Abstract
Measurement of perfusion in longitudinal studies allows for the assessment of tissue integrity and the detection of subtle pathologies. In this work, the feasibility of measuring brain perfusion in rats with high spatial resolution using arterial spin labeling (ASL) is reported. A flow sensitive alternating recovery (FAIR) sequence, coupled with a balanced gradient fast imaging with steady state precession (TrueFISP) readout section was used to minimize ghosting and geometric distortions, while achieving high SNR. The quantitative imaging of perfusion using a single subtraction (QUIPSS) method was implemented to address the effects of variable transit delays between the labeling of spins and their arrival at the imaging slice. Studies in six rats at 7 T showed good perfusion contrast with minimal geometric distortion. The measured blood flow values of 152.5 ± 6.3 ml/100g/min in gray matter and 72.3 ± 14.0 ml/100g/min in white matter are in good agreement with previously reported values based on autoradiography, considered to be the gold standard.
Keywords: cerebral blood flow, arterial spin labeling, perfusion measurement, FAIR-TrueFISP
INTRODUCTION
Brain perfusion is the process of delivering fresh volume of arterial blood to the capillary bed for providing nutrients to the tissue. Perfusion is an important physiological measure that reflects tissue integrity and it can help detect subtle pathology that may not be evident on conventional imaging techniques.
Strategies to measure brain perfusion have been investigated since the 1940s [1]. Magnetic resonance imaging (MRI) has emerged as a noninvasive way of assessing tissue perfusion. MRI-based perfusion measurement strategies can be divided into two major categories: 1) use of exogenous tracers (e.g. paramagnetic contrast agents, such as those based on gadolinium chelates) as in dynamic contrast enhanced (DCE) [2] or dynamic susceptibility contrast (DSC) [3] and 2) use of magnetically tagged endogenous water protons as the tracer. This second approach is the basis for arterial spin labeling (ASL) [4,5]. In ASL, radiofrequency (RF) pulses are used to magnetically label protons that cause signal change due to flow. The labeling can be continuous (continuous ASL or CASL) or transient (pulsed ASL or PASL). Of these two methods, PASL has gained considerable popularity as evidenced by large number of publications. PASL MRI pulse sequences consist of an inversion recovery labeling section followed by a readout section. The perfusion information is obtained after the subtraction of two images: one acquired with a non-selective inversion and the other with slice selective inversion of magnetization. Various ASL-based perfusion techniques have been recently reviewed [6–9].
Variations in the strategy for labeling spins in ASL include echo planar imaging (EPI) and signal targeting with alternating radiofrequency (EPISTAR) [10], proximal inversion with control for off-resonance effects (PICORE) [11], and flow-sensitive alternating inversion recovery (FAIR) [12] among others. Moreover, there are variations in the type of readout section used in ASL. EPI [13] is mostly used as the default readout section because of its ultrafast acquisition. However, EPI is prone to ghosting and geometric distortions, mainly due to eddy current and magnetic susceptibility effects. Single shot fast imaging with steady state precession (FISP), with balanced gradient waveforms (TrueFISP) [14], can avoid most of the disadvantages of EPI while still allowing fast acquisition and providing high signal-to-noise ratio (SNR). Boss, et al [15] reported FAIR-TrueFisp to ASL-based perfusion measurements in human brain at 1.5 T and 3 T. These authors adapted a simplified model proposed by Martirosian, et al [16] for estimating perfusion. This technique requires mapping the longitudinal relaxation time (T1) for each slice, which increases the scan time. Furthermore, the delay between the application of the labeling and the arrival of labeled blood into the imaging slice (transit delay) is neglected. Because this delay is on the order of T1, it can significantly affect the calculated perfusion values. One way to eliminate these variable transit delays is to implement the quantitative imaging of perfusion using a single subtraction (QUIPSS) labeling scheme by Wong et al [11,17]. This modification involves the application of saturation pulses during the recovery time prior to acquisition, to reset the magnetization to zero in the imaging slice to truncate the labeled bolus. The effect of this strategy is that the measured change in the magnetization between the selective and non-selective acquisitions becomes independent of the transit delay; instead, it becomes dependent on time parameters associated with the application of the saturation pulses that can be measured. In QUIPPS I, the saturation pulse is applied to the imaging slice. On the other hand, in QUIPSS II, the saturation pulse is applied to the region outside the imaging slice. In addition, the long acquisition times for T1 mappings can be avoided by implementing the CBF quantification model described by Buxton [18], because a constant global T1 value of arterial blood can be used in this model.
