Abstract
The range and physical qualities of molecules that act as ligands for the γδ T cell receptors (TCRs) remain uncertain. Processed insulin is recognized by αβ T cells, which mediate diabetes in non-obese diabetic (NOD) mice. Here, we present evidence that γδ T cells in these mice recognize processed insulin as well. Hybridomas generated from NOD spleen and pancreatic lymph nodes included clones expressing γδ TCRs that responded specifically to purified islets of Langerhans and to an insulin peptide, but not to intact insulin. The γδ TCRs associated with this type of response are diverse, but a cloned γδ TCR was sufficient to transfer the response. The response to the insulin peptide was autonomous as demonstrated by stimulating single responder cells in isolation. This study reveals a novel specificity for γδ TCRs, and raises the possibility that γδ T cells become involved in islet-specific autoimmunity.
Keywords: T Cell, T Cell Receptor, Insulin, Diabetes
Introduction
Autoimmunity to insulin plays a central role in the pathogenesis of human type I diabetes and in the NOD mouse model (1). NOD mice spontaneously develop insulin auto-antibodies (IAA) starting around four to eight weeks of age, and progress to overt diabetes as early as eleven weeks of age (2). Among islet-infiltrating CD4+ T cells cloned from pre-diabetic NOD mice, more than 90% of the insulin-specific clones reacted with insulin peptide B:9–23 (3), and such clones were able to transfer diabetes to immune-compromised NOD.SCID mice (4). CD8+ T cells in NOD mice also recognize insulin, with a focus on B chain amino acids 15–23 (5). There are two insulin genes in mice, ins1, which is only expressed in the pancreas, and ins2, which is expressed in pancreas and thymus. NOD mice made deficient for both insulin genes, and rescued from hyperglycemia with a mutated insulin trans-gene that does not stimulate the B:9–23 reactive T cells (having alanine instead of tyrosine in position 16), failed to develop diabetes (6), and this protection was lost when the B:9–23 epitope was restored (7). Importantly, autoimmunity to against proinsulin also supports the expansion of diabetogenic IGRP-specific CD8+ T cells (8). Thus, insulin/pro-insulin provides a critical auto-antigen in initiating the immune response that leads to diabetes in NOD mice. Responses to other auto-antigens documented in NOD mice appear to be “downstream” of the immune response to insulin (9).
Several lines of evidence suggest a role for γδ T cells in diabetes. Circumstantial evidence in humans either at risk for or suffering from type I diabetes or having gestational diabetes has implicated γδ T cells (10–17). As well, the lyp mutation in BB rats, a marker of diabetes susceptibility, inhibits the development of γδ T cells (18). Finally, in NOD mice, γδ T cells were increased by comparison with BALB/c controls (19), and aerosolized inhaled insulin specifically induced γδ T cells capable of inhibiting spontaneous diabetes development (20). However, it was not resolved whether γδ T cells in these mice actually recognize insulin. We now have isolated NOD-derived hybridomas which express γδ TCRs that respond to purified islets of Langerhans, and to a synthetic insulin peptide, but not to intact native insulin. Our data indicate that the γδ TCR confers these responses, which do not require antigen presenting cells, and suggest that diverse insulin-specific γδ T cells develop in NOD mice.
RESULTS AND DISCUSSION
Insulin-specific γδ TCR using a new TCR-Vα/δ gene
NOD.BDC12-4.1-TCRαtgCα−/− mice, a mouse strain deficient in endogenous TCR-α but expressing a transgenic TCR-α chain from a CD4+ T cell clone specific for insulin B:9–23 (BDC12-4.1), contain insulin-peptide B:9–23-specific αβ T cells and develop insulin auto-antibodies (IAA) spontaneously (6). The αβ T cells in these mice express the transgenic TCR-α in combination with diverse endogenous TCR-β chains (21). To analyze the repertoire of TCR-β chains supporting the insulin response, we immunized these mice twice by i.p. injection of the ins2 B:9–23-peptide in IFA, in vitro re-activated their splenic T cells in the presence of plate-bound anti-CD3 and anti-CD28 mAbs, and hybridized them with the AKR-thymoma BW5147-derived BWZ.36 cells (22). This fusion line for T cells is modified to carry a β-galactosidase reporter transgene driven by the nuclear factor of activated T cells (NFAT) promoter, as a specific cytokine-independent means for detecting T cell activation. Of the insulin peptide-responsive hybridomas, all expressed αβ TCRs (21) except one, SP9D11, which was TCR-β-negative but TCR-δ and TRGV4(Vγ4)-positive (Fig. 1). Because available antibodies specific for individual Vδs failed to stain the SP9D11 hybridoma, we reverse transcribed its RNA and amplified the cDNA with a series of primers corresponding to the Vδ-gene repertoire discovered so far (23). TRDV10 primers amplified a transcript, but the sequence of the amplification product was more closely related to the TRAV4-2*01 gene than to the TRDV10 gene. Because the TRDV10 primer sequence resembles the TRAV4-2 sequence, it seemed likely that this primer was actually hybridizing with TRAV4-2 cDNA despite some mismatch. Therefore, we designed new primers specific for TRAV4-2 and TRDV10 and used them to re-amplify the SP9D11 cDNA. This time, only the TRAV4-2 primers yielded an amplification product, and the TRAV4-2 sequence derived from the SP9D11 gene transcript was identical to the published TRAV4-2*01 sequence (GenBank Accession AC004407). So far, in the literature 11 TRDV genes have been reported that rearrange to both (D)J segments of the TRD locus and TRAJ segments, indicating that these V genes are used to generate both TCR-δ and TCR-α chains. These genes are designated TRAV/DV; TRAV4-2 thus represents a previously unrecognized member of this group. To determine if SP9D11 cells indeed utilize TRAV4-2 to express TCR-δ, we sequenced the entire rearranged δ gene in the cDNA of SP9D11 cells. We found that the SP9D11 TCR-δ gene rearrangement utilizes the TRAV4-2 leader sequence followed by TRAV4-2*01, TRDD2*01, TRDJ1*01 and TRDC*01 gene segments. The DNA and predicted amino acid sequences of the entire SP9D11 TCR-δ and TCR-γ gene products are shown in supplemental Figure 2A. In cDNA from spleen and liver of the same mouse from which the SP9D11 hybridoma was derived, amplified with the same pair of primers, we found the same V-gene sequence, confirming that TRAV4-2*01 at least occasionally rearranges into the δ-locus (not shown). In summary, these data re-assign a gene previously assigned as TRAV gene instead as TRAV/DV gene, which is functionally expressed in the SP9D11 γδ TCR.
