Abstract
During infection, viruses hijack various host cell components and programs for their amplification, among which is the canonical ERK signaling pathway, mainly consisting of three tiered serine/threonine kinases, Raf, MEK and ERK. MEK1 and MEK2 are two isoforms of the kinase operating immediately upstream of ERK, and connecting Raf and ERK by phosphorylating ERK. Previous studies have suggested that different isoforms of MEK have distinct biological functions, although their in vitro kinase function may be redundant. However, little is known about the isoform-specific effects of these kinases on viral propagation. In this study, we showed that herpes simplex virus type 2 (HSV-2) infection of human embryonic kidney (HEK) 293 cells induced a sustained activation of ERK1/2. Inhibition of this ERK activation by U0126, a specific inhibitor of MEK1/2, severely impaired virus production. A similar reduction of virus production was also seen following transfection of cells with siRNAs for MEK1/2. Interestingly, a specific knockdown of MEK1 with siRNAs caused a marked inhibition of viral titers, viral proteins and virus-induced cytopathic effect (CPE), whereas silencing MEK2 had little effect. Therefore, our results demonstrate that MEK1 and MEK2 act differently and that HSV-2 hijacks host MEK1 for its own amplification. To our knowledge, this is the first report showing inhibition of HSV-2 replication by targeting human MEK1. This study also suggests that MEK1 could be a potential target for anti-HSV-2 therapy, which may minimize damage to the host cells engendered by targeting both MEK1 and MEK2.
Keywords: HSV-2, Viral replication, MEK1, MEK2, ERK, siRNA
1. Introduction
Human herpes simplex virus type 2 (HSV-2) belongs to the alpha herpesvirus subfamily, and its genes are divided into three broad groups termed immediate-early (IE or alpha) gene, early (beta) gene, and late (gamma) gene, according to their temporal order of expression and requirement for expression of the viral products (Hay and Ruyechan, 1992; Wagner et al., 1995; Ward and Roizman, 1994). HSV-2 is characterized by a short cytolytic replication cycle in permissive cells and an ability to establish latency in sensory ganglia (Brooks et al., 2004; Weir, 2001; Whitley, 2004). It accounts for about 2/3 of all new genital infections, causing mainly herpes lesions on external areas of the genital organs (Brooks et al., 2004; Sizemore et al., 2006), it is also the most common cause of neonatal herpes (Hill and Roberts, 2005; Yamashita and Morishima, 2006). Overall, infection with HSV-2 produces strong morbidity and shows broad prevalence, long latency, and difficult curability. To date, no preventive vaccines or effective therapeutic drugs for recurrent infections are available.
The extracellular signal regulated kinase (ERK) cascade is one of the three major mitogen-activated protein kinase (MAPK) cascades, and consists of three tiered serine/threonine kinases of Raf, MAPK/ERK(MEK) and ERK (Chang et al., 2003; Cohen, 1997; McCubrey et al., 2007; Zhang and Liu, 2002). Raf, consisting of three isoforms, Raf-1, B-Raf and A-Raf, activates very few partners other than MEKs, and no other substrates for MEK than ERK (i.e. ERK1, ERK2) have been found to date (Chambard et al., 2007). Thus, The second kinase comprising two isoforms, MEK1 and MEK2, plays a key role in connecting the Raf and ERK. The inhibition of MEK has been studied as the most effective way of investigating the importance of the Raf/MEK/ERK signaling cascade in various cellular physiological and pathological processes (Kelemen et al., 2002; Kohno and Pouyssegur, 2006; Luo et al., 2002; McCubrey et al., 2007; Shakibaei et al., 2001; Yip-Schneider and Schmidt, 2003; Zheng et al., 2003). In many scenarios, MEK1 and MEK2 are functionally interchangeable, however more recent studies have added complexity to the signaling functions of MEK1 and MEK2 in terms of their interactions with activators and substrates, responses to extracellular factors, and various cellular functions they regulate (Belanger et al., 2003; Skarpen et al., 2008; Ussar and Voss, 2004). For example, only 40% of identity is found in the N-terminal region of MEK containing the characteristic proline-rich inserts that are involved in interactions with activators and substrates (Enslen and Davis, 2001; Kolch, 2000). MEK1 but not MEK2 forms a signaling complex with Raf-1, suggesting that in some cells the Raf-1 signaling pathway preferentially activates ERKs via MEK1 (Jelinek et al., 1994). Furthermore, MEK1 and MEK2 have distinct ways to regulate ERK activity and cell cycle progression (Ussar and Voss, 2004). In addition, different phenotypes have been demonstrated in MEK1 and MEK2 knockout mice (Belanger et al., 2003; Giroux et al., 1999). All these studies indicate MEK1 and MEK2 exhibit different biological functions.
