Abstract
Treatment outcomes were compared across smokers enrolled in the COMPASS cessation trial with (PH+, n = 271) and without (PH-, n = 271) a diagnosis of psychiatric history based on medical record evidence of anxiety, depression, psychotic disorder, or bipolar disorder Everyone received behavioral counseling plus varenicline and was followed for 6 months post-quit date. PH+ smokers took varenicline for fewer days on average (59.4 vs. 68.5, P ≤ .01), but did not differ in their use of behavioral treatment. PH+ smokers were more likely to report anxiety and depression, but side-effect intensity ratings did not differ after adjusting for multiple comparisons. Overall, all side-effects were rated as moderate intensity or less. Groups had similar 30 day abstinence rates at 6 months (31.5% PH+ vs. 35.4% PH-, P = .35). In sum, having a psychiatric diagnosis in this trial did not predict worse treatment outcome or worse treatment side-effects.
Keywords: varenicline, smoking cessation, depression, anxiety, psychiatric illness, side-effects
1. Introduction
Rates of tobacco use and dependence are disproportionately high among those with psychiatric disorders (Diaz et al., 2009; Gonzalez-Pinto et al., 1998; Lasser et al., 2000; Ziedonis, Hitsman, Beckham, Zvolensky, Adler, Adurain-McGovern et al., 2008). In the U.S., persons with a psychiatric condition smoke at nearly twice the rate of the general population (Lasser et al., 2000; Rohde, Lewinsohn, Brown, Gau, & Kahler, 2003); and although approximately 1 in 5 (22%) adults in the U.S. have a current psychiatric disorder (Kessler, McGonagle, Zhao, & al., 1994; Regier et al., 1993; U.S. Department of Health and Human Services, 1999), this group consumes 44.3% of all cigarettes smoked (Lasser et al., 2000).
Smoking rates are particularly pronounced among persons with a history of anxiety, depression, bipolar disorder, and psychotic disorder (Ziedonis, Hitsman, Beckham, Zvolensky, Adler, Adurain-McGovern et al., 2008)..Providing effective cessation treatment to these individuals is important, but there is limited data on the effectiveness of cessation treatments among persons with these conditions (Hall & Prochaska, 2009). Some research supports the use of specialized treatment programs targeted to their unique needs (Fagerstrom & Aubin, 2009; Haas, Munoz, Humfleet, Reus, & Hall, 2004; McFall et al., 2005), yet existing treatment programs can still be more effective among psychiatric populations than no intervention at all (Hall & Prochaska, 2009). At present, more research is needed to understand the effectiveness of standard cessation interventions among those with psychiatric conditions (Ziedonis, Hitsman, Beckham, Zvolensky, Adler, Audrain-McGovern et al., 2008). This is particularly true for interventions involving varenicline.
Varenicline (aka, Chantix®) is the latest smoking cessation medication approved by the U.S. Food and Drug Administration (FDA). It significantly increases abstinence rates compared to placebo, bupropion, and NRT (Cahill, Stead, & Lancaster, 2008; Gonzales et al., 2006; Jorenby et al., 2006; Nides et al., 2006; Oncken et al., 2006; Tonstad et al., 2006; Williams, Reeves, Billing, Pennington, & Gong, 2007). However, post-marketing surveillance and case reports have raised concerns about potential adverse effects associated with use of this medication (Alhatem & Black, 2009; DiPaula & Thomas, 2009; FDA, 2008; Freedman, 2007; Kohen & Kremen, 2007; Kuehn, 2008; 2009; Pirmoradi, Roshan, & Nadeem, 2008; Pumariega, Nelson, & Rotenberg, 2008). Increased neuropsychiatric symptoms such as depressed mood, agitation, and suicidal ideation and behavior have been reported. As a result, the FDA added a black box warning in 2009 to alert physicians and patients to these risks. Persons with a psychiatric history might be particularly vulnerable to these side-effects (Kuehn, 2009), but empirical evidence is limited. It is also unclear whether varenicline is equally effective among persons with and without psychiatric history. If this medication is associated with worse side-effects among those with psychiatric histories, it may also be less effective. If not, cessation outcomes might not differ significantly.
The current study examined the association between psychiatric history and smoking cessation in a randomized effectiveness trial of three behavioral intervention programs. Smokers received one of 3 forms of cognitive-behavioral intervention (phone-based counseling, Web-based treatment, or a combination of phone and Web-based treatment) plus a standard 12 week course of varenicline. Additional detail about each behavioral program is reportedly separately (Swan et al., In press), but all treatment was consistent with best practice recommendations (Fiore et al., 2008). In a previous secondary data analysis, we reported that smokers with a likely history of depression based on a brief, single-item self-report screen had similar short-term treatment outcomes to smokers who did not screen positive for likely prior depression (McClure et al., 2009); however, depression screeners may not accurately reflect prior clinical depression and we were unable to comment on participants’ other psychiatric conditions. The current paper extends our prior report by examining long term treatment outcome; relying on documented diagnoses of depression, anxiety, psychosis, or bipolar disorder based on medical chart review; and examining process variables that could account for cessation differences (e.g., frequency and intensity of treatment utilization). Given our prior findings and the lower lifetime quit rates among psychiatric populations, it was unclear whether smokers with a history of psychiatric illness would have lower abstinence rates after receiving a combination of behavioral intervention and varenicline. However, based on FDA warnings, we hypothesized these individuals would report worse treatment side-effects. We also hypothesized they would be less adherent to treatment as a result of these side-effects.
