Abstract
Neurologic disease is a major cause of disability in resource-poor countries and a substantial portion of this disease is due to infections of the CNS. A wide variety of emerging and re-emerging viruses contribute to this disease burden. New emerging infections are commonly due to RNA viruses that have expanded their geographic range, spread from animal reservoirs or acquired new neurovirulence properties. Mosquito-borne viruses with expanding ranges include West Nile virus, Japanese encephalitis virus and Chikungunya virus. Zoonotic viruses that have recently crossed into humans to cause neurologic disease include the bat henipaviruses Nipah and Hendra, as well as the primate-derived human immunodeficiency virus. Viruses adapt to new hosts, or to cause more severe disease, by changing their genomes through reassortment (e.g. influenza virus), mutation (essentially all RNA viruses) and recombination (e.g. vaccine strains of poliovirus). Viruses that appear to have recently become more neurovirulent include West Nile virus, enterovirus 71 and possibly Chikungunya virus. In addition to these newer challenges, rabies, polio and measles all remain important causes of neurologic disease despite good vaccines and global efforts toward control. Control of human rabies depends on elimination of rabies in domestic dogs through regular vaccination. Poliovirus eradication is challenged by the ability of the live attenuated vaccine strains to revert to virulence during the prolonged period of gastrointestinal replication. Measles elimination depends on delivery of two doses of live virus vaccine to a high enough proportion of the population to maintain herd immunity for this highly infectious virus.
Abbreviations: CHIKV, Chikungunya virus; CNS, central nervous system; DALY, disability adjusted life year; EV71, enterovirus 71; HIV, human immunodeficiency virus; JEV, Japanese encephalitis virus; SSPE, subacute sclerosing panencephalitis; WNV, West Nile virus
Keywords: Viral encephalitis, Measles, Polio, Subacute sclerosing panencephalitis, Chikungunya, West Nile virus, Rabies, Japanese encephalitis
1. Introduction
The highest burden of neurologic diseases, as defined by disability adjusted life years (DALYs), occurs in countries with the lowest income levels (Johnston and Hauser, 2008). These countries have a disproportionate amount of disease due to neurologic damage associated with trauma and asphyxia at birth, but infections of the central nervous system (CNS) are also over-represented as a cause of death and long-term disability. Some infections, such as bacterial meningitis and human immunodeficiency virus (HIV)-associated dementia, are important causes of CNS disease worldwide, while infections such as cerebral malaria, African trypanosomiasis and viral encephalomyelitis are often more geographically restricted. Many of the important viral neurologic infections are new diseases that have recently expanded their geographic range, spread from animal reservoirs or evolved to become more neurovirulent, while others are old diseases that continue to be problematic because of difficulties with treatment, vaccine delivery or vector control.
2. Emerging virus infections of the CNS
New infectious diseases are continuously emerging in the human population. These infections are most commonly due to RNA viruses and half of these newly recognized infections cause neurologic disease (Olival and Daszak, 2005). Changes in vector populations, in human association with reservoir hosts and appearance of new viral variants that are more efficiently transmitted are associated with emerging viruses. Emergence of new viral diseases of the CNS can come through expansion of viruses to new geographic regions, spread from an animal reservoir or by the acquisition of increased neurovirulence by a previously non-neurotropic virus.
2.1. Expansion of viruses to new geographic regions
Important examples of the expansion of the range of encephalitic viruses have been seen with the arthropod-borne viruses. These viruses are maintained in natural cycles involving both vertebrate and invertebrate hosts and can increase their range and cause human disease if introduced into a new location that has appropriate hosts available to maintain the natural cycle. Modern transportation has introduced vectors that efficiently transmit arboviruses into new areas (e.g. the Asian tiger mosquito Aedes albopictus into North America and Europe) and pre-existing or new populations of competent vectors set the stage for successful establishment of viruses in new regions. The 1999 introduction of West Nile virus (WNV) into North America from the Middle East (Gubler, 2007), the spread of Japanese encephalitis virus (JEV) into India, Nepal and northern Australia (Solomon et al., 2003, van den Hurk et al., 2009) and the explosive 2005–2007 outbreaks of Chikungunya virus infection in the Indian Ocean islands and India (Powers and Logue, 2007, Renault et al., 2007) are examples of this mechanism.
Once introduced into New York, probably through air transport, WNV infected a variety of susceptible mosquito and bird species to establish an endemic cycle and spread rapidly across America. WNV has resulted in tens of thousands of cases of CNS disease in North America and is now endemic across the continent. WNV has the widest distribution of all flaviviruses as its geographic range now spans North and South America, Europe, the Middle East, Africa, Western Asia and Australia (Gubler, 2007). The lineage 1 strain of WNV introduced into the United States is a more neurovirulent strain that causes encephalitis than the lineage 2 African strains that cause febrile illness (Beasley et al., 2002).