Viruses ultimately depend on host cells for their replication, taking advantage of pre-existing signaling pathways in the host cells for their amplification and survival. Numerous studies have demonstrated that activation of the Raf/MEK/MAPK pathway is a specific response to virus infection and plays an important role in virus replication (Cai et al., 2007; Jacque et al., 1998; King et al., 1986; Ludwig et al., 2003; Luo et al., 2002; Panteva et al., 2003; Planz et al., 2001; Pleschka et al., 2001; Smith et al., 2000). For example, specific inhibitors for both MEK1 and MEK2 such as U0126 and PD98059 have been shown to severely impair the growth of a variety of viruses in cultured cell models (Cai et al., 2007; Jacque et al., 1998; Ludwig et al., 2003; Planz et al., 2001; Pleschka et al., 2001; Smith et al., 2000). However the specific contributions of MEK1 and MEK2 to virus replication have to our knowledge not been addressed to date. In the present study, RNAi technology has been used to specifically silence MEK1 and MEK2 expression in HEK 293 cells and the effect(s) on the subsequent replication of HSV-2 has been assessed.
2. Materials and methods
2.1. Antibodies
Antibodies were purchased from cell signaling (anti-phospho-ERK1/2 and anti-ERK1/2), BD Biosciences (anti-MEK1 and MEK2), Sigma (anti-β-actin) and East Coast Bio (HSV gB monoclonal antibody). The HSV UL30 monoclonal antibody was a kind gift of Dr. Roger Everett, University of Glasgow.
2.2. Cell culture
Human embryonic kidney (HEK) 293 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO) supplemented with 2 or 10% heat-inactivated fetal bovine serum (FBS, Hyclone) at 37 °C in a 5% CO2 humidified incubator.
2.3. Virus
HEK 293 cells were infected with strain 333 of HSV-2 (kindly provided by Dr. WX Jia) in different multiplicities of infection (MOI) as indicated in the figure legends. After 1 h at 37 °C, the inoculum was removed and incubation continued in DMEM containing 2% FBS at 37 °C for viral growth. Supernatants collected at different time post-infections (p.i.) were clarified by centrifugation (2000 × g) and viral titers determined by plaque assays carried out in triplicates.
2.4. siRNA and transient transfection
Specific short interfering RNAs (siRNAs) targeting sequences for human MEK1 and MEK2 mRNAs were selected based on previous reports (Ussar and Voss, 2004). Mutant siRNAs derived from the MEK1 or MEK2 target sequences but containing 2 base pair substitutions near their 3′ end (Table 1) were used as controls. All siRNAs used were commercially synthesized by Gene Chem, Ltd., and are listed in Table 1. Alternative siRNAs (Santa Cruz), consisting of pools of three–five target-specific 19–25 nt siRNAs, for human MEK1 and MEK2 mRNA each were used to confirm the effects seen on HSV-2 replication.
Table 1.
The siRNA target sequences (sense strand) in this study.
| Name | Target gene | siRNA target sequence | Positiona |
|---|---|---|---|
| siMEK1 | mek1 (Sa) | 5 ′ gcaactcatggttcatgct3′ | 1131–1149 |
| b siMut1 | Derived from mek1 (Sa) | 5 ′gcaactcatggttcaGTct3′ | 1131–1149 |
| siMEK2 | mek2 (Sa) | 5 ′gaaggagagcctcacagca3′ | 1116–1134 |
| b siMut2 | Derived from mek2 (Sa) | 5 ′ gaaggagagcctcacGAca3′ | 1116–1134 |
Positions of siMEK1 and siMEK2 target sequences are referred to as GenBank NM 002755.3 for human (Sa) mek1 and NM 030662.3 for human (Sa) mek2.
siMut1 and siMut2 have 2 base pair change as indicated in capital fonts.
HEK 293 cells were transfected in 12 well plates at 40 to 60% confluence with siRNAs at a concentration of 20 or 30 nM using INTERFER in (Polyplus-transfection) according to the manufacturer’s instructions. The cells were maintained in growth medium for 36 h before changing to medium containing only 0.5% FBS for 8–12 h, and then infected with HSV-2 at an MOI of 5 or 1.5. Following virus adsorption for 1 h, DMEM supplemented with 2% FBS was added and incubation continued for selected time. Virus titers in the medium at 24 h p.i. were measured by plaque assay; cell lysates after infection were used for Western blotting analysis of the early viral protein UL30, late protein gB and host cellular proteins.
To provide a positive control for the inhibition of MEK1/2, the inhibitor U0126 (MEK1/2 specific inhibitor, Promega) was freshly dissolved at 10 mM in DMSO, and added to culture medium at a final concentration of 50 μM for 1 h before and throughout the entire period of the infection.
2.5. Morphological analysis
HEK 293 cells were examined following HSV-2 infection at every 12 h intervals post-infection for the cytopathic effect (CPE) using phase-contrast microscopy.
2.6. Western blot analysis
Cells were lysed in a buffer containing 20 mM Hepes (pH 7.4), 100 mM NaCl, 5 mM EDTA (pH 7.4), 1 mM Na3VO4, 30 mM NaF, 5% glycerol, 0.1% SDS, 1% Triton X-100, 10 mM p-nitrophenylphosphate, 1 mM glycerophosphate, supplemented with complete protease inhibitors (Roche). Cell lysates were obtained by centrifugation at 4 °C, 13000 rpm and protein concentration was determined with the Bicinchoninic Acid Protein Assay Kit (Pierce). Proteins were resolved onto sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to PVDF membranes (Millipore). The membrane was blotted with specific primary antibodies, as indicated in figure legends, followed by incubation with secondary antibodies conjugated with horseradish peroxidase, and developed with an enhanced chemiluminescent substrate (ECL) or SuperSignal West Femto Maximum Sensitivity Substrate (Pierce).
