Abstract
Background
Detectable HIV-1 RNA at delivery is the strongest predictor of mother-to-child transmission. The risk factors for detectable HIV, including type of regimen, are unknown. We evaluated factors, including highly active antiretroviral (HAART) regimen, associated with detectable HIV-1 RNA at delivery in the Women and Infants Transmission Study.
Methods
Data from 630 HIV-1 infected women who enrolled from 1998–2005 and received HAART during pregnancy were analyzed. Multivariable analyses examined associations between regimens, demographic factors, and detectable HIV-1 RNA (>400 cp/ml) at delivery.
Results
Overall, 32% of the women in the cohort had detectable HIV-1 RNA at delivery. Among the subset of 364 HAART-experienced women, a lower CD4+ cell count at enrollment (adjusted odds ratio [AOR]=1.20 per 100 cells/μl, CI 1.04–1.37) and higher HIV-1 RNA at enrollment (AOR=1.52 per log10 cp/ml, CI 1.32–1.75) were significantly associated with detectable HIV-1 RNA levels at delivery. For the 266 HAART-naïve women, both lower CD4+ cell count at enrollment (AOR=1.24 per 100 cells/μl, CI 1.05–1.48) and higher HIV-1 RNA at enrollment (AOR=1.35 per log10 cp/ml, CI 1.12–1.63) were associated with detectable HIV-1 RNA at delivery. In addition, age at delivery (AOR=0.92 per 10y older, CI 0.86–0.99), and maternal illicit drug use (AOR=3.15, CI 1.34–7.41) were significantly associated with detectable HIV-1 RNA at delivery among HAART-naïve women. Type of HAART regimen was not significant in either group.
Conclusions
Lack of viral suppression at delivery was common in the WITS cohort, but differences by antiretroviral regimen were not identified. Despite a transmission rate below 1% in the last 5 years of the WITS cohort, improved measures to maximize HIV-1 RNA suppression at term among high-risk women are warranted.
Keywords: antiretrovirals, HIV, pregnancy, MTCT, HAART
INTRODUCTION
More than 6000 women in the United States living with human immunodeficiency virus (HIV) become pregnant each year.1 Prior to 1994, approximately 25% of their infants ultimately became infected with HIV. Results from the AIDS Clinical Trials Group study 076 (ACTG 076) established that administration of zidovudine (ZDV) to the mother during pregnancy and delivery, as well as to the infant post-partum reduced the rate of HIV transmission from mother to child (MTCT) by two-thirds.2 Highly active antiretroviral treatment (HAART), elective caesarian sections for women with HIV-1 RNA >1,000 cp/ml, and avoidance of breastfeeding have subsequently been shown to reduce MTCT rates to < 1–2% for women identified early in pregnancy.3 Unfortunately, the most recent surveillance data from the Centers for Disease Control (CDC) demonstrate up to 4% transmission at sentinel sites. 4 While these data may not be indicative of current trends, they suggest that we are not achieving optimal coverage of HIV-infected pregnant women. Despite the great success in the prevention of mother-to-child transmission (PMTCT) in the United States, more aggressive screening and implementation of effective interventions may be required.
The Women and Infants Transmission Study (WITS) cohort previously found that HIV-1 RNA at delivery is associated with risk for MTCT. 3 A prospective analysis from January 1990 to June 2000 showed the odds of transmission increased 2.4-fold (95% CI, 1.7–3.5) for every one log10 cp/ml increase in delivery viral load. Other studies support these findings, however, research to date has not correlated the use of HAART with the outcome of viral suppression.5–9 While data support the use of HAART for PMTCT,3, 10, 11 prior studies have not fully elucidated the impact of ritonavir boosting on maternal HIV-1 RNA at term.12,13 In addition, few studies have examined the association between specific HAART regimens and HIV-1 RNA during pregnancy while controlling for socio-demographic, racial and behavioral factors. Instead, most prior research has focused on the impact of these factors on HIV infected women outside of the context of pregnancy.14
Using data from one of the largest and well-characterized prospective cohort studies of infected pregnant women in the United States, we conducted an analysis to determine the clinical, socio-demographic, and biological risk factors associated with lack of maternal virologic suppression at term in women who were either “HAART-experienced” or “HAART-naïve” prior to pregnancy. We chose to subdivide our sample into these two categories to provide proxy measures for “duration of treatment” and because the approaches for managing determinants of lack of suppression might differ between these two groups. Our primary aim was to determine the relationship between HIV-1 RNA levels at delivery and HAART regimen in these two groups. Our a priori hypothesis was that more potent regimens containing ritonavir would be associated with improved HIV-1 RNA suppression at delivery. Our secondary aim was to examine the impact of known sociodemographic risk factors on HIV-1 RNA while controlling for HAART regimen. We hypothesized that sociodemographic factors that have previously been associated with lower adherence would increase the risk of having a detectable HIV-1 RNA level at delivery, independent of HAART regimen.15,16
METHODS
The Women and Infants Transmission Study (WITS) is a multicenter, prospective, natural history cohort study of the perinatal transmission of HIV-1 and the natural history of HIV-1 infection in pregnant women and their infants.17 Starting in December, 1989, HIV infected pregnant women and their infants were enrolled at centers in New York City, Boston, Worcester, San Juan, and Chicago. Additional sites were added in 1991 in Brooklyn and in 1993 in Houston. The study was approved by each site’s institutional review board, and all women provided informed consent for enrollment of themselves and their newborns. The women were enrolled at any time during pregnancy or up to seven days postpartum.