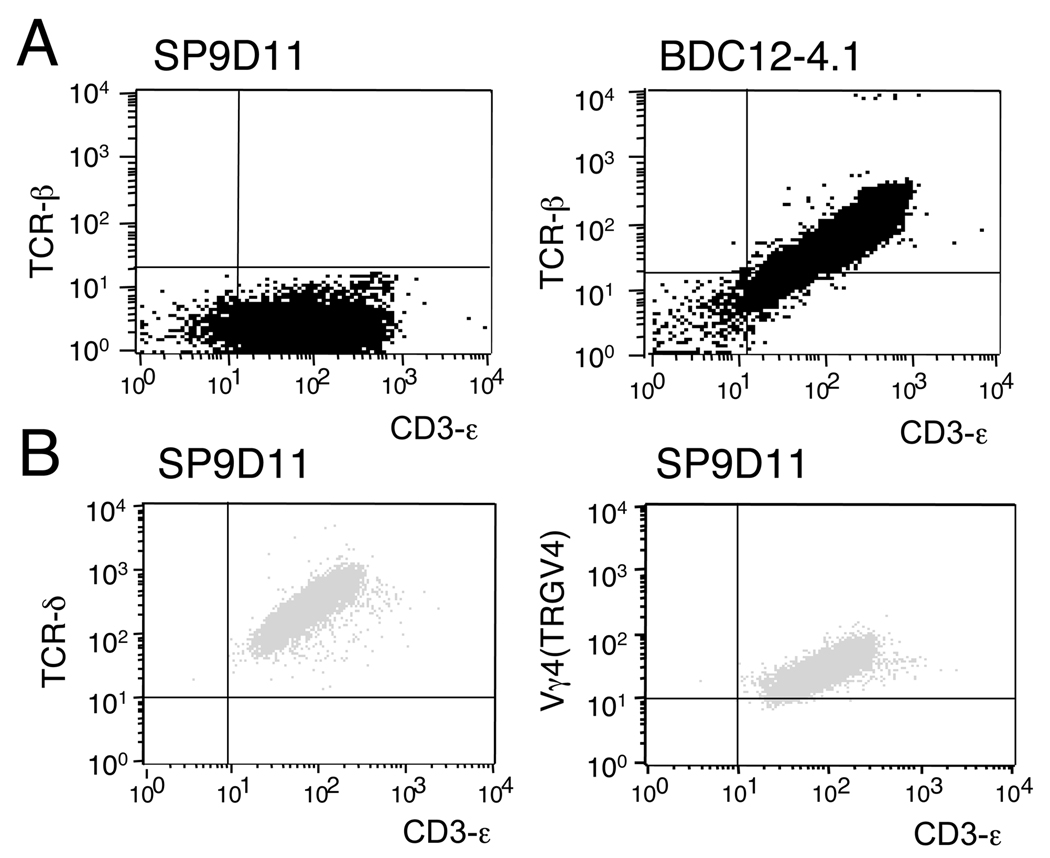
Figure 1. The SP9D11 hybridoma: absence of TCR-β expression.
A: SP9D11 cells and an ins2 B:9–23 reactive TCRαβ+ hybridoma (BDC12-4.1) were stained with mAbs specific for TCR-β and CD3ε, and analyzed by flow cytometry. B: SP9D11 cells were stained with mAbs specific for TCR-δ or TCR-Vγ4 (TRGV4) and CD3ε, and analyzed by flow cytometry. Data shown are representative of multiple (>3) independent staining experiments.
Specificity of an insulin-peptide reactive γδ TCR+ hybridoma
SP9D11 cells not only responded to the ins2 B:9–23 peptide (Fig. 2, Fig. 4, Fig. 5) but also to purified islets of Langerhans derived from 8wks old NOD mice (Fig. 2A). They did not respond, however, to equivalent amounts of adrenal or pituitary tissues derived from the same mice. Pancreatic islets derived from mice deficient in endogenous insulin but expressing transgenic insulin (6) elicited even stronger responses of the SP9D11 cells (Fig. 2B). However, incubation of SP9D11 with intact bovine or human insulin failed to induce a response (Fig. 2C). Together, these data suggested that SP9D11 cells recognize an insulin-derived antigen expressed by pancreatic islet cells.
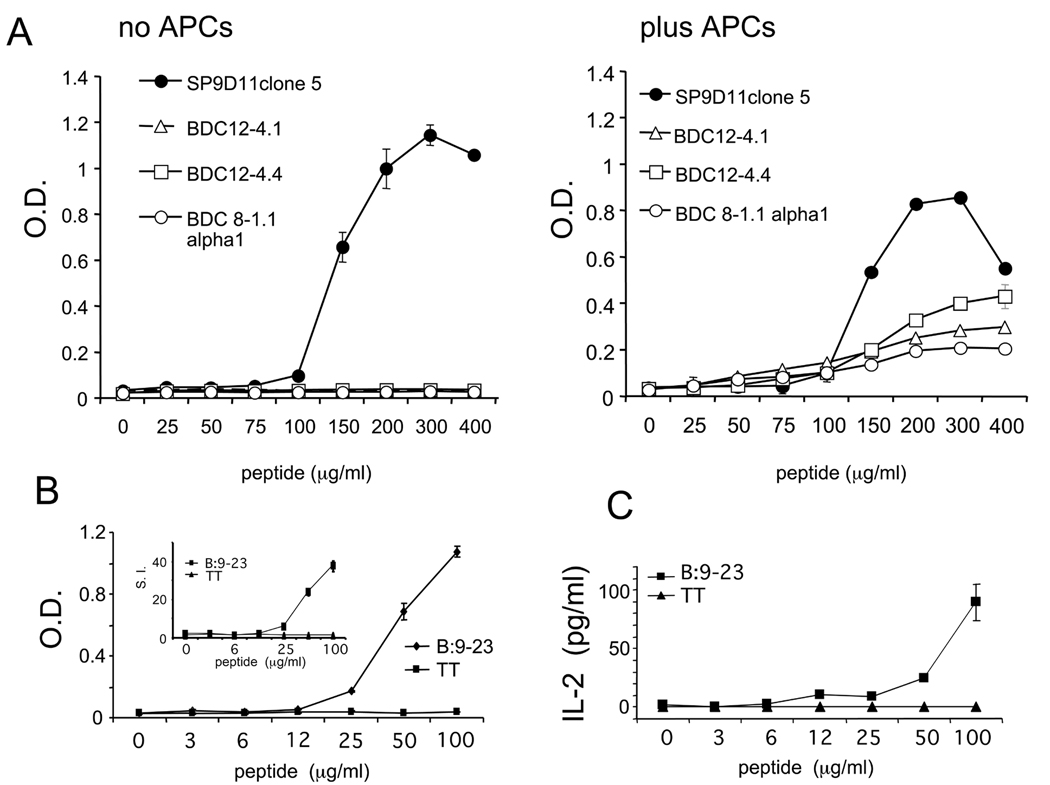
Figure 2. SP9D11 cells specifically respond to purified islets of Langerhans and to the insulin B:9–23 peptide, but not to intact insulin.