2.7. Statistics
The relative amounts of the phospho-ERK1/2 (pERK1/2) were normalized by densitometric scanning of actin using the band leader software (version 3.0). Statistical difference analysis of the pERK1/2 levels and of viral titers (PFU/ml) was carried out using ANOVA
3. Results
3.1. Activation of the ERK pathway was required for HSV-2 replication
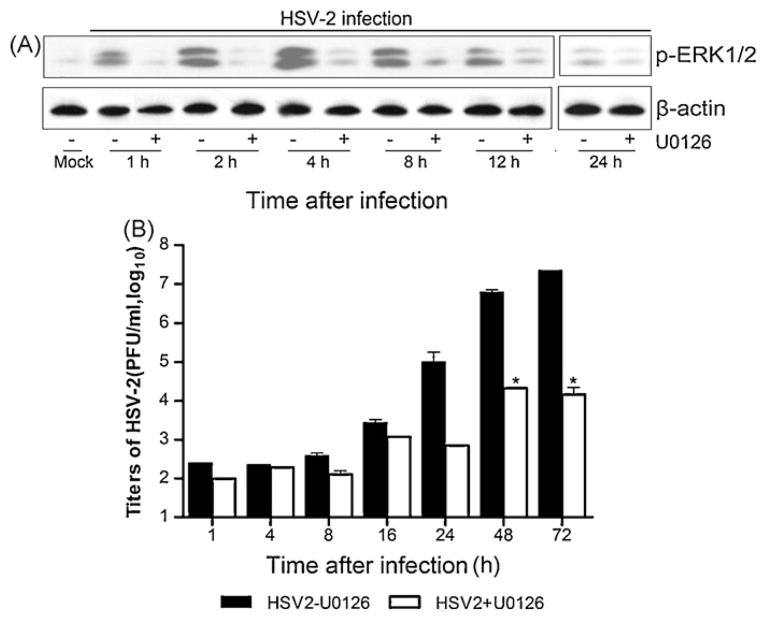
To determine if the ERK was activated by HSV-2, HEK 293 cells were infected with HSV-2 and cell extracts were blotted with anti-pERK1/2 antibody. As shown in Fig. 1A, ERK activation peaked around 4 h and sustained up to 24 h following HSV-2 infection. The activation was severely suppressed in the presence of the MEK-specific inhibitor U0126. To assess the contribution of ERK activation to HSV-2 replication, U0126 was added to the culture medium prior to infection. As shown in Fig. 1B, HSV-2 propagation increased progressively, which was significantly diminished by treatment with U0126. The inhibitory effect of U0126 lasted to 72 h. This result suggests that activation of the Raf/MEK/ERK cascade is necessary for HSV-2 replication. In consistency, our study also showed a similar inhibition when U0126 was added 2 h post-infection (data not shown).
Fig. 1.
Dependency of virus production on ERK activation. (A) HEK 293 cells were pre-incubated without or with U0126 (50 μM) for 1 h, followed by infection with HSV-2 at an MOI of 1.5. The cells and medium were collected separately at different time points. Cell lysates were blotted with phospho-ERK1/2 (pERK1/2) antibody and β-actin antibody. (B) Virus titers in the medium were measured by plaque assay. Data shown were the means ± standard deviations (n = 3). The titers on y-axis represented logarithmic values. The values obtained with U0126 were significantly different from those in its absence indicated by the asterisk (*P < 0.01 by ANOVA).
3.2. HSV-2 propagation was impaired by siRNAs for MEK1/2
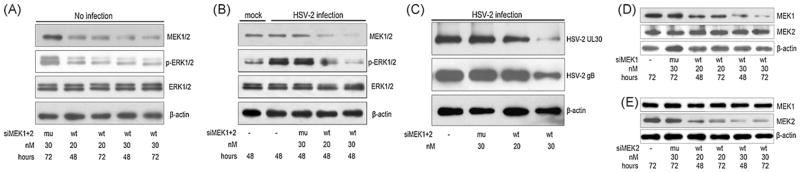
To confirm the results with the MEK inhibitor, we transfected siRNAs specific for MEK1 and MEK2 (siMEK1+2) and examined their effect on virus production. As shown in Fig. 2A, MEK1/2 expression was diminished by siMEK1+2 at 48 to 72 h post-transfection in a dose-dependent manner. The silencing effect was very specific, as no cross-silencing by each other’s siRNA was observed (Fig. 2D and E) or by mutated siRNA (mu) where 2 base pairs were replaced (Table 1). In addition, ERK1/2 expression was not affected by co-transfection of siMEK1+2 (Fig. 2A and B). In a titration experiment we did not found evident cell toxicity even at 30 nM siRNA which achieves the maximal knockdown effect (data not shown).