HIV infected women were assessed, based upon their time of enrollment, at or before 20 weeks of gestation, at 25 +/− 2 weeks, at 32 +/− 2 weeks, at delivery, and at two and six month post-partum visits. At each visit, women completed detailed medical and behavioral questionnaires, underwent physical examination, and gave a sample of blood. CD4+ cell counts were determined in fresh blood by flow cytometry, in accordance with protocols developed by the Division of AIDS of the National Institute of Allergy and Infectious Diseases. Plasma HIV-1 RNA was measured in five laboratories from repository samples (stored at −70°C). Obstetric data were obtained from abstraction of the medical record. Antiretroviral therapy was not prescribed as part of the study. Start and stop dates for medications were recorded as the month and year of the first or last use. Only one regimen was categorized per pregnancy, based upon a woman’s earliest regimen initiated.
The nested cohort used for this analysis consisted of the subset of women who received HAART during pregnancy and gave birth to infants between June 1998 and December 2005. We chose June 1998 to December 2005 because that corresponded to the funding periods for WITS III and IV, when there were larger numbers of women on 3 drug regimens for PMTCT. We restricted our primary analysis to women who had an HIV-1 RNA level available from delivery. We excluded all women whose “delivery HIV-1 RNA” was recorded as their “enrollment HIV-1 RNA,” in order to eliminate any women who enrolled during or immediately prior to delivery. HAART was defined as a regimen of 3 or more antiretroviral drugs. In all cases, women received at least two nucleoside reverse-transcriptase inhibitors (NRTIs) combined with either: 1) a non-nucleoside reverse-transcriptase inhibitor (NNRTI); 2) an unboosted (without ritonavir) protease inhibitor (PI); 3) a boosted (with ritonavir) PI; or 4) a third NRTI. Two subpopulations of women were analyzed: those who were HAART-experienced prior to pregnancy and those who were HAART-naïve prior to pregnancy. This information was determined based upon self-report from patients. Women classified as HAART-naïve prior to pregnancy could have started on treatment prior to study enrollment (given they were allowed to enroll at any point during their pregnancy). Given this, a minority of the HAART-naïve subset (11%) had undetectable levels of HIV-1 RNA upon study enrollment.
Univariate analyses of associations were performed using the chi squared test, Fisher’s exact test, Wilcoxon rank sum test, or F-test from analysis of variance, as appropriate. Covariates considered as potential risk factors for detectable HIV-1 RNA at delivery included sociodemographic characteristics (maternal age at delivery, race, insurance status), factors associated with birth (parity, number of live births), factors associated with disease acuity (CDC clinical classification18 enrollment CD4+ count, and enrollment HIV-1 RNA level), maternal illicit drug use (defined as use of heroine/opiates, methadone, cocaine, or any injection drug use during the current pregnancy), timing of HIV diagnosis and of HAART initiation, and HAART regimen. Logistic regression models estimated the odds of having a detectable HIV-1 RNA level at delivery (>400 cp/ml) as a function of these characteristics. We chose an HIV-1 RNA cutoff of >400 cp/ml because it was the standard assay limit of detection used during the WITS study.
We generated 2 separate multivariable models that examined women who were either HAART-experienced or HAART-naïve prior to pregnancy. For each multivariable model, we first used stepwise logistic regression with p<0.05 as the entry and stay criteria for variable selection. We then fit a model for each of the two populations which included all variables which were significant in one or both of the models identified using stepwise variable selection. Finally, as we were interested in evaluating potential differences between HAART regimens, we added this variable in the model. Statistical analyses were performed with SAS version 9.1 (SAS Institute Inc, Cary, North Carolina).
RESULTS
Of 935 HIV-1 infected women enrolled between June 1998 and December 2005, 630 (67%) met eligibility criteria for this analysis (Table 1). The majority of women in the analysis cohort were young (59% were < 30 years old), black (57%), on public insurance (80%), not illicit drug users (80%), and in either CDC class A or B (93%). Among all 630 women, 109 (17%) received a boosted PI-based regimen (64 women were on lopinavir/ritonavir and the remainder received either indinavir/ritonavir or saquinavir/ritonavir), 402 (65%) received an unboosted PI-based regimen (all containing nelfinavir), 87 (14%) received an NNRTI-based regimen (all containing nevirapine), and 25 (4%) received a triple NRTI-based regimen (the majority with abacavir). Most regimens contained zidovudine (ZDV) (89%) and lamivudine (95%) as part of the NRTI backbone.
TABLE 1.