Despite the many advantages that ASL presents, the technique is not yet standardized for general use but several variations are reported to better fit investigational conditions, mainly because of the noisy nature and short tag life that compromise the quality of perfusion images. In translational research, animal models for human disease are commonly used. In addition, animal studies are commonly performed at higher magnetic fields such as 7 T. Since the quality of ASL-based perfusion improves with increasing magnetic field strength, ASL-based perfusion measurements can potentially yield high quality perfusion data in rodents at high fields due to increased SNR and T1.
The main purpose of our work was to investigate the feasibility of implementing a FAIR-TrueFISP with QUIPSS ASL technique to perform fast, high-resolution, quantitative brain perfusion measurements in rats with minimal geometric distortions at 7 T. The performance of the TrueFISP was compared with the commonly used FAIR-EPI sequence in terms of SNR per unit acquisition time.
METHODS
Animal Preparation
All imaging and animal procedures were approved by our Center for Laboratory Animal Medicine and Care, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Six male Sprague-Dawley rats (300 – 350 g) were used in these studies. Animals were anesthetized with isoflurane with and induction dose of 4% and were placed in the prone position on a custom designed Plexiglas bed. Ear plugs secured the animal head to the bed. During the scans, the animals were anesthetized with 2% isoflurane in a mixture of air (68%) and oxygen (30%) delivered by a rodent ventilator (Harvard Apparatus, South Natic, MA) through a mask. The respiratory rate and rectal temperature were monitored throughout the experiment with a physiologic monitoring unit (Model 1025, SA Instruments, Inc., Stonybrook, NY). A pulse oximeter (Model 8600V, Nonin Medical Inc., Plymouth, MN) was used to monitor the heart rate and oxygen saturation levels. An air flow warmer activated by a temperature probe (Model 11007B, SA Instruments, Inc., Stonybrook, NY) maintained the body temperature at ~36 °C. The careful replication of preparation procedures and close monitoring of the physiologic parameters helped to ensure consistent conditions in all scans.
Magnetic Resonance Imaging
A 7 T/30-cm USR MRI scanner (Bruker BioSpin, Karlsruhe, Germany) with an actively shielded, water-cooled gradient coil system (Model BGA12; 116 mm diameter) capable of producing a maximum gradient amplitude of 200 mT m−1 with 80 μs rise time was used for image acquisition. The vendor-supplied birdcage RF coil (Bruker BioSpin, Billerica, MA) with 72 mm internal diameter and 112 mm effective length was used for transmitter. An in-house designed, remotely tunable, receiver surface RF coil (15 mm diameter) was placed over the head and its holder was fixed to the imaging bed using plastic screws.
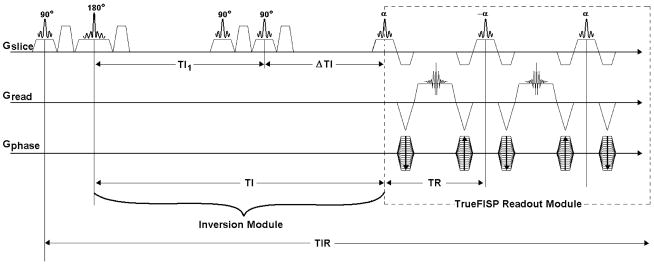
The FISP pulse sequence that is provided by the scanner manufacturer was modified to include an inversion section to convert it to FAIR-TrueFISP ASL pulse sequence (Fig. 1). The option to turn the gradients off or on during the inversion pulse was implemented to allow for non-selective or selective labeling of the spins, respectively. Slice selective saturation pulses were also included for implementing the QUIPSS I method. Two adjacent saturation pulses and associated crusher gradients were included to improve the saturation efficiency. The capability to manipulate the delay between the inversion and saturation pulses was added to allow for controlling the remaining inversion time after saturation. An in-plane pre-saturation pulse was also added before the inversion pulse to achieve a consistent initial magnetization state in the imaging plane.