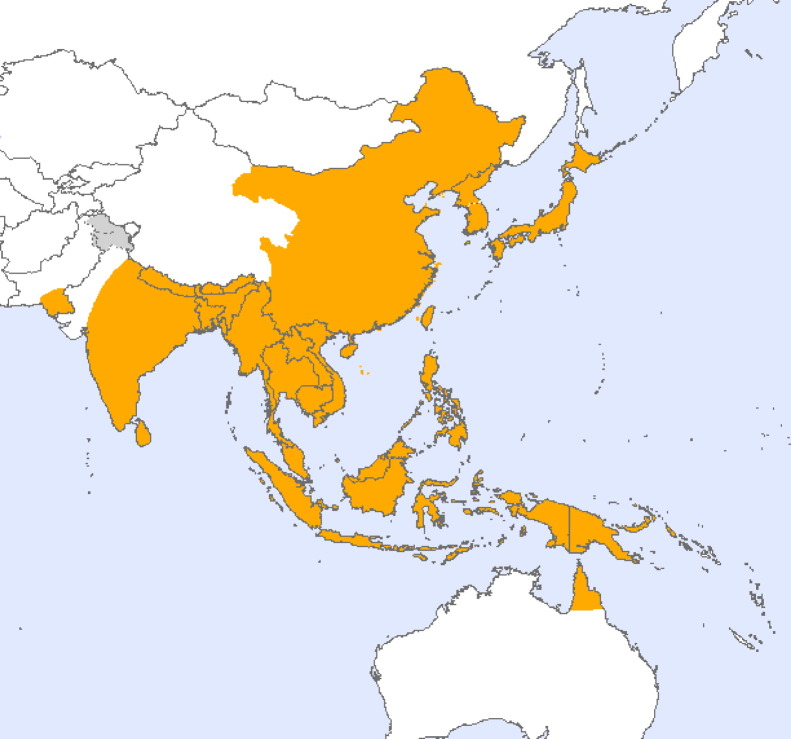
Japanese encephalitis is the leading cause of viral encephalitis in Asia, with an annual incidence of 30,000–50,000 cases of neurologic disease. JEV is distributed in temperate and tropical areas of eastern and southern Asia and maintained in a zoonotic cycle between Culex mosquitoes and wading birds or pigs (van den Hurk et al., 2009). Recent geographic extension to Pakistan, Papua New Guinea and the Torres Strait of northern Australia (Fig. 1 ) may have involved viremic migratory birds or transport of infected mosquitoes by wind, land vehicles or planes. Emergence is also associated with the increases in endemic areas of population, pig production and irrigated rice farming (Erlanger et al., 2009). The case fatality rate is typically between 20% and 40% with children and the elderly at greatest risk. Effective vaccines are increasingly available and appropriate use should decrease the disease burden (Solomon, 2006).
Fig. 1.
Current geographic distribution of Japanese encephalitis virus. Source: World Health Organization.
An additional recent dramatic example of arbovirus expansion has been the appearance of explosive outbreaks of rash and arthritis due to CHIKV infection on islands in the Indian Ocean with subsequent spread to India and Europe (Powers and Logue, 2007). The introduced CHIKV strain is a variant of the Central/East African genotype that is efficiently transmitted by Aedes albopictus (Chevillon et al., 2008). Although occasionally recognized previously, large numbers of neurologic complications have been reported only recently, although the actual frequency of neurologic disease is still unclear (Arpino et al., 2009). Neurologic complications are age-dependent with the greatest susceptibility in the very young and the elderly. Encephalopathy is the main manifestation in newborns, while meningitis and encephalitis occur in older children and adults (Arpino et al., 2009, Chandak et al., 2009).
2.2. Spread of viruses into humans from animal reservoirs
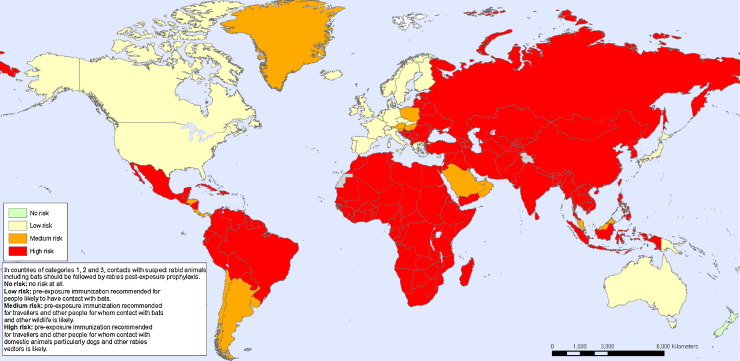
Exposure of humans to animals provides the opportunity for animal viruses to spread into human populations. This may result only in infection of the exposed human (e.g. rabies after a bite from a rabid animal) or in infection that has or gains the ability to spread within the human population (e.g. HIV, SARS coronavirus) (Wolfe et al., 2007). For instance, lentiviruses of primates have been transmitted on multiple occasions to humans, but only a few strains acquired the ability for human-to-human transmission to become the HIV strains that are currently prevalent in human populations around the world (Sharp et al., 2001). If, such as rabies, the virus remains a zoonosis with intermittent transmission to humans, then control of infection in animal populations is necessary to prevent human disease (Zinsstag et al., 2007). For rabies, regular vaccination of domestic dogs is the most important intervention, but much of the world remains at risk (Fig. 2 ) (Hampson et al., 2009, Rupprecht et al., 2008).