Characteristics of HIV Positive Pregnant Women Meeting Inclusion Criteria by History of HAART ExposurePrior to Pregnancy
| Characteristic | All women (n=630) | HAART experienced (n=364) | HAART naïve (n=266) | p-valuea |
|---|---|---|---|---|
| Age at delivery | <0.001 | |||
| <30 years | 372 (59) | 187 (51) | 185 (70) | |
| ≥30 years | 258 (41) | 177 (49) | 81 (30) | |
| Race/Ethnicity | 0.109 | |||
| White | 47 (7) | 34 (9) | 13 (5) | |
| Black | 361 (57) | 205 (56) | 156 (59) | |
| Hispanic and Other | 222 (35) | 125 (35) | 97 (36) | |
| Insurance status | 0.020 | |||
| Public Insurance(Medicaid, etc.) | 507 (80) | 305 (84) | 202 (76) | |
| Private Insurance | 87 (14) | 45 (12) | 42 (16) | |
| Other/unknown | 36 (6) | 14 (4) | 22 (8) | |
| Parity | <0.001 | |||
| Nulliparous | 157 (25) | 63 (17) | 94 (36) | |
| Parous | 469 (74) | 299 (83) | 170 (64) | |
| Number of live births (n=606) | 0.424 | |||
| Singleton | 591 (98) | 337 (98) | 254 (97) | |
| Twins or + | 15 (2) | 7 (2) | 8(3) | |
| Maternal illicit drug use | 0.165 | |||
| No illicit drug use | 504 (80) | 284 (78) | 220 (83) | |
| Illicit drug use | 125 (20) | 79 (22) | 46 (17) | |
| Timing of HIV diagnosis (n=622) | <0.001 | |||
| Antenatal | 168 (27) | 4 (1) | 164 (62) | |
| Prepregnancy | 454 (73) | 354 (99) | 100 (38) | |
| Median duration of HIV diagnosis, Yrs | 2.92 | 5.04 | 0.59 | <0.001 |
| CDC Class | <0.001 | |||
| Class A | 390 (62) | 197 (54) | 193 (73) | |
| Class B | 193 (31) | 131 (36) | 62 (23) | |
| Class C | 47 (7) | 36 (10) | 11 (4) | |
| CD4+ cell count at enrollment (n=598) | <0.001 | |||
| <200 cells/μl | 71 (12) | 56 (16) | 15 (6) | |
| 200–399 cells/μl | 180 (30) | 97 (28) | 83 (33) | |
| ≥400 cells/μl | 347 (58) | 190 (55) | 157 (62) | |
| Median CD4+ cell count at enrollment, cells/μl | 435 | 433 | 447 | 0.525 |
| HIV-1 RNA at enrollment | 0.943 | |||
| Undetectable (<400cp/ml) | 295 (47) | 170 (47) | 125 (47) | |
| Detectable (≥400 cp/ml) | 335 (53) | 194 (53) | 141 (53) | |
| HAART regimen (n=623) | <0.001 | |||
| PI with ritonavir | 109 (17) | 81 (22) | 28 (11) | |
| PI without ritonavir | 402 (65) | 207 (57) | 195 (73) | |
| NNRTI | 87 (14) | 55 (15) | 32 (12) | |
| NRTI | 25 (4) | 18 (5) | 7 (3) |
Note: Data are no. (column%) of patients unless otherwise indicated.
Cumulative percentage may not exactly equal 100% based upon rounding to nearest decimal.
p-values were calculated with χ2 test (for categorical variables) or non-parametric statistic for median to compare distributions of baseline characteristics
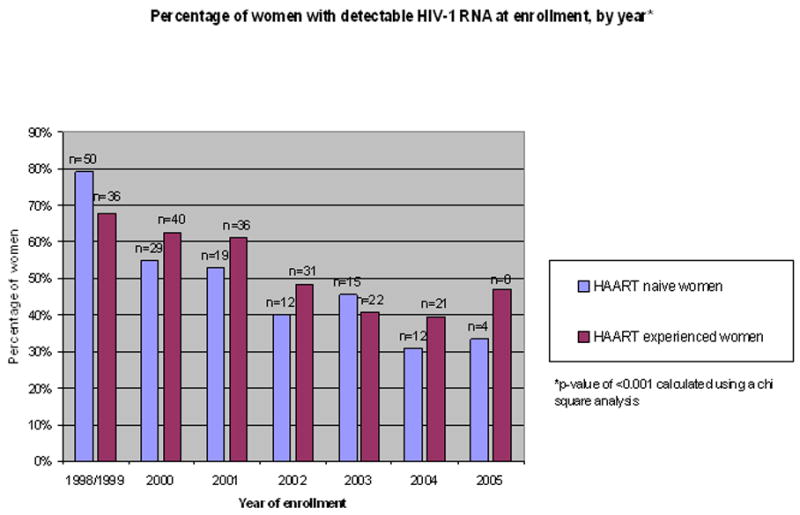
Of these, 364 (58%) were HAART-experienced prior to pregnancy and 266 (42%) were HAART-naïve prior to pregnancy. A comparison of enrollment characteristics by history of HAART exposure is shown in Table 1. There were significant differences between HAART-experienced and HAART-naïve women in age, insurance status, parity, and time since HIV diagnosis (median of 5 years in the HAART-experienced group versus 6 months in the HAART-naïve group). The median CD4+ cell count overall was 435 cells/μl (IQR 294, 640), however, women who were naïve to HAART prior to pregnancy were more likely to have a first CD4+ cell count measurement >200 cells/μl (95% vs. 83%, p<0.001) and lower CDC class (73% vs. 54% Class A, p<0.001). Among both HAART-experienced and HAART-naïve women, 53% had a detectable HIV-1 RNA at first study measurement in pregnancy. There was a significant difference between HAART-experienced and HAART-naïve women in the proportion of women using the different types of regimens (p<0.001, Table 1). This primarily reflected a higher proportion of HAART-experienced compared to HAART-naïve women who received a boosted PI-regimen. We also examined the distribution of women by year of study enrollment (Figure 1). The proportion of women with detectable HIV-1 RNA at enrollment varied significantly by calendar year of enrollment, reflecting declining trends over time, both among women who were HAART-experienced prior to pregnancy (p<0.001) and among women who were naïve prior to pregnancy (p<0.001).
