypoglycemia in healthy men. Psychoneuroendocrinology. 2004;30:149–161. doi: 10.1016/j.psyneuen.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Cryer PE. Hypoglycaemia-associated autonomic failure. In: Frier BM, Fisher BM, editors. Hypoglycaemia and Diabetes: Clinical and Physiological Aspects. Edward Arnold; London: 1993. pp. 275–283. [Google Scholar]
- 40.McGaugh JL, Gold PE, Van Buskirk R, Haycock J. Modulating influences of hormones and catecholamines on memory storage processes. Progress in Brain Research. 1975;42:151–62. doi: 10.1016/S0079-6123(08)63656-0. [DOI] [PubMed] [Google Scholar]
- 41.Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–34. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Reger M, Watson G, Frey W, Baker L, Cholerton B, Keeling M, Belongia D, Fishel M, Plymate S, Schellenberg G, Cherrier M, Craft S. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiology of Aging. 2006;27:451–458. doi: 10.1016/j.neurobiolaging.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Park CR, Seeley RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive avoidance task. Physiology and Behaviour. 2000;68:509–514. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- 44.McNay E. The impact of recurrent hypoglycemia on cognitive function in aging. Neurobiology of Aging. 2005;26:S76–79. doi: 10.1016/j.neurobiolaging.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Inouye K, Shum K, Chan O, Mathoo J, Matthews SG, Vranic M. Effects of recurrent hyperinsulinemia with and without hypoglycemia on counterregulation in diabetic rats. American Journal of Physiology - Endocrinology & Metabolism. 2002;282:E1369–E1379. doi: 10.1152/ajpendo.00480.2001. [DOI] [PubMed] [Google Scholar]
- 46.Cotero V, Routh V. Insulin blunts the response of glucose-excited neurons in the ventrolateral-ventromedial hypothalamic nucleus to decreased glucose. American Journal of Physiology, endocrinology and metabolism. 2009;296:E1101–1109. doi: 10.1152/ajpendo.90932.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Izumi Y, Yamada K, Matsukawa M, Zorumski C. Effects of insulin on LTP in hippocampal slices from diabetic rats. Diabetologia. 2003;46:1007–12. doi: 10.1007/s00125-003-1144-2. [DOI] [PubMed] [Google Scholar]
- 48.Jauch-Chara K, Hallschmid M, Gais S, Schmid S, Oltmanns K, Colmorgen C, Born J, Schultes B. Hypoglycemia During Sleep Impairs Consolidation of Declarative Memory in Type 1 Diabetic and Healthy Humans. Diabetes Care. 2007;30:2040–2045. doi: 10.2337/dc07-0067. [DOI] [PubMed] [Google Scholar]
- 49.McNay EC, Williamson A, McCrimmon RJ, Sherwin RS. Cognitive and Neural Hippocampal Effects of Long-Term Moderate Recurrent Hypoglycemia. Diabetes. 2006;55:1088–1095. doi: 10.2337/diabetes.55.04.06.db05-1314. [DOI] [PubMed] [Google Scholar]
- 50.Stefani M, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behavioral Neuroscience. 2003;117:728–737. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- 51.Colman R. Mortality in Rhesus Monkeys Caloric Restriction Delays Disease Onset and Mortality in Rhesus Monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Witte A, Fobker M, Gellner R, Knecht S, Floel A. Caloric restriction improves memory in elderly humans. PNAS. 2009;106:1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumagai A, Yang Y, Boado R, Pardridge W. Upregulation of blood-brain barrier GLUT 1 glucose transporter protein and mRNA in experimental chronic hypoglycemia. Diabetes. 1995;44:1399–1404. doi: 10.2337/diab.44.12.1399. [DOI] [PubMed] [Google Scholar]
- 54.Uehara Y, Nipper A, McCall A. Chronic insulin hypoglycemia induces GLUT-3 protein in rat brain neurons. American Journal of Physiology. 1997;272:E716–E719. doi: 10.1152/ajpendo.1997.272.4.E716. [DOI] [PubMed] [Google Scholar]
- 55.Simpson I, Appel N, Hokari M, Oki J, Holman G, Maher F, Koehler-Stec E, Vannucci S, Smith Q. Blood-brain barrier glucose transporter: effects of hypo- and hyperglycemia revisited. Journal of Neurochemistry. 1999;72:238–247. doi: 10.1046/j.1471-4159.1999.0720238.x. [DOI] [PubMed] [Google Scholar]
