Abstract
Vascular smooth muscle cells (VSMCs) are important targets in the treatment of atherosclerosis. However, the arterial media, where majority of VSMCs reside, has proven to be a difficult target for drug/gene delivery. We have demonstrated that ultrasound enhances drug/gene delivery to VSMCs in vitro using echogenic immunoliposomes (ELIP) as vector. This study aimed to evaluate whether ultrasound can similarly enhance delivery of an agent to VSMCs, particularly within the arterial media, in vivo, using ELIP. Anti-smooth muscle cell actin-conjugated calcein-loaded ELIP were injected into the peripheral arteries of Yucatan miniswine (n=8 arterial pairs). The right-sided porcine arteries were treated with 1-MHz continuous wave ultrasound at a peak-to-peak pressure amplitude of 0.23 ± 0.05 MPa for two minutes. The contralateral arteries served as controls. Arteries were harvested after 30 minutes and imaged with fluorescence microscopy. Image data were converted to gray scale, and analyzed using computer-assisted videodensitometry. There was significant improvement in calcein uptake in all three arterial layers in the arteries exposed to ultrasound (p < 0.05 vs. no ultrasound), with a more marked increase in uptake in the arterial media (> 300%). This enhanced uptake was site specific and appeared limited to the ultrasound-treated arterial segment. We have demonstrated enhanced delivery of a small molecule to VSMCs in all arterial wall layers particularly the arterial media, using ultrasound and targeted ELIP. The combined effect of ultrasound exposure and ELIP as a contrast agent and a drug/gene-bearing vector has the potential for site-specific therapy directed at VSMC function.
Keywords: Ultrasound Contrast Agents, Targeted Delivery
INTRODUCTION
Vascular smooth muscle cells (VSMCs) are implicated in the pathogenesis of coronary atherosclerosis, including neointimal formation following percutaneous angioplasty or stent placement, bypass graft failure, and transplant arteriopathy (Ross, 1986; Virmani and Farb, 1999; Horlitz et al., 2002; Sarjeant and Rabinovitch, 2002; Hillebrands and Rozing, 2003). In response to vascular injury, VSMCs increase their rate of cellular proliferation, migration, and synthetic capacity, thereby playing a critical role in vascular repair (Dzau et al., 2002; Newby, 2000). The VSMC response is regulated by many factors and is implicated in atheroma formation, with a competition between favorable vascular repair, and detrimental neointimal formation and plaque development (Geng and Libby, 2002; Hedin et al., 2004). The ability to regulate or manipulate VSMC function is an important goal in the treatment of atherosclerosis.
One well accepted concept regarding the origins of intimal involvement in atherosclerosis is that the majority of VSMCs are derived from preexisting medial smooth muscle cells (SMCs) that undergo migration into the intima and phenotypic transformation (Campbell et al., 1988; Schwartz and Murry, 1998). More recent evidence suggests that VSMC involvement in atheroma formation is also derived from pre-existing intimal clones and adventitial fibroblasts that undergo transdifferentiation (Hedin et al., 2004; Owens et al., 2004). Due to the probable diverse origins of intimal SMC involvement, treatments designed to impact VSMC function must ideally be able to reach all layers of the arterial wall (Hao et al., 2003). In particular, the arterial media, where the majority of VSMCs reside, is an important target. Many investigators have demonstrated effective drug or gene delivery to the arterial intima and adventitia (Lemarchand et al., 1993; Maeda et al., 1997; Yao and Wang, 1995). However, there has been less success with delivery of drugs or genes to the arterial media (Lemarchand et al., 1993; Maeda et al., 1997; Yao and Wang, 1995), mainly because of anatomic barriers such as the internal elastic lamina. Rome et al. (1994) has even suggested that the arterial media is virtually inaccessible to vectors larger than 70–100 nm, and disruption of the internal elastic laminae seems to be necessary for larger drug or gene vectors such as liposomes.
Our group has described development of an agent that can be used as a targeted ultrasound contrast agent, which we term echogenic immunoliposomes (ELIP) (Alkan-Onyuksel et al., 1996; Lanza et al., 1997; Demos et al., 1999; Hamilton et al., 2002)). These liposomes can be manipulated to entrap drugs or genes, and can be targeted to specific structures by the attachment of ligands (Kopechek et al., 2008; Tiukinhoy-Laing et al., 2007; Tiukinhoy et al., 2004; Tiukinhoy et al., 2000). We have shown that ultrasound enhances drug and gene delivery to VSMCs in vitro using ELIP as the vector (Huang et al., 2002a; Huang et al., 2002b). We hypothesize that ultrasound exposure can similarly enhance delivery of a small molecule to the VSMC in vivo, including the arterial media, using ELIP as the vector. For the clinical application of ultrasound therapy for drug or gene delivery, it may be advantageous and simpler to use a vector that also encapsulates micro- or nanobubbles to nucleate acoustic cavitation in order to effect drug or gene delivery and enhance uptake across the vascular endothelium. Hence, this study aims to explore the potential of calcein-loaded ELIP delivery to VSMCs in vivo using anti-SMC-actin targeted ELIP in combination with 1-MHz ultrasound exposure.
MATERIALS AND METHODS
Liposome Formulation
ELIP were prepared by the sonication-lyophilization-rehydration method as described previously (Huang et al., 2001). The liposomal composition used was DPPC:DPPG:Chol:MPB-PE: in a 69:8:15:8 molar ratio [DPPC = dipalmitoylphosphatidylcholine; DPPG = dipalmitoylphosphatidylglycerol; Chol = Cholesterol (all 3 lipids from Sigma-Aldrich Inc., St. Louis, MO); MPB-PE = 4-(p-maleimidolphenyl) butyrate phosphatidylethanolamine (Avanti Polar Lipids Inc., Alabaster, AL)]. The component lipids were mixed in 4 ml glass vials and dissolved in chloroform (Fisher Scientific, Pittsburgh, PA) after which the solvent was allowed to evaporate completely under argon while the vial was rotated in a 50 °C water bath. The resulting lipid film was then placed under vacuum for 4 hours at ≤100 mTorr pressure for full removal of the solvent. The lipid film was then hydrated with distilled, deionized water and the dispersion was sonicated in a water bath for 5 minutes. An equal volume of 0.2 M D-mannitol was then added to the liposome suspension and the sample was frozen at −70 °C. The samples were lyophilized for 24 hours and resuspended with 0.1 M phosphate-buffered saline (PBS). The final concentration used for dilution was 10 mg lipid/ml PBS.
Anti-smooth muscle cell actin antibody (R&D Systems; Minneapolis, MN) was conjugated to the liposomes as described previously (Demos et al., 1997). Antibody was reacted with 3-(2-pyridyl dithio) propionic acid N-hydroxysuccinimide ester (SPDP) in a 5:1 SPDP:protein molar ratio for 30 minutes. The protein was separated from the reactants using a Sephadex G-50 column and reduced with 2.5 M dithiothreitol (DTT). The thiolated protein was then separated using a Sephadex G-50 column (Sigma-Aldrich Inc., St. Louis, MO), mixed with the liposomes, and allowed to react overnight with gentle stirring. Unbound protein was separated from the liposomes on a Sepharose 4B column (Sigma-Aldrich Inc., St. Louis, MO). Conjugated ELIP were then relyophilized at −50°C for 24 hours.
