Abstract
Background
Conditional survival (CS) has emerged as a clinically relevant measure of prognosis for cancer survivors. The objective of this analysis was to provide melanoma-specific CS estimates to help clinicians promote more informed patient decision-making.
Methods
Patients with melanoma and at least 5 years of follow-up were identified from the Surveillance Epidemiology and End Results (SEER) registry (1988–2000). Using the methods of Kaplan and Meier, stage-specific 5-year CS estimates were independently calculated for survivors for each year following diagnosis. Stage-specific multivariate Cox regression models including baseline survivor functions were used to calculate adjusted melanoma-specific CS for different subgroups of patients further stratified by age, gender, race, marital status, anatomic tumor location, and tumor histology.
Results
Five-year CS estimates for stage I patients remained constant at 97% annually, while for patients with stages II, III and IV disease, 5-year CS estimates from time 0 (diagnosis) to 5 years improved from 72% to 86%, 51% to 87%, and 19% to 84%, respectively. Multivariate CS analysis revealed that differences in stages II through IV CS based on age, gender and race decreased over time.
Conclusions
Five-year melanoma-specific CS estimates improve dramatically over time for survivors with advanced stages of disease. These prognostic data are critical to patients for both treatment and non-treatment related life decisions.
Keywords: melanoma, conditional survival, SEER
INTRODUCTION
In 2008, 62,480 Americans were diagnosed with melanoma, making it the sixth and seventh most common malignancy among men and women, respectively.1 The risk of developing metastatic melanoma and dying of disease is directly related to the stage of disease at presentation, which in-turn is based on primary tumor thickness, ulceration, and the presence of metastatic nodal or distant disease.2 Traditional Kaplan-Meier 5-year survival estimates for melanoma patients presenting with American Joint Committee on Cancer (AJCC) stages I, II, III and IV disease, have been reported to be 93%, 68%, 45% and 11%, respectively.3
It has become evident that many traditional survival estimates become less meaningful and less accurate for ongoing cancer survivors. As a result, conditional survival (CS), which represents the probability that a cancer patient survives additional years, given that the patient has already survived a given number of years, has emerged as a more clinically relevant measure of prognosis for patients who have survived several years beyond diagnosis and treatment. CS has been reported for patients with breast,4–6 lung,6–8 gastric,9 colon,6, 10 rectal,11 gallbladder,12, 13 ovarian,14 prostate,6, 15 head/neck16 and brain cancers.17–19 However, CS estimates have not been published for patients with melanoma. The objective of this analysis was to determine melanoma-specific CS estimates for patients with various stages of disease in order to provide information that would be valuable for discussions between clinicians and patients with respect to both treatment and non-treatment related decisions.
METHODS
Data from the Surveillance Epidemiology and End Results (SEER) database program of the National Cancer Institute (released May, 2008) was used in this study. During the inclusion period of our study (1988–2000), SEER consisted of approximately 14% of the U.S. population and includes patient demographics, tumor site and morphology, detailed pathologic information, as well as data on the first course of treatment (surgical and radiation therapy).
Patients with invasive cutaneous malignant melanoma diagnosed between 1988 and 2000 and with survival data available up to 2005 were identified from SEER (n=67,410). The 1988 study date was chosen as the earliest study date for the cohort because it was the first year SEER coded pathological fields that would allow restaging according to the AJCC 6th edition2, and 2000 was selected as the latest study date for the cohort as it was the last year of diagnosis that would allow for at least 5 years of available follow-up data. All patients were manually re-staged using updated pathological information from SEER and the staging criteria of the AJCC 6th edition staging manual.2 The study cohort was limited to patients whose invasive melanoma was their first and only cancer diagnosis (n = 48,625). Subsequently, patients with incomplete pathological data were excluded, and the final study cohort consisted of 40,520 patients with stages I to IV melanoma.
Statistical Analysis
Using the traditional methods of Kaplan and Meier20, disease-specific survival (DSS), overall survival (OS), and relative survival (the ratio of observed survival in a population to the expected survival rate) estimates at year 1 through 10 were calculated for each stage-specific cohort. Separately, for those patients with a minimum of 5 years of follow-up data available, 5-year disease-specific CS, overall CS, and relative CS estimates were calculated for each stage-specific cohort using previously described statistical methods.9 Conditional survival represents the probability that a cancer patient survived additional years, given that the patient has already survived a given number of years. For example, to compute the 5-year conditional survival for patients who have survived one year, the 6-year disease-specific survival is divided by the 1-year disease-specific survival. Accordingly, we calculated the stage-specific univariate 5-year CS for melanoma patients, given that the patients had already survived 1, 2, 3, 4, or 5 years.