2. Methods
2.1 Setting
This research was conducted by Group Health (GH), SRI International (SRI), and Free & Clear, Inc. (F&C). GH is a large, non-profit, consumer-governed health plan in the U.S. SRI is a non-profit independent research organization, and F&C is a for-profit vendor of tobacco quitline services. All study activities were approved by the appropriate Institutional Review Boards (IRBs). Study enrollment began in October, 2006 and completed in October, 2007.
2.2 Recruitment, Intervention, and Sample Procedures
Adult smokers (n= 1202) were recruited from GH to participate in a randomized effectiveness trial, called the COMPASS study. Participants were randomized to one of three behavioral treatment programs (phone-based counseling, Web-based intervention, or combined phone and Web-based intervention) and all received a standard 12 week course of varenicline. Holding the medication constant across all three conditions allowed the independent effects of the behavioral treatment programs to be evaluated, which was the primary intent of COMPASS (Swan et al., in review). This paper reports on a secondary analysis of the data examining differential outcomes by pre-randomization psychiatric history.
Prior psychiatric history was determined from participants’ medical records based on ICD-9-CM codes in the 5 years preceding the date of study enrollment. Conditions thought to be most relevant to either smoking cessation or varenicline adverse events were examined and included anxiety disorders (e.g., panic disorder, generalized anxiety disorder, phobic disorders), major depression (single or recurrent), bipolar disorder, and psychotic disorders (e.g., schizophrenia, schizoaffective disorder, paranoid psychosis). For ease of interpretation, diagnoses were collapsed into 5 categories: anxiety, depression, bipolar disorder, or psychotic disorder and a sixth category of any psychiatric diagnosis.
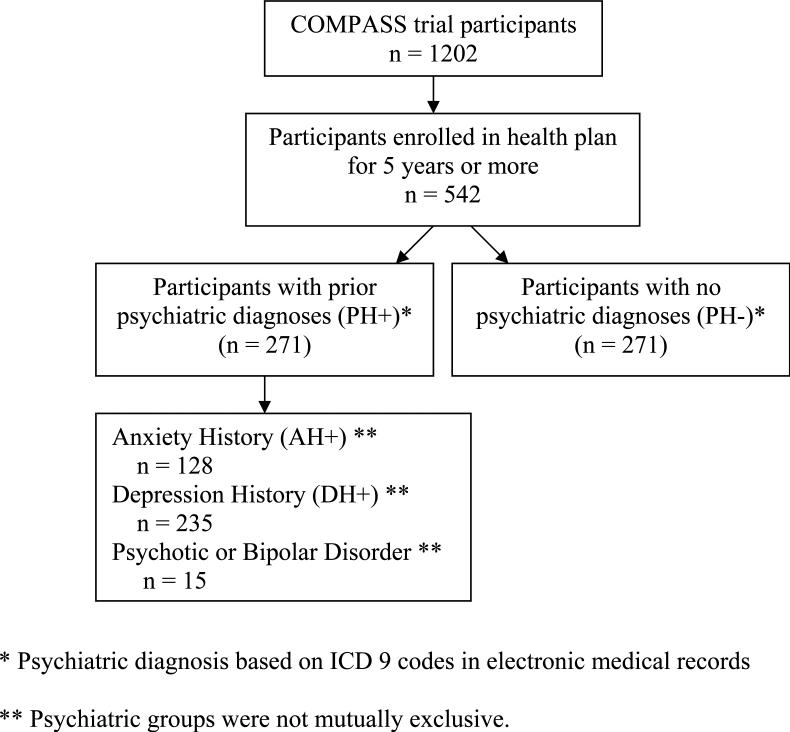
To compare participants with and without a documented psychiatric history, the sample was restricted to persons who had been enrolled in GH for at least 5 years prior to study enrollment (n = 542). The timeframe was chosen to allow adequate time for significant psychiatric diagnoses to be documented. Persons with less than 5 years of GH enrollment (n = 660) were excluded because persons enrolled for a shorter period may have had a prior psychiatric diagnosis which was not documented in the medical chart. Therefore, including these individuals could bias the sample of smokers thought not to have a mental health history. Conditions occurring more than 5 years prior with no evidence of recent diagnosis or treatment were considered less relevant as prognostic indicators and therefore, less biasing. The derivation of the final analytic samples are presented in Figure 1. Coincidentally, the sample included an equal number of participants with (PH+, n = 271) and without (PH-, n = 271) a prior psychiatric diagnosis.
Figure 1.
Study Sample
2.3 Participants
COMPASS participants were screened for eligibility by phone. Individuals were eligible if they: were 18 years or older; smoked ≥ 10 cigarettes per day over the past year and ≥ 5 cigarettes per day over the past week; were ready to quit smoking; could read and speak English; had telephone and Internet access; and were a member of GH. Individuals were excluded if they self-reported medical contraindications for varenicline use; poor health; severe heart disease; COPD; current dialysis treatment or kidney disease; high frequency alcohol intake or binge drinking; current use of other cessation treatment; and current use of investigational or recreational/street drugs or drugs thought to potentially interfere with renal clearance of varenicline. Study enrollment preceded FDA warnings about the use of varenicline in psychiatrically vulnerable populations. Persons who self-reported a diagnosis of or treatment for schizophrenia, bipolar disorder, or mania were excluded from the trial, but diagnostic interviews and medical record review were not conducted to screen out individuals with a psychiatric history. As a result, the final study sample included individuals with these psychiatric diagnoses who did not self-disclose their history at intake.
