Abstract
Traumatic brain injury (TBI) is a leading cause of morbidity in children and boys are disproportionately represented. Hypotension is common and worsens outcome after TBI. Previous studies show that adrenomedullin, a cerebrovasodilator, prevented sex dependent impairment of autoregulation during hypotension after piglet fluid percussion brain injury (FPI). We hypothesized that this concept was generalizable and that administration of another vasodilator, sodium nitroprusside (SNP), may equally improve CBF and cerebral autoregulation in a sex dependent manner after FPI. SNP produced equivalent percent cerebrovasodilation in male and female piglets. Reductions in pial artery diameter, cortical CBF, and cerebral perfusion pressure (CPP) concomitant with elevated intracranial pressure (ICP) after FPI were greater in male compared to female piglets during normotension which was blunted by SNP. During hypotension, pial artery dilation (PAD) was impaired more in the male than the female after FPI. However, SNP did not improve hypotensive PAD after FPI in females and paradoxically caused vasoconstriction in males. SNP did not prevent reductions in CBF, CPP or autoregulatory index during combined hypotension and FPI in either sex. SNP aggravated ERK MAPK upregulation after FPI. These data indicate that despite prevention of reductions in CBF after FPI, SNP does not prevent impairment of autoregulation during hypotension after FPI. These data suggest that therapies directed at a purely hemodynamic increase in CPP will fail to improve outcome during combined TBI and hypotension.
Keywords: brain injury, cerebral circulation, newborn, autoregulation, signal transduction
1. Introduction
Pediatric traumatic brain injury (pTBI) is a global public health concern (14,15). Boys are disproportionately represented and young children have devastating outcomes (14). Hypotension is common and worsens outcome after TBI (7). By definition, cerebral autoregulation denotes constant cerebral blood flow (CBF) during changes in mean arterial blood pressure. Hypotension can lead to cerebral ischemia when cerebral autoregulation is impaired. Since impaired autoregulation renders CBF dependent on cerebral perfusion pressure (CPP) and CBF may contribute to neuronal cell integrity, optimal management of CPP to limit tissue hypoxia with low CPP is critical. Administration of a cerebrovasodilator such as sodium nitroprusside (SNP) can improve cerebral hemodynamics after TBI by reducing intracranial pressure (ICP) and increasing CPP. However, CBF can increase in response to cerebrovasodilator administration in the absence of impaired cerebral autoregulation in the setting of TBI. Since ethical constraints preclude mechanistic studies of cerebral autoregulation in children, we have used an established porcine model of fluid percussion injury (FPI) that mimics many of the pathophysiological features of pTBI to corroborate clinical observations after pTBI (3-5). Piglets offer the unique advantage of a gyrencephalic brain containing substantial white matter, which is more sensitive to ischemic/TBI damage, similar to humans.
It is unclear whether beneficial outcome depends on increases in CPP or on signaling pathways which link improved cerebral autoregulation to limited tissue injury after pTBI. Mitogen activated protein kinase (MAPK), a family of at least 3 kinases, extracellular signal-related kinase (ERK), p38, and c-Jun N-terminal kinase (JNK) is upregulated and may contribute to injury after TBI (2-4,13). For example, activation of ERK MAPK contributes to hypoperfusion after FPI to greater extent in male compared to female pigs (4). Our recent studies show that adrenomedullin, a cerebrovasodilator, prevented sex dependent impairment of autoregulation during hypotension after piglet FPI through inhibition of ERK MAPK upregulation (4,5). Inhibition of ERK MAPK upregulation also prevents reductions of CBF after FPI (3). However, it is unknown whether SNP modulates ERK MAPK upregulation. We hypothesized that the concept that administration of a cerebrovasodilator improves cerebroautoregulation in the setting of TBI was generalizable and that administration of another cerebrovasodilator, such as SNP, may equally improve CBF and cerebral autoregulation in a sex dependent manner after piglet FPI. If this is found to be the case, then the ability of SNP to modulate ERK MAPK upregulation will also be explored.
2. Results
2.1 SNP prevents sex dependent reductions in pial artery diameter and CBF after FPI
SNP (0.1 mg/kg iv) in the absence of FPI produced equivalent decreases in mean arterial blood pressure (37 ± 4 and 35 ± 2% respectively) and increases in pial artery diameter (12 ± 2 and 17 ± 4% respectively) in male and female pigs. Changes in pial artery diameter and mean arterial blood pressure were short (1-3 min) after acute bolus administration of SNP. FPI produced greater pial small artery vasoconstriction and reduction in CBF in male compared with female newborn pigs (Fig. 1). SNP treatment 30 min prior to FPI (pre-treatment) or 30 min post-injury (post-treatment) blunted pial artery vasoconstriction and reductions in CBF at 1 and 4h post insult (Figs. 1,2). On a percentage basis, the protection afforded by SNP on reductions in pial diameter and CBF were greater in the male compared with the female.
