Introduction
Fibro-osseous lesions of the jaws are a complex and diverse group of lesions characterized by a remarkably similar histological appearance—replacement of normal bone with a fibroblastic stroma containing varying amounts of mineralized substance, which may be bone and/or cementum-like in appearance 1. Although the nomenclature and classification of these lesions is controversial, this generic term generally includes three broad categories of clinically and radiographically distinct entities: (a) Fibrous dysplasia (b) cemento-ossifying fibroma and (c) cemento-osseous dysplasia 2. Fibrous dysplasia (FD) is a genetic disease with skeletal manifestations. The lesions may be single (monostotic) or may involve multiple bones (polyostotic). The most frequently involved sites are the craniofacial bones and the proximal femur. Occasionally, polyostotic FD is a manifestation of the McCune-Albright syndrome (MAS) 3. The majority of FD lesions, especially those in the craniofacial region are established at a young age and stabilize by age 15 4. Most monostotic FD lesions are asymptomatic and discovered as incidental findings during radiographic examination. These lesions pose little risk for pathological fracture or deformity and for most part, are managed by clinical observation 5. Symptomatic patients may be treated with bisphosphonates to relieve bone pain 6 or may require surgical treatment to correct a deformity 5.
Cemento-ossifying fibroma (COF) is a benign neoplastic lesion of the jaw that progressively expands the affected bone, often causing considerable cosmetic and functional deformity. These lesions require complete surgical removal. Notably, this tumor is a frequent manifestation of the hyperparathyroidism-jaw tumor syndrome, caused by germline mutations in the HRPT2 gene 7. The third category of fibro-osseous lesions, cemento-osseous dysplasia (COD), comprises a group of common benign lesions that occur exclusively in the tooth-bearing areas of the jaws and are presumed to arise from cells of the periodontal ligament 1. The terms periapical-, focal and florid- COD have been used to describe this group of lesions, depending on the site of occurrence and the extent of jaw involvement. These lesions are most often detected as incidental findings during routine dental radiographic examinations. Except for florid COD, the COD lesions seldom expand the jaws and do not displace teeth and require no treatment.
Despite their similar and often indistinguishable histological appearances, the clinical manifestations, radiographic appearances and most importantly, the biological behaviors of the various fibro-osseous lesions are dramatically different. Although there is no evidence of a common molecular pathogenesis, some groups have considered that these fibro-osseous lesions represent a spectrum of the same disease entity 8, 9. It is well established that MAS is caused by postzygotic activating mutations of the GNAS gene 10, which encodes the α-subunit of the stimulatory G-protein Gs. These mutations, at codon 201, replace an arginine residue with either histidine or cysteine. The mutations are also present in FD lesions not associated with MAS 11, suggesting that monostotic FD, polyostotic FD and MAS share a common molecular etiology. Two previous reports have demonstrated lack of GNAS mutations in gnathic COF lesions 12, 13. However, it is not known whether somatic mutations of GNAS contribute to the pathogenesis of COD. Here we report that mutations of the GNAS gene are absent in COD and COF of the jaws and highlight a clear molecular distinction between FD and other fibro-osseous lesions of the jaws.
Materials and Methods
Tissue samples
Formalin-fixed, paraffin-embedded tissue blocks from 8 cemento-ossifying fibromas and 24 cemento-osseous dysplasias (11 focal- and 13 florid cemento-osseous dysplasias) were used in this study. All cases were diagnosed histologically as fibro-osseous lesions and the final diagnosis was established after considering the clinical manifestations and radiographic features. As positive control, we used genomic DNA from a MAS-associated fibrous dysplasia lesion, with a previously confirmed R201H mutation in the GNAS gene 14. This study was approved by the Institutional Review Boards at the University of Connecticut Health Center, University of Michigan and the Medical College of Georgia.