FIGURE 1.
FAIR-TrueFISP ASL pulse sequence. Enclosed in the dashed box is the TrueFISP readout section. The inversion section is shown on the left. Saturation pulses complete the sequence with the QUIPSS implementation.
Test Phantom
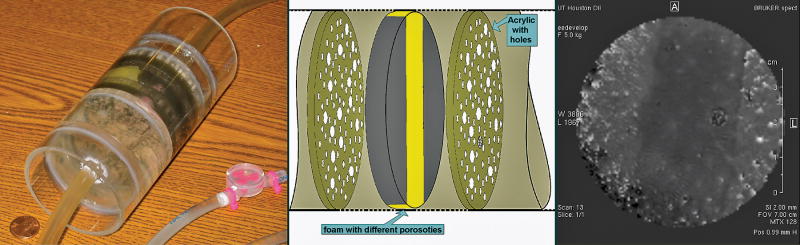
A multi-compartment cylindrical (7 cm diameter × 16 cm long) perfusion phantom was designed and built using foam layers of various porosities, holes, and fine tubing strips (Fig. 2). The phantom was used to test the sequence prior to performing the in vivo studies and to optimize parameters like the margin. The margin refers to the thickness augmentation of the imaging slice during selective inversion. The margin is necessary to minimize the effects from an imperfect inversion pulse profile [19]. By subtracting images acquired with selective and non-selective labeling in a non-flowing experiment, the margin was increased until such subtraction rendered the signal to the noise level. This helps to eliminate the residual systematic differences between acquisitions with selective and non-selective labeling, thus providing confidence in the detection of actual perfusion contrast during the in vivo studies.
FIGURE 2.
Left: Perfusion phantom showing the various compartments separated by acrylic machined pieces with holes of various diameters. Foam layers of different porosities were used in three of the five compartments. Some holes between compartments were interconnected and others converged to a common end using fine tubes. The feeding tubes came from each end of the magnet and were connected to a water pump (not shown) for continuous water circulation. Middle: Schematic of the center part of the phantom showing the structure in this region. Right: Perfusion image acquired axially at the middle section of the phantom with the FAIR-TrueFISP ASL sequence. The hypointense area in the perfusion map corresponds to the phantom’s yellow foam (central stripe), which has finer porosity than its surroundings and is shown in the figure on the left. In this area, hyperintense spots show larger in-plane spread consistent with a relatively lower flow in this region.
In vivo Imaging
Initially tri-pilot images were acquired to position the animal and locate the region of interest. Global shimming was performed over the entire brain region. ASL data was acquired from 2 mm contiguous brain slices using the single shot FAIR-TrueFISP ASL pulse sequence. Slices were acquired as separate scans. For each slice the scan with selective labeling followed the scan with non-selecting labeling. An adiabatic secant hyperbolic pulse (4.89 ms) was used for inversion and a Hermite pulse (0.5 ms, 70° flip angle) was used for excitation. Other imaging parameters for the TrueFISP readout section were: pulse repetition time (TR) = 2.68 ms, echo time (TE) = 1.34 ms, acquisition matrix = 128 ×128 pixels, field of view = 35 × 35 mm, spectral bandwidth = 100 kHz, and number of dummy echoes = 64. The readout section, including the dummy echoes, was 520.17 ms long. The imaging parameters used for the FAIR inversion section using the QUIPPS method were: inversion time TI = 1400 ms, post-labeling delay time (ΔTI) suitable for any location in the rat brain [20], ΔTI = 700 ms, inversion slab thickness = 12 mm, and repetition time for inversion = 5000 ms. The number of averages for each scan was 30. The total imaging time per scan was 2.5 minutes.
After covering the whole brain (typically 7 slices), a final scan of a centric slice was acquired to determine the equilibrium magnetization of the arterial blood. Imaging parameters for this scan were the same as those used in the non-selective labeling scan, but with the inversion and saturation pulses turned off, and a TR value of 12000 ms. The signal intensity from the most intense pixel in the carotid artery was calculated and rescaled according to the flip angle used.