Fig. 2.
Suppression of ERK activation and HSV-2 propagation by co-transfection of siMEK1 and siMEK2. (A) and (B) Cells were co-transfected with siMEK1 and siMEK2 (siMEK1+2, wt) at 20 or 30 nM of each, or with their mutated versions (mu), siMut1 and siMut2 (Table 1). The cells were challenged without (mock) or with HSV-2 (MOI = 5) at 44 h post-transfection. Protein lysates were prepared at 4 h p.i. (the peak time of ERK activation) and blotted with MEK1, MEK2, pERK1/2 and ERK antibodies. (C) The experiments were performed as described in panel A except for virus infection with MOI of 1.5. Cell lysates collected at 24 p.i. were assayed for viral protein expressions by Western blot with monoclonal antibodies specific to HSV-2 UL30 or gB protein. (D) and (E) HEK293 cells were transfected with specific siRNAs (wt) or its mutated version (mu) as indicated and blotted with anti-MEK2 or anti-MEK1 antibody indicated. The results show specific knockdown and no cross-silencing effect and represent one of a triplicate experiment. β-Actin served as a loading control. The transfection efficiency in current study was more than 85%, as evaluated by transfection of GFP and its siRNAs (data not shown).
In control cells with mock infection, basal phosphorylation of ERK1/2 was inhibited by siMEK1+2 by about 60% (Figs. 2A and 3E), in virus-infected cells, the activation of ERK1/2 was reduced by about 40 and 85% by transfection with 20 or 30 nM siMEK1+2, respectively (Fig. 2B, *P < 0.01 by ANOVA). We then measured HSV-2 UL30 and gB protein expression and virus titers. As shown in Fig. 2C, the expression of these two viral proteins was significantly reduced. Concurrently HSV-2 replication, as measured by plaque assay, was inhibited in a range from 40- to 55-fold, as compared to viral titers seen in siMut1+2 transfected cells (Fig. 4A, *P < 0.01). Taken together, these results indicate that HSV-2 infection induces activation of the host ERK pathway, which is in turn used for virus replication.
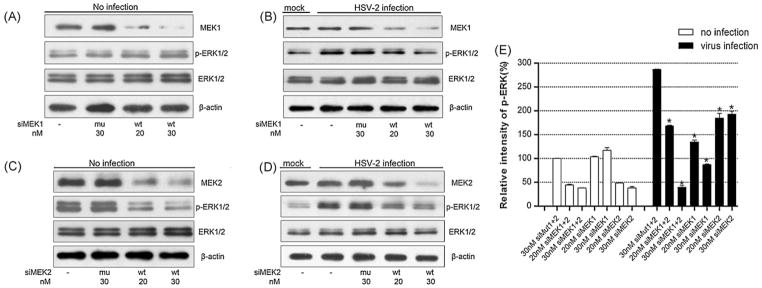
Fig. 3.
The effects of MEK1 and MEK2 knockdown on ERK activation by HSV-2. (A) and (B) HEK 293 cells were transiently transfected with the siMEK1 (wt) or siMut1 (mu) at the indicated doses. The cells were then infected with HSV-2 (MOI = 5) at 44 h post-transfection. Protein lysates were prepared from mock-infected and virus-infected cells, at 4 h p.i. MEK1, pERK1/2 and ERK1/2 were examined by Western blots with specific antibodies. (C) and (D) The experiment was performed as in panel A and B except that siMEK2 or siMut2 was used to examine the effect of MEK2 on ERK activation (n = 3). β-Actin was used for the loading control. (E) Quantification of pERK1/2 bands (4 h p.i.) relative to ERK1/2 bands in panels A and B of Fig. 2, panels A–D of the figure was determined by densitometric scanning. The relative intensity of pERK1/2 on y-axis was presented by percentage of intensity of mock transfection (100%). The data were the average of three independent experiments, and error bars denoted the standard deviations. *P < 0.01 versus 30 nM siMut1+2 by ANOVA.
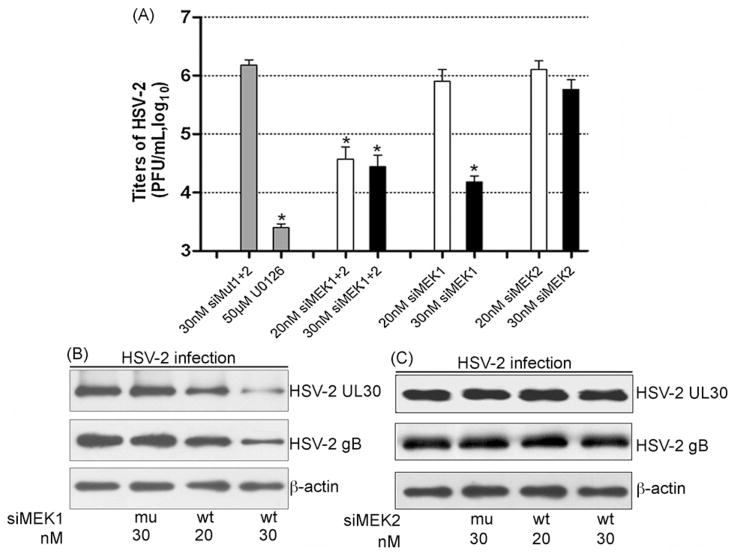
Fig. 4.