To account for the potential covariate influences on the survival function, additional estimates were adjusted for age, gender, race, marital status, anatomic tumor location, and tumor histology. Specifically, the computation of the 1 to 10 year-specific DSS were completed using the adjusted survival functions for age, gender, race, anatomic tumor location, and tumor histology.21 To account for potential covariate influences, adjusted survival estimates and standard errors were obtained using the coefficient estimates from the final Cox model and the baseline survival function.21 For example, stage III CS for patients age <50 years versus age ≥50 years was calculated based on the adjusted survival functions for these two groups from the stage III cohort using the baseline hazard incorporating the influence of gender, race, marital status, anatomic tumor location, and tumor histology simultaneously. The proportional hazards assumption was assessed graphically on the basis of Schoenfeld residuals.22 The test of slope23 was also performed to assess whether the CS improvements differed by categories of age, sex, race, histology and location.
We used the following formula, a variation of the usual “Greenwood’s formula”, to calculate the standard errors of CS estimates.17
Given m time intervals, dk is the number of deaths during interval k; rk is the number at risk for interval k; S (tj|ti) is the probability of survival past tj given survival past ti.
The majority of the statistical calculations were performed using Stata/MP (version 10.0; StataCorp, College Station, TX). Prism version 5.02 (GraphPad Software, La Jolla, CA) was used to examine the significance of the rates of change for conditional survival. All P values were 2-sided, and P values ≤ 0.05 were considered significant.
RESULTS
The median follow-up for the entire cohort (n= 40,520) was 9 years, and the majority of patients were from western states. Most of the patients were diagnosed with stage I (80%), followed by stage II (12.6%), stage III (4.8%) and stage IV (2.6%) disease. There was a larger proportion of older patients (≥ 50 years) in the stages II and IV cohorts than in the stages I and III cohorts (69.8% and 71.9% v 51% and 52.4%, respectively, P < 0.001). About two-thirds of the stage IV patients were male, and there were more African American (2.5%) patients in the stage IV group than in the other three less advanced stage groups (0.4% for stage I, and 1% for stages II