DNA extraction
Genomic DNA was extracted as described previously 15. Approximately 15-20 10-μm thick sections were deparafinnized twice in xylenes for 10 minutes each and dehydrated in 100% ethanol twice for 10 minutes each. The samples were dried at room temperature and resuspended in 500 μl DNA extraction buffer (100mM sodium chloride, 10mM Tris-hydrochloride, pH 8.0, 25mM EDTA, pH 8.0, 0.5% SDS and 0.3 mg/ml proteinase K, Sigma, St. Louis, MO). Resuspended samples were incubated at 55°C in a shaking water bath for 72 hours. At 24 and 48 hours after the start of incubation, additional proteinase K (200μg) was added to the extraction reaction. DNA was extracted with phenol-chloroform isoamyl alcohol, precipitated with ammonium acetate and ethanol and pelleted by centrifugation using standard methods. DNA pellets were washed with 70% ethanol, dissolved in 20-30 μl of Tris–EDTA buffer (10mM Tris, 1 mM EDTA) and stored at 4°C.
Mutation analysis
To detect mutations of the Arg201 codon of the GNAS gene, we used two previously described PCR-restriction fragment length polymorphism (RFLP) strategies 16, 17 with minor modifications. Primer sequences and PCR reaction conditions are shown in Table I. All PCR amplifications were performed using a Mastercycler EPS (Eppendorf, Westbury, NY). Approximately 50ng of genomic DNA was amplified by PCR using primers P7 and P5 and 1μl of this amplification reaction was used as template for subsequent nested PCR-RFLP assays to detect GNAS Agr201 mutations. We performed two independent nested PCR reactions to yield amplicons encompassing the Arg201 codon (CGT).
Table I.
Oligonucleotide primers and primer combinations used for PCR amplification. The temperatures and times for denaturation, annealing and extension are indicated
| Primer Sequences | |
| P7 (Sense): | 5′- CCATTGACCTCAATTTTGTTTCAG -3′ |
| P5 (Antisense): | 5′- GGTAACAGTTGGCTTACTGGAAGTTG -3′ |
| P1 (Sense): | 5′- TTTTGTTTCAGGACCTGCTTCGCGGC -3′ |
| P6 (Antisense): | 5′- ACTTTGTCCCACCTGGAACTTGGTCTC -3′ |
| GNAS F (Sense): | 5′- TTGTTTCAGGACCTGCTTCGCAGC-3′ |
| GNAS R (Antisense): | 5′- AGGTAACAGTTGGCTTACTGGAAG-3′ |
| Mismatches in primers P1 and GNAS F are underlined. | |
| Primer Pair | PCR Conditions | Amplicon Size (bp) |
|---|---|---|
| P7 & P5 | Pfu DNA polymerase, 20 cycles | 115 |
| 94°C, 60 sec; 55°C, 60 sec; 75°C, 60 sec | ||
|
| ||
| Primer set A | Taq DNA polymerase, 30 cycles | 102 |
| P1 & P5 | 94°C, 30 sec; 55°C, 30 sec; 72°C, 30 sec | |
|
| ||
| Primer set A | Taq DNA polymerase, 30 cycles | 76 |
| P1 & P5 | 94°C, 30 sec; 55°C, 30 sec; 72°C, 30 sec | |
|
| ||
| Primer set C | Taq DNA polymerase, 30 cycles | 101 |
| GNAS F & GNAS R |
94°C, 30 sec; 55°C, 30 sec; 72°C, 30 sec | |
The first nested PCR reaction used primer set A (primers P1 and P5) to amplify a 102 bp region of GNAS. The sequence of primer P1 is altered from the native GNAS sequence to introduce a unique EagI restriction site in the amplified product. As a result, EagI cleaves amplicons from the normal GNAS alleles into 79- and 23 bp fragments. In contrast, the presence of any mutation at the Arg201 codon eliminates this EagI site and thus, amplification products from mutant alleles are resistant to EagI digestion. Twenty μl of each PCR reaction was digested overnight at 37°C with EagI. Digested products were analyzed by electrophoresis on a 3% agarose gel. To rule out the presence of a low frequency of EagI-resistant mutant alleles, the digested product was diluted 20 fold and used as a template for a PCR reaction with primer set B (primers P1 and P6) that flanks the EagI restriction site. Undigested (mutant) alleles yield an amplification product of 76 bp, whereas digested (normal) alleles do not yield any amplification product.