Figure 1.
Among all 630 women, the overall rate of detectable HIV-1 RNA at delivery was 32%. In univariate analyses, significant predictors for detectable HIV-1 RNA at delivery included pre-pregnancy HAART exposure, younger age at delivery, black race, public insurance, maternal illicit drug use, HIV diagnosis prior to the current pregnancy, detectable HIV-1 RNA at enrollment, and earlier year of enrollment (Table 2). Parity was not significant and type of HAART regimen was of borderline significance (p=0.051). In separate analyses of HAART-experienced and HAART-naïve women, the directions of associations with these factors remained the same as in the overall analysis, but for HAART-experienced women, only age, race, HIV-1 RNA at enrollment and year of enrollment remained significant, while for HAART-naïve women, only maternal illicit drug use, timing of HIV diagnosis and HIV-1 RNA at enrollment were significant. Of 295 women who entered the study with an undetectable HIV-1 RNA, 39 (13%) ultimately had detectable levels of HIV-1 RNA at delivery; 15% among women in the HAART-experienced group, and 11% among women in the HAART-naïve group. Conversely, 162 (48%) of the 335 women who were detectable at enrollment remained detectable at delivery; 57% among women in the HAART-experienced group and 36% among women in the HAART-naïve group.
TABLE 2.
Univariate Analysis of Factors Associated With HIV-1 RNA >400 cp/ml at Delivery by History of HAART Exposure Prior to Pregnancy
| Characteristic | All women | p-value | HAART experienced | p-value | HAART naïve | p-value |
|---|---|---|---|---|---|---|
| N (%)detectable HIV RNA* | N (%)detectable HIV RNA* | N (%)detectable HIV RNA* | ||||
| Overall detection rate | 201/630 (32) | N/A | 136/364 (37) | N/A | 65/266 (24) | N/A |
| HAART prior to pregnancy | 0.001 | ------- | N/A | ------- | N/A | |
| Experienced | 136/364 (37) | |||||
| Naïve | 65/266 (24) | |||||
| Age at delivery** | 0.013 | 0.009 | 0.073 | |||
| <30 years | 133/372 (36) | 82/187 (44) | 51/185 (28) | |||
| ≥30 years | 68/258 (26) | 54/177 (31) | 14/81 (17) | |||
| Race/Ethnicity | <0.001 | 0.010 | 0.085 | |||
| White | 7/47 (15) | 6/34 (18) | 1/13 (8) | |||
| Black | 123/361 (34) | 80/205 (39) | 43/156 (28) | |||
| Hispanic | 59/199 (30) | 42/112 (38) | 17/87 (20) | |||
| Insurance status | 0.015 | 0.14 | 0.12 | |||
| Public | 173/507 (34) | 119/305 (39) | 54/202 (27) | |||
| Private/Other | 28/123 (23) | 17/59 (29) | 11/64 (17) | |||
| Parity | 0.13 | 0.51 | 0.59 | |||
| Nulliparous | 42/157 (27) | 21/63 (33) | 21/94 (22) | |||
| Parous | 156/469 (33) | 113/299 (38) | 43/170 (25) | |||
| Maternal illicit drug use | 0.016 | 0.22 | 0.030 | |||
| No illicit drug use | 149/504 (30) | 101/284 (36) | 48/220 (22) | |||
| Illicit drug use | 51/125 (41) | 34/79 (43) | 17/46 (37) | |||
| Timing of HIV diagnosis | 0.001 | N/A | 0.045 | |||
| Antenatal | 35/168 (21) | *** | 33/164 (20) | |||
| Prepregnancy | 161/454 (35) | ---- | 31/100 (31) | |||
| HIV-1 RNA at enrollment** | <0.001 | <0.001 | <0.001 | |||
| Undetectable (<400 cp/ml) | 39/295 (13) | 25/170 (15) | 14/125 (11) | |||
| Detectable (≥ 400 cp/ml) | 162/335 (48) | 111/194 (57) | 51/141 (36) | |||
| HAART regimen | 0.051 | 0.081 | 0.32 | |||
| PI with ritonavir | 37/109 (34) | 30/81 (37) | 7/28 (25) | |||
| PI without ritonavir | 137/402 (34) | 86/207 (42) | 51/195 (26) | |||
| NNRTI | 18/87 (21) | 13/55 (24) | 5/32 (16) | |||
| NRTI | 5/25 (20) | 5/18 (28) | 0/7 (0) | |||
| Year of Enrollment | <0.001 | <0.001 | 0.063 | |||
| 1998/1999 | 56/116 (48) | 32/53 (60) | 24/63 (38) | |||
| 2000 | 45/117 (38) | 32/64 (50) | 13/53 (25) | |||
| 2001 | 38/95 (40) | 29/59 (49) | 9/36 (25) | |||
| 2002 | 23/94 (24) | 20/64 (31) | 3/30 (10) | |||
| 2003 | 17/87 (20) | 10/54 (19) | 7/33 (21) | |||
| 2004 | 17/92 (18) | 9/53 (17) | 8/39 (21) | |||
| 2005 | 5/29 (17) | 4/17 (24) | 1/12 (8) |
Percentage reflects women with detectable HIV viral load within each category (e.g. black women with detectable viral load/all black women)
All numerical cutpoints (age, years of HIV diagnosis and enrollment) are derived from the median value
4 women were diagnosed during pregnancy, but were classified as HAART-experienced because they were already on medications prior to enrollment in the WITS.