Calcein Incorporation
Calcein (2’, 7’–[{bis[carboxymethyl]-amino} methyl]-fluorescein; MW=623; Sigma Chemical Co., St. Louis, MO) was used as a surrogate for a small molecule drug and loaded into the liposomes. Calcein (0.1 mmole) was added to the lipid film after the initial dehydration process. The encapsulation efficiency of calcein was 15% (Huang et al., 2008). Prior studies have also confirmed retention of echogenicity of the ELIP with calcein loading (Huang and MacDonald, 2004; Huang et al., 2008).
1-MHz Ultrasound Transducer Calibration
A 1-MHz ultrasound transducer (Sonitron 1000, Rich-Mar Corp, Inola, OK) which has a 1 cm aperture, was used to expose the porcine arteries to ultrasound. This transducer was calibrated to determine the acoustic pressure output and beam characteristics using a 0.5 mm-diameter polyvinylidene difloride (PVDF) needle hydrophone (Precision Acoustics, Dorchester, England). Briefly, calibration measurements were performed in a water-filled 36 cm × 24 cm × 20 cm acrylic tank at room temperature (20 to 23 °C). The Sonitron transducer was submerged using a rigidly supported positioner, and the PVDF needle hydrophone was mounted on a motorized three-axis translation system (Velmex NF90 Series, Velmex Inc, Bloomfield, NY) and aligned with the transducer. The hydrophone was inserted through a 2.0 cm × 3.5 cm slab of acoustic absorber material (Precision Acoustics, Dorchester, England) in order to minimize standing wave interference from the positioner. Hydrophone measurements were digitized using a digital oscilloscope (WaveRunner, LeCroy Corp, Chestnut Ridge, NY) and transferred to a desktop computer. All calibration measurements were automated and controlled with a collection of visual interface programs written in LabVIEW (National Instruments, Austin, TX) on the desktop computer. The Sonitron output dial setting was 2.0 W/cm2 with a center frequency of 1 MHz and 100% duty cycle (continuous wave). The beam profiles were characterized and plotted in the axial and transverse planes using MATLAB (Mathworks Inc, Natick, MA).
Animal Protocol
All animal studies were approved by the Northwestern University Animal Care and Use Committee. Three Yucatan miniswine (female; 15–20 kg; Sinclair Research, Inc., Wyndham, ME) were anesthetized, beginning with glycopyrrolate (0.004 mg/kg IM) 15–20 minutes prior to administering an injection of TelazolR (10 mg/kg IM). The miniswine were maintained on a mixture of low-level isoflurane (1.5–3%) and oxygen (4.5 ml/kg/min) during the procedure. For the first miniswine, only the carotid and axillary arteries were used (n=2 pairs) as the iliofemoral arteries were of very small caliber and did not accommodate the delivery catheter. For the subsequent two miniswine, the carotid, iliofemoral, and axillary arteries (n=6 pairs) were surgically exposed and used (total of n=8 pairs available for evaluation).
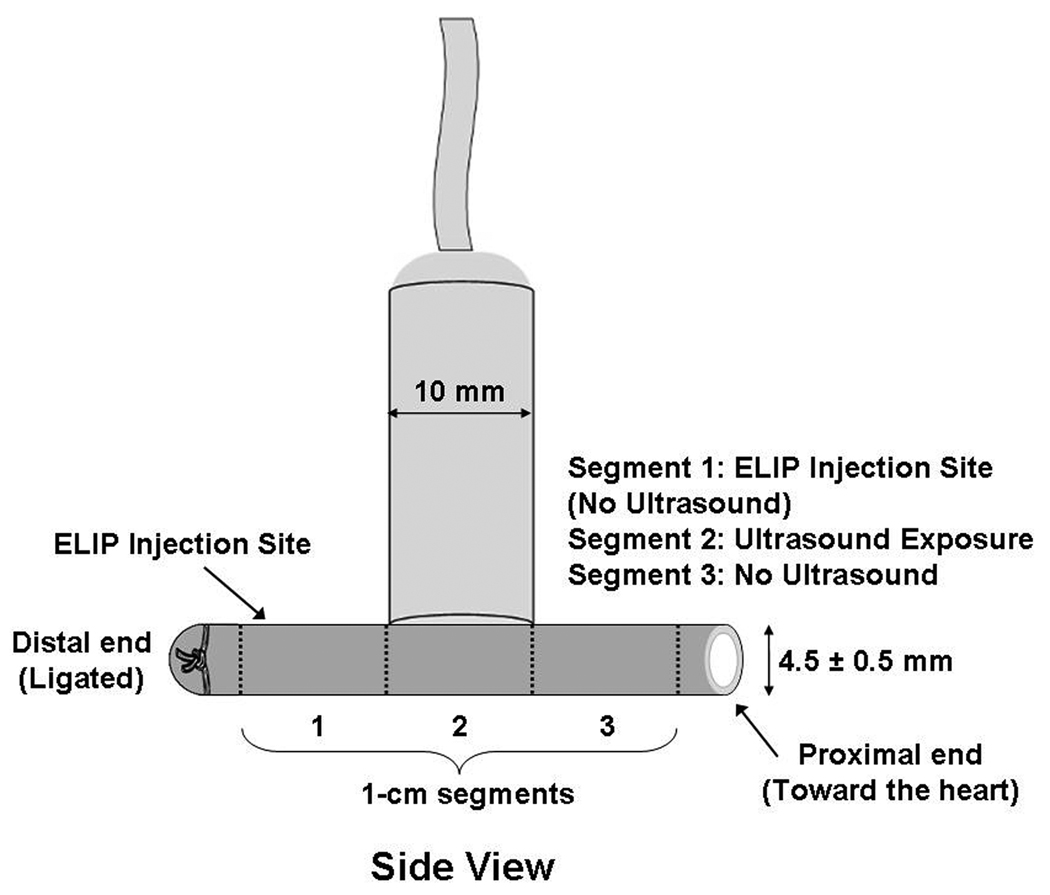
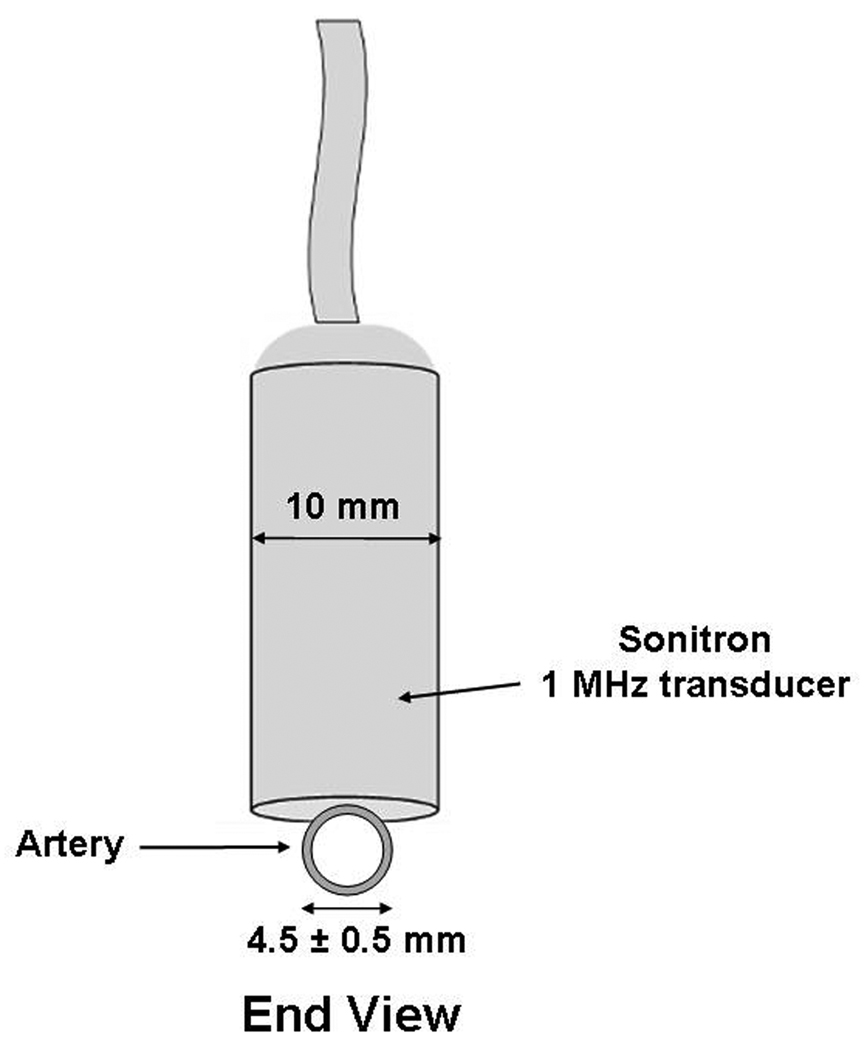
The portion of the arteries distal to the heart was ligated and calcein-loaded ELIP (5 mg lipid) were injected at this (distal) end, just proximal to the ligature (see Figure 1a and b). The right-sided arteries were exposed to ultrasound with the probe placed directly on the artery 1 cm away from the site of ELIP injection (towards the proximal end of the artery, directed towards the heart; Figure 1). The incision site was filled with warm saline as the coupling medium. Treatment included 2 minutes of 1 MHz ultrasound exposure at a peak-to-peak pressure amplitude of 0.23 ± 0.05 MPa. Intraoperative ultrasound was delivered in the surgically exposed saline-filled cavity surrounding the artery from the