Responses were measured using the LacZ enzyme activity assay and are shown as optical density (O.D.) values; the fold increase over the signal produced by the responders alone (SI) is also shown over each column. Data shown represent mean values +/− standard error of the mean of 3–4 separate determinations in a single experiment, representative of at least 3 similar stimulation experiments.
A: 2×104 SP9D11 cells were incubated overnight alone, with NOD islets (100 size-balanced islets/well), with an equivalent amount of adrenal tissue (based on DNA content), or with the ins2 B:9–23 peptide (100 µg/ml). The background signal of NOD islets alone is also shown.
B: 2×104 SP9D11 cells were incubated overnight alone, or with islets derived from ins1/ins2 double gene knockout mice expressing an insulin transgene with the wild-type nucleotide sequence (100 size-balanced islets/well).
C: 2×104 SP9D11 cells were incubated overnight alone (PBS), with intact bovine insulin (100 µg/ml) or with ins2 B:9–23 peptide (100 µg/ml).
D: 2×104 SP9D11 cells were incubated overnight alone (PBS), with intact human insulin (100 µg/ml) or with ins2 B:9–23 peptide (100 µg/ml).
Figure 4. The SP9D11 hybridoma: response to the insulin peptide B:9–23.
A: 2×104 SP9D11 cells (subclone 5) and equal numbers of three αβ TCR+ hybridomas with known specificity for peptide B:9–23 (BDC12-4.1; BDC12-4.4; BDC8-1.1alpha1) were cultured with peptide either alone (no APCs) or in the presence of 1×105 NOD spleen cells (plus APCs). Responses were measured with the lacZ activity assay and are shown as optical density values (O.D.). B: 2×104 SP9D11 cells were cultured with peptide for 24 hours (no accessory cells). Responses were measured with the lacZ activity assay and are shown as optical density values (O.D.). The insert shows the stimulation index (S.I.) calculated as fold increase over background responses.
C: 1×105 SP9D11 cells were cultured with peptide for 24 hours (no accessory cells), then the supernatant was harvested for an IL-2 ELISA assay. The cells secreted IL-2 in response to the B:9–23 peptide. Data shown represent mean values +/− standard error of the mean of 3 separate determinations in a single experiment, representative of >3 similar stimulation experiments.
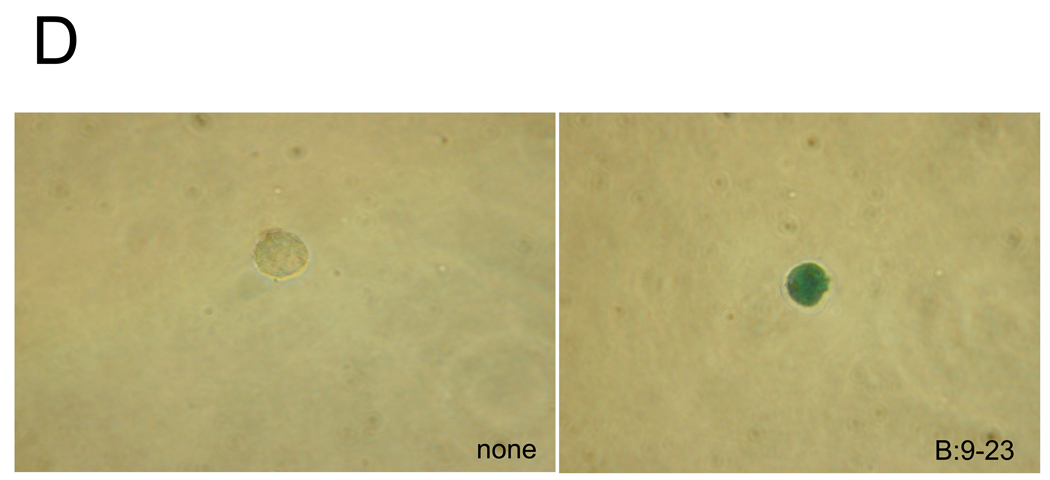
D: Individual SP9D11 cells were cultured alone (left panel) or with peptide (right panel), then fixed, overlaid with X-gal, and examined microscopically. Magnification: ×40
Figure 5. Responses of the hybridoma SP9D11 and the transfectoma expressing the SP9D11 γδ TCR to the insulin peptide B:9–23 and derivatives.
A: 2×104 SP9D11 cells were incubated overnight alone (PBS), with the indicated peptide antigens at 100 µg peptide/ml or with 1 µg/ml anti CD3ε mAb in solution. Responses were measured using the LacZ enzyme activity assay, and are expressed as fold increase over the signal produced by the responders alone (SI). The OD value of SP9D11 cells without antigen was 0.029±0.004 at 4 hours. B: 3×104 5KC-TCR:SP9D11 cells were incubated overnight either alone (open columns) or together with NOD spleen cells (1×105) (black columns) and the indicated peptide antigens. (1) = 100 µg/ml; (2) = 200 µg/ml. Responses were measured by IL-2 ELISA. Data shown represent mean values +/− standard error of the mean of 3 separate determinations in a single experiment, representative of multiple (>3) similar stimulation experiments.