and III). Nodular melanoma was more commonly noted in patients with stages II (33.5%) and III (24.4%) disease, while superficial spreading melanoma was the predominant histologic subtype in the stage I group (50.9%). About two-thirds of stages I and II patients received treatment in the form of wide local excision (WLE) and/or sentinel node biopsy (SNB), while over 70% percent of the stage III patients underwent lymph node dissection (LND). For those with stage IV disease, radiation therapy was more often used (33.9%) than in the other three stage-specific cohorts.
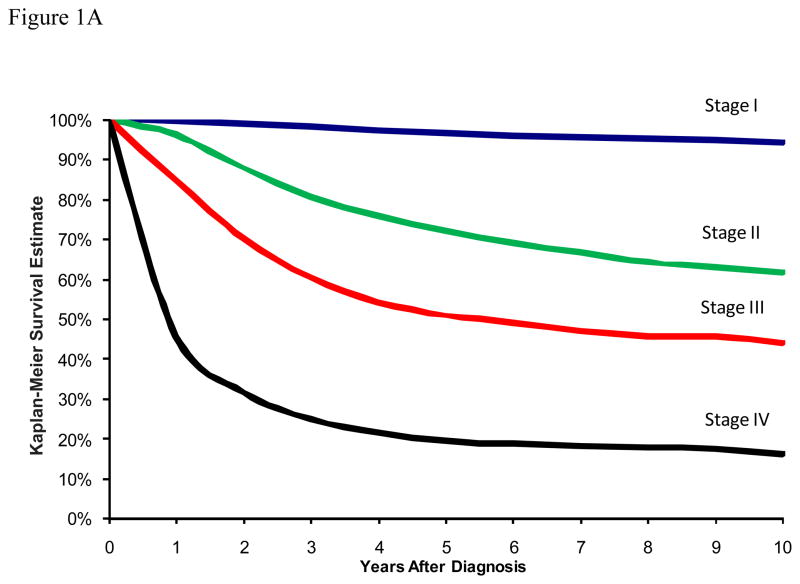
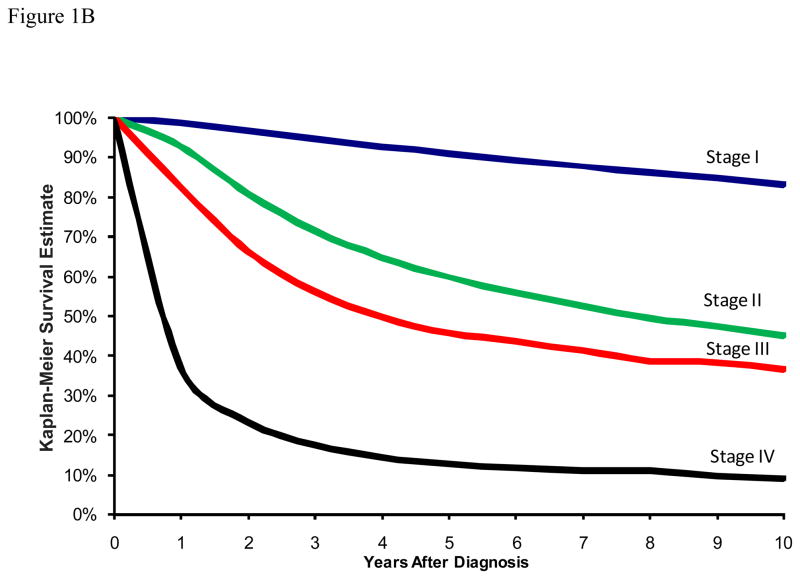
Figure 1A depicts the traditional DSS curves for patients in this cohort with stages I through IV melanoma. The 10-year DSS estimates were 94.3% (95% CI: 94.0 – 94.6) for stage I, 61.5% (95% CI: 59.9–63.1) for stage II, 44.1% (95% CI: 41.4–46.7) for stage III, and 16.1% (95% CI: 12.7–19.7) for stage IV. In terms of traditional overall survival, the 10-year survival estimates were 83.0% (95% CI: 82.5 – 83.5) for stage I, 45.0% (95% CI: 43.5–46.6) for stage II, 36.5% (95% CI: 34.0–39.0) for stage III, and 9.1% (95% CI: 7.0–11.6) for stage IV (Figure 1B).
Figure 1.
Figure 1A: Traditional Kaplan-Meier estimate of Melanoma Disease-Specific Survival at the time of diagnosis stratified by disease stage.
Figure 1B: Traditional Kaplan-Meier estimate of Overall Survival at the time of diagnosis stratified by disease stage.
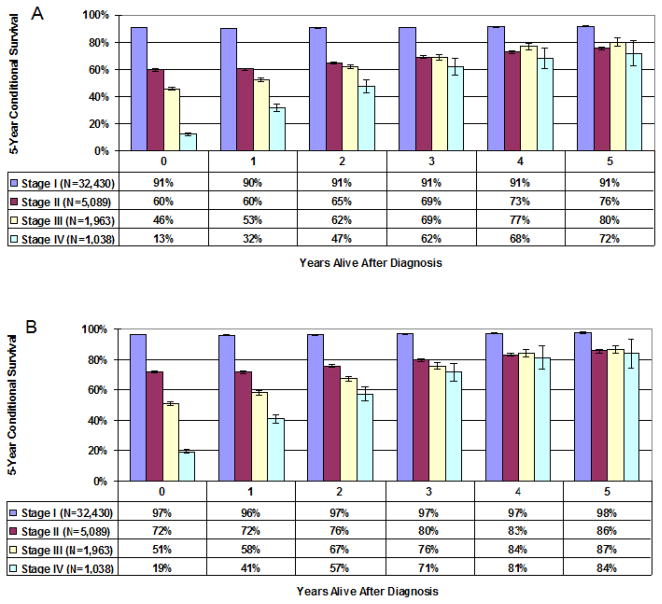
Stage-specific 5-year disease-specific melanoma CS is presented as a bar graph in Figure 2A. For patients with stage I melanoma, 5-year CS estimates remained constant over time at 97%, reflecting the high likelihood of cure at the time of diagnosis. For patients with stage II melanoma, 5-year CS estimates increased over time, from 72% at time 0 (diagnosis) to 86% for patients surviving 5 years. With more advanced melanoma (stages III and IV), there were even greater changes in 5-year CS over time. For patients with stage III disease, 5-year CS estimates increased from 51% at time 0 to 87% for survivors at 5 years. Five-year CS estimates for patients with Stage IV melanoma also improved dramatically for survivors over time, from 19% at time 0 to 84% for survivors at 5 years (Figure 2A). Similar trends were found for 5-year overall CS (Figure 2B). For example, 5-year CS estimates increased from 46% at time 0 to 80% for stage III survivors at 5 years.