Sonitron 1-MHz transducer, placed directly next to the artery (Figure 1a and b). The left-sided arteries were also surgically exposed, calcein-loaded ELIP were injected, but no ultrasound was administered, such that these arteries served as controls. Five minutes after ultrasound exposure (or sham exposure on the left-sided arteries), the proximal end of each artery was occluded with a suture. Thirty minutes after ultrasound or sham exposure, the animals were sacrificed with saturated KCL (10–20 ml) administered intravenously and the arteries were harvested for histological analysis.
Figure 1.
Transducer position during intraoperative exposure of the arteries, including segmentation of the artery for analysis (1 cm segments). Segment 1 represents the area of ELIP injection (the arrow refers to the injection site); Segment 2 represents the portion where ultrasound treatment was delivered; and Segment 3 represents the portion of the artery which was untreated by ultrasound. (A) Side View; (B) End View.
Histological Studies
The ventral side of the arteries (the side exposed to ultrasound) were labeled with India ink prior to harvesting. The arteries were sectioned into 3 one-centimeter segments for analysis (Figure 1a): Segment 1 represents the most distal (i.e., distal to the heart) segment which included the site of injection, Segment 2 was the middle segment which was the site of ultrasound treatment, and Segment 3 was the proximal (i.e., most proximal to the heart) segment which was the area just adjacent to ultrasound exposure. Arteries were removed, immersed in Optimal Cutting Temperature (OCT) compound, and sectioned using a microtome-cryostat. Arteries were stored at −80 °C and imaged within 24 hours using a fluorescence microscope (Olympus BX61, Olympus Imaging America Inc., Center Valley, PA) at 40-x and 100-x magnification. Image data (for the whole image on the slide) were converted to gray scale, and analyzed using computer-assisted videodensitometry. All image processing and analyses were performed with Image-Pro Plus Software (v. 4.1, Media Cybernetics, Silver Spring, MD). Data are reported as mean gray scale values (MGSV).
Twenty randomly chosen sections per arterial segment were also analyzed for apoptosis using in situ labeling of deoxyribonucleic acid (DNA) 3’ ends (Gavrieli et al., 1992). Briefly, the enzyme terminal deoxynucleotidyl transferase (TdT), which catalyzes a template-independent addition of deoxyribonucleotide to the 3’-OH ends of DNA, was used to incorporate digoxigenin-conjugated 2’-deoxyuridine 5’-triphosphate (dUTP) to the ends of DNA fragments. The signal of TdT-mediated dUTP nick end labeling (TUNEL technique) was then detected by an anti-digoxigenin antibody conjugated with peroxidase, a reporter enzyme that catalytically generates a brown color product from the chromogenic substrate diaminobenzidine (TUNEL Labeling Kit, R&D Systems, Inc., Minneapolis, MN). TUNEL-positive sections consisted of apoptotic cells. TUNEL-negative sections represented viable tissue. Analysis was performed by a pathologist who was blinded to the treatment groups.
Statistics
The presence of calcein within the arterial wall layers was reported as mean ± standard deviation (n = 8 arterial pairs). A paired t-test was used to compare the presence of calcein in each vascular layer for those arteries exposed to ultrasound versus sham exposed arteries. Multiple groups were compared using the analysis of variance with pairwise multiple comparison performed using the Student-Newman-Keuls Method. A p-value of less than 0.05 was considered significant. All analyses were performed using SigmaStat software (Systat Software Inc., Chicago, IL).