Knockdown of MEK1 and MEK2 differentially impacts on HSV-2 replication. Cells were transiently transfected with the siMEK1+2, siMEK1 and siMEK2 at the dose indicated, respectively. The cells transfected with siMut1+2, or siMut1, or siMut2 served as negative controls and those treated with U0126 1 h before and throughout the entire period as a positive control. The cells were infected with HSV-2 (MOI = 1.5) at 44 h post-transfection, and culture supernatant and cells collected at 24 h p.i. (A) Supernatants were assayed for viral titers (PFU/ml). The data represent the average of three independent experiments and shown as the means ± standard deviations (n = 3). P < 0.01 compared with 30 nM siMut1+2 by ANOVA. (B) and (C) Protein lysates from siMEK1 (B) or siMEK2 (C) transfected cells were blotted with anti-HSV UL30 and gB monoclonal antibodies to detect viral protein expression. The data are one representative of three independent experiments.
3.3. Differential effects of MEK1 and MEK2 on ERK activation in mock- and virus-infected cells
To further determine the isoform-specific effect of MEK on ERK activation by HSV-2, HEK 293 cells were transfected with siMEK1 and siMEK2, respectively, and then infected with HSV-2. As shown in Fig. 3A–D, transfection of siMEK1 or siMEK2 effectively silenced the expression of MEK1 or MEK2 in a dose-dependent manner. Furthermore, in mock-infected cells, reduced expression of MEK1 did not significantly alter ERK phosphorylation at a basal level (Fig. 3A and E), whereas reduced expression of MEK2 led to about 50 or 60% inhibition of ERK1/2 phosphorylation, compared with those of respective mock transfection group (Fig. 3C and E). Moreover, in virus-infected cells, ERK activations were diminished to some degrees by siRNAs for two respective MEKs, as compared with their cognate mutated siRNAs (siMut1+2) (Fig. 3B, D and E, *P < 0.01 by ANOVA). In addition, identical results on ERK inhibition were obtained with alternative siRNAs designed on different sequence segments (data not shown). Hence, our data point to distinct functions for MEK1 and MEK2 in ERK activation in mock- and virus-infected cells.
3.4. MEK1 and MEK2 depletion exhibited different effects on HSV-2 replication
To investigate whether the two MEK subtypes acted differently in HSV-2 replication, HEK293 cells transfected with siMEK1 or siMEK2 were infected with HSV-2, as compared with control cells infected with HSV-2 alone or after transfection with siMut1+2, or MEK1/2 specific inhibitor U0126, and the virus production was examined. As Fig. 4A shown, in the cells transfected with 30 nM siMEK1, HSV-2 titers were markedly inhibited by about 100-fold, as compared to siMut1+2 treated cells (*P < 0.01 by ANOVA), but no significant difference with the cells treated by U0126 or siMEK1+2 (P > 0.05 by ANOVA). In contrast, transfection with siMEK2 did not display a significant effect. Consistently, MEK1 knockdown resulted in a clear reduction of HSV-2 UL30 and gB protein expression (Fig. 4B), while MEK2 knockdown showed no change in HSV-2 UL30 and gB protein levels (Fig. 4C). Similar results were obtained with alternative siRNAs (data not shown). Thus, these results demonstrate that MEK1 but not MEK2 plays an important role in HSV-2 amplification, although both are activated and contributes to ERK activation during virus infection.
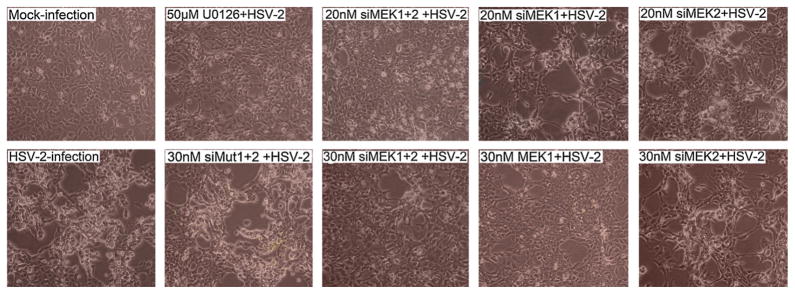
3.5. HSV-2-induced CPE was inhibited by knockdowns of MEK1 and MEK1/2 but not MEK2
To determine the effects of siMEK1, siMEK2, or both on cytopathic effect (CPE) of HSV-2, HEK293 cells transfected with siMEK1, siMEK2, or both were infected with HSV-2 (Fig. 5), and then monitored with phase-contrast microscopy every 12 h for 3 days p.i. In this experiment, mock infection, HSV-2 infection, siMut1+2 transfection and U0126 were used as controls. At 24 h p.i., the cells transfected with 30 nM siMEK1, 20 and 30 nM siMEK1+2 or U0126 were manifested much less CPE, as compared to HSV-2 and siMut1+2 controls. Of note, the cells pretreated with siMEK2 showed an obvious sign of CPE. Similarly, the same result of CPE was shown using alternative siRNAs (data not shown). These results indicate that MEK1 is required for HSV-2-induced CPE.