Fig. 2.
Map of the geographic areas of the world where rabies is considered a high risk. Red areas are high risk from contact with rabid dogs and orange areas are high risk from contact with infected bats and other wildlife. Gray and green areas are low or no risk. Source: World Health Organization.
Often, there may be an intermediate host for the virus on the way to becoming a human disease. Bats are increasingly recognized as important hosts for a number of zoonoses that cause CNS infection (e.g. lyssaviruses, henipaviruses, coronaviruses and filoviruses) (Halpin et al., 2007). Disruption of the environment with changing agricultural practices has increased the likelihood that these viruses will be transmitted to humans. Recent outbreaks of neurologic disease in humans due to the pteropid bat viruses Hendra and Nipah illustrate this process.
Hendra is a paramyxovirus that was first identified when horses, and a few humans in close association with the horses in Australia, became ill with respiratory and neurologic disease that was often fatal. The related Nipah virus was recognized in Malaysia because of an outbreak of respiratory disease in swine and encephalitis in humans exposed to infected swine. For each, horses and pigs became infected through exposure to infected bats, and humans became infected through exposure to the infected horses and pigs. The viruses do not cause disease in the bat reservoir hosts. Recently, in Bangladesh, humans have developed fatal neurologic disease directly from exposure to infected bats with evidence of subsequent human-to-human spread (Epstein et al., 2006, Gurley et al., 2007).
2.3. Genetic mechanisms for virus adaptation
Adaptation to a new host requires the virus to change. Viruses, particularly RNA viruses, are adept at change and have several mechanisms for altering their genomes. The basic mechanisms are: shuffling gene segments by reassortment (applies to viruses with segmented genomes), mutation (applies to all viruses) and recombination (applies to most viruses).
Influenza virus has eight gene segments and is the most important virus that evolves by reassortment. When two different strains of influenza (e.g. an avian strain and a human strain) infect the same host cell, the new progeny viruses can reassort the two sets of eight genes with a potential for 256 different combinations of segments in individual virus particles. This process results in viruses with new properties. Most will be less “fit” for human infection than the original viruses, but others may be able to replicate well in humans that do not have immunity to the new virus. This is the process that resulted in the antigenic shift that occurred prior to the influenza pandemics of 1957, 1968 and, most recently, 2009 (Garten et al., 2009). Although most of the disease is confined to the respiratory tract, neurologic complications of influenza occur, particularly in children (CDC, 2009) and for H5N1 avian influenza virus, invasion of the CNS has been documented (De et al., 2005). After new strains are established in the human population, they evolve slowly by mutation (antigenic drift).
Mutation is an important mechanism of change for all RNA viruses because viral RNA-dependent RNA polymerases are error-prone and have no built in mechanism for correcting mistakes (Domingo et al., 2006). Polymerase errors, plus the lack of proofreading, results in one mistake approximately every 10,000 nucleotides, an average of one error in every genome. Furthermore, progeny viruses are produced in large numbers by infected cells. Most of these mutations will be deleterious to virus growth, but will occasionally be advantageous. It has recently been shown in animal models of polio and foot and mouth disease that this ability to vary is important for virulence (Vignuzzi et al., 2006, Sanz-Ramos et al., 2008).
An additional mechanism for virus evolution is recombination. In this case, two similar viruses that infect the same cell can recombine different portions of their genomes to produce new viruses. This is commonly observed for enteroviruses that can infect and be shed from the gastrointestinal tract for long periods of time. This is of current importance for poliovirus where the live virus vaccine includes three strains of polio that not only mutate, but also recombine with themselves and other enteroviruses to produce new vaccine-derived strains that may have increased virulence (Cherkasova et al., 2003).
2.4. Acquisition of increased virulence
Several recently emergent viruses, including WNV and CHIKV, appear to be more neurovirulent than their predecessors, although specific mutations responsible for this have not been identified. In general, increased neurovirulence is associated with improved ability of a virus to enter the CNS and usually with more efficient replication in neurons that can result in neuronal cell death (Griffin, 1998). Another important current example is enterovirus 71 (EV71). EV71, along with other closely related type A enteroviruses, causes hand, foot and mouth disease. However, EV71 has gained the ability to invade the CNS and cause a spectrum of serious neurologic syndromes, including meningitis, encephalitis and paralysis, in young children (Modlin, 2007, Chang et al., 2007). Outbreaks in Southeast Asia began in 1997 in Malaysia. Epidemics have subsequently occurred in multiple countries in the region (Anonymous, 2009, Chang et al., 2007). In 2008, China had 500,000 cases and 200 deaths due to EV71 subgenotype C4a (Zhang et al., 2009, Anonymous, 2009). Severe disease in Asia may be related to genetic susceptibility (Chang et al., 2008). The epidemiology is reminiscent of the epidemics of polio in Europe and North America in the first half of the 20th century and suggests the need for development of a vaccine for EV71 (Modlin, 2007).