Figure 1.
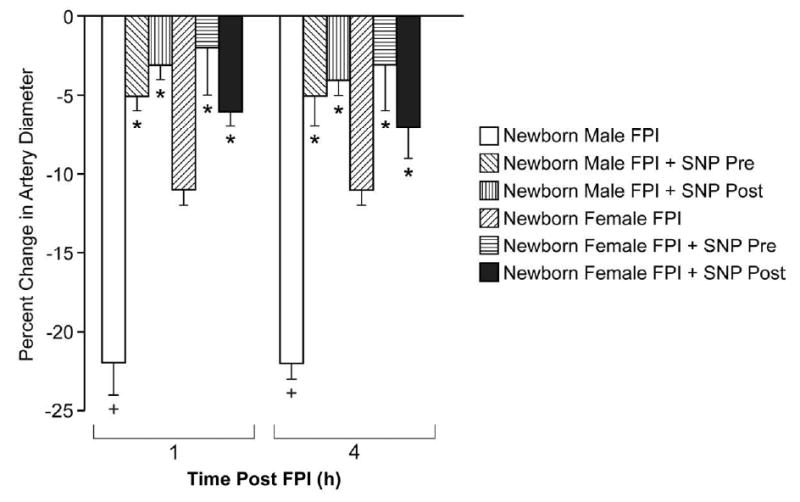
Influence of FPI (1,4h) on pial artery diameter in vehicle (FPI) and SNP (0.1 mg/kg iv) pretreated newborn male and female pigs, n=6. *p<0.05 compared with corresponding FPI vehicle value +p<0.05 compared with corresponding female value.
Figure 2.
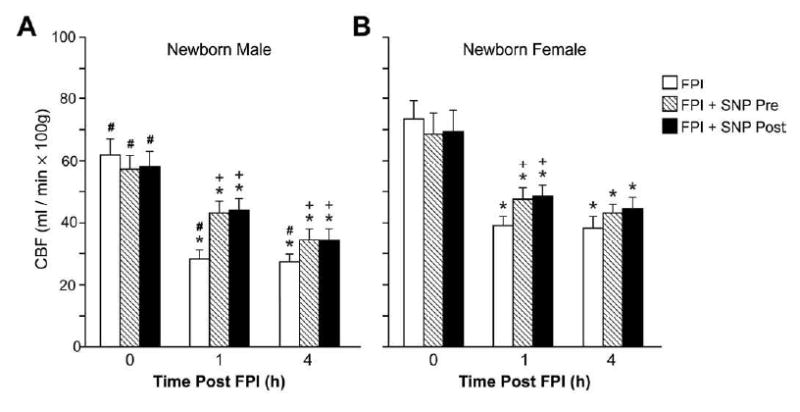
Influence of FPI (1,4h) on total CBF (ml/min. 100g) in vehicle (FPI) and SNP (0.1 mg/Kg iv) pre-treated newborn male (A) and female (B) pigs, n=6. *p<0.05 compared with corresponding 0 time (pre-injury) value +p< 0.05 compared with corresponding vehicle pre-treated value #p<0.05 compared with corresponding female value.
2.2 SNP aggravates sex dependent loss of pial artery dilation during hypotension after FPI
Moderate and severe hemorrhagic hypotension and papaverine (10-8, 10-6 M) elicited reproducible dilation of pial small arteries and arterioles. Prior to FPI, hemorrhagic hypotensive pial artery dilation was significantly less in male than female pigs (Fig. 3), similar to that recently published (4). Within 1h of FPI, hypotensive pial artery dilation was impaired in both sexes, but the degree of impairment was greater in the male compared with the female (Fig. 3). However, SNP pre-treatment and post-treatment did not augment hypotensive pial artery dilation after FPI in the female and paradoxically caused vasoconstriction after FPI in the male (Fig. 3). The numeric values for mean arterial blood pressure during normotension, moderate and severe hypotension were no different between FPI vehicle and FPI SNP treated piglets (72 ± 8, 55 ± 6, and 41 ± 4 versus 67 ± 7, 52 ± 5, and 38 ± 4 mm Hg, respectively). Absolute values for pial artery diameter for normotension, moderate, and severe hypotension conditions before and after FPI in the male were: 137 ± 7, 151 ± 8, and 165 ± 9 versus 134 ± 9, 130 ± 10, and 126 ± 12 μm, respectively). In the female, these values were: 122 ± 7, 141 ± 8, and 157 ± 10 versus 123 ± 7, 132 ± 8, and 143 ± 9 μm, respectively. Autoregulatory pial artery dilation during hypotension was unchanged by SNP in the absence of FPI (Fig. 3). Papaverine induced pial artery vasodilation was unchanged by FPI and SNP (Fig. 4), indicating that impairment of cerebral autoregulatory pial artery dilation by SNP is not an epiphemonenon.