However, because the SP9D11 hybridoma was derived from BDC12-4.1 TCRα transgenic mice, the possibility remained that the transgenic TCR-α chain is essential to its peptide response. To address this, we transduced a TCR-α and β-loss variant T cell hybridoma, 5KC-73.8.20 α−β− (24), with the SP9D11 TCR-γ and TCR-δ genes, using a retroviral vector (25). The virally transduced 5KC cells expressed the SP9D11 γδ TCR on the cell surface (Fig. 3A), and they responded specifically to the insulin peptide B:9–23, in contrast to non-transduced controls (Fig. 3B). These data indicate that the SP9D11 γδ TCR confers the response to the insulin peptide independently of the “inherited” TCR-α transgene, which might still be expressed in the SP9D11 hybridoma. As with the response of the γδ hybridoma to pancreatic islets, the response to the insulin peptide was dose-dependent and specific (Fig. 4). However, unlike αβ T cell hybridomas responding to the same peptide, the γδ hybridoma did not require additional antigen-presenting cells (Fig. 4A, B). Moreover, individual hybridoma cells in isolation still responded to the peptide, eliminating any requirement for cell-cell interaction in this response (Fig. 4C and supplemental Fig. 1). We also examined truncated and mutated insulin B:9–23 peptides for their stimulatory potential on SP9D11 cells (Fig. 5A). C-terminal truncations were much better tolerated than N-terminal ones, and a change to the ins1 sequence (proline instead of the serine at the N-terminus) slightly reduced the response. In clear contrast to the αβ T cells (21), SP9D11 cells did not respond to a peptide in which the cysteine in position 19 was replaced with an alanine (ins2 B:9–23/B19C to ins2 B:9–23/B19A). SP9D11 also failed to respond to the BDC-2.5 TCR mimetope peptide HRPI-RM. In accordance with the distinctive response pattern of hybridoma SP9D11 (Fig. 5A), the TCR transfectoma also failed to respond to the modified insulin peptide ins2 B:9–23/B19A, but unlike the hybridoma which did not benefit from accessory cells, the transfectoma showed better reactivity in the presence of added NOD splenocytes (Fig. 5B).
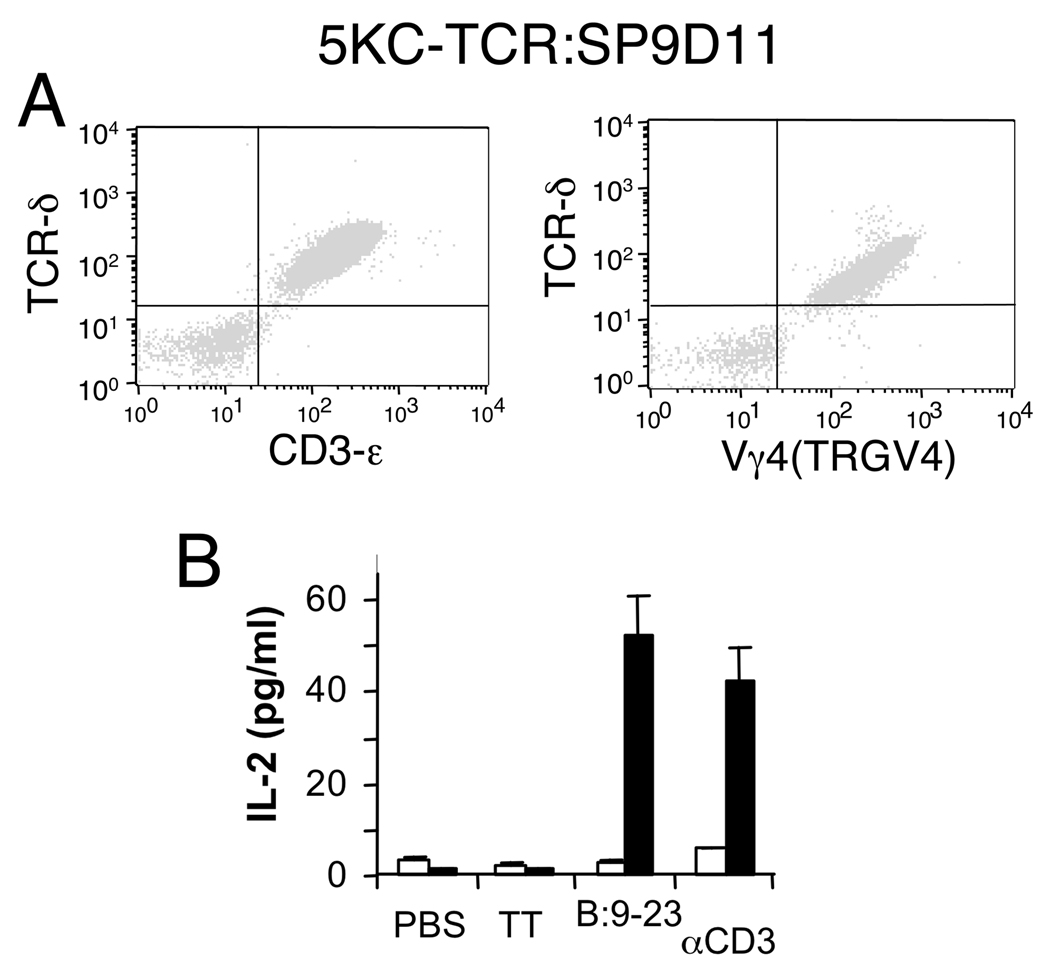
Figure 3. The SP9D11 γδ TCR confers responsiveness to insulin peptide B:9–23.
A: SP9D11 TCR-γ and δ genes were expressed by viral transduction in a TCRαβ-loss variant of hybridoma 5KC-73.8.20 to generate transfectoma 5KC-TCR:SP9D11. The transfectoma cells were selected for GFP-expression (not shown), then stained with mAbs specific for TCR-δ and CD3ε or TCR-Vγ4 (TRGV4), and analyzed cytofluorimetrically. Data shown are representative of 3 independent staining experiments. B: The transfectoma 5KC-TCR:SP9D11 (black columns) and the recipient cell line 5KC-73.8.20α−β− (open columns) were incubated at 4×104 cells/well (no APCs) overnight with the insulin peptide B:9–23 (200 µg/ml), TT (200 µg/ml) or anti CD3ε mAbs in solution (10 µg/ml), and responses were measured by IL-2 ELISA. Data shown represent mean values +/− standard error of the mean of 3–4 separate determinations in a single experiment, representative of at least 3 similar stimulation experiments.