RESULTS
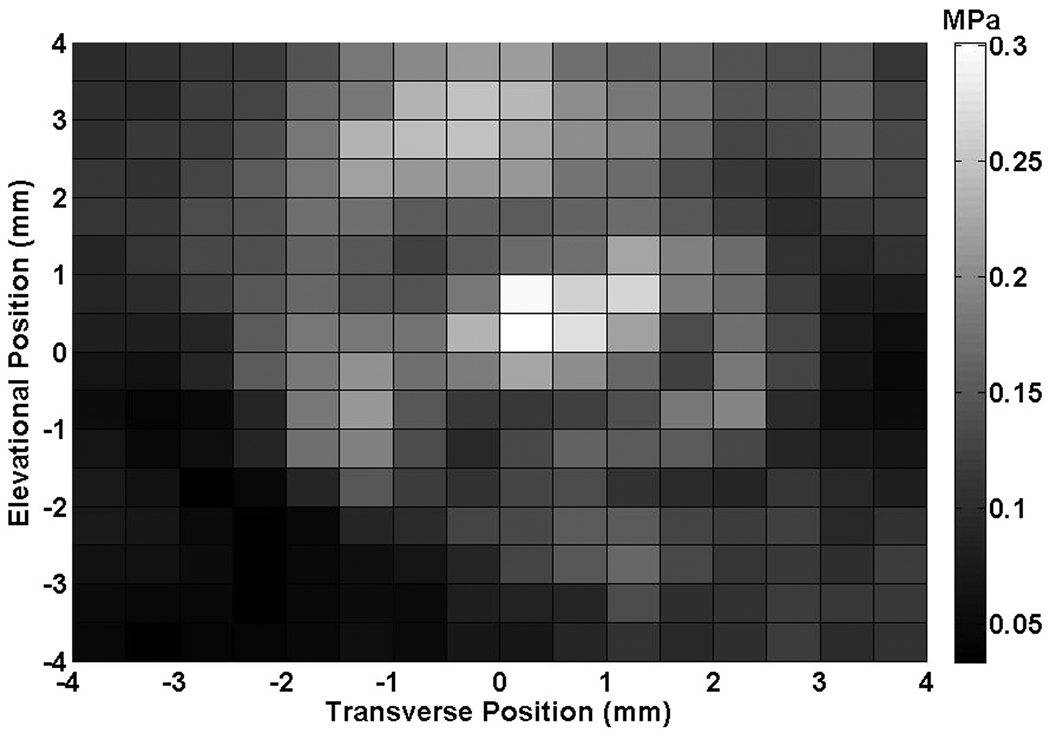
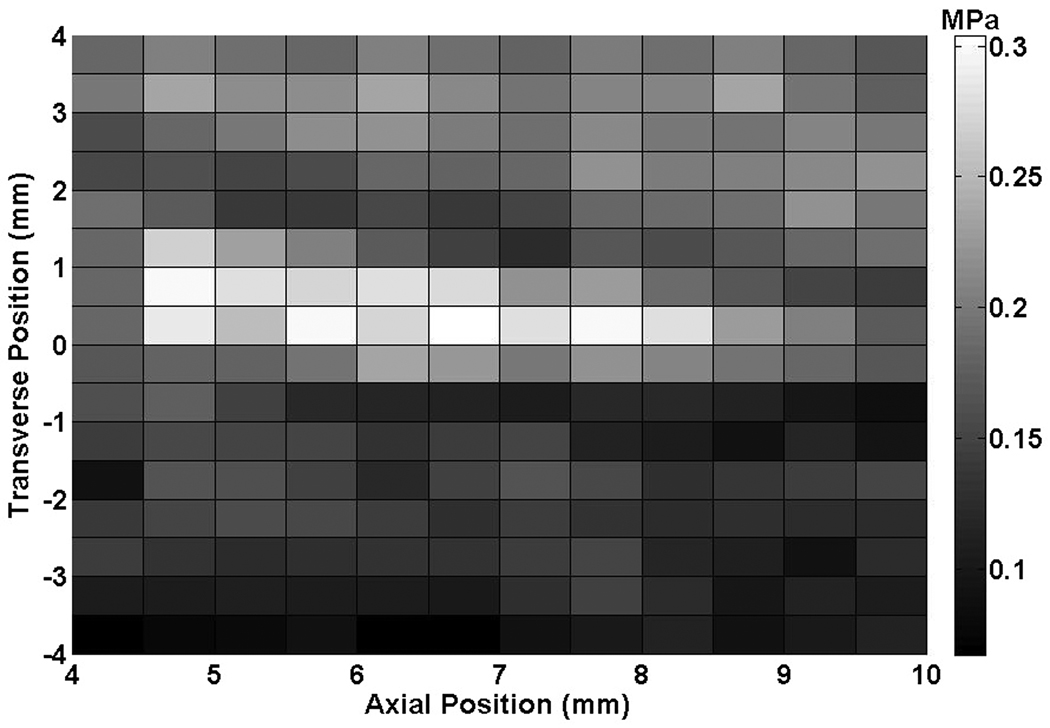
The axial and transverse beam profiles for the Sonitron 1000 are shown in Figure 2. The peak-to-peak acoustic pressure amplitude at a distance of 1 to 5 mm from the transducer was 0.23 ± 0.05 MPa.
Figure 2.
(A) Transverse beam profile produced by the Sonitron ultrasound transducer (1 MHz, 2.0 W/cm2, 100% duty cycle) at a distance of 5 mm from the transducer, (B) Axial beam profile.
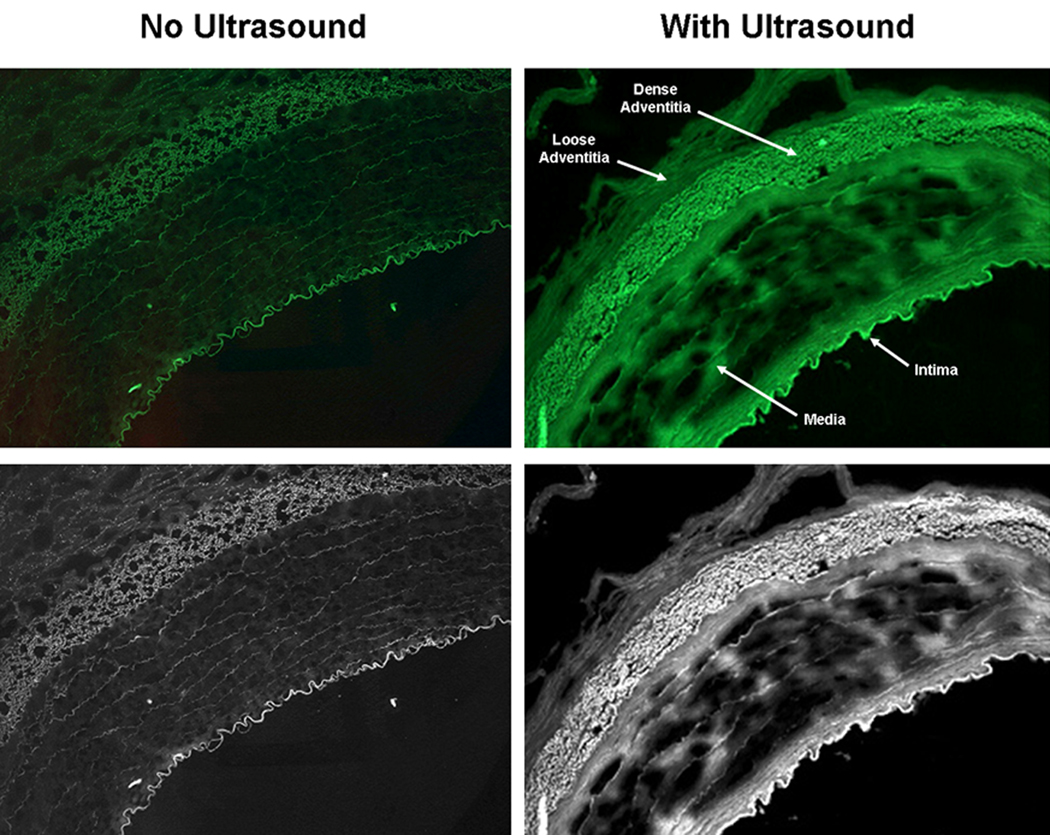
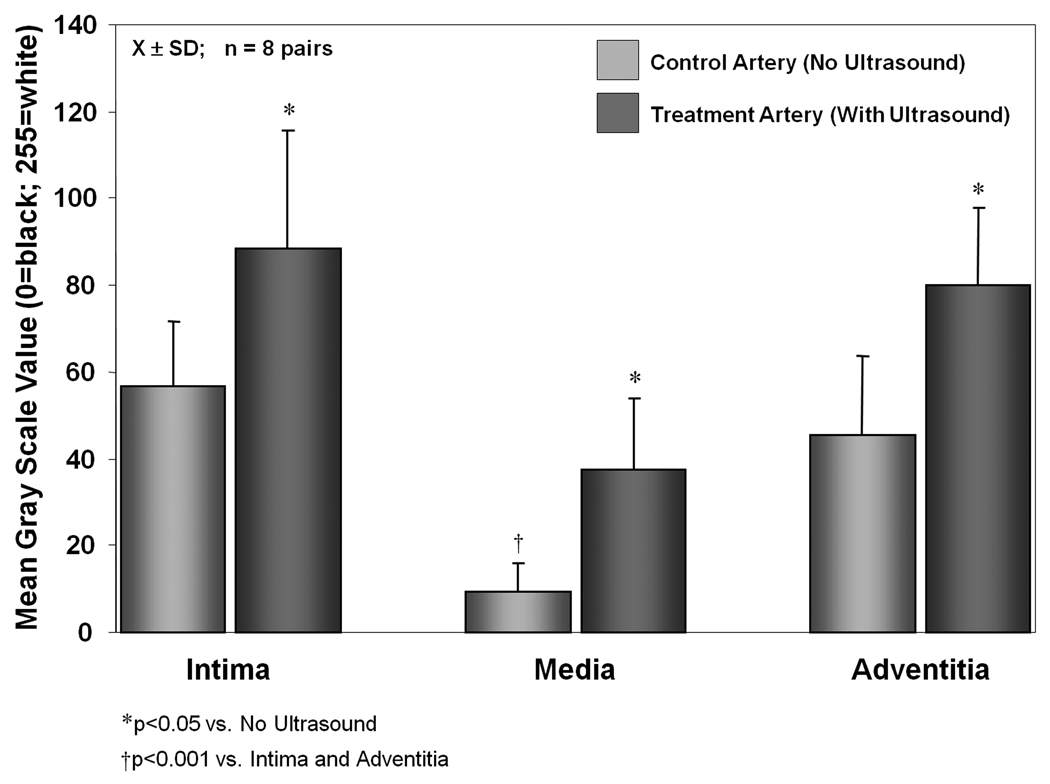
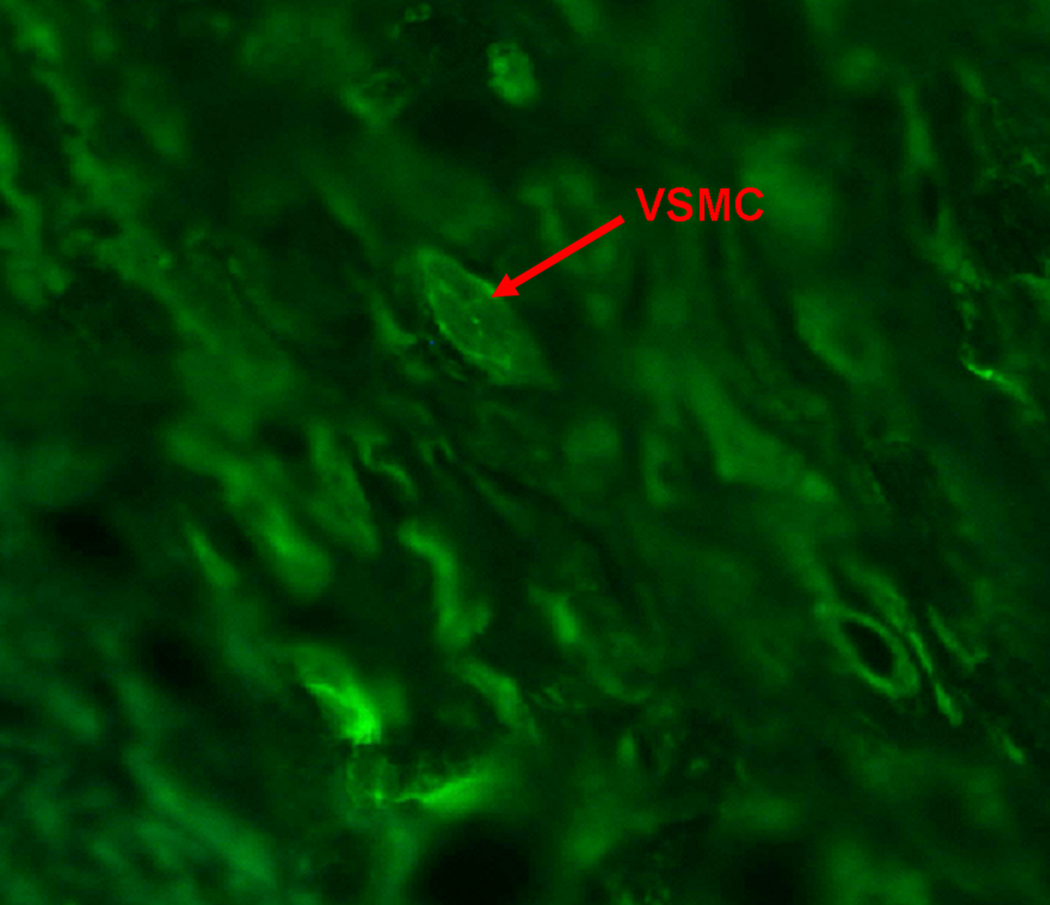
Figure 3 illustrates calcein delivery to the three layers of the arteries with and without ultrasound treatment. As demonstrated by other investigators, we similarly found very little calcein uptake in the arterial media (MGSV = 9.2 ± 6.7) without ultrasound treatment [Figure 4; p<0.001 compared to the intima (MGSV = 56.8 ± 14.9) and adventitia (MGSV = 45.3 ± 18.2)]. There was significant improvement in calcein uptake in all three arterial layers for those arteries exposed to 1-MHz ultrasound (p<0.05 compared to no ultrasound) (Figure 3 & Figure 4; Table 1). There was 56% improvement in calcein uptake in the intima (MGSV = 88.4 ± 27.3; p<0.05 vs. no ultrasound), and 76% improvement in calcein uptake in the adventitia (MGSV = 80.0 ± 17.9; p<0.05 vs. no ultrasound). Most marked was the increase in calcein uptake in the media (MGSV = 37.7 ± 16.3; p<0.001 vs. no ultrasound), which represents a 309% increase in calcein uptake. Despite this marked improvement however, calcein uptake was still significantly lower in the arterial media compared to the intima and adventitia (p<0.001 vs. intima and adventitia) despite ultrasound treatment, highlighting the difficulty for entry of even small molecules to this arterial layer. Figure 5 demonstrates calcein uptake in a vascular smooth muscle cell within the arterial media in a right axillary artery treated with ultrasound and our anti-SMC-actin antibody-conjugated calcein-loaded ELIP.
Figure 3.
Images of arteries treated with and without ultrasound under fluorescence microscopy and after conversion to gray scale images.
Figure 4.
Calcein delivery to the three arterial layers in the treatment artery (ultrasound treatment) and the control artery (no ultrasound treatment) [X±SD; n=8 arterial pairs].
Table 1.