The second nested PCR reaction used primer set C (primers GNAS F and GNAS R) to yield a 101 bp amplicon. The GNAS F primer contains a mismatch at the 3′ end—this introduces a Nla III restriction site only in the presence of an R201H mutation. Thus, digestion of amplicons from R201H mutant alleles are cleaved into 73bp and 28 bp fragments, whereas the amplicons from normal alleles are resistant to digestion. Twenty μl of each PCR reaction was digested overnight at 37°C with NlaIII. Digested products were analyzed by electrophoresis on a 3% agarose gel.
Sequencing analysis
PCR amplification products generated by primer set C were cloned into the pCR2.1-TOPO vector (Invitrogen, Carlsbad, CA), following the manufacturer’s protocols. The ligation reaction was used to transform DH5-α competent E.coli and grown on Luria Broth plates containing 50μg/ml ampicillin. Twenty discrete bacterial colonies from each ligation reaction were innoculated into Luria Broth containing 50μg/ml amplicillin and grown at 37°C overnight. Plasmid DNA was extracted and purified using the QIAquick Miniprep Kit, following the manufacturer’s protocols. Purified plasmid DNA was sequenced by direct sequencing using the M13-forward primer.
Results
Genomic DNA from all fibro-osseous lesions was successfully amplified and both PCR strategies yielded amplification products of expected sizes. PCR products amplified with primer set A yielded a 102 bp fragment, whereas primer set C yielded a 101 bp amplicon. PCR-RFLP analyses of positive control DNA from the fibrous dysplasia lesion demonstrated the presence of an R201H mutation in GNAS gene, thereby validating our assays. Digestion of the positive control PCR products with the appropriate restriction enzyme showed the presence of both normal and mutant alleles (Figure 1), consistent with the frequency of the mutant allele in this DNA sample, as determined previously 14. In contrast, we did not detect GNAS mutations in any of the cemento-ossifying fibromas and cemento-osseous dysplasias examined. PCR products generated using primer set A were completely cleaved by EagI (Figure 1A). Further PCR amplification of EagI-digested products showed amplification only in the positive control, but not in any of the COF/COD lesions (Figure 1B), confirming the absence of mutant alleles. Likewise, PCR amplification products generated using primer set C were resistant to NlaIII digestion (Figure 1C).
Figure 1.
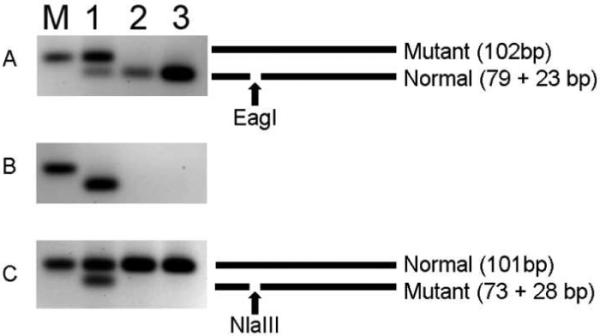
Representative examples of the PCR-RFLP strategy used to detect Arg201 mutations in GNAS. PCR reactions and restriction digests were performed as described in the Methods section and analyzed on a 3% agarose gel. The gray scales of the ethidium bromide-stained gel are reversed to enhance visualization. A schematic of the PCR products is shown to the right. Lane M: 100bp band of the DNA size marker is shown. Lane 1: Positive control (FD), lane 2: COF, lane 3: COD. Panel A: PCR amplification of DNA from COF/COD is completely digested by EagI. Panel B: PCR amplification of EagI digested products from panel A yields amplicons only for the mutant alleles. COF and COD lesions do not yield an amplification product. Panel C: PCR amplification of DNA from COF/COD is resistant to digestion by NlaIII.