2.4 Assessment
Enrolled participants were interviewed by phone at baseline and 21 days, 3 months, and 6 month post-target quit date (TQD). Baseline assessment included demographics, smoking history, nicotine dependence assessed via the Fagerström Test of Nicotine Dependence (FTND) (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), and current depressive symptoms. Current depressive symptoms were assessed using a brief measure derived from the Hopkins Symptom Checklist (Derogatis, Lipman, Rickels, Uhlenhuth, & Covi, 1974). This measure evaluates depressive symptoms (disturbed sleep, low energy, blaming self, feeling blue, feeling everything is an effort, hopelessness about the future). Respondents rated how much they were bothered by each symptom in the past month on a 4- point scale ranging from ‘not at all’ to ‘extremely.’ Mean score was calculated. Scores on this symptom screen have been found to be inversely predictive of smoking cessation (Swan et al., 2003; Swan, Jack, Javitz, McAfee, & McClure, 2008).
At follow-up, participants reported their current smoking status, varenicline use, and presence/intensity of common side-effects associated with nicotine withdrawal and/or varenicline use. Use of the behavioral treatment programs was monitored using automated telephonic service delivery records and data captured during each visit to the online program. Non-smoking was defined as a self-report of not smoking, even a puff, for the last 7 days (point prevalent abstinence, PPA) and the last 30 days at each follow-up. For each potential treatment side-effect, participants endorsed whether they had experienced it in the prior 30 days. If so, symptom severity was rated on a 5-point Likert scale ranging from “very mild” to “very severe.”
2.5 Data Analyses
As would be expected clinically, psychiatric history groups were not mutually exclusive. Some participants had more than one diagnosis (see footnote in Table 1). Additionally, only a small number of participants had diagnoses of bipolar disorder (n = 11) or a psychotic disorder (n = 4), since self-report with these disorders was an exclusion criteria in the COMPASS trial. Consequently, these individuals were included among those with any psychiatric history, but not examined as separate diagnostic groups. Also, diagnostic groups were not compared against one another due to the overlap in group membership. We considered comparing mutually-exclusive subgroups (e.g., AH+ with no DH+ against DH+ with no AH+) against one another, but ruled this out due to the added complexity of the presentation and interpretation of pairwise comparisons among subgroups and the minimal marginal gain from the clinical relevance of these findings. Rather, persons with a positive psychiatric history (PH+), depression history (DH+), or anxiety history (AH+) were compared against people without a prior psychiatric diagnosis (PH-).
Table 1.
Participant Characteristics
| Baseline Characteristic | Anxiety (AH+) n = 128 (23.6%) | Depression (DH+) n = 235 (43.4%) | Any Psychiatric Diagnosis (PH+) n = 271 (50.0%) | No Psychiatric Diagnosis (PH-) n = 271 (50.0%) |
|---|---|---|---|---|
| Age, years (M) | 51.15 (9.4) | 51.80 (10.2) | 51.53 (10.0) | 52.02(9.4) |
| Gender (% Female) | 80.5** | 77.4** | 77.1** | 53.5 |
| Race (% White) | 90.6 | 90.6 | 88.9 | 88.6 |
| Marital status (% married) | 64.1 | 59.2 | 61.0* | 72.2 |
| Years of formal schooling (M, SD) | 14.06 (2.1) | 14.44 (2.4) | 14.33(2.0) | 14.09 (2.1) |
| Cigarettes per day (M, SD) | 19.41 (8.5) | 20.38 (8.9) | 19.82 (8.8) | 19.55(8.8) |
| FTND (M, SD) | 5.12(2.0) | 5.03 (2.2) | 4.98 (2.4) | 4.81 (2.2) |
| Motivation for quitting (M, SD) | 4.61 (0.6) | 4.64 (0.6) | 4.64 (0.6) | 4.58 (0.6) |
| Depression score | 1.03 (0.7)** | 1.07 (0.7)** | 1.01 (0. 7)** | 0.73 (0.6) |
| Treatment group | ||||
| Phone counseling program (%) | 35.9 | 34.5 | 33.9 | 33.9 |
| Web-based program (%) | 34.4 | 32.8 | 32.8 | 32.1 |
| Phone + Web (%) |
29.7 |
32.8 |
33.2 |
33.9 |
| Follow-up completion | ||||
| 21 day (n,%) | 112 (87.5) | 201 (85.5) | 235(86.7) | 235(86.7) |
| 3 month (n,%) | 106(82.8) | 191(81.3) | 209(77.1) | 216(79.7) |
| 6 month (n,%) | 100 (78.1) | 181 (77.0) | 223(82.3) | 221(81.5) |
Each diagnostic group was compared against persons with no psychiatric diagnosis (PH-). AH+ and DH+ groups were not compared to one another due to participant overlap. Ninety-three people had concurrent diagnoses of depression and anxiety, 10 had concurrent diagnoses of depression and bipolar disorder, 7 had concurrent diagnoses of anxiety and bipolar disorder, 4 had concurrent diagnoses of depression and a psychotic disorder, 3 had concurrent diagnoses of anxiety and a psychotic disorder, and 1 had a concurrent diagnosis of bipolar disorder and a psychotic disorder.