Mean gray scale values (MGSV) calculated from the fluorescent images of the three arterial layers in the treatment artery (ultrasound treatment) and the control artery (no ultrasound treatment) [X±SD; n=8 arterial pairs]. Absolute change in MGSV calculated as MGSV with ultrasound exposure minus MGSV without ultrasound exposure. Percent change in MGSV calculated as absolute change in MGSV divided by MGSV without ultrasound (baseline).
| Mean Gray Scale Values (MGSV) |
No Ultrasound | With Ultrasound |
Absolute Change in MGSV |
% Change in MGSV |
|---|---|---|---|---|
| Intima | 56.8 ± 14.9 | 88.4 ± 27.3* | 31.7 | 56 |
| Media | 9.2 ± 6.7† | 37.7 ± 16.3*† | 28.5 | 309 |
| Adventitia | 45.3 ± 18.2 | 80.0 ± 17.9* | 34.8 | 76 |
p<0.05 vs. No Ultrasound
p<0.001 vs. Intima and Adventitia
Figure 5.
High power image of arterial media treated with ultrasound demonstrating uptake of calcein by vascular smooth muscle cells.
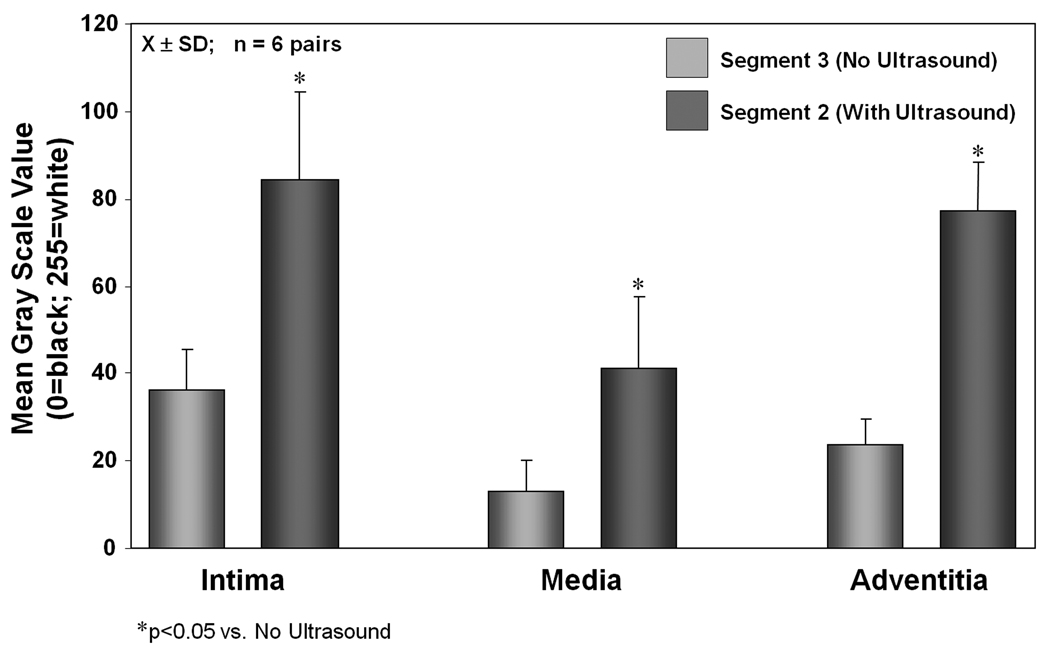
Figure 6 illustrates calcein delivery to different segments of the arterial wall (within the same artery). Note that one segment was exposed to 1-MHz ultrasound (Segment 2) and the other segment (Segment 3) was outside the 3-dB beamwidth of the transducer (Figure 1). For two of the eight arterial pairs, Segment 3 could not be analyzed related to technical problems with tissue storage, resulting in n=6 arterial pairs for this analysis. Similar to the control artery, there is negligible delivery to the media in the arterial segment (Segment 3) which was not exposed to ultrasound (MGSV = 12.8 ± 7.2; p<0.05 vs. intima and adventitia). There was a significant increase in calcein uptake in all three layers in the arterial segment exposed to 1-MHz ultrasound compared to the adjacent segment which was not exposed to ultrasound (Figure 5; Table 2).
Figure 6.
Calcein delivery to the three arterial layers in the treatment artery. Segment 2 represents the discrete area of ultrasound treatment while Segment 3 represents the area adjacent to this (within the same artery) which was untreated [X±SD; n=6 arterial pairs].
Table 2.
Mean gray scale values calculated from the fluorescent images of the three arterial layers in the treatment artery. Segment 2 represents the discrete area of ultrasound treatment while Segment 3 represents the area adjacent to this (within the same artery) which was untreated [X±SD; n=6 arterial pairs].
| Mean Gray Scale Values (MGSV) | Segment 3 No Ultrasound |
Segment 2 With Ultrasound |
|---|---|---|
| Intima | 36.2 ± 9.2 | 88.4 ± 27.3* |
| Media | 12.8 ± 7.2† | 37.7 ± 16.3*† |
| Adventitia | 23.6 ± 5.8 | 80.0 ± 17.9* |
p<0.05 vs. Segment 3 (No Ultrasound)
p<0.05 vs. Intima and Adventitia
No TUNEL-positive tissues were demonstrated in random representative sections (n=20 per segment) of the arteries evaluated, consistent with no apoptosis seen with ultrasound exposure. There was also no damage to the intimal layer at the region of ultrasound treatment.
DISCUSSION
This study demonstrates ultrasound-enhanced delivery of a small molecule, calcein, into all layers of the arterial wall using ELIP as the vector. There was increased uptake in the arterial media, a region notorious for poor drug and gene uptake. This enhanced drug uptake was seen even after only a brief, two-minute exposure to 1-MHz continuous wave ultrasound in the presence of calcein-loaded ELIP.
Ultrasound has been demonstrated to enhance the entry of small molecules into cells (sonoporation), an effect that has been exploited by many investigators for drug and gene delivery (Bao et al., 1997; Kim et al., 1996; Koch et al., 2000; Tachibana and Tachibana, 2001). The improvement in cell membrane permeabilization and hence, drug and gene uptake, is generally thought to be related to cavitation effects (Miller et al., 1996). As cavitation is nucleated by microbubbles, conceptually drug or gene vectors can be mixed with contrast agents, injected locally, and insonified selectively to effect directed drug or gene delivery (Greenleaf et al., 1998).
VSMCs are an important target for disease modulation in atherosclerosis. The majority of intimal SMCs involved in atherogenesis are thought to originate from the arterial media. However, the arterial media has proven to be difficult with regards to drug delivery or gene transduction in vivo, requiring high viral titers and access to the subendothelial layer by compromising endothelial integrity (Work et al., 2004). Even with disruption of the internal elastic lamina, the reported efficiency of transduction of the subendothelial layer only includes approximately one-third of the arterial media in vivo (Richter et al., 2000).
We have demonstrated effective drug and gene uptake to VSMCs in vitro using ELIP as a vector in combination with exposure to 1-MHz continuous wave ultrasound at a peak-to-peak amplitude of 0.26 MPa (Huang et al., 2002a; Huang et al., 2002b). In the present study, we hypothesized that this technique would similarly allow access to the arterial media with demonstration of drug uptake for eventual modulation of VSMC function. We demonstrated enhanced calcein uptake using targeted ELIP and ultrasound in all three layers of the artery, with a 300-fold improvement in calcein uptake within the arterial media (Table 1). This ultrasound-mediated effect appears to be amenable to selective site delivery as the segment adjacent to ultrasound treatment within the same artery did not show enhanced calcein uptake. We hypothesize that ultrasound exposure mediated a transient “loosening” of the gap junctions in the endothelial layer to promote passage of calcein-loaded ELIP into the vascular media. Alternatively, or perhaps in concert as well, the ELIP were delivered to the media via the adventitial vasa vasorum. It should be noted that there was also improvement in calcein uptake in the intimal and adventitial layers. Potentially, preventive and therapeutic strategies for treating atherosclerosis will also target the VSMC progenitor cells in all three arterial layers using this technique.