Table 3 shows gestational age at delivery as well as number of women with detectable HIV-1 RNA at enrollment and delivery by treatment regimen for both HAART-experienced and HAART-naïve women. In both cohorts, women on PI-based therapies were more likely to have a detectable viral load both at enrollment and at delivery. In univariate analysis, there was no apparent advantage to boosting PI-based regimens with ritonavir.
TABLE 3.
Gestational age at delivery and number of women with detectable HIV-1 RNA by history of HAART Exposure Prior to Pregnancy*
| Characteristic | HAART experienced prior to pregnancy (n=361)* | HAART naïve prior to pregnancy (n=262)* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PI with ritonavir n=81 | PI no ritonavir n=207 | NNRTI n=55 | NRTI n=18 | p-value | PI with ritonavir n=28 | PI no ritonavir n=195 | NNRTI n=32 | NRTI n=7 | p-value | |
| Gestational age at delivery** | 38.0 (36.0,40.0) | 38.0 (36.0, 40.0) | 38.0 (35.0, 41.0) | 38.5 (35.5, 41.5) | 0.69 | 38.0 (36.0, 40.0) | 38.0 (36.0, 40.0) | 38.0 (35.0, 41.0) | 38.0 (35.0, 41.0) | 0.22 |
| HIV-1 RNA at enrollment | n= 191 for Experienced women | n= 138 for Naïve women | ||||||||
| Detectable*** | 46 (57) | 118 (57) | 22 (40) | 5 (28) | <0.001 | 13 (46) | 111 (57) | 12 (38) | 2 (29) | <0.001 |
| HIV-1 RNA at delivery | n=134 for Experienced women | n=63 for Naïve Women | ||||||||
| Detectable*** | 30 (37) | 86 (42) | 13 (24) | 5 (28) | <0.001 | 7 (25) | 51 (26) | 5 (16) | 0 (0) | <0.001 |
Note: Data are no. (column % where applicable) of patients unless otherwise indicated
p-value calculated using Chi Square or Fisher’s Exact Test, as relevant
Total n for this table is 623 due to incomplete data on regimens for 1% of cohort
Values are median wks (interquartile range)
Detectable is ≥400 cp/ml
Results from the multivariable analysis are displayed in Table 4. The variables that were selected for inclusion in the model (see Statistical Methods) are reported in the footnote in Table 4. Among HAART-experienced women, detectable levels of HIV-1 RNA levels at delivery were significantly associated with a higher enrollment HIV-1 RNA (adjusted odds ratio [AOR] 1.52 per log10 cp/ml, 95% confidence interval [CI] 1.32 to 1.75, p<0.001) and a lower enrollment CD4+ count (AOR 1.20 per 100 cells/μl, CI 1.04 to 1.37, p=0.009) at first study measurement. Among HAART-naïve women, detectable levels of HIV-1 RNA levels at delivery were also associated with a higher HIV-1 RNA at enrollment (AOR 1.35 per log10 cp/ml, CI 1.12 to 1.63, p=0.002) and a lower CD4+ count at enrollment (AOR 1.24 per 100 cells/μl, CI 1.05 to 1.48, p=0.014). In addition, maternal age (AOR 0.92 per 10y older, CI 0.86 to 0.99, p=0.022) and maternal illicit drug use (AOR 3.15, CI 1.34 to 7.41, p=0.008) were significantly associated with detectable HIV-1 RNA among HAART-naïve women. Type of regimen was not associated with detectable HIV-1 RNA at delivery for either HAART-experienced or HAART-naïve women. After adjustment for the other variables included in the model, there was a significant association among HAART-experienced women (p=0.001), but not among with HAART-naïve women (p=0.77), with calendar year of enrollment reflecting a decreasing trend over time in the odds of detectable HIV-1 RNA at delivery.
TABLE 4.
Multivariable Analysis of Factors Associated with HIV-1 RNA >400 cp/ml at Delivery by History of HAART Exposure Prior to Pregnancy*
| Characteristic | HAART experienced (n=364) | HAART naïve (n=266) | ||
|---|---|---|---|---|
| AOR** | 95% CI | AOR | 95% CI | |
| CD4+ cell count at enrollment, per 100 cells/μl lower | 1.20 | 1.04–1.37 | 1.24 | 1.05–1.48 |
| HIV-1 RNA load at enrollment, per 1 log10 copies/ml higher | 1.52 | 1.32–1.75 | 1.35 | 1.12–1.63 |
| HAART regimen (using PI-based without ritonavir as the reference group) | ||||
| PI-based with ritonavir | 1.21 | 0.46–3.19 | 0.90 | 0.18–4.50 |
| NNRTI-based | 2.23 | 0.48–10.38 | 1.13 | 0.35–3.62 |
| NRTI-based | 1.30 | 0.57–2.97 | --- | --- |
| Age at delivery per 10 years older | 0.98 | 0.93–1.03 | 0.92 | 0.86–0.99 |
| Maternal illicit drug use | 0.75 | 0.39–1.46 | 3.15 | 1.34–7.41 |
| Year of enrollment (as compared to 2004/2005) | ||||
| 1998/1999 | 5.56 | 2.01–15.42 | 0.90 | 0.29–2.80 |
| 2000 | 3.57 | 1.39–9.13 | 0.61 | 0.18–2.04 |
| 2001 | 2.94 | 1.14–7.55 | 1.03 | 0.31–3.46 |
| 2002 | 2.09 | 0.81–5.38 | 0.41 | 0.09–1.87 |
| 2003 | 0.79 | 0.28–2.29 | 0.91 | 0.24–3.50 |
The following variables were considered for inclusion in the logistic regression model, with selection using stepwise variable selection: race, SES, number of live births, gestational age, side effects, age at delivery, maternal illicit drug use, year of enrollment (as a categorical variable), enrollment CD4 count, enrollment HIV RNA, and ART regimen. A variable was retained in the model if significant at a level of p<0.05 in either the HAART-experienced population or the HAART-naïve population.