P < .05, not adjusted for multiple comparisons
P < . 001, not adjusted for multiple comparisons
The primary outcome of interest was abstinence at 6 month follow-up. Abstinence results are reported using an intent to treat (ITT) methodology where missing participants were conservatively counted as smokers. Secondary outcomes included days to relapse, treatment utilization (an indicator of adherence), and the presence and intensity of treatment side-effects.
Based on previous findings, we were unsure whether PH groups would have differential abstinence rates, but hypothesized that PH+ participants would have greater side-effects and would be less adherent to behavioral and pharmaceutical treatment. Unadjusted group differences were examined using chi-square analyses for categorical variables and t-tests for continuous variables. Logistic regression models were run for unadjusted and adjusted analyses of binary outcomes. Generalized linear models were run for adjusted analyses of Likert-scale variables (e.g. side-effect intensity scores). Adjusted analyses controlled for treatment group and baseline differences in marital status and gender. We did not adjust for baseline differences in depression score since depression is in the causal pathway between psychiatric diagnosis and treatment outcome. Finally, we applied the Benjamini-Hochberg (BH) procedure (Benjamini and Hochberg, 1995; Benajmini and Yekutieli, 2001) to control for false discovery across multiple comparisons of treatment side-effect outcomes. The procedure was applied at the 5% False Discovery Rate level to comparisons within six sets of comparisons: 1) the proportion of AH+ vs. PH- reporting symptoms, 2) the proportion of DH+ vs. PH- reporting symptoms, 3) the proportion of PH+ vs. PH- reporting symptoms, 4) average symptom intensity rating of AH+ vs. PH-, 5) average symptom rating of DH+ vs. PH-, and 6) the average symptom rating of PH+ vs. PH-. The use of this procedure assures that in each of these six sets of comparisons, we expect that no more than 5% of the symptoms that are identified as statistically significant by the BH procedure are false positives.
3. Results
3.1 Participants
Participant characteristics at baseline are presented in Table 1. Half (n = 271) were PH+, 23.6% were AH+, and 43.4% were DH+. PH+ smokers were more likely to be female, less likely to be married, and had higher baseline depression scores. Groups were otherwise similar. An equal number were randomized to each behavioral treatment group.
3.2 Abstinence & Time to Relapse
Intent to treat PPA rates at 6 month follow-up by psychiatric history group are indicated in Table 2. Abstinence rates did not differ between PH groups at 6 month follow-up (7 day PPA PH+ 32.5% vs. PH- 39.5%; OR = 1.36, CI =0.95- 1.93; 30 day PPA PH+ 31.0% vs. PH- 35.4%, OR = 1.22, CI=0.85 – 1.75). Outcomes were not significantly changed in analyses adjusting for treatment group and baseline differences in gender and marital status (7 day PPA OR = 0.77, CI = 0.53 – 1.11; 30 day PPA OR = 1.18, CI = 0.81 – 1.72).
Table 2.
Abstinence at 6 Month Follow-up by Baseline Psychiatric History
| Abstinence | Anxiety (AH+) | Depression (DH+) | Any Psychiatric Diagnosis (PH+) | No Psychiatric Diagnosis (PH-) |
|---|---|---|---|---|
| 7 day PPA (%) | 33.6 | 32.8 | 32.5 | 39.5 |
| 30 day PPA (%) | 31.3 | 31.5 | 31.0 | 35.4 |
Each diagnostic group was compared against persons with no psychiatric diagnosis. No comparisons were statistically significant in unadjusted or adjusted comparisons.
PPA = point prevalent abstinence.
Groups also did not differ in their self-reported time to relapse (Table 3). PH+ smokers relapsed after 51.1 days on average, compared to 59.5 days for persons with no psychiatric diagnosis (P = 0.23). Results were unchanged after controlling for treatment group, gender, and marital status (P = .24)
Table 3.
Days to Relapse and Treatment Utilization by Psychiatric History
| Outcomes | Anxiety (AH+) M(SE)a | Depression (DH+) M(SE)a | Any Psychiatric Diagnosis (PH+) M(SE)a | No Psychiatric Diagnosis (PH-) M(SE)a |
|---|---|---|---|---|
| Days to relapse | 49.0 (6.8) | 52.2 (5.3) | 51.1 (4.8) | 59.5 (5.0) |
| Days varenicline duration | 56.4 (3.0)* | 60.0 (2.4)* | 59.4 (2.2)* | 68.5 (2.2) |
| Total phone counseling callsb | 3.3 (0.2) | 3.5 (0.1) | 3.4 (0.1) | 3.5 (0.1) |
| Total web log-insc | 4.7 (9.9) | 3.7 (9.2) | 3.9 (9.3) | 5.6 (21.0) |
| Duration of phone counseling (total minutes) | 46.0 (4.1) | 47.6 (3.0) | 47.1 (2.8) | 43.4 (2.4) |
| Duration web treatment (total minutes) | 94.3 (1.1) | 46.7 (9.2) | 65.6 (0.7) | 81.8 (1.6) |
M = mean; SE = standard error.
Total number of proactive and reactive phone calls completed. Maximum proactive calls (i.e., counselor initiated calls to participants) attempted to be completed was 5 and was limited to participants in Phone and Phone + Web treatment groups. Additional participant initiated (i.e., reactive) calls allowed among all participants.
Total number of log-ins to online treatment program. Applicable to participants in Web and Phone + Web groups.
P ≤ .01 comparison to persons with no psychiatric diagnosis (PH-) in analyses that are unadjusted for covariates or multiple comparisons. Results of all comparisons were unchanged controlling for treatment group and baseline differences in gender and marital status.