Figure 2.
Figure 2A–B: Melanoma-specific (A) and Overall (B) 5-year Conditional Survival estimates stratified by disease stage. Error bars represent the standard error.
The Cox proportional hazards model was used to identify prognostic factors associated with DSS for stages I to IV melanoma patients (Table 1). For stage I, the model demonstrated that age, gender, race, marital status, anatomic tumor location, and tumor histology were all significant prognostic factors. For stages II and III, all aforementioned variables except race were significant. While for stage IV, only age, marital status, and anatomic tumor location were statistically significantly associated with DSS.
Table 1.
Stage-specific multivariate Cox regression on disease-specific survival for melanoma patients (n=40,520) from SEER (1988–2000)
| I (n=32,430) | II (n=5,089) | III (n=1,963) | IV (n=1,038) | |
|---|---|---|---|---|
| Characteristic | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) |
| Age | ||||
| < 50 years (ref) | 1 | 1 | 1 | 1 |
| ≥ 50 | 1.60(1.44–1.78) | 1.56(1.39–1.74) | 1.60(1.40–1.82) | 1.31(1.11–1.56) |
| Sex | ||||
| Female (ref) | 1 | 1 | 1 | 1 |
| Male | 1.50(1.34–1.67) | 1.49(1.33–1.67) | 1.24(1.07–1.42) | 1.08(0.92–1.27) |
| Race | ||||
| White (ref) | 1 | 1 | 1 | 1 |
| Non-White | 0.37(0.25–0.55) | 0.83(0.62–1.12) | 0.92(0.64–1.31) | 0.74(0.48–1.15) |
| Marital status | ||||
| Married (ref) | 1 | 1 | 1 | 1 |
| Unmarried | 1.29(1.15–1.45) | 1.16(1.04–1.29) | 1.24(1.09–1.42) | 1.25(1.06–1.46) |
| Unknown | 0.40(0.34–0.47) | 0.66(0.55–0.80) | 0.98(0.69–1.40) | 1.00(0.67–1.49) |
| Histology | ||||
| Superficial (ref) | 1 | 1 | 1 | 1 |
| Nodular | 4.32(3.72–5.03) | 1.11(0.97–1.27) | 1.36(1.12–1.65) | 1.09(0.65–1.84) |
| Acral | 2.48(1.59–3.86) | 1.28(0.97–1.69) | 1.47(1.00–2.15) | 1.06(0.45–2.45) |
| Other/Unknown | 1.26(1.13–1.40) | 0.86(0.75–0.98) | 1.21(1.02–1.45) | 1.19(0.75–1.87) |
| Location | ||||
| Upper Extremity (ref) | 1 | 1 | 1 | 1 |
| Lower Extremity | 1.06(0.89–1.26) | 1.29(1.10–1.52) | 1.11(0.89–1.38) | 0.87(0.58–1.29) |
| Trunk | 1.54(1.34–1.77) | 1.46(1.27–1.68) | 1.13(0.92–1.38) | 1.37(0.97–1.94) |
| Head & Neck | 1.68(1.44–1.96) | 1.30(1.11–1.51) | 1.36(1.09–1.70) | 1.09(0.76–1.57) |
| Other | 1.54(0.82–2.89) | 0.94(0.46–1.90) | 1.42(1.07–1.89) | 1.29(0.95–1.75) |
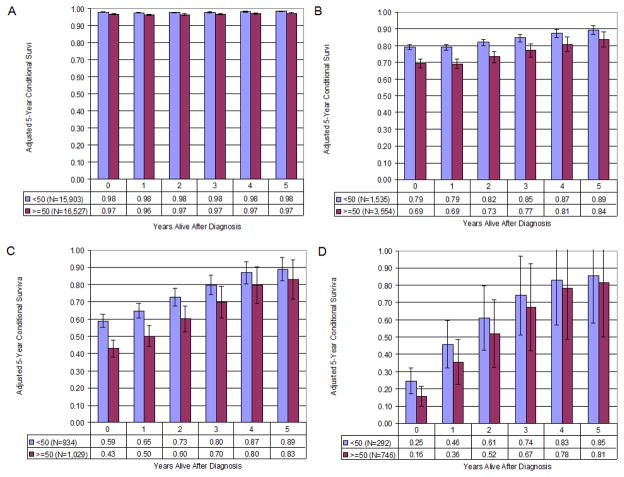
To account for their covariate influences on the survival function, additional CS estimates were adjusted for age, gender, race, marital status, anatomic tumor location, and tumor histology. The adjusted CS was calculated for subgroups of patients further stratified by age. Figures 3A through 3D are generated from the adjusted survival function for these two age groups from each stage cohort, while controlling for the influence of above covariates. Overall, patients who were less than 50 years of age had much higher estimated survival at the time of diagnosis compared to patients who were 50 years of age or older. The differences between melanoma-specific survival at the time of diagnosis were 1% (stage I), 10% (stage II), 16% (stage III), and 9% (stage IV). In terms of CS, younger patients tended to have better outcomes, and of note, these age-related differences in CS generally diminished over time for the stages II and III cohorts.