In order to compare the performance of our FAIR-TrueFISP technique with the commonly used FAIR-EPI method in terms of the SNR per unit acquisition time, images were also acquired using FAIR-EPI sequence. The acquisition parameters were the same as those used for the TrueFISP except for TE of 63.0 ms. The total scan time was 3.67 minutes per scan.
Perfusion Calculation
Offline reconstruction and data analysis were performed using IDL software (ITT industries, Boulder, CO). CBF maps (ml/100g/min) were obtained using the following equation [12,18]:
| (1) |
where λ = 90 ml/100g [21] is the tissue-blood partition coefficient, MoB is the equilibrium magnetization of the blood, T1B = 2070 ms is the T1 relaxation time of the blood at 7 T [22], and 6×10−4 is the conversion factor from ms to min. In the above equation, Mselective and Mnon-selective represent the signals with selective and non-selective inversion, respectively.
The CBF values for gray and white matter were calculated from the maps using the regions of interest (ROIs) drawn by hand in the cerebral cortex and in the Genu of corpus callosum, respectively (Fig. 3). The average value for each animal was calculated from the slices (around three) that depicted most clearly the gray and white matter.
FIGURE 3.
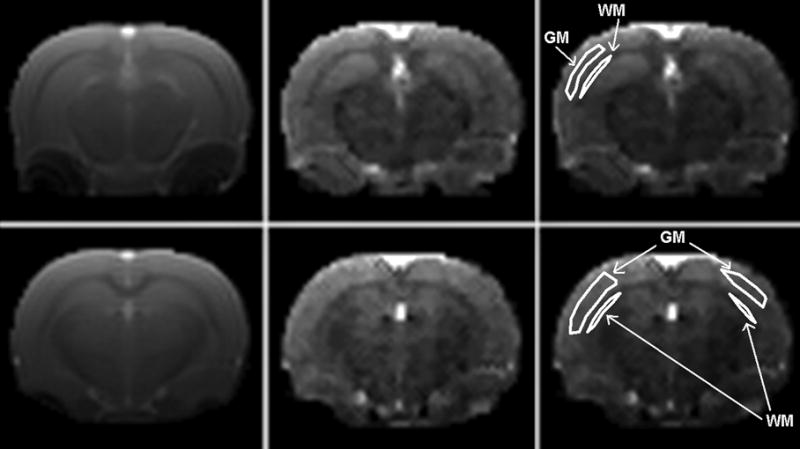
Representative brain ASL images acquired using the FAIR-TrueFISP with QUIPSS sequence with non-selective inversion (left column), and corresponding CBF maps (central column). Right column is the replicated CBF map showing representative ROI drawings for the gray (GM) and white matter (WM) definition. Imaging parameters were: matrix size = 128×128, FOV = 35 × 35 mm, slice thickness = 2.0 mm, TR = 2.68 ms, TE = 1.34 ms, TI = 1400 ms, ΔTI (QUIPSS) = 700 ms, slab thickness (inversion) = 12 mm, and repetition time for inversion = 5000 ms.
SNR Calculation
SNR was calculated for each ROI as S/N, where S is the tissue signal or M in eq. (1) and N is the standard deviation of the signal in the region outside the image. The SNR of the subtracted image was calculated as CNR=(Sselective−Snon-selective)/N.
RESULTS
A typical perfusion image from the phantom is shown in Fig. 2. Images from selective-non-selective ASL acquisitions confirmed the performance of the FAIR-TrueFISP pulse sequence. Images show relative perfusion contrast in accordance with the location of the holes, the different foam porosities, and tubing arrangements in the phantom. The effect of the foam with finer porosity than its surroundings (central stripe, colored yellow in Fig. 2) is clearly seen as a hypointense area on MRI because of reduced flow. The relatively hyperintense blurred areas within this hypointense region represent the flow through the holes in the acrylic sheet passing through the foam.