3. Problems with old or re-emerging viruses that cause neurologic disease
Viruses, such as smallpox, that have humans as the only host, offer the opportunity for eradication and this is a current goal for poliovirus. Features of smallpox that were important for the eradication effort included a distinctive rash that facilitated identifications of all infections, and the availability of an easily administered vaccine that conferred long-lasting protective immunity. Other factors that affect the possibility of virus eradication include the infectiousness of the virus and an international commitment to the goal of eradication.
Two human viruses that cause substantial neurologic disease, for which there are ongoing international eradication or elimination efforts are poliovirus and measles virus. For each, problems have arisen that have made these lofty goals more difficult than originally envisioned.
3.1. Poliovirus
Poliovirus is an enterovirus that is spread by the oral route through contaminated food and water and causes asymptomatic infection in the majority of people. It is approximately two times more infectious than smallpox virus, but only one in 100–200 will develop virus spread to motor neurons in the CNS followed by acute flaccid paralysis due to motor neuron death or dysfunction. Thus, unlike smallpox, poliovirus can circulate widely in the population in the absence of symptoms. Furthermore, virus can continue to be shed from the gastrointestinal tract for weeks after infection. Therefore, laboratory tests are needed to identify poliovirus infection and surveillance relies on identifying cases of acute flaccid paralysis associated with poliovirus in the stool.
There are three poliovirus serotypes and immunity must be induced to all three for full protection from paralytic disease. This can be accomplished either through the use of an inactivated vaccine or a live attenuated virus vaccine. The inactivated vaccine induces serum antibody that prevents virus spread to the CNS, but does not necessarily prevent infection of the gut. The live attenuated vaccine is essential for eradication efforts because it is delivered orally, infects and induces immunity in the intestinal tract and protects against subsequent wild type virus infection and thus interrupts silent spread of wild type virus from person-to-person.
The original poliovirus eradication goal was 2000. At that time, two of six WHO regions, the Americas and Western Pacific, were certified free of wild type poliovirus and only a few countries in the other regions had continuing wild type virus circulation. Confirmed cases of poliovirus-induced paralysis worldwide had decreased from 350,000 to 2971.
However, in the same year a new problem was recognized. Viruses isolated from a cluster of children with paralysis on the Caribbean island of Hispaniola were identified as mutated vaccine viruses that had circulated in an under vaccinated population for about 2 years and had reacquired neurovirulence. Subsequently, several other polio outbreaks were identified as due to vaccine-derived polioviruses that can circulate widely (Wringe et al., 2008). The strains of live attenuated vaccine viruses have only a small number of genetic changes from the wild type viruses from which they were derived (Kew et al., 2005). Revertants that re-gain a neurovirulent phenotype are selected rapidly in the intestine and shed in stool (Minor et al., 1986, Chumakov et al., 2007). Despite this, disease in vaccinees is rare because infants are protected from spread to the CNS by maternal antibody. However, spread to nonprotected individuals can occur and result in paralytic disease. Recombination between the vaccine strains, as well as other related enteroviruses, such as Coxsackie A viruses, is also frequent, although importance of this for acquisition of virulence is less clear (Chumakov et al., 2007, Cherkasova et al., 2003, Minor et al., 1986).
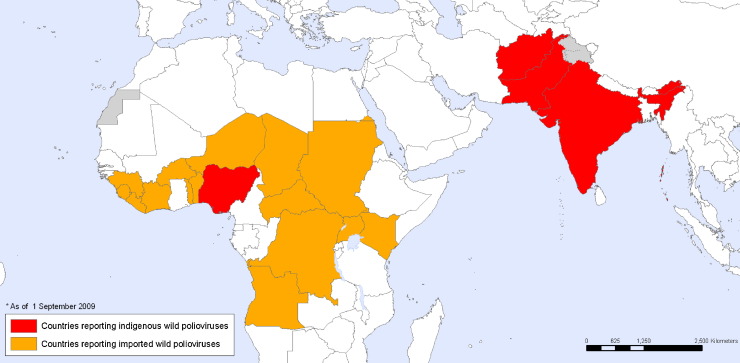
In 2002, only India, Nigeria, Pakistan and Afghanistan had endemic circulation of wild type poliovirus, but since that time there has been spread from these countries, particularly Nigeria, to previously polio-free regions and the eradication effort has stalled (Fig. 3 ) (Pallansch and Sandhu, 2006, Chumakov et al., 2007). In addition, it appears that in some populations (e.g. the northern regions of India), protective immunity is not always achieved even after multiple doses of vaccine (Grassly et al., 2009). Because of prolonged shedding of vaccine virus, particularly by immunodeficient individuals, the current endgame strategy is unclear (Kew et al., 2005, Minor, 2009), but will probably require the development of a new type of polio vaccine (Chumakov and Ehrenfeld, 2008).
Fig. 3.
September 2009 map of countries reporting indigenous wild poliovirus (red) or imported wild poliovirus (orange). Source: World Health Organization.