Figure 3.
Figure 3A–D. Stages I–IV Melanoma-specific 5-year Adjusted Conditional Survival stratified by age (≥50 v <50). Error bars represent the standard error. (A: stage I; B: stage II; C: stage III; D: stage IV)
When stage-specific subgroups were stratified by gender, males were noted to have lower 5-year melanoma-specific survival than females at the time of diagnosis (stage I: 96% v 97%, stage II: 69% v 78%, stage III: 48% v 56%, and stage IV: 17% v 20%, respectively). The CS estimates for both genders increased over time for each year of follow-up; specifically, at year 5, CS for males and females was 98% v 98% for stage I, 83% v 88% for stage II, 85% v 88% for stage III, and 82% v 83% for stage IV patients, respectively.
We also examined the impact of race, anatomic tumor location, and histology on stage-specific melanoma CS, and differing patterns emerged across disease stage groups. Because of the favorable prognosis for all patients with stage I melanoma, there were no major differences in CS among patients when stratified by race, tumor location, and histology. However, for the stages II–IV cohorts, we observed that the effects of race, anatomic tumor location, and histology decreased over time for stage-specific melanoma CS over time. For example, 5-year survival estimates for Caucasian patients with stage IV melanoma were lower than estimates for stage IV non-Caucasians by 9% at the time of diagnosis (18% v 27%) and by 4% at year 5 (82% v 86%), demonstrating a trend that race-related differences in CS decreased over time (p=0.06). In terms of histologic subtype, acral lentiginous melanomas were associated with worse CS than the superficial and nodular groups, regardless of the length of follow-up. But this subgroup always had the largest gain in CS over the 5 year follow-up period for stages I–III patients (improvements of 3%, 16%, and 40% for stages I, II, and III, respectively). With respect to tumor location, stages I–III patients who had primary tumors in the upper extremity were noted to have the most favorable CS, followed by the lower extremity, head/neck, and truncal groups, while stage IV patients who had primary tumors in the lower extremity had the highest CS among different locations
By testing whether the trends of the survival curves were different among various subgroups (i.e. age, race, sex, histology, and tumor location), in other words, we found that sex and histology were determinates of CS improvement for stages I, age, sex, and location for stage II, age, sex and histology for stage III patients (all p-values ≤0.05), and that none of the variables were determinates of CS improvement for the stage IV cohort.
DISCUSSION
Our analysis demonstrates that 5-year melanoma-specific CS improves dramatically over time for surviving patients with stages III and IV melanoma. For survivors with advanced (stage III and IV) melanoma, these estimates provide critical prognostic information; and for clinicians, it provides quantitative data for what is often qualitatively observed – a subset of patients continuing with biologically favorable disease who survive beyond what would have been clinically predicted at the time of diagnosis. It is these unanticipated long-term survivors who provide the ongoing impetus for clinicians to continue to pursue aggressive treatment approaches despite having advanced melanoma at diagnosis.