AOR is Adjusted Odds Ratio
NB: NRTI-based regimen was removed from the analysis for HAART-naïve women due to the small sample size (n=7)
DISCUSSION
The use of HAART for PMTCT is one of the most successful public health interventions of the HIV era in the United States.10 Despite rates of transmission remaining below 1% for the last 5 years of WITS,19 we found that 32% of the women who received HAART between 1998 and 2005 had a detectable HIV-1 RNA at delivery. This discrepancy may indicate successful peripartum prophylaxis efforts of mothers and infants, but it also highlights the fact that one of the cornerstones of PMTCT – maternal virologic suppression–was difficult to achieve in this cohort. MTCT continues to occur in the United States, and this transmission is at least in part due to factors associated with detectable maternal HIV-1 RNA at delivery. While rates of detectable viral load declined over time, a finding which is also consistent with CDC data,4 the high numbers of women on treatment with detectable HIV-1 RNA highlights the need for increased counseling of known HIV-infected women and aggressive treatment for the most vulnerable groups regardless of pregnancy status, both for PMTCT as well as overall maternal health.
In the current study, women who were most at risk were those who presented with a higher HIV-1 RNA and lower CD4+ count at enrollment. This was seen among women who were HAART–experienced and among women who were HAART-naïve prior to their pregnancy. For women who were HAART-naïve prior to pregnancy, this may have reflected more advanced disease and/or initiation of therapy later in pregnancy. For women who were HAART-experienced prior to pregnancy, this may have reflected either more advanced disease possibly refractory to antiretrovirals, or suboptimal use of antiretrovirals prior to pregnancy that was ultimately more resistant to subsequent regimens. Younger age and illicit drug use also significantly increased the odds of having detectable HIV-1 RNA at delivery in women who were HAART-naïve prior to pregnancy.
The type of HAART treatment did not significantly impact HIV-1 RNA levels at delivery, both before and after adjusting for enrollment factors, including HIV-1 RNA and CD4+ count. This finding was consistent for all women. The lack of superiority for boosted PI’s was a surprising finding in our evaluation of the impact of antiretroviral regimens on suppression of maternal HIV-1 RNA at term. While these findings are novel, it is important to interpret them with caution, given the relatively small numbers in each treatment group, and the observational design that may be prone to residual confounding. In addition, boosted PI’s may have been used more commonly in patients with more advanced disease, in whom antiretrovirals may have diminished effectiveness. We therefore believe that current treatment practices that recommend the use of ritonavir boosting when using PI’s in pregnancy should continue, pending further study of this question, ideally in randomized studies.
The loss of viral load suppression during pregnancy that occurred among 13% of women who initially had undetectable levels of HIV-1 RNA is of concern. It is unknown whether pregnancy itself increases the risk for loss of suppression. Factors specific to pregnancy may include increased medication intolerance (especially gastrointestinal), increased medication side effects (anemia’s or other), or altered pharmacokinetics. 20, 21, 22 Several studies have reported lower area under the plasma concentration time curve (AUC) in the third trimester with both lopinavir/ritonavir 23,24 and nelfinavir, 24 but the association between lower drug levels during pregnancy and virologic outcome at delivery has not been studied. Many experts, however, consider it to be reasonable to increase the dose of certain PI’s during the third trimester when viral load remains unsuppressed in the setting of good medication adherence. 25 Whether or not such dose increases might have reduced the loss of suppression in this cohort is unknown. We believe our findings warrant further study in trials where information on medication adherence, pharmacokinetics, and resistance can be obtained.
Women who were HAART-experienced prior to pregnancy differed from those who were HAART-naïve prior to pregnancy, both in enrollment characteristics as well as likelihood of virologic suppression at delivery. In general, women who were HAART-experienced were significantly more likely to be older, on public insurance, parous, have a more advanced CDC class, a longer duration of HIV diagnosis, and a lower CD4 count at enrollment. HAART-naïve women appeared to have generally less advanced disease at enrollment and were more likely to be virologically suppressed at term. This may in fact reflect disease stage, but could also reflect unmeasured sociodemographic factors, or lower rates of resistant virus among HAART-naïve women.