Figure 3.
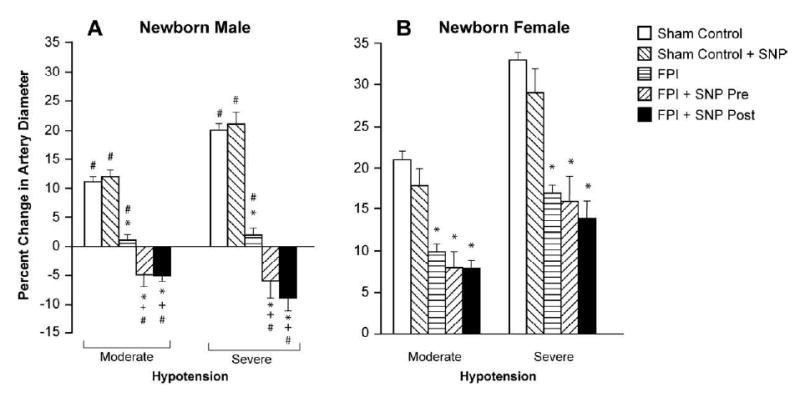
Influence of moderate and severe hypotension on pial artery diameter in absence and presence of SNP (0.1 mg/kg iv) under sham control conditions, pre-treatment or post-treatment after FPI in newborn male (A) and female (B) pigs, n=6. *p<0.05 compared with corresponding sham control value +p<0.05 compared with corresponding FPI vehicle treated value #p<0.05 compared with corresponding female value.
Figure 4.
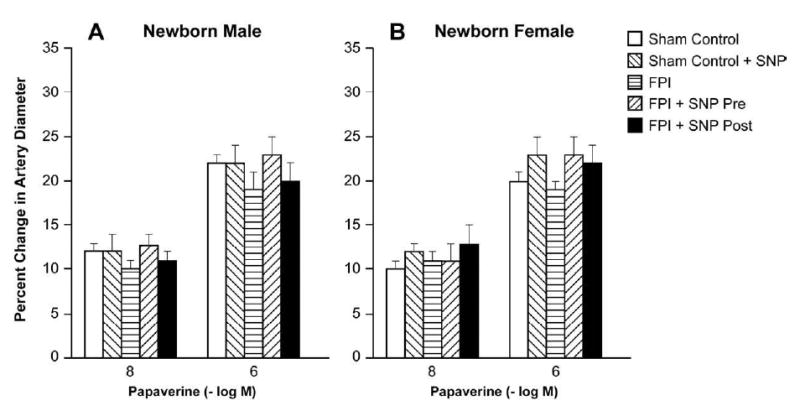
Influence of papaverine (10-8, 10-6 M) on pial artery diameter in absence and presence of SNP (0.1 mg/kg iv) under sham control conditions, pre-treatment or post-treatment after FPI in newborn male (A) and female (B) pigs, n=6.
2.3 SNP aggravates sex dependent loss of CBF autoregulation during hemorrhagic hypotension after FPI
CBF in the parietal cortex was unchanged during hypotension prior to FPI (2.0 ± 0.1 atm), supportive of intact cerebral autoregulation pre insult (Fig. 5). After FPI, CBF in the cortex was reduced more in the male compared to the female under equivalent insult level conditions (Fig. 5). CBF was further reduced during severe hypotension, but such reductions were greater in the male compared to the female (Fig. 5). SNP (0.1 mg/kg iv) post-treatment did not prevent reductions in cortical CBF observed during hemorrhagic hypotension after FPI in the female (Fig. 5). In contrast, SNP post-treatment aggravated reduction of CBF during concomitant hypotension and FPI in the male compared to comparable vehicle treated males post injury (Fig. 5). Similar observations were made when SNP was administered as a pre-treatment.