γδ T cells expressing insulin peptide-specific TCRs develop in non-immunized NOD mice
Since the insulin peptide-responsive SP9D11 hybridoma was derived from B:9–23-immunized mice with an unusual engineered genetic background, and because it expresses a γδ TCR containing a newly assigned TRAV/DV gene, the insulin-response of this cell conceivably might be exceptional. We therefore examined γδ T cell insulin-responses in experiments with two additional sources of γδ T cells. In the first experiment, we analyzed non-immunized NOD.SCID mice that had been reconstituted with bone marrow from NOD.Cα−/− mice. Prior to the reconstituting cell-transfer, the bone marrow cells were infected with a virus transducing NOD.BDC12-4.4 TCRα. These mice represent a similar cell source to the one we used previously, but eliminate the possible influence of peptide immunization. Here, from a fusion with pancreatic lymph node cells, four additional B:9–23-responsive γδ TCR+ hybridomas were generated (Table 1). We analyzed TCR expression of these cells by flow cytometry and gene sequencing (Table 1 and supplemental Figure 2B). All of the γδ T cell hybridomas expressed TCRs different from that of SP9D11, and different from each other. In the second experiment, we generated hybridomas from non-immunized wild-type NOD mice, at an age of 12 wks, again with pancreatic lymph node cells. In this fusion, three additional insulin peptide-responsive γδ TCR+ hybridomas were generated (Table 1), which expressed TCRs that differed from all the others. In all, three different Vγs and 5 different Vδs were expressed (Table 1 and supplemental Figure 2). However, among the nine γδ TCR+ hybridomas responsive to insulin peptide B:9–23 we have generated so far, six express TRGV1 (Vγ1). Other NOD-derived γδ TCR+ hybridomas (not shown), and γδ TCR+ hybridomas from other sources having partially matched TCR-V expression, failed to respond to the insulin peptide (Table 1). Taken together, these data indicate that γδ TCRs capable of responding to the insulin B:9–23 peptide develop naturally in NOD mice, and that the repertoire of these TCRs is diverse, though perhaps biased in favor of TRGV1(Vγ1)-expression.
Table 1.
TCR γδ expressing hybridomas responsive to insulin peptide B:9–23
| Mouse strain; tissue | Immunization | Hybridoma | TCR-Vγ/Vδ | B:9–23 Response& |
|
|---|---|---|---|---|---|
| NOD.BDC12-4.1 TCR-αtg/Cα−/−; spleen |
Yes | SP9D11 | TRGV4/TRAV4-2 | Vγ4/ Vδ10-like | + |
| NOD.BDC12-4.4 TCR-αtg/Cα−/− retrogenic NOD.scid; pancreatic lymph node |
No | Pln1B5.2 | TRGV1/TRAV15D1DV6D-1 | Vγ1/ Vδ6.3 | + |
| Pln2B8.5 | TRGV1/TRAV6-7/DV9 | Vγ1/ Vδ2.3(Vα4) | + | ||
| Pln5B8.2 | TRGV1/TRAV15D-1DV6D1 | Vγ1/ Vδ6.3 | + | ||
| Pln1B11.13 | TRGV2/TRDV5 | Vγ2/ Vδ5 | + | ||
| Pln3C5.3 | TRGV4/? | Vγ4/ TCR-δ+, Vδ? | + | ||
| NOD; pancreatic lymph node |
No | Pln1B8.1 | TRGV1/? | Vγ1/ TCR-δ+, Vδ? | + |
| Pln1B11.2 | TRGV1/TRAV15D-2/DV6D-2*05 | Vγ1/ Vδ6(λ12-like) | + | ||
| Pln2C11.5 | TRGV1/TRV2-2 | Vγ1/ Vδ4 | + | ||
| C57BL/10; liver | No | 96BLT-8 | TRGV4/TRAV13-4/DV7 | Vγ4/ Vδ7 | − |
| 96BLT-15 | TRGV4/TRDV5 | Vγ4/ Vδ5 | − | ||
| 96BLT-14 | TRGV1/TRAV13-4/DV7 | Vγ1/ Vδ7 | − | ||
| 96BLT-13 | TRGV4/TRDV2-2 | Vγ4/ Vδ4 | − | ||
Immunization: B:9–23/IFA i.p., 2×
Nomenclature: according to IMGT (* designates alleles) and Heilig & Tonegawa (see Methods).
All hybridomas responded to TCR cross-linking with anti CD3ɛ mAbs. Responses were measured using a LacZ activity assay and IL-2 ELISA.
Conclusions
In this study, we present evidence that γδ T cells develop in NOD mice whose TCRs confer a specific response to an insulin antigen. Several aspects of this study seem noteworthy. First, a series of fusion experiments revealed that the repertoire of γδ TCRs supporting this response is diverse, and includes a previously unassigned Vα/δ gene. Second, using transgenic expression of one of these γδ TCRs, we confirmed TCR-dependence of the insulin response. Third, characterization of one of these hybridomas showed a specific response also to purified islets of Langerhans as well as the synthetic insulin peptide (ins2 B:9–23) representing a dominant insulin epitope recognized by CD4+ αβ T cells in NOD mice (1). This implies that NOD mice harbor insulin-specific γδ T cells capable of recognizing this auto-antigen in some processed form. Consistently, there was no response to intact native insulin. This was unexpected, considering the prevailing view that γδ T cells recognize native ligands such as the T10/22 cell surface molecules (26), but is consistent with earlier observations of γδ T cell responses to small peptides (27). Fourth, the hybridoma response to the insulin peptide revealed clear specificity differences compared to insulin-reactive αβ T cells, including a requirement for the B19 cysteine, which αβ T cells lack (21), and the lack of a requirement for antigen presenting cells (APCs), which are needed for a response by αβ T cells. Based on these observations, the mechanism underlying this cell-autonomous response mediated by the γδ TCR must be fundamentally different from that of αβ T cells, and clearly deserves further characterization. Intriguingly, the portion of insulin recognized by γδ T cells overlaps with dominant epitopes for both insulin-specific CD4+ and CD8+ αβ T cells (1), perhaps revealing an auto-antigenic “hot spot”.
The physiological significance of this new γδ TCR specificity is still unclear. Conceivably, insulin peptide-specific γδ TCRs might serve as sensors of insulin levels, in addition and perhaps complementary to the receptors that bind intact insulin. However, it seems more likely that insulin-specific γδ T cells become involved in the auto-immune response that leads to NOD diabetes. Involvement of γδ T cells in auto-immunity has been already documented (28–30). Furthermore, based on studies in models of other diseases (31–34), their role is likely to be complex, encompassing both pathogenic and protective components. Consistently, we recently found that γδ T cells representing one particular subset, TRGV1(Vγ1)+ cells, preferentially infiltrate NOD islets (JM Wands, unpublished), suggesting a role of this subset in the development of insulitis, whereas others reported that splenic γδ T cells induced by insulin inhalation and transferred to secondary recipients are capable of suppressing diabetes development (20).