3.3 Treatment Utilization
Psychiatric groups significantly differed in the mean duration of their varenicline use compared to persons with no psychiatric diagnosis (Table 3). Persons with no psychiatric history reportedly used varenicline the longest, an average of 68.5 days compared to 59.4 days for smokers with any of the psychiatric diagnoses of interest (P ≤ .01). Persons with anxiety or depression diagnoses reported using varenicline for significantly fewer days than smokers with no psychiatric diagnosis. All group comparisons were still statistically significant after controlling for controlling for treatment group, gender, and marital status (P ≤ .01).
On the contrary, there were no indications that persons with a psychiatric diagnosis differed in their use of behavioral counseling services. Compared to persons with no prior psychiatric history, each psychiatric group exhibited a similar total number of counseling calls completed, total web log-ins, and similar overall duration of counseling by phone (in minutes) or time spent logged into the web program (Table 3). Results were unchanged in analyses adjusting for treatment group and baseline differences in gender and marital status.
3.4 Treatment Side-Effects
Self-reported side-effects were evaluated at 21 day follow-up (corresponding to approximately one month post-varenicline initiation). An equal proportion of smokers with (n = 235) and without (n = 235) a psychiatric history endorsed experiencing side-effects in the prior month. Smokers with a psychiatric history were more likely to report tension/agitation, depression, anxiety, confusion, nausea, retching, and difficulty concentrating in both unadjusted analyses and analyses controlling for treatment group and baseline differences in gender and marital status; however, after applying the BH procedure to control for multiple comparisons, none of the comparisons were statistically significant. Comparisons significant at the P ≤ .01 level in adjusted analyses are indicated in Table 4 for interested readers.
Table 4.
Proportion of Participants Reporting Each Symptom at 21 Day Follow-up
| Symptoma | Anxiety (AH+) % | Depression (DH+) % | Any Psychiatric Diagnosis (PH+) % | No Psychiatric Diagnosis (PH-) % |
|---|---|---|---|---|
| Nauseab | 63.4 | 59.2 | 59.6 | 48.1 |
| Retchingb | 13.4 | 13.4 | 12.8 | 6.4 |
| Vomitingb | 9.0 | 12.5 | 11.1 | 7.3 |
| Gasb | 61.6 | 56.7 | 57.4 | 54.5 |
| Dysgeusiab | 43.8 | 39.8 | 41.3 | 40.4 |
| Change in appetiteb,c | 46.4 | 47.8 | 47.7 | 40.7 |
| Change in dreamingb | 59.8 | 56.2 | 56.6 | 55.3 |
| Difficulty sleepingb | 39.3 | 39.8 | 39.6 | 38.3 |
| Constipationb,c | 35.7 | 30.8 | 32.3 | 32.3 |
| Desire to smokec | 73.2 | 77.6 | 77.9 | 80.4 |
| Tension/agitationc | 51.8 | 51.7 | 50.6 | 38.7 |
| Irritability/ angerb,c | 37.5 | 43.3 | 41.7 | 37.9 |
| Anxietyb,c | 45.5 | 43.8* | 42.6* | 30.2 |
| Depressionb,c | 25.0 | 30.8* | 28.1 | 17.9 |
| Difficulty concentratingc | 33.9 | 34.3 | 33.6 | 23.8 |
| Confusionb | 19.6 | 20.9 | 19.6 | 12.8 |
Self-reported symptoms experienced in the past month, regardless of intensity.
Symptom commonly associated with varenicline use.
Symptom commonly associated with nicotine withdrawal.
P ≤ .01 compared to persons with no psychiatric diagnosis after adjusting for treatment group, gender, and marital status. However, no comparisons were statistically significant after applying the Benjamini-Hochberg procedure to control for multiple comparisons.
Disparities in symptom intensity were significant for depression, anxiety, and constipation in adjusted analyses. However, after controlling for multiple comparisons only the average symptom rating for constipation was significant between AH+ and PH- smokers. Mean ratings and items significant at the P ≤ .01 level are indicated in Table 5.
Table 5.
Average Symptom Rating Among Those Reporting Each Symptom at 21 Day Follow-up
| Symptomb | Anxiety (AH+) M (SD)a | Depression (DH+) M (SD)a | Any Psychiatric Diagnosis (PH+) M (SD)a | No Psychiatric Diagnosis (PH-) M (SD)a |
|---|---|---|---|---|
| Nauseac | 2.9 (1.1) | 2.8 (1.1) | 2.8 (1.1) | 2.5 (1.2) |
| Retchingc | 2.9 (1.2) | 2.9 (1.1) | 2.8 (1.1) | 2.7 (1.2) |
| Vomitingc | 2.8 (1.0) | 3.2 (1.1) | 3.1 (1.1) | 2.6 (1.0) |
| Gasc | 2.9 (0.9) | 2.9 (0.9) | 2.9 (0.9) | 2.8 (1.0) |
| Dysgeusiac | 2.7 (0.9) | 2.7 (1.1) | 2.7 (1.0) | 2.7 (0.8) |
| Change in appetitec,d | 2.7 (0.8) | 2.6 (1.1) | 2.6 (1.0) | 2.7 (1.0) |
| Change in dreamingc | 2.8 (1.0) | 3.0 (1.0) | 3.0 (1.1) | 3.1 (1.2) |
| Difficulty sleepingc | 2.8 (0.9) | 2.8 (0.9) | 2.8 (0.9) | 2.9 (0.9) |
| Constipationc,d | 3.1 (1.0)* | 2.9 (1.0)* | 2.8 (1.0)* | 2.5 (0.9) |
| Desire to smoked | 2.7 (1.0) | 2.9 (1.1) | 2.8 (1.1) | 2.6 (1.2) |
| Tension/agitationd | 2.4 (0.8) | 2.5(0.9) | 2.5 (0.9) | 2.2 (0.8) |
| Irritability/ angerc,d | 2.6 (0.9) | 2.6 (0.9) | 2.6 (0.9) | 2.4 (1.1) |
| Anxietyc,d | 2.5 (0.9) | 2.6 (1.0)* | 2.6 (0.9)* | 2.2 (0.8) |
| Depressionc,d | 2.9 (1.0) | 2.8 (1.0) | 2.7 (0.9) | 2.3 (1.1) |
| Difficulty concentratingd | 2.5 (1.0) | 2.6 (0.8) | 2.5 (0.9) | 2.3 (0.8) |
| Confusionc | 2.1 (0.8) | 2.0 (0.8) | 2.0 (0.8) | 2.1 (0.9) |
M = mean; SD = standard deviation.