Representative FAIR-TrueFISP images with corresponding CBF maps of a rat brain are shown in Fig. 3. As seen from these images, the excellent contrast allows for a clear distinction between gray matter and white matter structures. As expected, gray matter with higher perfusion appears hyperintense relative to white matter with lower perfusion. As can be observed from this figure, these images exhibit little geometric distortion.
The CBF values for the gray and white matter from each animal study are shown in Fig. 4. These values represent the average over various slices for each animal. Averaged over all six animals, gray matter perfusion was 152.5 ± 6.3 ml/100g/min and white matter perfusion was 72.3 ± 14.0 ml/100g/min.
FIGURE 4.
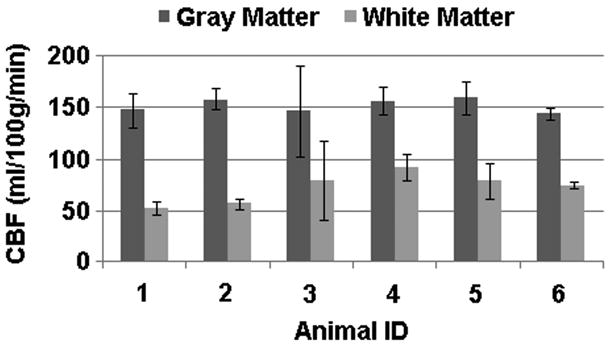
Calculated CBF values for six rats. Error bars represent the standard deviation over all the slices. Mean CBF values were 152.5 ± 6.3 ml/100g/min for gray matter and 72.3 ± 14.0 ml/100g/min for white matter. Representative images in Fig. 3 correspond to animal ID 4.
Table 1 shows the results of SNR calculations for the FAIR-TrueFISP and FAIR-EPI. Images with FAIR-TrueFISP provided higher SNR relative to conventional FAIR-EPI by a factor of about 4 and for the subtracted image by a factor of about 2.
TABLE 1.
SNR values from acquired images and subtracted image using FAIR-TrueFISP and conventional FAIR-EPI.
| SNR | SNR (Subtracted Image) | |||
|---|---|---|---|---|
| Acquisition Method | Gray Matter | White Matter | Gray Matter | White Matter |
| FAIR-EPI | 267.1+42.1 | 159.2+23.6 | 23.1+9.7 | 20.6+0.6 |
| FAIR-TrueFISP | 852.6+62.3 | 738.3+36.5 | 46.7+17.8 | 36.3+16.2 |
DISCUSSION AND CONCLUSION
In these preliminary studies, we have implemented a FAIR-TrueFISP with QUIPSS ASL technique for quantitative measurements of perfusion in rat brain. The high SNR and lack of image distortion (Fig. 3) demonstrate the effectiveness of TrueFISP as an alternative to EPI for the ASL readout section. The use of QUIPSS allowed for elimination of the variable transit delay effects [11,17]. Furthermore, the use of QUIPSS allowed the reduction in the scan time since no T1 maps are required.
Most MRI-based CBF studies have been performed on human brain. In contrast, very few ASL-based CBF values in rodent brains have been reported [23,24]. Most of animal studies were performed on the whole brain. Therefore, it is difficult to compare the published results with ours. In a recent ASL-based perfusion measurements, Carr et al [25] reported average CBF values of 140–150 ml/100g/min over the whole brain using FAIR-Turbo FLASH. A reason for reporting the average values over the entire brain is the noisy nature of ASL, which poses problems in acquiring CBF maps with high spatial resolution from small rat brains. For example, Carr et al [25] reported an SNR of 273 per mm3, with 25 minutes per acquisition. The SNR reported by Carr et al [25] translates to 11 per unit time compared to 13 with our FAIR-TrueFISP and with 6 with FAIR-EPI. Our findings of a higher SNR with TrueFISP relative to to FAIR-EPI are also in agreement with the findings by Boss et al. [15]. The ASL technique with EPI readout further aggravates this problem because of geometric distortions. Nevertheless, small animal models are an important part of translational medicine. Here, we demonstrated that some of these problems can be overcome by combining the intrinsic high SNR at high fields with TrueFISP. Voucher et al. [26] used autoradiography to measure CBF in various structures. Their reported average value of CBF was 137 ml/100g/min in gray matter structures and 50 ml/100g/min in white matter. These autoradiographic measurements, considered to be the gold standard, are in good agreement with our results.