Materials and Methods
Animals
The strains of mice used include the regular NOD.ShiLT/J strain, NOD.Cα−/− mice expressing a transgenic TCR-α chain from the B:9–23 peptide-specific CD4+ T cell clone, BDC12-4.1 (NOD.BDC12-4.1TCRαtg/Cα−/−), and NOD.scid mice that had received a reconstituting dose of NOD.Cα−/− bone marrow cells transduced with a virus carrying the TCR-α gene of a second B:9–23 peptide-specific CD4+ T cell clone, BDC12-4.4 (BDC12-4.4 TCRαtg/Cα−/− retrogenic mice). The retrogenic mice were produced following a published protocol (25, 35). Mice were housed and bred under specific pathogen-free conditions at the University of Colorado Denver Center of Comparative Medicine (CCM) with an approved protocol from the University of Colorado Denver Animal Care and Use Committee.
Hybridomas
For cell fusion experiments, cells from two adult female mice (12 wk) were pooled in each fusion experiment. The NOD.BDC12-4.1TCRαtg/Cα−/− mice were immunized twice (one week apart) by i.p. injection with 100 µg insulin2 B:9–23 peptide in 100 µl IFA. Five days after the second immunization, spleen cells were activated in vitro with plate-bound anti-CD3 and anti-CD28 mAbs for 48 to 72 hours. For wild-type NOD mice and BDC12-4.4 TCRαtg/Cα−/− retrogenic mice, pancreatic lymph node cells were merely activated in vitro, without prior immunization. Activated T cells were harvested, and separated on a one-step Ficoll-Hypaque™ Plus (GE Healthcare) gradient. The purified cells were mixed with an equal number of log phase BWZ.36 cells (at least 107). BWZ.36 cells lack functional TCR-α and β genes (36), and have been transfected with a nuclear factor of activated T (NFAT) cells–LacZ reporter construct (22). T cell fusions were carried out as previously described (37). After the fusion, cells were plated and selected in HAT, and 2× hygromycin B (2mg/ml, Sigma Cat# H0654), and insulin B:9–23 peptide responsive cells were identified using the LacZ enzymatic assay.
LacZ enzymatic activity assay
Specific antigen stimulation of the T cell hybridomas was measured as LacZ enzymatic activity by using chlorophenol red-β-D-galactopyranoside (CPRG) (32;33), and ins2 B:9–23 peptide (SHLVEALYLVCGERG) as antigen. 2×104 hybridoma cells were cultured in medium with or without 100 µg/ml B:9–23 peptide or other peptides, in 96-well flat bottom microtiter plates. After overnight incubation, cells were washed with PBS to remove the medium, and 100 µl of CPRG reagent (91mg/L CPRG, 0.125% NP-40,1mM MgCl2 in 10mM phosphate buffer) was added. Cleavage of CPRG by active LacZ results in production of chlorophenol red. The cells were lysed after 4 hours at 37 °C, and absorbencies were read at 575 nm and 655 nm (reference wavelength), immediately or after 24 hrs for weak responses. The stimulation index (SI) was calculated as LacZ activity compared to background without antigen stimulation. For visualizing peptide responses of single cells, individual cells were placed in V-bottom plates and incubate overnight with the B:9–23 peptide (200 µg/ml). Cell were then fixed, incubated with X-gal, and examined microscopically (22). Hybridomas identified as B:9–23 responders were tested repeatedly with this peptide, as well as with negative control peptides, including tetanus toxoid peptide (TT, QYIKANSKFIGITEL), a BDC2.5 mimotope HRPI-RM peptide (EKAHRPIWARMDAKK) (34), and B:9–23/B16A peptide (7). Stimulation with anti-CD3 antibody (10 µg/ml added to the cultures) was used as a positive control. None of the negative control peptides induced responses higher than two-fold over background (SI = 2).
Flow cytometry assay
To characterize the B:9–23 peptide responsive hybridomas, cells were stained with specific antibodies using standard methods. Briefly, 1×106 cells were washed in FACS buffer (PBS containing 1% BSA and 0.1% sodium azide), resuspended in 100µl FACS buffer containing 1:100 diluted Fc-Block antibodies, washed again and incubated with specific anti TCR mAbs including anti-mouse CD3ε (clone 500A2); pan anti-mouse TCR-β chain (mAb H57–597); pan anti-mouse TCR-δ (mAb GL3), anti-mouse CD4 (mAb RM4–5), anti-mouse CD8α (mAb 53-6.7), along with and isotype controls. Further mAbs specific for mouse Vγs and Vδs, which were included also have been listed in earlier publications (38). Cell staining was analyzed on a FACSCalibur cytometer, and the data processed using CellQuest software.
TCR gene-sequencing
Rearranged TCR gene cDNAs were obtained by RT-PCR of mRNA, followed by specific amplification of TCR gene transcripts using V and C-specific primers, as described previously (23). The amplified products were purified on 2% agarose, extracted using the QIAquick Gel Extraction kit, and sequenced. For designating TCR genes, we used nomenclatures available at the website of the International ImmunoGene Tics information system (IMGT) (39), and a shorter nomenclature for TCR-V genes (40).
Expression of the SP9D11 TCR in TCR-αβ-deficient cells
The complete rearranged SP9D11 TCR gamma gene was linked with the delta gene via the P2A sequence (25), and the linked genes subcloned into the vector pMIG II. The plasmid DNA was transfected into Phoenix cells along with a helper virus plasmid for virus production. The retrovirus-containing supernatant of these cells was used to transduce the TCR-deficient hybridoma 5KC-73.8.20 α−β− (24). 5KC cells expressing the γδ TCR of the SP9D11 hybridoma (5KC-TCR:SP9D11) were identified as GFP+ cells, purified by cell-sorting, and then cloned and propagated for stimulation experiments.
IL-2 ELISA
Antigen-specific activation of the 5KC TCR-transfectoma and the hybridomas was measured using an IL-2 ELISA. For this assay, 105 cells/well were incubated with or without 105 antigen presenting cells (APC)/well, for 24 hours, and supernatants analyzed for cytokine content using a rat anti mouse anti IL-2 antibody (mAb JES6-1A12) for capture and a second biotinylated rat anti-mouse IL-2 antibody (mAb JES6-5H4) for detection.