Fig. 5.
CPE induced by HSV-2 in HEK 293 cells. Cells were transfected with siMEK1, or siMEK2 or their combination at the indicated doses, and infected at 44 h post-transfection with 1.5 MOI of HSV-2. The effects of MEK siRNA on CPE were examined from 8 h p.i., and images were taken at 24 h p.i., as opposed to the cells with mock infection, HSV-2 infection, U0126 control and siMut1+2 control.
4. Discussion
Activation of the Raf/MEK/MAPK pathway has been agreed as one of the specific responses to virus infection indicative of the complex interactions that occur between viruses and their hosts. It has been demonstrated in cultured cells infected with a variety of different viruses (Jacque et al., 1998; King et al., 1986; Ludwig et al., 2003; Luo et al., 2002; Panteva et al., 2003; Planz et al., 2001; Pleschka et al., 2001; Smith et al., 2000), and in the case of HSV-2 has been seen in both neuronal and non-neuronal cells (Hunter et al., 1995; Perkins et al., 2002; Smith et al., 2000). These studies have usually made use of two chemical inhibitors of MEK1/2, U0126 and PD98059, to block the activation of ERK cascade (Kelemen et al., 2002; Planz et al., 2001; Pleschka et al., 2001; Sturgill, 2008). However these inhibitors have some limitations; for example, they may produce off-target effects and importantly are unable to distinguish if the effect is mediated by MEK1 or MEK2. To circumvent these limitations and allow distinction of its individual involvement in virus replication, this study made use of RNA interference to knock down expression of the individual MEK isoforms. Thus, we have demonstrated that expression of viral proteins (viral DNA polymerase, UL30 and envelope glycoprotein, gB), the yield of infectious virus and HSV-2-induced CPE are markedly decreased when ERK activation is blocked by pre-treatment of cells with either of MEK siRNAs or U0126 (Figs. 1A, 2B and C, 4A, and 5). This is consistent with previous findings using chemical MEK1/2 inhibitors (Perkins et al., 2002; Smith et al., 2000). Furthermore, our results have shown that MEK1-medicated activation of ERK plays an essential role in viral production, whereas MEK2 is dispensable although the latter also contributes to the virus-induced ERK activation (Figs. 4 and 5). To our knowledge, this is the first report that MEK1 activity rather than its isoform MEK2 is important for HSV-2 replication. This finding not only adds new information to our understanding of how the host cell ERK signaling pathway is utilized by virus, but also provides new evidence for isoform-specific function of MEK in the virus propagation.
Thus far, it remains unclear how HSV-2 infection causes activation of ERK. (Smith et al., 2000, 2002) has reported that HSV-2 infection induces expression of ICP10 PK, the large subunit of the HSV-2 ribonucleotide reductase 1(RR1), which has an intrinsic protein kinase activity. This in turn was found to lead to an increase in Ras-GTP and subsequent activation of Raf1, which lies immediately upstream of MEK1/2 in the ERK signaling cascade. However these studies provided no clues as to why MEK1 but not MEK2 is required for the virus production, despite both being activated by the virus infection. It is possible that a MEK1/ERK complex may associate with either component necessary for virus replication. Of relevance to such possibilities are earlier studies that have shown that Raf1 forms a signaling complex with MEK1, but not MEK2 (Jelinek et al., 1994), and that MEK and ERK form a complex together with a scaffold protein that apparently has a preference for MEK1 (Morrison and Davis, 2003; Schaeffer et al., 1998). Clearly future studies will be required to unravel the detailed molecular events linking the activation of the ERK signaling cascade and MEK1s involvement in it with effects on the growth of HSV-2 in virus-infected cells.
In an attempt to explore the possibility of therapy for viral infection, cellular proteins important for the virus life cycle have been considered. It is also noteworthy that many small molecular compounds inhibiting MAPK pathways, such as ARRY-142886 (MEK1/2 inhibitor), have reached the clinical trial stage for cancer therapy (English and Cobb, 2002; Kohno and Pouyssegur, 2006). Our data add new information in considering only MEK1 as a potential antiviral molecular target which may be safer because of fewer side effects produced by targeting than targeting two.
In conclusion, this study is the first to identify the key role played by MEK 1 in the activation of the ERK signaling cascade required to maximize HSV-2 replication in viral infected cells. This finding raises the possibility that the identification of specific small molecule inhibitors of the MEK1 kinase will lead to the development of a new class of anti-virals with which to combat HSV-2-induced disease. Clearly further studies will be needed to fully characterize the benefits and limitations of blocking MEK1 based activation of the ERK pathway for HSV-2-induced disease.
Acknowledgments
This work was supported by grants from National High-Tech Research and Development Program of China (863 Program, no. 2007AA02Z317), the National Natural Science Foundation of China (no. 30470084) and we thank Dr. Roger Everett (University of Glasgow, Scotland) and Dr. Wen-Xian Jia (Sichuan University, China) for generous gifts of HSV UL30 antibody and virus. We also thank Dr. Malcolm A. McCrae for critical reading and revision on the manuscript.