There were some significant trends within the cohort over time. There appeared to be fewer women who were HAART-naïve at enrollment in later years. This may reflect expanded screening for HIV and earlier treatment outside of pregnancy or more known HIV-infected women choosing to get pregnant. An important observation seen in our multivariable model was that the odds of having a detectable HIV-1 RNA at delivery declined with calendar year in the HAART-experienced group, even after adjustment for HIV-1 RNA and CD4+ cell count at enrollment. This may be reflective of increasing knowledge of the importance of viral load suppression at delivery and greater ability to achieve this in the HAART- experienced group as treatment options expanded, despite minimal changes over time with the HAART-naïve group. We did not find, however, that calendar year confounded associations with other factors included in the model.
This was an observational analysis, and prone to biases, including potential selection bias, and unmeasured confounding. We had limited information on medication dosage, patterns of resistance, adherence, and the exact timing of HAART initiation. In addition, we were only able to measure the initial regimen used in pregnancy, and viral suppression at delivery may have been affected by subsequent regimen changes. Because most women had started HAART before study entry, our first measured CD4+ cell count and HIV-1 RNA were not true “baseline” values, even for women who were HAART naïve prior to pregnancy. Despite these limitations, our study is one of the first U.S.-based observational analyses comparing the association between regimens and virologic suppression at delivery in the current era of HAART availability. Virologic suppression at delivery is an important risk factor for MTCT, and one that can be clinically measured. HIV-1 RNA suppression at delivery is a reasonable surrogate measure of success for antiretroviral therapy as an intervention to prevent MTCT.
In conclusion, our analysis suggests that women with lower CD4+ counts and higher HIV-1 RNA during pregnancy are at an increased risk for a detectable HIV-1 RNA at delivery. Persistently detectable HIV-1 RNA is particularly common among HAART-experienced women presenting with detectable HIV-1 RNA in pregnancy (57% of whom remained detectable at delivery). Of additional concern was the loss of virologic suppression by delivery among 13% of women with undetectable HIV-1 RNA at entry. Our findings suggest that efforts to improve suppression at term are warranted, particularly among women with lower CD4+ cell count and higher HIV-1 RNA.
Acknowledgments
The authors gratefully acknowledge the women and their families who have participated in the WITS and the efforts of the dedicated study personnel at all sites throughout the study who have made this analysis possible. Special thanks to Erin George for help in manuscript preparation as well as David R. Bangsberg and Alexi Wright for editorial suggestions.
Support: Please see appendix for support information for individual investigators. Additional funding includes the following: Ingrid Katz (NIAID AI 007433); Roger Shapiro (4U01AI066454-0509); and Michael Hughes (5U01AI068634-03)
APPENDIX
Principal investigators, study coordinators, program officers, and funding include the following: Clemente Diaz and Edna Pacheco-Acosta (University of Puerto Rico, San Juan, PR; U01-AI-34858); Ruth Tuomala, Ellen Cooper, and Donna Mesthene (Boston/Worcester site, Boston, MA; U01-DA-15,054); Phil La Russa and Alice Higgins (Columbia-Presbyterian Hospital, New York, NY; U01-DA-15053); Sheldon Landesman, Edward Handelsman, and Ava Dennie (State University of New York, Brooklyn, NY; U01-HD-36117); Kenneth Rich and Delmyra Turpin (University of Illinois at Chicago, Chicago, IL; U01-AI-34841); William Shearer, Susan Pacheco, and Norma Cooper (Baylor College of Medicine, Houston, TX; U01-HD-41,983); Joana Rosario (National Institute of Allergy and Infectious Diseases, Bethesda, MD); Kevin Ryan, (National Institute of Child Health and Human Development, Bethesda, MD); Vincent Smeriglio and Katherine Davenny (National Institute on Drug Abuse, Bethesda, MD); and Bruce Thompson (Clinical Trials and Surveys Corporation, Baltimore, MD; N01-AI-85339). The scientific leadership core includes Kenneth Rich (Principal Investigator) and Delmyra Turpin (Study Coordinator) (1-U01-AI-50274-01).