Ultrasound-enhanced membrane permeabilization is limited by the need to balance this desired bioffect with cellular viability. Cellular viability decreases with increasing acoustic pressure amplitude (Sundarum et al., 2003). Contrast microbubbles lower the pressure amplitude threshold for cavitation nucleation (Holland and Apfel, 1990). In this study, by limiting the ultrasound exposure duration to two minutes, the acoustic energy was also limited. With exposure to 1-MHz continuous wave ultrasound at a pressure amplitude of 0.23 ± 0.05 MPa and concomitant administration of ELIP, there was no detectable damage to the endothelial cell layer and no apoptosis seen in any of the ultrasound-treated arteries.
A limitation of this study is the methodology used to deliver ultrasound in the animal model. We applied ultrasound therapy directly on the external surface of the artery, a protocol with limited applicability for clinical use. Transcutaneous ultrasound delivery would be desirable as a treatment strategy in humans. For coronary arteries, we envision delivering the ultrasound treatment intravascularly during percutaneous angioplasty and stent placement. Although this study demonstrates markedly enhanced delivery to the arterial media with the combination of ELIP and ultrasound exposure, calcein uptake in the media was still less than the uptake seen in the intima and adventitia (Table 1), highlighting the difficulty of therapeutic delivery to this region of the arterial wall. This study was not performed to optimize drug delivery to the media alone, but aimed to provide proof-of-concept that delivery to the arterial media can be enhanced using ELIP and 1-MHz continuous wave ultrasound.
CONCLUSIONS
In summary, we have demonstrated enhanced delivery to all arterial wall layers including the arterial media using ultrasound and SMC actin-targeted ELIP. The combined effect of 1-MHz ultrasound exposure and ELIP as a contrast agent as well as a vehicle for drug delivery has the potential for specific therapy directed at VSMC function. Over the next decade, the role of ultrasound is likely to extend beyond the diagnostic realm to that of therapeutics. Ultrasound-mediated therapeutic delivery using targeted ELIP may have potential for modulation of VSMC function and the treatment of atherosclerosis.
ACKNOWLEDGMENTS
We would also like to acknowledge the invaluable help of Bonnie Kane and Janet Martinez for the performance of the animal experiments.
This work was supported in part by the National Institutes of Health RO1 HL-059586 and HL-074002.
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Alkan-Onyuksel H, Murer SE, Lanza GM, Vonesh MJ, Klegerman ME, McPherson DD. Development of inherently echogenic liposomes as an ultrasonic contrast agent. J Pharm Sci. 1996;85:486–490. doi: 10.1021/js950407f. [DOI] [PubMed] [Google Scholar]
- Bao S, Thrall BD, Miller DL. Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound Med Biol. 1997;23:953–959. doi: 10.1016/s0301-5629(97)00025-2. [DOI] [PubMed] [Google Scholar]
- Campbell GR, Campbell JH, Maderson JA, Horrigan S, Rennick RE. Arterial smooth muscle: a multifunctional mesenchymal cell. Arch Pathol Lab Med. 1988;112(10):977–986. [PubMed] [Google Scholar]
- Coussios CC, Holland CK, Jakubowska L, Huang SL, MacDonald RC, Nagaraj A, McPherson DD. In vitro characterization of liposomes and Optison by acoustic scattering at 3.5 MHz. Ultrasound Med Biol. 2004;30:181–190. doi: 10.1016/j.ultrasmedbio.2003.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos SM, Alkan-Onyuksel H, Kane BJ, Ramani K, Nagaraj A, Greene R, Klegerman M, McPherson DD. In-vivo targeting of acoustically reflective liposomes for intravascular ultrasonic enhancement. J Am Coll Cardiol. 1999;33:867–875. doi: 10.1016/s0735-1097(98)00607-x. [DOI] [PubMed] [Google Scholar]
- Demos SM, Onyuksel H, Gilbert J, Roth SI, Kane B, Jungblut P, Pinto JV, McPherson DD, Klegerman ME. In vitro targeting of antibody-conjugated echogenic liposomes for site-specific ultrasonic image enhancement. J Pharm Sci. 1997;86:167–171. doi: 10.1021/js9603515. [DOI] [PubMed] [Google Scholar]
- Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8:1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng YJ, Libby P. Progression of atheroma: A struggle between death and procreation. Arterioscler Thromb Vasc Biol. 2002;22:1370–1380. doi: 10.1161/01.atv.0000031341.84618.a4. [DOI] [PubMed] [Google Scholar]
- Greenleaf WJ, Bolander ME, Sarkar G, Goldring MB, Greenleaf JF. Artificial cavitation nuclei significantly enhance acoustically induced cell transfection. Ultrasound Med Biol. 1998;24:587–595. doi: 10.1016/s0301-5629(98)00003-9. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Huang SL, Warnick D, Stein A, Rabbat M, Madhav T, Kane B, Nagaraj A, Klegerman M, MacDonald R, McPherson D. Left ventricular thrombus enhancement following intravenous injection of echogenic immunoliposomes: studies in a new experimental model. Circulation. 2002;105:2772–2778. doi: 10.1161/01.cir.0000017500.61563.80. [DOI] [PubMed] [Google Scholar]
- Hao H, Gabbiani G, Bochaton-Piallat ML. Arterial smooth muscle cell heterogeneity: Implications for atherosclerosis and restenosis development. Arterioscler Thromb Vasc Biol. 2003;23:1510–1520. doi: 10.1161/01.ATV.0000090130.85752.ED. [DOI] [PubMed] [Google Scholar]
- Hedin U, Roy J, Tran PK. Control of smooth muscle cell proliferation in vascular disease. Curr Opin Lipidol. 2004;15(5):559–565. doi: 10.1097/00041433-200410000-00010. [DOI] [PubMed] [Google Scholar]
- Hillebrands JL, Rozing J. Chronic transplant dysfunction and transplant arteriosclerosis: new insights into underlying mechanisms. (A contemporary review on the pathology of transplant arteriosclerosis.) Expert Rev Mol Med. 2003;2003:1–23. doi: 10.1017/S146239940300557X. [DOI] [PubMed] [Google Scholar]
- Holland CK, Apfel RE. Thresholds for transient cavitation produced by pulsed ultrasound in a controlled nuclei environment. J Acoust Soc Am. 1990;88:2059–2069. doi: 10.1121/1.400102. [DOI] [PubMed] [Google Scholar]
- Horlitz M, Sigwart U, Niebauer J. Fighting restenosis after coronary angioplasty: contemporary and future treatment options. Int J Cardiol. 2002;83:199–205. doi: 10.1016/s0167-5273(02)00033-5. [DOI] [PubMed] [Google Scholar]