Supplementary Material
Acknowledgments
The authors would like to acknowledge helpful discussions with Dr. John Kappler.
Non-standard abbreviations
- IDDM
insulin dependent diabetes mellitus
- IAA
insulin autoantibody
- IGRP
islet-specific glucose-6-phosphatase catalytic subunit-related protein
- GDM
gestational diabetes mellitus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported by grants from the National Institutes of Health (R01DK55969), the NIH Autoimmunity Prevention Center (2U19A1050864), the Diabetes Endocrine Research Center grant from the National Institute of Diabetes and Digestive and Kidney Diseases (P30 DK57516), the American Diabetes Association, the Juvenile Diabetes Foundation (1-2006-16 and 4-2007-1056), and by two separate NIH Autoimmunity Center Pilot Project grants (to W.K.B. and to R.L.O., respectively). Li Zhang is supported by a fellowship grant from the Juvenile Diabetes Foundation (3-2008-107) and an ADA postdoctoral fellowship (7-06-MN-17).
References
- 1.Zhang L, Nakayama M, Eisenbarth GS. Insulin as an autoantigen in NOD/human diabetes. Current Opinion in Immunology. 2008;20:111–118. doi: 10.1016/j.coi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu L, Robles DT, Abiru N, Kaur P, Rewers M, Kelemen K, Eisenbarth GS. Early expression of antiinsulin autoantibodies of humans and the NOD mouse:evidence for early determination of subsequent diabetes. Proc. Natl. Acad. Sci (USA) 2000;97:1701–1706. doi: 10.1073/pnas.040556697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniel D, Gill RG, Schloot N, Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clonesisolated from NOD mice. Eur. J. Immunol. 1995;25:1056–1062. doi: 10.1002/eji.1830250430. [DOI] [PubMed] [Google Scholar]
- 4.Jasinski JM, Yu L, Nakayama M, Li MM, Lipes MA, Eisenbarth GS, Liu E. Transgenic insulin (B:9–23) T-cell receptor mice develop autoimmune diabetes dependent upon RAG genotype, H-2g7 homozygosity, and insulin 2 gene knockout. Diabetes. 2006;55:1978–1984. doi: 10.2337/db06-0058. [DOI] [PubMed] [Google Scholar]
- 5.Wong FS, Karttunen J, Dumont C, Wen L, Visintin I, Pilip IM, Shastri N, Pamer EG, Janeway CAJ. Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat. Med. 1999;5:1026–1031. doi: 10.1038/12465. [DOI] [PubMed] [Google Scholar]
- 6.Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, Eisenbarth GS. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;43:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakayama M, Beilke JN, Jasinski JM, Kobayashi M, Miao D, Li M, Coulombe MG, Liu E, Elliott JF, Gill RG, Eisenbarth GS. Priming and effector dependence on insulin B:9–23 peptide in NOD islet autoimmunity. J. Clin. Invest. 2007;117:1835–1843. doi: 10.1172/JCI31368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnamurthy B, Mariana L, Gellert SA, Colman PG, Harrison LC, Lew AM, Santamaria P, Thomas HE, Kay TW. Autoimmunity to both proinsulin and IGRP is required for diabetes in nonobese diabetic 8.3 TCR transgenic mice. J. Immunol. 2008;180:4458–4464. doi: 10.4049/jimmunol.180.7.4458. [DOI] [PubMed] [Google Scholar]
- 9.Krishnamurthy B, Dudek NL, McKenzie MD, Purcell AW, Brooks AG, Gellert S, Colman PG, Harrison LC, Lew AM, Thomas HE, Kay TW. Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J. Clin. Invest. 2006;116:3258–3265. doi: 10.1172/JCI29602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang FP, Schatz DA, Pollock BH, Riley WJ, Maclaren NK, Dumont-Driscoll M, Barrett DJ. Increased T lymphocytes bearing the gammadelta T cell receptor in subjects at high risk for insulin dependent diabetes. J. Autoimmun. 1991;4:925–933. doi: 10.1016/0896-8411(91)90055-h. [DOI] [PubMed] [Google Scholar]
- 11.Lang FP, Pollock BH, Riley WJ, Maclaren NK, Barrett DJ. The temporal association between gamma delta T cells and the natural history of insulin-dependent diabetes. J. Autoimmun. 1993;6:107–119. doi: 10.1006/jaut.1993.1009. [DOI] [PubMed] [Google Scholar]
- 12.Auricchio R, Paparo F, Maglio M, Franzese A, Lombardi F, Valerio G, Nardone G, Percopo S, Greco L, Troncone R. In vitro-deranged intestinal immune response to gliadin in type 1 diabetes. Diabetes. 2004;53:1680–1683. doi: 10.2337/diabetes.53.7.1680. [DOI] [PubMed] [Google Scholar]
- 13.Santamaria P, Nakleh RE, Sutherland DE, Barbosa JJ. Characterization of T lymphocytes infiltrating human pancreas allograft affected by isletitis and recurrent diabetes. Diabetes. 1992;41:53–61. doi: 10.2337/diab.41.1.53. [DOI] [PubMed] [Google Scholar]
- 14.Gyarmati J, Szekeres-Bartho J, Fischer B, Soltesz G. Fetal type lymphocytes in insulin dependent diabetes mellitus. Autoimmunity. 1999;30:63–69. doi: 10.3109/08916939908994762. [DOI] [PubMed] [Google Scholar]
- 15.Kretowski A, Mysliwiec J, Kinalska I. Abnormal distribution of gammadelta T lymphocytes in Grave's disease and insulin-dependent diabetes type 1. Arch Immunol Ther (Warsz) 2000;48:39–42. [PubMed] [Google Scholar]
- 16.Lapolla A, Sanzari M, Betterle C, Dalfra MG, Masin M, Zanchetta R, Zancanaro F, Capovilla F, Toniato R, Plebani M, Fedele D. Evaluation of T-cell receptor CD3+ gamma delta in gestational diabetes mellitus. Acta Diabetol. 2000;37:207–211. doi: 10.1007/s005920070007. [DOI] [PubMed] [Google Scholar]
- 17.Lapolla A, Dalfra MG, Sanzari M, Fedele D, Betterle C, Masin M, Zanchetta R, Faggian D, Masotti M, Nucera V, Plebani M. Lymphocyte subsets and cytokines in women with gestational diabetes mellitus and their newborn. Cytokine. 2005;31:280–287. doi: 10.1016/j.cyto.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Ramanathan S, Marandi L, Poussier P. Evidence for the extrathymic origin of intestinal TCRgammadelta(+) T cells in normal rats and for impairment of this differentiation pathway in BB rats. J.Immunol. 2002;168:2182–2187. doi: 10.4049/jimmunol.168.5.2182. [DOI] [PubMed] [Google Scholar]