Footnotes
These data were presented in poster form at the Conference on Retroviruses and Opportunistic Infections, Montreal, 2009
References
- 1.Centers for Disease Control and Prevention. Progress toward elimination of perinatal HIV infection–Michigan 1993–2000. MMWR Morb Mortal Wkly Rep. 2002;51(5):93–97. [PubMed] [Google Scholar]
- 2.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994 Nov 3;331(18):1173–80. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 3.Cooper ER, Charurat M, Mofenson L, et al. Women and Infants’ Transmission Study Group. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002 Apr 15;29(5):484–94. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Enhanced Perinatal Surveillance -Participating Areas in the United States and Dependent Areas, 2000–2003. HIV/AIDS Surveillance Supplemental Report. 2008;13(4):1–35. [Google Scholar]
- 5.Garcia PM, Kalish LA, Pitt J, et al. Women and Infants Transmission Study Group. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. N Engl J Med. 1999 Aug 5;341(6):394–402. doi: 10.1056/NEJM199908053410602. [DOI] [PubMed] [Google Scholar]
- 6.Mofenson LM, Lambert JS, Stiehm ER, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med. 1999;341:385–93. doi: 10.1056/NEJM199908053410601. [DOI] [PubMed] [Google Scholar]
- 7.Magder LS, Mofenson L, Paul ME, et al. Risk factors for in utero and intrapartum transmission of HIV. J Acquir Immune Defic Syndr. 2005 Jan 1;38(1):87–95. doi: 10.1097/00126334-200501010-00016. [DOI] [PubMed] [Google Scholar]
- 8.European Collaborative Study. Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40:458–65. doi: 10.1086/427287. [DOI] [PubMed] [Google Scholar]
- 9.Thea DM, Steketee RW, Pliner V, et al. The effect of maternal viral load on the risk of perinatal transmission of HIV-1. New York City Perinatal HIV Transmission Collaborative Study Group. AIDS. 1997 Mar 15;11(4):437–44. doi: 10.1097/00002030-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Public Health Service Taskforce. [Accessed June 5, 2008.];Recommendations for use of antiretroviral drugs in pregnant IV-1 infected women for maternal health and Interventions to Reduce Perinatal HIV transmission in the United States. 2007 November 2; Available at: http://aidsinfo.nih.gov/
- 11.European Collaborative Study. Time to undetectable Viral Load after Highly Active Antiretroviral Therapy Initiation among HIV-Infected Pregnant Women. Clin Infect Dis. 2007;44:1647–56. doi: 10.1086/518284. [DOI] [PubMed] [Google Scholar]
- 12.Bartlett JA, Buda JJ, von Scheele B, et al. Minimizing resistance consequences after virologic failure on initial combination therapy: a systematic overview. J Acquir Immune Defic Syndr. 2006 Mar;41(3):323–31. doi: 10.1097/01.qai.0000197070.69859.f3. Review. Erratum in: J Acquir Immune Defic Syndr. 2006 Nov 1;43(3):381. [DOI] [PubMed] [Google Scholar]
- 13.Walmsley S, Bernstein B, King M, et al. M98–863 Study Team. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002 Jun 27;346(26):2039–46. doi: 10.1056/NEJMoa012354. [DOI] [PubMed] [Google Scholar]
- 14.Anastos K, Schneider M, Gange S, et al. for the Women’s Interagency HIV. The Association of Race, Sociodemographic, and Behavioral Characteristics with Response to Highly Active Antiretrovial Therapy in Women. J Acquir Immune Defic Syndr. 2005 Aug 15;39(5):537–544. [PubMed] [Google Scholar]
- 15.Bardeguez AD, Lindsey JC, Shannon M, et al. for the PACTG 1025 Protocol Team. Adherence to antiretrovirals among US women during and after pregnancy. J Acquir Immune Defic Syndr. 2008 Aug 1;48(4):408–17. doi: 10.1097/QAI.0b013e31817bbe80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mellins CA, Chu C, Malee K, et al. Adherence to antiretroviral treatment among pregnant and postpartum HIV-infected women. AIDS Care. 2008 Sep;20(8):958–68. doi: 10.1080/09540120701767208. [DOI] [PubMed] [Google Scholar]
- 17.Sheon AR, Fox HE, Rich KC. The Women and Infants Transmission Study (WITS) of maternal-infant HIV transmission: study design, methods, and baseline data. J Women’s Health. 1996;5:69–78. [Google Scholar]
- 18.National Center for Infectious Diseases Division of HIV/AIDS. Castro KG, Ward JW, Slutsker L, Buehler JW, Jaffe HW, Berkelman RL, Curran JW. Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults. [Accessed October 8, 2009.];MMWR. 1993 December 18; 1992/41(RR-17). http://www.cdc.gov/mmwr/preview/mmwrhtml/00018871.htm.
- 19.Personal Communication with Dr. Ruth Tuomala.
- 20.Stek AM. Antiretroviral medications during pregnancy for therapy or prophylaxis. Curr HIV/AIDS Rep. 2009 May;6(2):68–76. doi: 10.1007/s11904-009-0011-2. [DOI] [PubMed] [Google Scholar]
- 21.Cressey TR, Lallemant M. Pharmacogenetics of antiretroviral drugs for the treatment of HIV-infected patients: an update. Infect Genet Evol. 2007 Mar;7(2):333–42. doi: 10.1016/j.meegid.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Clark R. Sex differences in antiretroviral therapy-associated intolerance and adverse events. Drug Saf. 2005;28(12):1075–83. doi: 10.2165/00002018-200528120-00003. [DOI] [PubMed] [Google Scholar]
- 23.Mirochnick M, Best BM, Stek AM, Capparelli E, Hu C, Burchett SK, Holland DT, Smith E, Gaddipati S, Read JS PACTG 1026s Study Team. Lopinavir exposure with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2008 Dec 15;49(5):485–91. doi: 10.1097/QAI.0b013e318186edd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stek AM, Mirochnick M, Capparelli E, Best BM, Hu C, Burchett SK, Elgie C, Holland DT, Smith E, Tuomala R, Cotter A, Read JS. Reduced lopinavir exposure during pregnancy. AIDS. 2006 Oct 3;20(15):1931–9. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 25.Read JS, Best BM, Stek AM, Hu C, Capparelli EV, Holland DT, Burchett SK, Smith ME, Sheeran EC, Shearer WT, Febo I, Mirochnick M. Pharmacokinetics of new 625 mg nelfinavir formulation during pregnancy and postpartum. HIV Med. 2008 Nov;9(10):875–82. doi: 10.1111/j.1468-1293.2008.00640.x. Epub 2008 Sep 14. [DOI] [PMC free article] [PubMed] [Google Scholar]