To rule out the possibility of a low frequency of mutant alleles that had evaded detection, the PCR products from 1 cemento-ossifying fibroma and 2 cemento-osseous dysplasias were cloned into plasmid vectors and used to transform E. coli. For each lesion, plasmid DNA from twenty independent transformed colonies was extracted and sequenced. Sequence analysis confirmed that the appropriate region of GNAS was amplified. All amplified sequences contained wild-type GNAS sequence (Figure 2).
Figure 2.
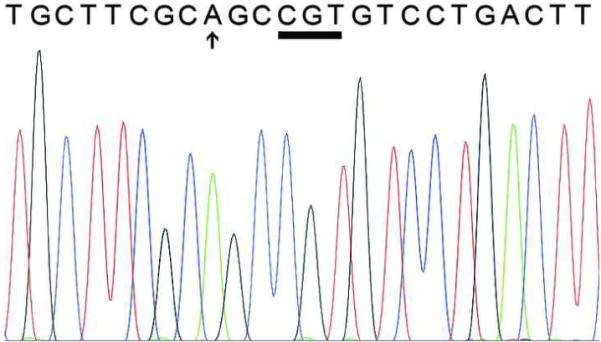
Representative sequence from one of the cloned PCR products showing wild-type GNAS sequence. The Arg201 codon (CGT) is underlined. The arrow indicates the base alteration in the PCR primer.
Discussion
Maxillofacial fibro-osseous lesions are a complex group of lesions that share a similar histological appearance. These lesions often pose a diagnostic challenge and the eventual diagnosis has to be based on careful consideration of the clinical and radiographic features. Despite their histological similarity, these lesions differ dramatically in their clinical manifestations and their biological behaviors and thus, are managed by different approaches. Given their similarities, it has been suggested that COF is a variant of FD 8 and that these two lesions represents opposing ends of a morphological spectrum of a single disease entity 9. Here, we have demonstrated a clear molecular distinction between FD and other fibro-osseous lesions of the jaws. Using 2 independent PCR-RFLP assays, we showed that GNAS mutations are absent in COF and COD lesions. FD lesions are mosaic and thus, contain a mixture of normal and mutant cells and highly sensitive methods to detect an extremely low frequency of mutant alleles have been developed 18. It is not known whether COF and COD lesions are also mosaic in nature. Of note, one of our nested PCR-RFLP assays is designed to selectively enrich amplification of mutant alleles 16 and thus, would have enabled us to detect the presence of a low frequency of mutant alleles. Furthermore, we sequenced multiple clones of amplified PCR products from a subset of lesions to eliminate the possibility that our assays had failed to detect a low frequency of mutant alleles.
The absence of GNAS Arg201 mutations in any of the COF and COD lesions suggests that GNAS does not play a role in the pathogenesis of these lesions. Our findings are consistent with recent reports that showed absence of GNAS mutations in COF 12, 13. Distinction between true GNAS-positive FD and other fibro-osseous lesions of the jaws has important clinical implications, especially in clinical situations where the fibro-osseous lesions present atypical clinical and/or radiographic features. This is underscored by a recent report of a case of gnathodiaphyseal dysplasia--a syndrome with cemento-osseous lesions of jaws, sclerosis, bowing of tubular bones and overall bone fragility--that was misdiagnosed as polyostotic fibrous dysplasia 19. Although primary hyperparathyroidism is an uncommon feature of McCune-Albright syndrome, there have been reported cases of sporadic primary hyperparathyroidism in association with fibrous dysplasia of the bone, including craniofacial FD 20, 21. Similarly, primary hyperparathyroidism and COF are the major manifestations of the hyperparathyroidism-jaw tumor syndrome 7, 22. Distinction between these two conditions has significant clinical implications—patients with FD may require further assessment to evaluate endocrine status or overall skeletal metabolism, and depending on the symptoms the FD lesions may or may not require treatment. In contrast, the COF lesions in patients with hyperparathyroidism-jaw tumor syndrome require complete removal to prevent recurrence. Such patients also need to be tested to confirm the presence of germline HRPT2 (CDC73) mutations and may benefit from genetic counseling. Thus, an inaccurate diagnosis of the co-existing fibro-osseous lesion in this regard may significantly influence patient management.