Self-reported symptoms experienced in the past month. Symptoms rated on 5-point Likert scale from “very mild” to “very severe.”
Symptom commonly associated with varenicline use.
Symptom commonly associated with nicotine withdrawal.
P ≤ .01 compared to persons with no psychiatric diagnosis after adjusting for treatment group, gender, and marital status. However, only constipation (AH+ vs. PH-) remained significant after applying the Benjamini-Hochberg procedure to control for multiple comparisons.
4. Discussion
Having a prior psychiatric diagnosis was not associated with worse outcomes in the COMPASS trial. As a group, PH+ smokers were equally likely to be abstinent at 6 month follow-up as persons with no documented psychiatric history. This is despite PH+ participants’ reports that they used less varenicline. The results were the same for persons with a diagnosis of anxiety (AH+), depression (DH+), or any of the monitored psychiatric conditions (PH+; anxiety, depression, bipolar disorder, or psychosis). It is also noteworthy that smokers with a pre-existing psychiatric diagnosis were no more likely to report treatment side-effects and the only significant difference in side-effect intensity ratings was for constipation. Differences in affect-related symptoms were not significant after controlling for multiple comparisons.
In epidemiological trials, persons with psychiatric history have lower lifetime quit rates (Lasser et al., 2000), suggesting they may have a more difficult time quitting smoking than persons with no psychiatric history, although this has not been borne out in some clinical cessation trials, at least for depression (Hitsman, Borelli, McChargue, Spring, & Niaura, 2003; Ziedonis, Hitsman, Beckham, Zvolensky, Adler, Adurain-McGovern et al., 2008). The lack of a significant difference in cessation rates in the COMPASS trial might be due to the groups’ use of behavioral services. AH+, DH+, and PH+ smokers each utilized the phone and web counseling services in an equivalent manner to smokers with no documented psychiatric history (PH-). One goal of the behavioral interventions was to encourage participants to remain focused on their abstinence goals in spite of medication side-effects or nicotine withdrawal symptoms. These results underscore the Clinical Practice Guideline for Treating Tobacco Dependence recommendation that all smokers receive a combination of behavioral and pharmacological treatment, when medically appropriate (Fiore et al., 2008). Finally, while there was no main effect for psychiatric history on cessation, it is possible that psychiatric history could interact with other individual characteristics such as age or gender to influence outcome. This possibility is worthy of future study.
This study's findings are significant for several reasons. Not only are they consistent with prior meta-analyses showing that a positive history of depression does not decrease one's odds of quitting smoking in clinical trials (Hitsman et al., 2003; Ziedonis, Hitsman, Beckham, Zvolensky, Adler, Adurain-McGovern et al., 2008), they extend this finding to smokers with other psychiatric diagnoses. Almost no smoking cessation clinical trials have been conducted among smokers with anxiety disorders (Ziedonis, Hitsman, Beckham, Zvolensky, Adler, Adurain-McGovern et al., 2008), so these data in particular provide valuable insight into the effectiveness of behavioral counseling plus varenicline in this population. However, readers are cautioned not to draw definitive conclusions about this treatment's effectiveness in populations with bipolar or psychotic disorders. Because we attempted to screen out individuals with these diagnoses from the COMPASS trial, the resulting subsamples were too small to analyze independently.
These data also add to the growing evidence base on the safety of varenicline. FDA warnings associate varenicline use with potential neuropsychiatric side-effects such as depressed mood, agitation, and suicidal ideation and behavior (FDA, 2008; Kuehn, 2008; 2009). We did not systematically and proactively assess suicidal ideation, but we did monitor changes in mood and agitation during the active varenicline treatment phase. Although one might expect PH+ smokers to experience more and more intense side-effect symptoms, particularly affect-related symptoms, this was not the case. It is also worth noting that none of the symptoms were rated as more than moderately severe on average. These findings do not undermine the FDA concerns about varenicline, but should provide some re-assurance to providers who prescribe this drug to patients with pre-existing depression or anxiety diagnoses. However, we cannot comment on differential treatment side-effects among participants with psychosis or bipolar disorder versus those with no psychiatric history. Case reports have raised the possibility that varenicline may increase psychiatric symptoms among some of these patients (Alhatem & Black, 2009; DiPaula & Thomas, 2009; Kohen & Kremen, 2007), while other research has found no change in psychopathology among patients with psychosis taking varenicline when compared to persons with similar psychiatric diagnoses not taking this drug (Smith et al., 2009). Until more definitive, well-controlled clinical trials have been conducted to examine this specific issue, it is best to use caution when prescribing varenicline to anyone with psychosis or mania.