The results of this CS analysis are consistent with other studies in which similar trends in 5-year CS have been reported in patients with advanced stages of lung,6–8 gastric,9 colon,6, 10 reactal,11 gallbladder,12, 13 ovarian,14 head/neck16 and brain cancers.17–19 However, the patterns for CS in this cohort differed from those found in patients with breast and prostate cancer as reported by Kato et al.6 Specifically, there was a less dramatic increase in median overall CS for patients with breast cancer who only gained an additional 4–7 months in CS every year following diagnosis6 while patients with advanced prostate cancer were noted to have CS estimates which plateaued over time. These disparate results may be explained in part by the multivariate models employed and/or the varying efficacy of adjuvant treatment in different malignancies. Our study demonstrated that for stage III melanoma, while some adverse prognostic factors for survival are significant at the time of initial diagnosis, such as age and gender, these factors become less influential over time. This is in contrast to the findings of Wang et al. who reported that the effects of gender, age (≥65 years v <65 years), and race (African-American v Caucasian) on survival persisted over time for patients with rectal cancer.11 Other studies have investigated the effects of tumor grade and histology on CS, and for patients with poor grade, serous, and undifferentiated epithelioid ovarian cancer, CS improved much more rapidly with time.14
Estimates for 5-year overall survival in this cohort at the time of diagnosis for this cohort of patients with stages I, II, III and IV disease were 91%, 60%, 46% and 13%, respectively, which are similar to estimates reported by the AJCC.3 Because there have been some questions related to the accuracy of cause of death data reported in SEER, Choi et al.14 have recommended relative survival as a more appropriate outcome measure to avoid this potential problem.24 However, with respect to melanoma, the cause of melanoma-related death data field has been examined by Weinstock et al.25 and noted to be 93% accurate. In addition, relative survival estimates were examined in this cohort and determined to be similar to the reported estimates of disease-specific survival (data not shown).
Conditional survival estimates provide critical quantitative information for patients and their families that may reduce unnecessary anxiety. Fear of cancer recurrence, which is distinct from general patient concerns for future health,26 has been well-documented as an increasingly distressful concern for survivors,27–30 Conditional survival estimates may also serve to alleviate the associated anxiety of cancer caregivers. A recent study by Mellon et al. indicated that survivors and caregivers often influence each others’ fears, and that caregivers often experience significantly more fear of recurrence than survivors.31 Furthermore, recognition of the needs of cancer caregivers has led to the recent development of adjunctive scales such as the Cancer Survivors’ Partners Unmet Needs (CaSPUN) measure to augment those for cancer survivors like the Cancer Survivors’ Unmet Needs (CaSun) measure.32, 33 Additionally, CS estimates revised annually accounting for additional years of survival may impact the economic decisions of survivors and their families. A recent study of the Cancer Problems in Living Scale (CPILS) from the American Cancer Society’s Studies of Cancer Survivors revealed that the economic and fiscal distress of cancer survivors is distinct from other forms of emotional distress.26 The concerns of cancer survivors relating to the domains of control, uncertainty, and the future have also been shown to be important factors in models predicting overall quality of life.34 It is likely that understanding the increased likelihood of continued survival over time will help alleviate anxiety in each of these domains. Finally, given that quality of life has been shown to be an independent predictor of survival in melanoma patients with advanced disease,35, 36 reduced anxiety and emotional distress may impact survival outcomes.
There are several limitations associated with the current study. SEER currently consists of comprehensive demographic and pathological data from 14 population-based cancer registries. First, SEER does not provide information on comorbidities, social economics status, nor complete treatment-related information. Because the final multivariate model could not incorporate these variables, our CS estimates might be biased and potentially limited to SEER regions only. In addition, SEER does not include data pertaining to disease recurrence, thus limiting our ability to examine disease-free CS outcomes in this analysis. The pathological data provided by SEER also do not designate micrometastatic nodal disease which is required to stratify stage III patients (who are known to have widely varying traditional survival estimates) into more homogeneous groups based on substage (i.e. IIIA, IIIB, and IIIC). Furthermore, the sample size for the stage IV cohort is comparatively small (n=1,038) and the small fraction of survivors in this group resulted in wider ranges of error for the 5-year CS estimates. As a means of highlighting this point, we have included error bars on our graphic representations (Figure 3D).
Compared to traditional survival estimates, such as median survival, DSS, and overall survival from the time of diagnosis, CS estimates provide more relevant quantitative information for cancer survivors as their risk profiles change over time. For clinicians, this information should be taken into account when making ongoing treatment decisions and when considering post-treatment surveillance intervals following annual milestones. These data are also critical for cancer survivors and their families when planning non-treatment related life decisions.
Acknowledgments
This project was supported by Grant Number 1 R01 CA127328-01 (Cormier, PI) from National Cancer Institute, National Institutes of Health. The contents of this manuscripts are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Health.
We would like to acknowledge Debbie Dunaway for assistance with manuscript preparation.
Footnotes
Presented at the 4th Annual Academic Surgical Congress, Fort Myers, FL, February, 2009.