References
- Belanger LF, Roy S, Tremblay M, Brott B, Steff AM, Mourad W, Hugo P, Erikson R, Charron J. Mek2 is dispensable for mouse growth and development. Mol Cell Biol. 2003;23:4778–4787. doi: 10.1128/MCB.23.14.4778-4787.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GF, Brutel JS, Morse SA. Jaweta, Melnick, & Adelberg’s Medical Microbiology. 23. McGraw Hill; New York: 2004. [Google Scholar]
- Cai Y, Liu Y, Zhang X. Suppression of coronavirus replication by inhibition of the MEK signaling pathway. J Virol. 2007;81:446–456. doi: 10.1128/JVI.01705-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambard JC, Lefloch R, Pouyssegur J, Lenormand P. ERK implication in cell cycle regulation. Biochim Biophys Acta. 2007;1773:1299–1310. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Chang F, Steelman LS, Shelton JG, Lee JT, Navolanic PM, Blalock WL, Franklin R, McCubrey JA. Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway. Int J Oncol. 2003;22:469–480. (review) [PubMed] [Google Scholar]
- Cohen P. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol. 1997;7:353–361. doi: 10.1016/S0962-8924(97)01105-7. [DOI] [PubMed] [Google Scholar]
- English JM, Cobb MH. Pharmacological inhibitors of MAPK pathways. Trends Pharmacol Sci. 2002;23:40–45. doi: 10.1016/s0165-6147(00)01865-4. [DOI] [PubMed] [Google Scholar]
- Enslen H, Davis RJ. Regulation of MAP kinases by docking domains. Biol Cell. 2001;93:5–14. doi: 10.1016/s0248-4900(01)01156-x. [DOI] [PubMed] [Google Scholar]
- Giroux S, Tremblay M, Bernard D, Cardin-Girard JF, Aubry S, Larouche L, Rousseau S, Huot J, Landry J, Jeannotte L, Charron J. Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr Biol. 1999;9:369–372. doi: 10.1016/s0960-9822(99)80164-x. [DOI] [PubMed] [Google Scholar]
- Hay J, Ruyechan WT. Regulation of herpes simplex virus type 1 gene expression. Curr Top Microbiol Immunol. 1992;179:1–14. doi: 10.1007/978-3-642-77247-4_1. [DOI] [PubMed] [Google Scholar]
- Hill J, Roberts S. Herpes simplex virus in pregnancy: new concepts in prevention and management. Clin Perinatol. 2005;32:657–670. doi: 10.1016/j.clp.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Hunter JC, Smith CC, Bose D, Kulka M, Broderick R, Aurelian L. Intracellular internalization and signaling pathways triggered by the large subunit of HSV-2 ribonucleotide reductase (ICP10) Virology. 1995;210:345–360. doi: 10.1006/viro.1995.1351. [DOI] [PubMed] [Google Scholar]
- Jacque JM, Mann A, Enslen H, Sharova N, Brichacek B, Davis RJ, Stevenson M. Modulation of HIV-1 infectivity by MAPK, a virion-associated kinase. EMBO J. 1998;17:2607–2618. doi: 10.1093/emboj/17.9.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek T, Catling AD, Reuter CW, Moodie SA, Wolfman A, Weber MJ. RAS and RAF-1 form a signalling complex with MEK-1 but not MEK-2. Mol Cell Biol. 1994;14:8212–8218. doi: 10.1128/mcb.14.12.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelemen BR, Hsiao K, Goueli SA. Selective in vivo inhibition of mitogen-activated protein kinase activation using cell-permeable peptides. J Biol Chem. 2002;277:8741–8748. doi: 10.1074/jbc.M108459200. [DOI] [PubMed] [Google Scholar]
- King CS, Cooper JA, Moss B, Twardzik DR. Vaccinia virus growth factor stimulates tyrosine protein kinase activity of A431 cell epidermal growth factor receptors. Mol Cell Biol. 1986;6:332–336. doi: 10.1128/mcb.6.1.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Pouyssegur J. Targeting the ERK signaling pathway in cancer therapy. Ann Med. 2006;38:200–211. doi: 10.1080/07853890600551037. [DOI] [PubMed] [Google Scholar]
- Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351:289–305. [PMC free article] [PubMed] [Google Scholar]
- Ludwig S, Planz O, Pleschka S, Wolff T. Influenza-virus-induced signaling cascades: targets for antiviral therapy? Trends Mol Med. 2003;9:46–52. doi: 10.1016/s1471-4914(02)00010-2. [DOI] [PubMed] [Google Scholar]
- Luo H, Yanagawa B, Zhang J, Luo Z, Zhang M, Esfandiarei M, Carthy C, Wilson JE, Yang D, McManus BM. Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J Virol. 2002;76:3365–3373. doi: 10.1128/JVI.76.7.3365-3373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- Panteva M, Korkaya H, Jameel S. Hepatitis viruses and the MAPK pathway: is this a survival strategy? Virus Res. 2003;92:131–140. doi: 10.1016/s0168-1702(02)00356-8. [DOI] [PubMed] [Google Scholar]