The frequency of the inversion pulse in the FAIR sequence, in contrast to EPISTAR or PICORE, is the same as the frequency of the imaging slice and does not exhibit off-resonance effects. Nevertheless, the in-plane pre-saturation pulse in our sequence (Fig. 1) was used to ensure consistent initial magnetization in the imaging plane. As demonstrated by Wong et al [11], the difference signal is completely independent of the tissue contrast in the original images, whether an inversion recovery or saturation recovery is employed.
Further improvements to the present technique may include the application of QUIPSS II with thin-slice TI1 periodic saturation (Q2TIPS) [27] to minimize errors arising from incomplete saturation of spins and spatial mismatch between the saturation and the inversion slice profiles. Furthermore, as a FAIR based sequence that is robust against magnetization transfer effects (because no off resonance irradiation is applied with respect to the imaging slice), thicker imaging slabs may be prescribed for multi-slice acquisitions to further reduce the scanning time. Although the studies reported here used single slice acquisitions, multi-slice scan acquisitions could also be used. However, at 520 ms readout length per slice, up to three slice packages may be achievable before there is a significant reduction in the contrast results. In conclusion, we showed the feasibility of using a FAIR-TrueFisp with QUIPPS ASL sequence at 7 T for CBF calculations in the rat brain.
Acknowledgments
This work was supported in part by NINDS/NIH, Grant R01 NS045624, awarded to PAN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kety SS, Schmidt CF. The Nitrous Oxide Method for the Quantitative Determination of Cerebral Blood Flow in Man: Theory, Procedure and Normal Values. J Clin Invest. 1948;27(4):476–483. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brix G, Semmler W, Port R, Schad LR, Layer G, Lorenz WJ. Pharmacokinetic parameters in CNS Gd-DTPA enhanced MR imaging. J Comput Assist Tomogr. 1991;15(4):621–628. doi: 10.1097/00004728-199107000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Rosen BR, Belliveau JW, Vevea JM, Brady TJ. Perfusion imaging with NMR contrast agents. Magn Reson Med. 1990;14(2):249–265. doi: 10.1002/mrm.1910140211. [DOI] [PubMed] [Google Scholar]
- 4.Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23(1):37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- 5.Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A. 1992;89(1):212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golay X, Petersen ET. Arterial spin labeling: benefits and pitfalls of high magnetic field. Neuroimaging Clin N Am. 2006;16(2):259–268. x. doi: 10.1016/j.nic.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Golay X, Petersen ET, Zimine I, Lim TC. Arterial Spin Labeling: a one-stop-shop for measurement of brain perfusion in the clinical settings. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:4320–4323. doi: 10.1109/IEMBS.2007.4353292. [DOI] [PubMed] [Google Scholar]
- 8.Petersen ET, Zimine I, Ho YC, Golay X. Non-invasive measurement of perfusion: a critical review of arterial spin labelling techniques. Br J Radiol. 2006;79(944):688–701. doi: 10.1259/bjr/67705974. [DOI] [PubMed] [Google Scholar]
- 9.van Laar PJ, van der Grond J, Hendrikse J. Brain perfusion territory imaging: methods and clinical applications of selective arterial spin-labeling MR imaging. Radiology. 2008;246(2):354–364. doi: 10.1148/radiol.2462061775. [DOI] [PubMed] [Google Scholar]
- 10.Edelman RR, Siewert B, Darby DG, Thangaraj V, Nobre AC, Mesulam MM, Warach S. Qualitative mapping of cerebral blood flow and functional localization with echo-planar MR imaging and signal targeting with alternating radio frequency. Radiology. 1994;192(2):513–520. doi: 10.1148/radiology.192.2.8029425. [DOI] [PubMed] [Google Scholar]