References
- 1.Cancer Facts & Figures 2008. American Cancer Society; 2008. [Google Scholar]
- 2.Greene FL, Page DL, Fleming ID, et al., editors. American Joint Committee on Cancer. AJCC Cancer Staging Handbook. 6. New York: Springer; 2002. p. 421. [Google Scholar]
- 3.Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–48. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 4.Henson DE, Ries LA, Carriaga MT. Conditional survival of 56,268 patients with breast cancer. Cancer. 1995;76(2):237–42. doi: 10.1002/1097-0142(19950715)76:2<237::aid-cncr2820760213>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 5.Meng L, Maskarinec G, Lee J. Ethnicity and conditional breast cancer survival in Hawaii. J Clin Epidemiol. 1997;50(11):1289–96. doi: 10.1016/s0895-4356(97)00183-2. [DOI] [PubMed] [Google Scholar]
- 6.Kato I, Severson RK, Schwartz AG. Conditional median survival of patients with advanced carcinoma: surveillance, epidemiology, and end results data. Cancer. 2001;92(8):2211–9. doi: 10.1002/1097-0142(20011015)92:8<2211::aid-cncr1565>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 7.Merrill RM, Henson DE, Barnes M. Conditional survival among patients with carcinoma of the lung. Chest. 1999;116(3):697–703. doi: 10.1378/chest.116.3.697. [DOI] [PubMed] [Google Scholar]
- 8.Skuladottir H, Olsen JH. Conditional survival of patients with the four major histologic subgroups of lung cancer in Denmark. J Clin Oncol. 2003;21(16):3035–40. doi: 10.1200/JCO.2003.04.521. [DOI] [PubMed] [Google Scholar]
- 9.Wang SJ, Emery R, Fuller CD, Kim JS, Sittig DF, Thomas CR. Conditional survival in gastric cancer: a SEER database analysis. Gastric Cancer. 2007;10(3):153–8. doi: 10.1007/s10120-007-0424-9. [DOI] [PubMed] [Google Scholar]
- 10.Merrill RM, Henson DE, Ries LA. Conditional survival estimates in 34,963 patients with invasive carcinoma of the colon. Dis Colon Rectum. 1998;41(9):1097–106. doi: 10.1007/BF02239430. [DOI] [PubMed] [Google Scholar]
- 11.Wang SJ, Fuller CD, Emery R, Thomas CR. Conditional survival in rectal cancer: a SEER database analysis. Gastrointest Cancer Res. 2007;1(3):84–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Fuller CD, Wang SJ, Kachnic L, Wong A, Thomas CR. Conditional survival of gallbladder adenocarcinoma treated with radiotherapy: analysis from the SEER database. Int J Radiat Oncol Biol Phys. 2005;63:S274. [Google Scholar]
- 13.Thomas C, Fuller C, Starling G, Wang S. Conditional survival in gallbladder carcinoma: Results from the SEER 11 dataset. 2006 ASCO Annual Meeting Proceedings, vol. 24. J Clin Oncol; 2006. p. 4130. [Google Scholar]
- 14.Choi M, Fuller CD, Thomas CR, Jr, Wang SJ. Conditional survival in ovarian cancer: results from the SEER dataset 1988–2001. Gynecol Oncol. 2008;109(2):203–9. doi: 10.1016/j.ygyno.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 15.Merrill RM, Bird JS. Effect of young age on prostate cancer survival: a population-based assessment (United States) Cancer Causes Control. 2002;13(5):435–43. doi: 10.1023/a:1015764507609. [DOI] [PubMed] [Google Scholar]
- 16.Fuller CD, Wang SJ, Thomas CR, Jr, Hoffman HT, Weber RS, Rosenthal DI. Conditional survival in head and neck squamous cell carcinoma: results from the SEER dataset 1973–1998. Cancer. 2007;109(7):1331–43. doi: 10.1002/cncr.22563. [DOI] [PubMed] [Google Scholar]
- 17.Davis FG, McCarthy BJ, Freels S, Kupelian V, Bondy ML. The conditional probability of survival of patients with primary malignant brain tumors: surveillance, epidemiology, and end results (SEER) data. Cancer. 1999;85(2):485–91. [PubMed] [Google Scholar]
- 18.Hwang SL, Yang YH, Lieu AS, Chuang MC, Chang SJ, Chang YY, et al. The conditional survival statistics for survivors with primary supratentorial astrocytic tumors. J Neurooncol. 2000;50(3):257–64. doi: 10.1023/a:1006484220764. [DOI] [PubMed] [Google Scholar]