In summary, our study has for the first time systematically evaluated fibro-osseous lesions of the jaws and demonstrated a clear distinction between COF and COD lesions from the GNAS-positive FD lesions.
Acknowledgements
This work was supported by NIH grants DE0014773 (SM) and DE007302 (MP and SM) and by institutional funds from the University of Connecticut School of Dental Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Waldron CA. Fibro-osseous lesions of the jaws. J Oral Maxillofac Surg. 1993 Aug;51(8):828–35. doi: 10.1016/s0278-2391(10)80097-7. [DOI] [PubMed] [Google Scholar]
- 2.Slootweg PJ. Lesions of the jaws. Histopathology. 2008;54(4):401–18. doi: 10.1111/j.1365-2559.2008.03097.x. [DOI] [PubMed] [Google Scholar]
- 3.Weinstein LS. G(s)alpha mutations in fibrous dysplasia and McCune-Albright syndrome. J Bone Miner Res. 2006 Dec;21(Suppl 2):P120–4. doi: 10.1359/jbmr.06s223. [DOI] [PubMed] [Google Scholar]
- 4.Hart ES, Kelly MH, Brillante B, Chen CC, Ziran N, Lee JS, et al. Onset, progression, and plateau of skeletal lesions in fibrous dysplasia and the relationship to functional outcome. J Bone Miner Res. 2007 Sep;22(9):1468–74. doi: 10.1359/jbmr.070511. [DOI] [PubMed] [Google Scholar]
- 5.DiCaprio MR, Enneking WF. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. The Journal of bone and joint surgery. 2005 Aug;87(8):1848–64. doi: 10.2106/JBJS.D.02942. [DOI] [PubMed] [Google Scholar]
- 6.Chapurlat RD. Medical therapy in adults with fibrous dysplasia of bone. J Bone Miner Res. 2006 Dec;21(Suppl 2):P114–9. doi: 10.1359/jbmr.06s222. [DOI] [PubMed] [Google Scholar]
- 7.Carpten JD, Robbins CM, Villablanca A, Forsberg L, Presciuttini S, Bailey-Wilson J, et al. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat Genet. 2002;32(4):676–80. doi: 10.1038/ng1048. [DOI] [PubMed] [Google Scholar]
- 8.Sissons HA, Steiner GC, Dorfman HD. Calcified spherules in fibro-osseous lesions of bone. Archives of pathology & laboratory medicine. 1993 Mar;117(3):284–90. [PubMed] [Google Scholar]
- 9.Voytek TM, Ro JY, Edeiken J, Ayala AG. Fibrous dysplasia and cemento-ossifying fibroma. A histologic spectrum. Am J Surg Pathol. 1995 Jul;19(7):775–81. [PubMed] [Google Scholar]
- 10.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991 Dec 12;325(24):1688–95. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 11.Bianco P, Riminucci M, Majolagbe A, Kuznetsov SA, Collins MT, Mankani MH, et al. Mutations of the GNAS1 gene, stromal cell dysfunction, and osteomalacic changes in non-McCune-Albright fibrous dysplasia of bone. J Bone Miner Res. 2000 Jan;15(1):120–8. doi: 10.1359/jbmr.2000.15.1.120. [DOI] [PubMed] [Google Scholar]
- 12.Hasselblatt M, Jundt G, Greiner C, Rama B, Schmal F, Iglesias-Rozas JR, et al. Juvenile psammomatoid ossifying fibroma of the neurocranium. Report of four cases. Journal of neurosurgery. 2005 Jun;102(6):1151–4. doi: 10.3171/jns.2005.102.6.1151. [DOI] [PubMed] [Google Scholar]