3.2. Measles
Measles virus is a paramyxovirus that is spread through the respiratory route and causes a rash disease. Despite the availability of an excellent attenuated live virus vaccine, measles continues to cause the deaths of hundreds of thousands of children in developing countries every year (Ferrari et al., 2008, Wolfson et al., 2009, Wolfson et al., 2007, Grais et al., 2007).
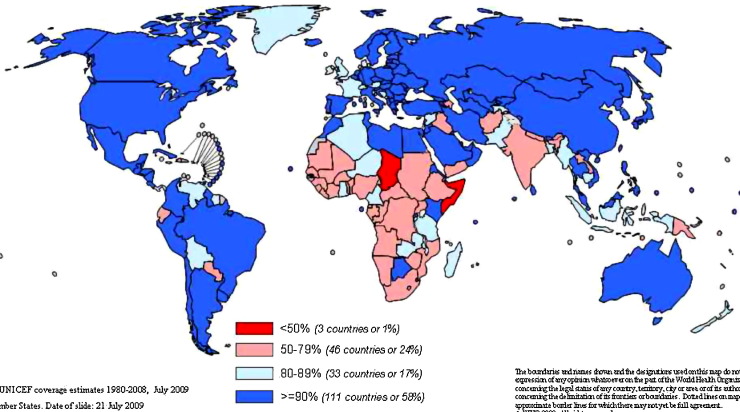
Like polio and smallpox viruses, measles is a human virus that has been identified as a candidate for eradication (Sencer et al., 1967). Although infection usually leads to a readily identifiable rash disease, virus transmission begins prior to the onset of the rash and is very efficient. The infectiousness of measles virus has proven to be one of the barriers to elimination, as the threshold for herd immunity requires 93–95% of the population to be immune. Achieving this level of immunity with a vaccine that has a 95% seroconversion rate requires delivery of two doses of the vaccine (Moss and Griffin, 2006). Achieving and maintaining this level of coverage in developing countries of Africa and Asia has been challenging (Fig. 4 ).
Fig. 4.
UNICEF estimates of measles vaccine coverage in July 2009. Red countries are below 50%, pink countries 50–79%, light blue countries 80–89% and dark blue countries greater than 90%. Source: World Health Organization.
The first dose is generally given as part of a routine vaccination program. With the Expanded Program on Immunization and the Measles Vaccine Initiative, coverage in many developing countries has improved. In developed countries, the second dose is generally given at school entry. In resource-poor countries, the most effective delivery has been through mass campaigns or national immunization days, a strategy pioneered by the Pan American Health Organization (Moss and Griffin, 2006). These campaigns target large proportions of the population, quickly interrupt endemic measles virus transmission and are credited with a steady decrease in the annual mortality due to measles. If campaigns are not repeated every 3–4 years, susceptible individuals will build up and measles will again become endemic as virus is inevitably imported from countries with continued measles transmission.
The neurological complications of measles include the autoimmune disease, acute disseminated encephalomyelitis (1:1000, primarily older children) and the progressive CNS infection subacute sclerosing panencephalitis (SSPE) (1:10,000, primarily children infected before the age of 2 years) appearing 7–10 years after acute infection (Halsey et al., 1980). Although these complications are relatively rare, measles in Africa often occurs in young infants most at risk for SSPE (Halsey et al., 1980, Moss et al., 2002, Grais et al., 2007, Moss et al., 2008). However, there are few reports of SSPE from Africa and those that have appeared suggest that the epidemiology may be different from that reported for the United States and Europe (Schoub et al., 1992, Carman and Johnson, 1989). Because of the difficulty of the diagnosis and a shortage both of neuroimaging facilities and neurologists, the disease may not always be recognized or reported. Alternatively, cases may be being diagnosed, but reports have not appeared in the literature (Johnston and Hauser, 2008). More research on neurologic complications of measles in developing countries will help to determine if manifestations and susceptibility differ between populations and may shed light on the poorly understood pathogenesis of SSPE.
4. Contributions of immunosuppression to increased neurologic disease due to infection
The nervous system can be a frequent site for opportunistic infections associated with immunosuppression due to HIV infection (Simpson and Tagliati, 1994) or treatment of malignancy or autoimmune disease with immunomodulatory drugs (Major, 2009). These opportunistic neurologic infections can be new primary infections (e.g. cryptococcus, syphilis) or reactivation of infections that were previously controlled by the host immune response (e.g. tuberculosis, toxoplasmosis, cytomegalovirus, varicella zoster virus, progressive multifocal encephalopathy). Furthermore, there is a risk of CNS immune reconstitution inflammatory syndrome when antiretroviral treatment is initiated in HIV-infected individuals with profound immunosuppression. With widespread prevalence of HIV infection and increasing availability and use of antiretroviral and immunomodulatory drugs for treatment of HIV and autoimmune diseases, the importance of this source of neurologic disease is likely to increase.
5. Conclusions
Virus infections of the CNS are an important, but likely under-diagnosed cause of neurological disease in many developing countries. Because viruses are constantly evolving, there is a continuing opportunity for infection of new hosts, extending range, increasing virulence and producing new diseases. Furthermore, HIV and drug-induced immunosuppression has increased susceptibility and incidence of neurologic infections. Application of currently available control measures can lessen the burden of neurological disease in many vulnerable populations.