- 56.Boyle P, Nagy R, O’Connor A, Kempers S, Yeo R, Qualls C. Adaption in brain glucose uptake following recurrent hypoglycemia. Proceedings of the National Academy of Science USA. 1994;91:9352–9356. doi: 10.1073/pnas.91.20.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Segel S, Fanelli C, Dence C, Markham J, Videen T, Paramore D, Powers W, Cryer P. Blood-to-brain glucose transport, cerebral glucose metabolism and cerebral blood flow are not increased after hypoglycemia. Diabetes. 2001;50:1911–1917. doi: 10.2337/diabetes.50.8.1911. [DOI] [PubMed] [Google Scholar]
- 58.Cranston I, Reed L, Marsden P, Amiel S. Changes in regional brain 18F-fluorodeoxyglucose uptake at hypoglycemia in type 1 diabetic men associated with hypoglycemia unawareness and counterregulatory failure. Diabetes. 2001;50:2329–2366. doi: 10.2337/diabetes.50.10.2329. [DOI] [PubMed] [Google Scholar]
- 59.Pelligrino D, Segil LJ, Albrecht RF. Brain glucose utilization and transport and conrtical function in chronic vs. acute hypoglycemia. American journal of Physiology. 1990;259:E729–E735. doi: 10.1152/ajpendo.1990.259.5.E729. [DOI] [PubMed] [Google Scholar]
- 60.Jiang L, Herzog R, Mason G, de Graaf R, Rothman D, Sherwin R, Behar K. Recurrent Antecedent Hypoglycemia Alters Neuronal Oxidative Metabolism In Vivo. Diabetes. 2009;58:1266–1274. doi: 10.2337/db08-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bingham E, Hopkins D, Smith D, Pernet A, Hallett W, Reed L, Marsden P, Amiel S. The role of insulin in human brain glucose metabolism. Diabetes. 2002;51:3384–3390. doi: 10.2337/diabetes.51.12.3384. [DOI] [PubMed] [Google Scholar]
- 62.Piroli GG, Grillo CA, Charron MJ, McEwen BS, Reagan LP. Biphasic effects of stress upon GLUT8 glucose transporter expression and trafficking in the diabetic rat hippocampus. Brain Research. 2004;1006:28–35. doi: 10.1016/j.brainres.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 63.Spanswick D, Smith MA, Mirshamshi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese, rats. Nature Neuroscience. 2000;3:757–758. doi: 10.1038/77660. [DOI] [PubMed] [Google Scholar]
- 64.Gruetter R. Glycogen: the forgotten cerebral energy store. Journal of Neuroscience Research. 2003;74:179–83. doi: 10.1002/jnr.10785. [DOI] [PubMed] [Google Scholar]
- 65.Herzog R, Chan O, Yu S, Dziura J, McNay E, Sherwin R. Effect of Acute and Recurrent Hypoglycemia on Changes in Brain Glycogen Concentration Endocrinology. 2008;149:1499–1504. doi: 10.1210/en.2007-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leino R, Gerhart D, Duelli R, Enerson B, Drewes L. Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain Neurochemistry International. 2001;38:519–527. doi: 10.1016/s0197-0186(00)00102-9. [DOI] [PubMed] [Google Scholar]
- 67.Sakurai T, Yang B, Takata T, Yokono K. Synaptic Adaptation to Repeated Hypoglycemia Depends on the Utilization of Monocarboxylates in Guinea Pig Hippocampal Slices. Diabetes. 2002;51:430–438. doi: 10.2337/diabetes.51.2.430. [DOI] [PubMed] [Google Scholar]
- 68.Page K, Williamson A, Yu N, McNay E, Dziura J, McCrimmon R, Sherwin R. Medium-Chain Fatty Acids Improve Cognitive Function in Intensively Treated Type 1 Diabetic Patients and Support In Vitro Synaptic Transmission During Acute Hypoglycemia Diabetes. 2009;58:1237–1244. doi: 10.2337/db08-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tkacs N, Pan Y, Raghupathi R, Dunn-Meynell A, Levin B. Cortical Fluoro-Jade staining and blunted adrenomedullary response to hypoglycemia after noncoma hypoglycemia in rats. Cerebral Blood Flow and Metabolism. 2005;25:1645–1655. doi: 10.1038/sj.jcbfm.9600152. [DOI] [PubMed] [Google Scholar]
- 70.Yamada K, Rensing N, Izumi Y, de Erausquin G, Gazit V, Dorsey D, Herrera D. Repetitive Hypoglycemia in Young Rats Impairs Hippocampal Long-Term Potentiation Pediatric Research. 2004;55:372–379. doi: 10.1203/01.PDR.0000110523.07240.C1. [DOI] [PubMed] [Google Scholar]
- 71.Suh S, Aoyama K, Matsumori Y, Liu J, Swanson R. Pyruvate Administered After Severe Hypoglycemia Reduces Neuronal Death and Cognitive Impairment. Diabetes. 2005;54:1452–1458. doi: 10.2337/diabetes.54.5.1452. [DOI] [PubMed] [Google Scholar]
- 72.Yamada KA, Rensing N, Izumi Y, de Erausquin GA, Gazit V, Dorsey DA, Herrera DG. Repetitive hypoglycemia in young rats impairs hippocampal long-term potentiation. Pediatric research. 2004;55:372–379. doi: 10.1203/01.PDR.0000110523.07240.C1. [DOI] [PubMed] [Google Scholar]