- 13.Toyosawa S, Yuki M, Kishino M, Ogawa Y, Ueda T, Murakami S, et al. Ossifying fibroma vs fibrous dysplasia of the jaw: molecular and immunological characterization. Mod Pathol. 2007 Mar;20(3):389–96. doi: 10.1038/modpathol.3800753. [DOI] [PubMed] [Google Scholar]
- 14.Malchoff CD, Reardon G, MacGillivray DC, Yamase H, Rogol AD, Malchoff DM. An unusual presentation of McCune-Albright syndrome confirmed by an activating mutation of the Gs alpha-subunit from a bone lesion. J Clin Endocrinol Metab. 1994 Mar;78(3):803–6. doi: 10.1210/jcem.78.3.8126161. [DOI] [PubMed] [Google Scholar]
- 15.Isola J, DeVries S, Chu L, Ghazvini S, Waldman F. Analysis of changes in DNA sequence copy number by comparative genomic hybridization in archival paraffin-embedded tumor samples. Am J Pathol. 1994;145(6):1301–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Candeliere GA, Roughley PJ, Glorieux FH. Polymerase chain reaction-based technique for the selective enrichment and analysis of mosaic arg201 mutations in G alpha s from patients with fibrous dysplasia of bone. Bone. 1997 Aug;21(2):201–6. doi: 10.1016/s8756-3282(97)00107-5. [DOI] [PubMed] [Google Scholar]
- 17.Collins MT, Sarlis NJ, Merino MJ, Monroe J, Crawford SE, Krakoff JA, et al. Thyroid carcinoma in the McCune-Albright syndrome: contributory role of activating Gs alpha mutations. J Clin Endocrinol Metab. 2003 Sep;88(9):4413–7. doi: 10.1210/jc.2002-021642. [DOI] [PubMed] [Google Scholar]
- 18.Karadag A, Riminucci M, Bianco P, Cherman N, Kuznetsov SA, Nguyen N, et al. A novel technique based on a PNA hybridization probe and FRET principle for quantification of mutant genotype in fibrous dysplasia/McCune-Albright syndrome. Nucleic Acids Res. 2004;32(7):e63. doi: 10.1093/nar/gnh059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riminucci M, Collins MT, Corsi A, Boyde A, Murphey MD, Wientroub S, et al. Gnathodiaphyseal dysplasia: a syndrome of fibro-osseous lesions of jawbones, bone fragility, and long bone bowing. J Bone Miner Res. 2001 Sep;16(9):1710–8. doi: 10.1359/jbmr.2001.16.9.1710. [DOI] [PubMed] [Google Scholar]
- 20.Braccini F, Bacciu A, Bruzzo M, Pech-Gourg F, Thomassin JM. Craniofacial fibrous dysplasia associated with primary hyperparathyroidism. Acta Biomed Ateneo Parmense. 1999;70(1-2):5–11. [PubMed] [Google Scholar]
- 21.Kim SJ, Seok JW, Kim IJ, Kim YK, Jung DS, Kim DS. Fibrous dysplasia associated with primary hyperparathyroidism in the absence of the McCune-Albright syndrome: Tc-99m MIBI and Tc-99m MDP findings. Clinical nuclear medicine. 2003 May;28(5):416–8. doi: 10.1097/01.RLU.0000063418.87438.6D. [DOI] [PubMed] [Google Scholar]
- 22.Cavaco BM, Barros L, Pannett AA, Ruas L, Carvalheiro M, Ruas MM, et al. The hyperparathyroidism-jaw tumour syndrome in a Portuguese kindred. Qjm. 2001;94(4):213–22. doi: 10.1093/qjmed/94.4.213. [DOI] [PubMed] [Google Scholar]