- 11.Wong EC, Buxton RB, Frank LR. Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR Biomed. 1997;10(4–5):237–249. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<237::aid-nbm475>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 12.Kim SG. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med. 1995;34(3):293–301. doi: 10.1002/mrm.1910340303. [DOI] [PubMed] [Google Scholar]
- 13.Edelman RR, Wielopolski P, Schmitt F. Echo-planar MR imaging. Radiology. 1994;192(3):600–612. doi: 10.1148/radiology.192.3.8058920. [DOI] [PubMed] [Google Scholar]
- 14.Schmitt P, Griswold MA, Gulani V, Haase A, Flentje M, Jakob PM. A simple geometrical description of the TrueFISP ideal transient and steady-state signal. Magn Reson Med. 2006;55(1):177–186. doi: 10.1002/mrm.20738. [DOI] [PubMed] [Google Scholar]
- 15.Boss A, Martirosian P, Klose U, Nagele T, Claussen CD, Schick F. FAIR-TrueFISP imaging of cerebral perfusion in areas of high magnetic susceptibility differences at 1.5 and 3 Tesla. J Magn Reson Imaging. 2007;25(5):924–931. doi: 10.1002/jmri.20893. [DOI] [PubMed] [Google Scholar]
- 16.Martirosian P, Klose U, Mader I, Schick F. FAIR true-FISP perfusion imaging of the kidneys. Magn Reson Med. 2004;51(2):353–361. doi: 10.1002/mrm.10709. [DOI] [PubMed] [Google Scholar]
- 17.Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II) Magn Reson Med. 1998;39(5):702–708. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]
- 18.Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998;40(3):383–396. doi: 10.1002/mrm.1910400308. [DOI] [PubMed] [Google Scholar]
- 19.Bernstein Matt A, King Kevin F, Zhou Xiaohong J. Handbook of MRI Pulse Sequences. Oxford: Elsevier Academic Press; 2004. p. 811. [Google Scholar]
- 20.Thomas DL, Lythgoe MF, van der Weerd L, Ordidge RJ, Gadian DG. Regional variation of cerebral blood flow and arterial transit time in the normal and hypoperfused rat brain measured using continuous arterial spin labeling MRI. J Cereb Blood Flow Metab. 2006;26(2):274–282. doi: 10.1038/sj.jcbfm.9600185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herscovitch P, Raichle ME. What is the correct value for the brain-blood partition coefficient for water? J Cereb Blood Flow Metab. 1985;5(1):65–69. doi: 10.1038/jcbfm.1985.9. [DOI] [PubMed] [Google Scholar]
- 22.Dobre MC, Ugurbil K, Marjanska M. Determination of blood longitudinal relaxation time (T1) at high magnetic field strengths. Magn Reson Imaging. 2007;25(5):733–735. doi: 10.1016/j.mri.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Wegener S, Wong EC. Longitudinal MRI studies in the isoflurane-anesthetized rat: long-term effects of a short hypoxic episode on regulation of cerebral blood flow as assessed by pulsed arterial spin labeling. NMR Biomed. 2008;21(7):696–703. doi: 10.1002/nbm.1243. [DOI] [PubMed] [Google Scholar]
- 24.Pell GS, Lewis DP, Branch CA. Pulsed arterial spin labeling using TurboFLASH with suppression of intravascular signal. Magn Reson Med. 2003;49(2):341–350. doi: 10.1002/mrm.10373. [DOI] [PubMed] [Google Scholar]
- 25.Carr JP, Buckley DL, Tessier J, Parker GJM. What levels of precision are achievable for quantification of perfusion and capillary permeability surface area product using ASL? Magn Reson Med. 2007;58(2):281–289. doi: 10.1002/mrm.21317. [DOI] [PubMed] [Google Scholar]
- 26.Vaucher E, Borredon J, Seylaz J, Lacombe P. Autoradiographic distribution of cerebral blood flow increases elicited by stimulation of the nucleus basalis magnocellularis in the unanesthetized rat. Brain Res. 1995;691(1–2):57–68. doi: 10.1016/0006-8993(95)00601-l. [DOI] [PubMed] [Google Scholar]
- 27.Luh WM, Wong EC, Bandettini PA, Hyde JS. QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn Reson Med. 1999;41(6):1246–1254. doi: 10.1002/(sici)1522-2594(199906)41:6<1246::aid-mrm22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]