Acknowledgments
Work from the author's laboratory was supported by the National Institutes of Health (NS18596, NS038932 and AI023047) and the Bill and Melinda Gates Foundation (RG3522).
References
- Anonymous Watch your back. Nature. 2009;459:751–752. doi: 10.1038/459751b. [DOI] [PubMed] [Google Scholar]
- Arpino C., Curatolo P., Rezza G. Chikungunya and the nervous system: what we do and do not know. Rev. Med. Virol. 2009;19:121–129. doi: 10.1002/rmv.606. [DOI] [PubMed] [Google Scholar]
- Beasley D.W., Li L., Suderman M.T., Barrett A.D. Mouse neuroinvasive phenotype of West Nile virus strains varies depending upon virus genotype. Virology. 2002;296:17–23. doi: 10.1006/viro.2002.1372. [DOI] [PubMed] [Google Scholar]
- Carman W.F., Johnson S. Subacute sclerosing panencephalitis in South Africa. Trans. R. Soc. Trop. Med. Hyg. 1989;83:117–118. doi: 10.1016/0035-9203(89)90732-3. [DOI] [PubMed] [Google Scholar]
- CDC Neurologic complications associated with novel Influenza A (H1N1) virus infection in children—Dallas, Texas, May 2009. MMWR. 2009;58:773–778. [PubMed] [Google Scholar]
- Chandak N.H., Kashyap R.S., Kabra D., Karandikar P., Saha S.S., Morey S.H., Purohit H.J., Taori G.M., Daginawala H.F. Neurological complications of Chikungunya virus infection. Neurol. India. 2009;57:177–180. doi: 10.4103/0028-3886.51289. [DOI] [PubMed] [Google Scholar]
- Chang L.Y., Chang I.S., Chen W.J., Huang Y.C., Chen G.W., Shih S.R., Juang J.L., Shih H.M., Hsiung C.A., Lin T.Y., Huang L.M. HLA-A33 is associated with susceptibility to enterovirus 71 infection. Pediatrics. 2008;122:1271–1276. doi: 10.1542/peds.2007-3735. [DOI] [PubMed] [Google Scholar]
- Chang L.Y., Huang L.M., Gau S.S., Wu Y.Y., Hsia S.H., Fan T.Y., Lin K.L., Huang Y.C., Lu C.Y., Lin T.Y. Neurodevelopment and cognition in children after enterovirus 71 infection. N. Engl. J. Med. 2007;356:1226–1234. doi: 10.1056/NEJMoa065954. [DOI] [PubMed] [Google Scholar]
- Cherkasova E., Laassri M., Chizhikov V., Korotkova E., Dragunsky E., Agol V.I., Chumakov K. Microarray analysis of evolution of RNA viruses: evidence of circulation of virulent highly divergent vaccine-derived polioviruses. Proc. Natl. Acad. Sci. U.S.A. 2003;100:9398–9403. doi: 10.1073/pnas.1633511100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevillon C., Briant L., Renaud F., Devaux C. The Chikungunya threat: an ecological and evolutionary perspective. Trends Microbiol. 2008;16:80–88. doi: 10.1016/j.tim.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Chumakov K., Ehrenfeld E. New generation of inactivated poliovirus vaccines for universal immunization after eradication of poliomyelitis. Clin. Infect. Dis. 2008;47:1587–1592. doi: 10.1086/593310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumakov K., Ehrenfeld E., Wimmer E., Agol V.I. Vaccination against polio should not be stopped. Nat. Rev. Microbiol. 2007;5:952–958. doi: 10.1038/nrmicro1769. [DOI] [PubMed] [Google Scholar]
- De J., Bach V.C., Phan T.Q., Vo M.H., Tran T.T., Nguyen B.H., Beld M., Le T.P., Truong H.K., Nguyen V.V., Tran T.H., Do Q.H., Farrar J. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N. Engl. J. Med. 2005;352:686–691. doi: 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- Domingo E., Martin V., Perales C., Grande-Perez A., Garcia-Arriaza J., Arias A. Viruses as quasispecies: biological implications. Curr. Top. Microbiol. Immunol. 2006;299:51–82. doi: 10.1007/3-540-26397-7_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J.H., Field H.E., Luby S., Pulliam J.R., Daszak P. Nipah virus: impact, origins, and causes of emergence. Curr. Infect. Dis. Rep. 2006;8:59–65. doi: 10.1007/s11908-006-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlanger T.E., Weiss S., Keiser J., Utzinger J., Wiedenmayer K. Past, present, and future of Japanese encephalitis. Emerg. Infect. Dis. 2009;15:1–7. doi: 10.3201/eid1501.080311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari M.J., Grais R.F., Bharti N., Conlan A.J., Bjornstad O.N., Wolfson L.J., Guerin P.J., Djibo A., Grenfell B.T. The dynamics of measles in sub-Saharan Africa. Nature. 2008;451:679–684. doi: 10.1038/nature06509. [DOI] [PubMed] [Google Scholar]