- Huang SL, Hamilton AJ, Nagaraj A, Tiukinhoy SD, Klegerman ME, McPherson DD, Macdonald RC. Improving ultrasound reflectivity and stability of echogenic liposomal dispersions for use as targeted ultrasound contrast agents. J Pharm Sci. 2001;90(12):1917–1926. doi: 10.1002/jps.1142. [DOI] [PubMed] [Google Scholar]
- Huang SL, MacDonald RC. Acoustically-active liposomes for drug encapsulation and ultrasound-triggered release. Biochim Biophy Acta. 2004;1665:134–141. doi: 10.1016/j.bbamem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Huang SL, McPherson DD, MacDonald EC. A method to co-encapsulate gas and drugs in liposomes for ultrasound-controlled drug delivery. Ultrasound Med Biol. 2008;34(8):1272–1280. doi: 10.1016/j.ultrasmedbio.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SL, McPherson DD, MacDonald RC. Ultrasound in conjunction with an ultrasonic-reflective transfection agent enhances gene delivery to cells. Am Coll Cardiol. 2002a;39:228A. (abstr) [Google Scholar]
- Huang S, Tiukinhoy S, McPherson D, MacDonald R. Combined use of ultrasound and acoustic cationic liposomes results in improved gene delivery into smooth muscle cells. Molecular Ther. 2002b;5:S9. (abstr) [Google Scholar]
- Kim HJ, Greenleaf JF, Kinnick RR, Bronk JT, Bolander ME. Ultrasound-mediated transfection of mammalian cells. Hum Gene Ther. 1996;7:1339–1346. doi: 10.1089/hum.1996.7.11-1339. [DOI] [PubMed] [Google Scholar]
- Koch S, Phol P, Cobet U, Rainov NG. Ultrasound enhancement of liposome-mediated cell transfection is caused by cavitation effects. Ultrasound Med Biol. 2000;26:897–903. doi: 10.1016/s0301-5629(00)00200-3. [DOI] [PubMed] [Google Scholar]
- Kopechek JA, Abruzzo TM, Wang B, Chrzanowski SM, Smith DA, Kee PH, Huang S, Collier JH, McPherson DD, Holland CK. Ultrasound-mediated release of hydrophilic and lipophilic agents from echogenic liposomes. J Ultrasound Med. 2008;27:1597–1606. doi: 10.7863/jum.2008.27.11.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza GM, Alkan-Onyuksel H, Klegerman ME, Vonesh MJ, McPherson DD. Patent # 5,612,057. University of Illinois and Northwestern University; Acoustically Reflective Liposomes and Methods to Make and Use the Same. Issued 3/18/97.
- Lemarchand P, Jones M, Yamada I, Crystal RG. In vivo gene transfer and expression in normal uninjured blood vessels using replication-deficient recombinant adenovirus vectors. Circ Res. 1993;72:1132–1138. doi: 10.1161/01.res.72.5.1132. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Ikeda U, Ogasawara Y, Urabe M, Takizawa T, Saito T, Colosi P, Kurtzman G, Shimada K, Ozawa K. Gene transfer into vascular cells using adeno-associated virus (AAV) vectors. Cardiovas Res. 1997;35(3):514–521. doi: 10.1016/s0008-6363(97)00163-6. [DOI] [PubMed] [Google Scholar]
- Miller MW, Miller DL, Brayman AA. A review of in vitro bioeffects of inertial ultrasonic from a mechanistic perspective. Ultrasound Med Biol. 1996;22:1131–1154. doi: 10.1016/s0301-5629(96)00089-0. [DOI] [PubMed] [Google Scholar]
- Newby AC. An overview of the vascular response to injury: a tribute to the late Russell Ross. Toxicol Lett. 2000;112:519–529. doi: 10.1016/s0378-4274(99)00212-x. [DOI] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Porter TM, Smith DA, Holland CK. Acoustic techniques for assessing the Optison destruction threshold. J Ultrasound Med. 2006;25:1519–1529. doi: 10.7863/jum.2006.25.12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M, Iwata A, Nyhuis J, Nitta Y, Miller AD, Halbert CL, Allen MD. Adeno-associated virus vector transduction of vascular smooth muscle cells in vivo. Physiol Genomics. 2000;2:117–127. doi: 10.1152/physiolgenomics.2000.2.3.117. [DOI] [PubMed] [Google Scholar]
- Rome JJ, Shayani V, Flugelman MY, Newman KD, Farb A, Virmani R, Dichek DA. Anatomic barriers influence the distribution of in vivo gene transfer into the arterial wall. Modeling with microscopic tracer particles and verification with a recombinant adenoviral vector. Arterioscler Thromb. 1994;14:148–161. doi: 10.1161/01.atv.14.1.148. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: an Update. N Engl J Med. 1986;314:488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Sarjeant JM, Rabinovich M. Understanding and treating vein graft atherosclerosis. Cardiovasc Pathol. 2002;11:263–271. doi: 10.1016/s1054-8807(02)00125-4. [DOI] [PubMed] [Google Scholar]
- Schwartz SM, Murry CE. Proliferation and the monoclonal origin of atherosclerotic lesions. Annu Rev Med. 1998;49:437–460. doi: 10.1146/annurev.med.49.1.437. [DOI] [PubMed] [Google Scholar]
- Sundarum J, Mellein BR, Mitragotri S. An experimental and theoretical analysis of ultrasound-induced permeabilization of cell membranes. Biophys J. 2003;84:3087–3101. doi: 10.1016/S0006-3495(03)70034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana K, Tachibana S. The use of ultrasound for drug delivery. Echocardiography. 2001;18:323–328. doi: 10.1046/j.1540-8175.2001.00323.x. [DOI] [PubMed] [Google Scholar]
- Tiukinhoy-Laing SD, Huang S, Klegerman M, Holland CK, McPherson DD. Ultrasound-facilitated thrombolysis using tissue-plasminogen activator-loaded echogenic liposomes. Thromb Res. 2007;119:777–784. doi: 10.1016/j.thromres.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiukinhoy S, Khan A, Huang S, Klegerman M, MacDonald R, McPherson D. Novel Echogenic Drug-Immunoliposomes for Drug Delivery. Investig Radiol. 2004;39:104–110. doi: 10.1097/01.rli.0000111207.92580.44. [DOI] [PubMed] [Google Scholar]
- Tiukinhoy S, Mahowald M, Shively V, Nagaraj A, Kane B, Klegerman M, MacDonald R, McPherson D, Matsumura J. Development of echogenic, plasmid-incorporated,tissue-targeted cationic liposomes that can be used for directed gene delivery. Investig Radiol. 2000;12:732–738. doi: 10.1097/00004424-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Virmani R, Farb A. Pathology of in-stent restenosis. Curr Opin Lipidol. 1999;10:499–506. doi: 10.1097/00041433-199912000-00004. [DOI] [PubMed] [Google Scholar]
- Work LM, Nicklin SA, Brain NJ, Dishart KL, Von Seggern DJ, Hallek M, Buning H, Baker AH. Development of efficient viral vectors selective for vascular smooth muscle cells. Molecular Ther. 2004;9(2):198–208. doi: 10.1016/j.ymthe.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Yao A, Wang DH. Heterogeneity of adenovirus mediated gene transfer in cultured thoracic aorta and renal artery of rats. Hypertension. 1995;26(2):1046–1050. doi: 10.1161/01.hyp.26.6.1046. [DOI] [PubMed] [Google Scholar]