The present study has a number of noteworthy strengths including the fact that treatment side-effects were systematically assessed at follow-up during the active treatment phase, behavioral treatment utilization was based on automated service records, psychiatric diagnosis was based on participants’ actual medical records and not brief screening tools or self-report, and the sample was restricted to persons with at least 5 years of medical records, thereby ensuring that significant psychiatric conditions warranting treatment were identified. At the same time, several limitations should be noted. First, this is a secondary analysis of data from a randomized clinical trial designed to compare the effectiveness of three behavioral treatments. As such, all participants received varenicline. We did not have a no-medication placebo control and, therefore, treatment side-effects cannot be attributed to varenicline specifically. Additionally, abstinence was not biochemically verified at follow-up and medication use was based on self-report.
In sum, these data provide sound evidence that having a psychiatric diagnosis, particularly anxiety or depression, does not necessarily portend worse outcomes for smokers treated with varenicline and behavioral intervention. Future research is needed to inform the safety and efficacy of varenicline in other psychiatric populations. In the meantime, clinicians should continue to carefully monitor all patients taking varenicline for adverse effects, but there is no indication that the combination of behavioral counseling and varenicline will necessarily be less effective in persons with a prior psychiatric diagnosis.
Acknowledgments
The authors wish to thank the study staff at SRI and Group Health for their assistance with data collection; the staff of Free & Clear, Inc. for their assistance recruiting and counseling participants; and Sallie Dacey, MD and Abigail Halperin, MD for their assistance with medical oversight. Special thanks to Chester Pabiniak, MS for his programming assistance. The COMPASS trial was funded by the National Cancer Institute (NCI) (RO1 071358, G. Swan PI). Pfizer, Inc. donated medication and provided nominal financial support to SRI to help support data collection at that site. However, neither NCI nor Pfizer was involved in the collection or analysis of this data, had any influence on the study design, or had any influence over the content of this report. Group Health provided in-kind pharmacy support of this research. This study is registered at Clinicaltrials.gov (NCT00301145).
Drs. McAfee and Zbikowski and Ms. Deprey are employed by Free & Clear, Inc. Dr. Swan received financial support from Pfizer to attend a one-day advisory meeting in 2008. The authors have no other potential conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alhatem F, Black JE. Varenicline-induced mania in a bipolar patient. Clinical Neuropharmacology. 2009;32(2):117–118. doi: 10.1097/WNF.0b013e31816f75bc. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews. 2008 doi: 10.1002/14651858.CD006103.pub2. (3. Art No: CD006103) [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behavioral Science. 1974;19(1):1–15. doi: 10.1002/bs.3830190102. [DOI] [PubMed] [Google Scholar]
- Diaz FJ, James D, Botts S, Maw L, Susce MT, de Leon J. Tobacco smoking behaviors in bipolar disorder: A comparison of the general population, schizophrenia, and major depression. Bipolar Disorders. 2009;11(2):154–165. doi: 10.1111/j.1399-5618.2009.00664.x. [DOI] [PubMed] [Google Scholar]
- DiPaula BA, Thomas MD. Worsening psychosis induced by varenicline in a hospitalized psychiatric patient. Pharmacotherapy. 2009;29(7):852–857. doi: 10.1592/phco.29.7.852. [DOI] [PubMed] [Google Scholar]
- Fagerstrom K, Aubin HJ. Management of smoking cessation in patients with psychiatric disorders. Current Medical Research and Opinions. 2009;25(2):511–518. doi: 10.1185/03007990802707568. [DOI] [PubMed] [Google Scholar]
- FDA [June 9, 2008];Varenicline (marketed as Chantix) Information. 2008 from http://www.fda.gov/cder/drug/infopage/varenicline/
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Update. Clinical Practice Guideline. U.S. Department of Health and Human Services. Public Health Service; Rockville, MD: 2008. Treating Tobacco Use and Dependence; p. 2008. [Google Scholar]
- Freedman R. Exacerbation of schizophrenia by varenicline. American Journal of Psychiatry. 2007;164(8):1269. doi: 10.1176/appi.ajp.2007.07020326. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Pinto A, Gutierrez M, Ezcurra J, Aizpuru F, Mosquera F, Lopez P, et al. Tobacco smoking and bipolar disorder. Journal of Clinical Psychiatry. 1998;59(5):225–228. doi: 10.4088/jcp.v59n0503. [DOI] [PubMed] [Google Scholar]
- Haas AL, Munoz RF, Humfleet GL, Reus VI, Hall SM. Influences of mood, depression history, and treatment modality on outcomes in smoking cessation. Journal of Consulting and Clinical Psychology. 2004;72(4):563–570. doi: 10.1037/0022-006X.72.4.563. [DOI] [PubMed] [Google Scholar]