- Garten R.J., Davis C.T., Russell-Harde D. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grais R.F., Dubray C., Gerstl S.E. Unacceptably high mortality related to measles epidemics in Niger, Nigeria, and Chad. PLoS Med. 2007;4:0122–0129. doi: 10.1371/journal.pmed.0040016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassly N.C., Jafari H., Bahl S., Durrani S., Wenger J., Sutter R.W., Aylward R.B. Mucosal immunity after vaccination with monovalent and trivalent oral poliovirus vaccine in India. J. Infect. Dis. 2009;200:794–801. doi: 10.1086/605330. [DOI] [PubMed] [Google Scholar]
- Griffin D.E. A review of alphavirus replication in neurons. Neurosci. Biobehav. Rev. 1998;22:721–723. doi: 10.1016/s0149-7634(98)00010-4. [DOI] [PubMed] [Google Scholar]
- Gubler D.J. The continuing spread of West Nile virus in the western hemisphere. Clin. Infect. Dis. 2007;45:1039–1046. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- Gurley E.S., Montgomery J.M., Hossain M.J., Bell M., Azad A.K., Islam M.R., Molla M.A., Carroll D.S., Ksiazek T.G., Rota P.A., Lowe L., Comer J.A., Rollin P., Czub M., Grolla A., Feldmann H., Luby S.P., Woodward J.L., Breiman R.F. Person-to-person transmission of Nipah virus in a Bangladeshi community. Emerg. Infect. Dis. 2007;13:1031–1037. doi: 10.3201/eid1307.061128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin K., Hyatt A.D., Plowright R.K., Epstein J.H., Daszak P., Field H.E., Wang L., Daniels P.W. Emerging viruses: coming in on a wrinkled wing and a prayer. Clin. Infect. Dis. 2007;44:711–717. doi: 10.1086/511078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsey N.A., Modlin J.F., Jabbour J.T., Dubey L., Eddins D.L., Ludwig D.D. Risk factors in subacute sclerosing panencephalitis: a case-control study. Am. J. Epidemiol. 1980;111:415–424. doi: 10.1093/oxfordjournals.aje.a112916. [DOI] [PubMed] [Google Scholar]
- Hampson K., Dushoff J., Cleaveland S., Haydon D.T., Kaare M., Packer C. Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biol. 2009;7:0462–0471. doi: 10.1371/journal.pbio.1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S.C., Hauser S.L. Neurological disease on the global agenda. Ann. Neurol. 2008;64:A11–A12. doi: 10.1002/ana.21477. [DOI] [PubMed] [Google Scholar]
- Kew O.M., Sutter R.W., de Gourville E.M., Dowdle W.R., Pallansch M.A. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu. Rev. Microbiol. 2005;59:587–635. doi: 10.1146/annurev.micro.58.030603.123625. [DOI] [PubMed] [Google Scholar]
- Major E.O. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu. Rev. Med. 2009;17:8.1–8.13. doi: 10.1146/annurev.med.080708.082655. [DOI] [PubMed] [Google Scholar]
- Minor P. Vaccine-derived poliovirus (VDPV): impact on poliomyelitis eradication. Vaccine. 2009;27:2649–2652. doi: 10.1016/j.vaccine.2009.02.071. [DOI] [PubMed] [Google Scholar]
- Minor P.D., John A., Ferguson M., Icenogle J.P. Antigenic and molecular evolution of the vaccine strain of type 3 poliovirus during the period of excretion by a primary vaccinee. J. Gen. Virol. 1986;67:693–706. doi: 10.1099/0022-1317-67-4-693. [DOI] [PubMed] [Google Scholar]
- Modlin J.F. Enterovirus deja vu. N. Engl. J. Med. 2007;356:1204–1205. doi: 10.1056/NEJMp078016. [DOI] [PubMed] [Google Scholar]
- Moss W.J., Fisher C., Scott S., Monze M., Ryon J.J., Quinn T.C., Griffin D.E., Cutts F.T. HIV type 1 infection is a risk factor for mortality in hospitalized Zambian children with measles. Clin. Infect. Dis. 2008;46:523–527. doi: 10.1086/526525. [DOI] [PubMed] [Google Scholar]
- Moss W.J., Griffin D.E. Global measles elimination. Nat. Rev. Microbiol. 2006;4:900–908. doi: 10.1038/nrmicro1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss W.J., Monze M., Ryon J.J., Quinn T.C., Griffin D.E., Cutts F. Prospective study of measles in hospitalized, human immunodeficiency virus (HIV)-infected and HIV-uninfected children in Zambia. Clin. Infect. Dis. 2002;35:189–196. doi: 10.1086/341248. [DOI] [PubMed] [Google Scholar]
- Olival K.J., Daszak P. The ecology of emerging neurotropic viruses. J. Neurovirol. 2005;11:441–446. doi: 10.1080/13550280591002450. [DOI] [PubMed] [Google Scholar]
- Pallansch M.A., Sandhu H.S. The eradication of polio-progress and challenges. N. Engl. J. Med. 2006;355:2508–2511. doi: 10.1056/NEJMp068200. [DOI] [PubMed] [Google Scholar]