- 19.Funda D, Stenvang JP, Buschard K. Age-related changes in T gamma delta cells of NOD mice. Immunol. Lett. 1995;45:179–184. doi: 10.1016/0165-2478(95)00003-n. [DOI] [PubMed] [Google Scholar]
- 20.Harrison LC, Dempsey-Collier M, Kramer DR, Takahashi K. Aerosol insulin induces regulatory CD8 gdT cells that prevent murine insulin-dependent diabetes. J. Exp. Med. 1996;184:2167–2174. doi: 10.1084/jem.184.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Jasinski JM, Kobayashi M, Davenport B, Johnson K, Davidson H, Nakayama M, Haskins K, Eisenbarth GS. Analysis of T cell receptor beta chains that combine with dominant conserved TRAV5D*04 anti-insulin B:9–23 alpha chains. Journal of Autoimmunity. 2009;33:42–49. doi: 10.1016/j.jaut.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanderson S, Shastri N. LacZ inducible, antigen/MHC-specific T cell hybrids. Intl. Immunol. 1993;6:369–376. doi: 10.1093/intimm/6.3.369. [DOI] [PubMed] [Google Scholar]
- 23.O'Brien RL, Fu Y-X, Cranfill R, Dallas A, Reardon C, Lang J, Carding SR, Kubo R, Born W. Heat shock protein Hsp-60 reactive γδ cells: A large, diversified T lymphocyte subset with highly focused specificity. Proc. Natl. Acad. Sci. U.S.A. 1992;89:4348–4352. doi: 10.1073/pnas.89.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White J, Pullen A, Choi K, Marrack P, Kappler JW. Antigen recognition properties of mutant Vbeta3+ T cell receptors are consistent with an imunoglobulin-like structure for the receptor. J. Exp. Med. 1993;177:119–125. doi: 10.1084/jem.177.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holst J, Szymczak-Workman AL, Vignali KM, Burton AR, Workman CJ, Vignali DAA. Generation of T-cell receptor retrogenic mice. Nature Protocols. 2006;1:406–417. doi: 10.1038/nprot.2006.61. [DOI] [PubMed] [Google Scholar]
- 26.Adams EJ, Chien Y-H, Garcia KC. Structure of a gd T cell receptor in complex with the nonclassical MHC T22. Science. 2005;308:227–231. doi: 10.1126/science.1106885. [DOI] [PubMed] [Google Scholar]
- 27.Born W, Hall L, Dallas A, Boymel J, Shinnick T, Young D, Brennan P, O'Brien R. Recognition of a peptide antigen by heat shock reactive γδ T lymphocytes. Science. 1990;249:67–69. doi: 10.1126/science.1695022. [DOI] [PubMed] [Google Scholar]
- 28.Mukasa A, Born WK, O'Brien RL. Inflammation alone evokes the response of a TCR-invariant mouse γδ T cell subset. J. Immunol. 1998;162:4910–4913. [PubMed] [Google Scholar]
- 29.Wohler JE, Smith SS, Zinn KR, Bullard DC, Barnum SR. Gammadelta T cells in EAE: early trafficking events and cytokine requirements. Eur. J. Immunol. 2009;39:1516–1526. doi: 10.1002/eji.200839176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O'Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, iL-17-producing gd T cells. J.Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huber SA, Graveline D, Newell MK, Born WK, O'Brien RL. Vγ1+ T cells suppress and Vγ4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J. Immunology. 2000;165:4174–4181. doi: 10.4049/jimmunol.165.8.4174. [DOI] [PubMed] [Google Scholar]
- 32.Hahn Y-S, Taube C, Jin N, Sharp L, Wands JM, Kemal Aydintug M, Lahn M, Huber SA, O'Brien RL, Gelfand EW, Born WK. Different potentials of γδ T cell subsets in regulating airway responsiveness: Vγ1+ cells, but not Vγ4+ cells, promote airway hyperreactivity, TH2 cytokines, and airway inflammation. J.Immunol. 2004;172:2894–2902. doi: 10.4049/jimmunol.172.5.2894. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, Jin N, Roark CL, Aydintug MK, Wands JM, Huang H, O'Brien RL, Born WK. The influence of IgE-enhancing and IgE-suppressive gammadelta T cells changes with exposure to inhaled ovalbumin. J.Immunol. 2009;183:849–855. doi: 10.4049/jimmunol.0804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blink SE, Miller SD. The contribution of gammadelta T cells to the pathogenesis of EAE and MS. Curr. Mol. Med. 2009;9:15–22. doi: 10.2174/156652409787314516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi M, Jasinski J, Liu E, Li M, Miao D, Zhang L, Yu L, Nakayama M, Eisenbarth GS. Conserved T cell receptor alpha-chain induces insulin autoantibodies. Proc. Natl. Acad. Sci. (USA) 2008;105:10090–10094. doi: 10.1073/pnas.0801648105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White J, Blackman M, Bill J, Kappler J, Marrack P, Gold D, Born W. Two better cell lines for making hybridomas expressing specific T cell receptors. J. Immunol. 1989;143:1822–1825. [PubMed] [Google Scholar]
- 37.Kappler JW, Skidmore B, White J, Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981;153:1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roark CL, Aydintug MK, Lewis J, Yin X, Lahn M, Hahn Y-S, Born WK, Tigelaar RE, O'Brien RL. Subset-specific, uniform activation among Vγ6/Vδ1+ γδ T cells elicited by inflammation. J.Leukocyte Biol. 2004;75:68–75. doi: 10.1189/jlb.0703326. [DOI] [PubMed] [Google Scholar]
- 39.Giudicelli V, Chaume D, Lefranc M-P. IMGT/GENE-DB: a comprehensive database for human and mouse immunoglobulin and T cell receptor genes. Nucleic Acids Research. 2005;33:D256–D261. doi: 10.1093/nar/gki010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature. 1986;322:836–840. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.