- 19.Lin CL, Lieu AS, Lee KS, Yang YH, Kuo TH, Hung MH, et al. The conditional probabilities of survival in patients with anaplastic astrocytoma or glioblastoma multiforme. Surg Neurol. 2003;60(5):402–6. doi: 10.1016/s0090-3019(03)00322-7. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimator from incomplete observations. J American Statistical Association. 1958;53:457–81. [Google Scholar]
- 21.Cox DR. Regression models and life tables. J R Statistical Soc. 1972;B34:187–220. [Google Scholar]
- 22.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–41. [Google Scholar]
- 23.Zar J. Biostatistical Analysis. 4. Prentice Hall; 1999. [Google Scholar]
- 24.Hoel DG, Ron E, Carter R, Mabuchi K. Influence of death certificate errors on cancer mortality trends. J Natl Cancer Inst. 1993;85(13):1063–8. doi: 10.1093/jnci/85.13.1063. [DOI] [PubMed] [Google Scholar]
- 25.Weinstock MA, Reynes JF. Validation of cause-of-death certification for outpatient cancers: the contrasting cases of melanoma and mycosis fungoides. Am J Epidemiol. 1998;148(12):1184–6. doi: 10.1093/oxfordjournals.aje.a009607. [DOI] [PubMed] [Google Scholar]
- 26.Zhao L, Portier K, Stein K, Baker F, Smith T. Exploratory Factor Analysis of the Cancer Problems in Living Scale: A Report from the American Cancer Society’s Studies of Cancer Survivors. J Pain Symptom Manage. 2008 doi: 10.1016/j.jpainsymman.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Baker F, Denniston M, Smith T, West MM. Adult cancer survivors: how are they faring? Cancer. 2005;104(11 Suppl):2565–76. doi: 10.1002/cncr.21488. [DOI] [PubMed] [Google Scholar]
- 28.Ferrell BR, Grant MM, Funk B, Otis-Green S, Garcia N. Quality of life in breast cancer survivors as identified by focus groups. Psychooncology. 1997;6(1):13–23. doi: 10.1002/(SICI)1099-1611(199703)6:1<13::AID-PON231>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 29.Mehta SS, Lubeck DP, Pasta DJ, Litwin MS. Fear of cancer recurrence in patients undergoing definitive treatment for prostate cancer: results from CaPSURE. J Urol. 2003;170(5):1931–3. doi: 10.1097/01.ju.0000091993.73842.9b. [DOI] [PubMed] [Google Scholar]
- 30.Mullens AB, McCaul KD, Erickson SC, Sandgren AK. Coping after cancer: risk perceptions, worry, and health behaviors among colorectal cancer survivors. Psychooncology. 2004;13(6):367–76. doi: 10.1002/pon.751. [DOI] [PubMed] [Google Scholar]
- 31.Mellon S, Kershaw TS, Northouse LL, Freeman-Gibb L. A family-based model to predict fear of recurrence for cancer survivors and their caregivers. Psychooncology. 2007;16(3):214–23. doi: 10.1002/pon.1074. [DOI] [PubMed] [Google Scholar]
- 32.Hodgkinson K, Butow P, Hobbs KM, Hunt GE, Lo SK, Wain G. Assessing unmet supportive care needs in partners of cancer survivors: the development and evaluation of the Cancer Survivors’ Partners Unmet Needs measure (CaSPUN) Psychooncology. 2007;16(9):805–13. doi: 10.1002/pon.1138. [DOI] [PubMed] [Google Scholar]
- 33.Hodgkinson K, Butow P, Hunt GE, Pendlebury S, Hobbs KM, Lo SK, et al. The development and evaluation of a measure to assess cancer survivors’ unmet supportive care needs: the CaSUN (Cancer Survivors’ Unmet Needs measure) Psychooncology. 2007;16(9):796–804. doi: 10.1002/pon.1137. [DOI] [PubMed] [Google Scholar]
- 34.Ferrell BR, Dow KH, Grant M. Measurement of the quality of life in cancer survivors. Qual Life Res. 1995;4(6):523–31. doi: 10.1007/BF00634747. [DOI] [PubMed] [Google Scholar]
- 35.Butow PN, Coates AS, Dunn SM. Psychosocial predictors of survival: metastatic breast cancer. Ann Oncol. 2000;11(4):469–74. doi: 10.1023/a:1008396330433. [DOI] [PubMed] [Google Scholar]
- 36.Coates A, Gebski V, Signorini D, Murray P, McNeil D, Byrne M, et al. Prognostic value of quality-of-life scores during chemotherapy for advanced breast cancer. Australian New Zealand Breast Cancer Trials Group. [comment] Journal of Clinical Oncology. 1992;10(12):1833–8. doi: 10.1200/JCO.1992.10.12.1833. [DOI] [PubMed] [Google Scholar]