- Powers A.M., Logue C.H. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J. Gen. Virol. 2007;88:2363–2377. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- Renault P., Solet J.L., Sissoko D., Balleydier E., Larrieu S., Filleul L., Lassalle C., Thiria J., Rachou E., de V.H., Ilef D., Ledrans M., Quatresous I., Quenel P., Pierre V. A major epidemic of chikungunya virus infection on Reunion Island, France, 2005–2006. Am. J. Trop. Med. Hyg. 2007;77:727–731. [PubMed] [Google Scholar]
- Rupprecht C.E., Barrett J., Briggs D., Cliquet F., Fooks A.R., Lumlertdacha B., Meslin F.X., Muler T., Nel L.H., Schneider C., Tordo N., Wandeler A.I. Can rabies be eradicated? Dev. Biol. (Basel) 2008;131:95–121. [PubMed] [Google Scholar]
- Sanz-Ramos M., az-San S.F., Escarmis C., Domingo E., Sevilla N. Hidden virulence determinants in a viral quasispecies in vivo. J. Virol. 2008;82:10465–10476. doi: 10.1128/JVI.00825-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoub B.D., Johnson S., McAnerney J.M. Observations of subacute sclerosing panencephalitis in South Africa. Trans. R. Soc. Trop. Med. Hyg. 1992;86:550–551. doi: 10.1016/0035-9203(92)90106-m. [DOI] [PubMed] [Google Scholar]
- Sencer D.J., Dull H.B., Langmuir A.D. Epidemiologic basis for eradication of measles in 1967. Public Health Rep. 1967;82:253–256. [PMC free article] [PubMed] [Google Scholar]
- Sharp P.M., Bailes E., Chaudhuri R.R., Rodenburg C.M., Santiago M.O., Hahn B.H. The origins of acquired immune deficiency syndrome viruses: where and when? Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2001;356:867–876. doi: 10.1098/rstb.2001.0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D.M., Tagliati M. Neurologic manifestations of HIV infection. Ann. Intern. Med. 1994;121:769–785. doi: 10.7326/0003-4819-121-10-199411150-00008. [DOI] [PubMed] [Google Scholar]
- Solomon T. Control of Japanese encephalitis—within our grasp? N. Engl. J. Med. 2006;355:869–871. doi: 10.1056/NEJMp058263. [DOI] [PubMed] [Google Scholar]
- Solomon T., Ni H., Beasley D.W., Ekkelenkamp M., Cardosa M.J., Barrett A.D. Origin and evolution of Japanese encephalitis virus in southeast Asia. J. Virol. 2003;77:3091–3098. doi: 10.1128/JVI.77.5.3091-3098.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hurk A.F., Ritchie S.A., Mackenzie J.S. Ecology and geographical expansion of Japanese encephalitis virus. Annu. Rev. Entomol. 2009;54:17–35. doi: 10.1146/annurev.ento.54.110807.090510. [DOI] [PubMed] [Google Scholar]
- Vignuzzi M., Stone J.K., Arnold J.J., Cameron C.E., Andino R. Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature. 2006;439:344–348. doi: 10.1038/nature04388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe N.D., Dunavan C.P., Diamond J. Origins of major human infectious diseases. Nature. 2007;447:279–283. doi: 10.1038/nature05775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson L.J., Grais R.F., Luquero F.J., Birmingham M.E., Strebel P.M. Estimates of measles case fatality ratios: a comprehensive review of community-based studies. Int. J. Epidemiol. 2009;38:192–205. doi: 10.1093/ije/dyn224. [DOI] [PubMed] [Google Scholar]
- Wolfson L.J., Strebel P.M., Gacic-Dobo M., Hoekstra E.J., McFarland J.W., Hersh B.S. Has the 2005 measles mortality reduction goal been achieved? A natural history modelling study. Lancet. 2007;369:191–200. doi: 10.1016/S0140-6736(07)60107-X. [DOI] [PubMed] [Google Scholar]
- Wringe A., Fine P.E.M., Sutter R.W., Kew O.M. Estimating the extent of vaccine-derived poliovirus infection. PLoS ONE. 2008;3:1–11. doi: 10.1371/journal.pone.0003433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Tan X.J., Wang H.Y., Yan D.M., Zhu S.L., Wang D.Y., Ji F., Wang X.J., Gao Y.J., Chen L., An H.Q., Li D.X., Wang S.W., Xu A.Q., Wang Z.J., Xu W.B. An outbreak of hand, foot, and mouth disease associated with subgenotype C4 of human enterovirus 71 in Shandong, China. J. Clin. Virol. 2009;44:262–267. doi: 10.1016/j.jcv.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Zinsstag J., Schelling E., Roth F., Bonfoh B., De S.D., Tanner M. Human benefits of animal interventions for zoonosis control. Emerg. Infect. Dis. 2007;13:527–531. doi: 10.3201/eid1304.060381. [DOI] [PMC free article] [PubMed] [Google Scholar]