- Hall SM, Prochaska J. Treatment of smokers with co-occurrring disorders: Emphasis on integration in mental health and addiction treatment settings. Annual Review of Clinical Psychology. 2009;5:409–431. doi: 10.1146/annurev.clinpsy.032408.153614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hitsman B, Borelli B, McChargue DE, Spring B, Niaura R. History of depression and smoking cessation outcome: A meta-analysis. Journal of Consulting and Clinical Psychology. 2003;71(4):657–663. doi: 10.1037/0022-006x.71.4.657. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of Varenicline, an alpha4beta2 Nicotinic Acetylcholine Receptor Partial Agonist, vs Placebo or Sustained-Release Bupropion for Smoking Cessation: A Randomized Controlled Trial. JAMA. 2006;296(1):56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S. Lifetime and 12 month prevalence of DSM-III-R psychiatric disorders in the United States: Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. al., et. [DOI] [PubMed] [Google Scholar]
- Kohen I, Kremen N. Varenicline-induced manic episode in a patient with bipolar disorder. American Journal of Psychiatry. 2007;164(8):1269–1270. doi: 10.1176/appi.ajp.2007.07010173. [DOI] [PubMed] [Google Scholar]
- Kuehn BM. FDA warns of adverse events linked to smoking cessation drug and antiepileptics. JAMA. 2008;299(10):1121–1122. doi: 10.1001/jama.299.10.1121. [DOI] [PubMed] [Google Scholar]
- Kuehn BM. Varenicline gets stronger warnings about psychiatric problems, vehicle crashes. JAMA. 2009;302(8):834. doi: 10.1001/jama.2009.1153. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- McClure JB, Swan GE, Jack L, Catz SL, Zbikowski SM, McAfee TA, et al. Mood, Side-effects and Smoking Outcomes Among Persons With and Without Probable Lifetime Depression Taking Varenicline. Journal of General Internal Medicine. 2009 doi: 10.1007/s11606-009-0926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall M, Saxon AJ, Thompson CE, Yoshimoto D, Malte C, Straits-Troster K, et al. Improving the rates of quitting smoking for veterans with posttraumatic stress disorder. American Journal of Psychiatry. 2005;162(7):1311–1319. doi: 10.1176/appi.ajp.162.7.1311. [DOI] [PubMed] [Google Scholar]
- Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Archives of Internal Medicine. 2006;166(15):1561–1568. doi: 10.1001/archinte.166.15.1561. [DOI] [PubMed] [Google Scholar]
- Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Archives of Internal Medicine. 2006;166(15):1571–1577. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- Pirmoradi P, Roshan S, Nadeem SS. Neuropsychiatric disturbance after initiation of varenicline in a patient with a history of alcohol abuse and major depression. American Journal of Health-System Pharmacy. 2008;65(17):1624–1626. doi: 10.2146/ajhp070641. [DOI] [PubMed] [Google Scholar]
- Pumariega AJ, Nelson R, Rotenberg L. Varenicline-induced mixed mood and psychotic episode in a ptient with a past history of depression. CNS Spectrums. 2008;13(6):511–514. doi: 10.1017/s1092852900016746. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Myers JK, Kramer M, Robins LN, et al. One-month prevalence of mental disorders in the United States and sociodemographic characteristics: The Epidemiologic Catchment Area study. Acta Psychiatrica Scandinavica. 1993;88(1):35–47. doi: 10.1111/j.1600-0447.1993.tb03411.x. [DOI] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Brown RA, Gau JM, Kahler CW. Psychiatric disorders, familiar factors and cigarette smoking: I. Associations with smoking initiation. Nicotine & Tobacco Research. 2003;5:85–98. doi: 10.1080/1462220031000070507. [DOI] [PubMed] [Google Scholar]
- Smith RC, Lindenmayer JP, Davis JM, Cronwell J, Noth K, Gupta S, et al. Cognitive and anit-smoking effects of varenicline in patients with schizophrenia or schizoaffective disorder. Schizophrenia Research. 2009;110(1-3):149–155. doi: 10.1016/j.schres.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Swan GE, Jack LM, Curry S, Chorost M, Javitz H, McAfee T, et al. Bupropion SR and counseling for smoking cessation in actual practice: predictors of outcome. Nicotine & Tobacco Research. 2003;5(6):911–921. doi: 10.1080/14622200310001646903. [DOI] [PubMed] [Google Scholar]
- Swan GE, Jack LM, Javitz HS, McAfee T, McClure JB. Predictors of 12-month outcome in smokers who received bupropion sustained-release for smoking cessation. CNS Drugs. 2008;22(3):239–256. doi: 10.2165/00023210-200822030-00004. [DOI] [PubMed] [Google Scholar]
- Swan GE, McClure JB, Zbikowski S, McAfee T, Jack L, Catz S, et al. Behavioral counseling and varenicline treatment for smoking cessation. American Journal of Preventive Medicine. doi: 10.1016/j.amepre.2010.01.024. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . Mental health: A report of the Surgeon General. U.S. Department of Health and Human Services, Substance Abise and Mental Health Services Administration, Center for Mental Health Services, National Institutes of Health, National Institute of Mental Health; Rockville, MD: 1999. [Google Scholar]
- Williams KE, Reeves KR, Billing CB, Jr., Pennington AM, Gong J. A double-blind study evaluating the long-term safety of varenicline for smoking cessation. Current Medical Research Opinion. 2007;23(4):793–801. doi: 10.1185/030079907x182185. [DOI] [PubMed] [Google Scholar]
- Ziedonis DM, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Adurain-McGovern J, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine & Tobacco Research. 2008;10(12):1691–1715. doi: 10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]
- Ziedonis DM, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J, et al. Tobacco use and cessation in psychiatric disorders” Bational Institute of Mental Health report. Nicotine & Tobacco Research. 2008;10(12):1691–1715. doi: 10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]