- Perkins D, Pereira EF, Gober M, Yarowsky PJ, Aurelian L. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway. J Virol. 2002;76:1435–1449. doi: 10.1128/JVI.76.3.1435-1449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planz O, Pleschka S, Ludwig S. MEK-specific inhibitor U0126 blocks spread of Borna disease virus in cultured cells. J Virol. 2001;75:4871–4877. doi: 10.1128/JVI.75.10.4871-4877.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleschka S, Wolff T, Ehrhardt C, Hobom G, Planz O, Rapp UR, Ludwig S. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat Cell Biol. 2001;3:301–305. doi: 10.1038/35060098. [DOI] [PubMed] [Google Scholar]
- Schaeffer HJ, Catling AD, Eblen ST, Collier LS, Krauss A, Weber MJ. MP1: a MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science. 1998;281:1668–1671. doi: 10.1126/science.281.5383.1668. [DOI] [PubMed] [Google Scholar]
- Shakibaei M, Schulze-Tanzil G, de Souza P, John T, Rahmanzadeh M, Rahmanzadeh R, Merker HJ. Inhibition of mitogen-activated protein kinase induces apoptosis of human chondrocytes. J Biol Chem. 2001;276:13289–13294. doi: 10.1074/jbc.M010859200. [DOI] [PubMed] [Google Scholar]
- Sizemore JM, Jr, Lakeman F, Whitley R, Hughes A, Hook EW., 3rd The spectrum of genital herpes simplex virus infection in men attending a sexually transmitted disease clinic. J Infect Dis. 2006;193:905–911. doi: 10.1086/500841. [DOI] [PubMed] [Google Scholar]
- Skarpen E, Flinder LI, Rosseland CM, Orstavik S, Wierod L, Oksvold MP, Skalhegg BS, Huitfeldt HS. MEK1 and MEK2 regulate distinct functions by sorting ERK2 to different intracellular compartments. FASEB J. 2008;22:466–476. doi: 10.1096/fj.07-8650com. [DOI] [PubMed] [Google Scholar]
- Smith CC, Nelson J, Aurelian L, Gober M, Goswami BB. Ras-GAP binding and phosphorylation by herpes simplex virus type 2 RR1 PK (ICP10) and activation of the Ras/MEK/MAPK mitogenic pathway are required for timely onset of virus growth. J Virol. 2000;74:10417–10429. doi: 10.1128/jvi.74.22.10417-10429.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Herrero R, Bosetti C, Munoz N, Bosch FX, Eluf-Neto J, Castellsague X, Meijer CJ, Van den Brule AJ, Franceschi S, Ashley R. Herpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer. J Natl Cancer Inst. 2002;94:1604–1613. doi: 10.1093/jnci/94.21.1604. [DOI] [PubMed] [Google Scholar]
- Sturgill TW. MAP kinase: it’s been longer than fifteen minutes. Biochem Biophys Res Commun. 2008;371:1–4. doi: 10.1016/j.bbrc.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Ussar S, Voss T. MEK1 and MEK2, different regulators of the G1/S transition. J Biol Chem. 2004;279:43861–43869. doi: 10.1074/jbc.M406240200. [DOI] [PubMed] [Google Scholar]
- Wagner EK, Guzowski JF, Singh J. Transcription of the herpes simplex virus genome during productive and latent infection. Prog Nucleic Acid Res Mol Biol. 1995;51:123–165. doi: 10.1016/s0079-6603(08)60878-8. [DOI] [PubMed] [Google Scholar]
- Ward PL, Roizman B. Herpes simplex genes: the blueprint of a successful human pathogen. Trends Genet. 1994;10:267–274. doi: 10.1016/0168-9525(90)90009-u. [DOI] [PubMed] [Google Scholar]
- Weir JP. Regulation of herpes simplex virus gene expression. Gene. 2001;271:117–130. doi: 10.1016/s0378-1119(01)00512-1. [DOI] [PubMed] [Google Scholar]
- Whitley R. Neonatal herpes simplex virus infection. Curr Opin Infect Dis. 2004;17:243–246. doi: 10.1097/00001432-200406000-00012. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Morishima T. Neonatal herpes. Nippon Rinsho. 2006;64 (Suppl 3):268–271. [PubMed] [Google Scholar]
- Yip-Schneider MT, Schmidt CM. MEK inhibition of pancreatic carcinoma cells by U0126 and its effect in combination with sulindac. Pancreas. 2003;27:337–344. doi: 10.1097/00006676-200311000-00012. [DOI] [PubMed] [Google Scholar]
- Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- Zheng B, Fiumara P, Li YV, Georgakis G, Snell V, Younes M, Vauthey JN, Carbone A, Younes A. MEK/ERK pathway is aberrantly active in Hodgkin disease: a signaling pathway shared by CD30, CD40, and RANK that regulates cell proliferation and survival. Blood. 2003;102:1019–1027. doi: 10.1182/blood-2002-11-3507. [DOI] [PubMed] [Google Scholar]