Figure 5.
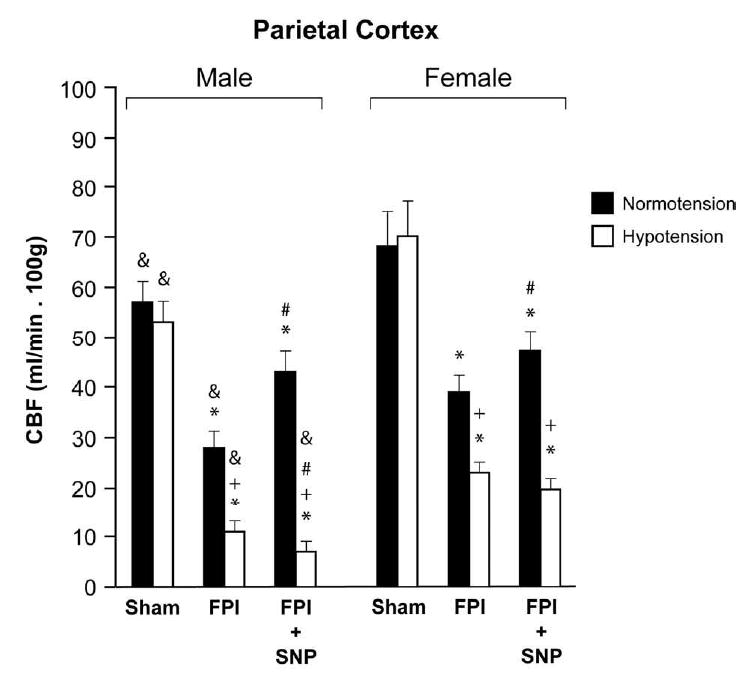
CBF (ml/min.100g) in parietal cortex during normotension and severe hypotension in sham, FPI and FPI + SNP (0.1 mg/kg iv) treated male and female pigs, n= 6. *p<0.05 compared to corresponding sham value +p<0.05 compared with corresponding normotension value #p<0.05 compared with corresponding FPI alone value & p<0.05 compared with corresponding female value.
2.4 SNP prevents sex dependent reduction in cerebral perfusion pressure (CPP) during normotension but has no effect on reductions in CPP during hypotension after FPI
ICP was increased after FPI more in the male than the female at an equivalent insult level (1.9 ± 0.1 atm) (Fig. 6A). Values of ICP during moderate and severe hypotension after FPI were also greater in the male compared to the female (Fig. 6A). Post-treatment with SNP blunted the elevation of ICP after FPI only during normotension and not during hypotension in the male (Fig. 6A). CPP was reduced after FPI more in the male than the female during normotension and hypotension due to higher ICP (Fig. 6B). Post-treatment with SNP prevented reductions in CPP during normotension in both males and females (Fig. 6B). However, reductions in CPP during hypotension after FPI were unchanged by SNP post-treatment in both males and females (Fig. 6B). Similar observations were made with SNP pre-treatment (data not shown).
Figure 6.
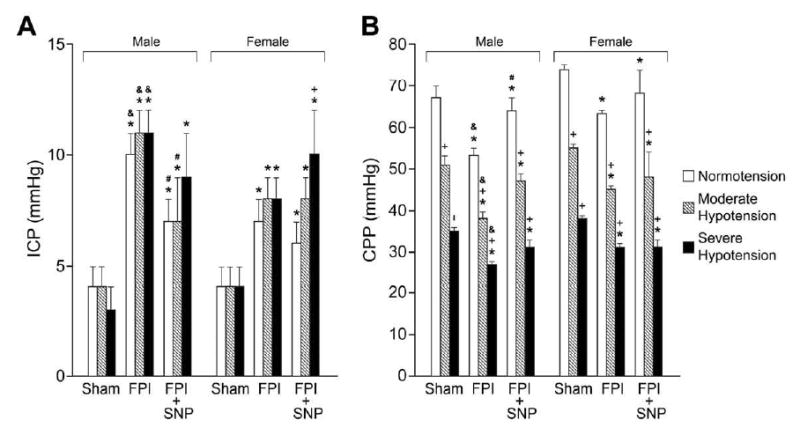
A. ICP (mm Hg) and B. CPP (mm Hg) during normotension, moderate and severe hypotension in sham, FPI and FPI + SNP (0.1 mg/kg iv) treated male and female pigs, n=6. *p<0.05 compared to corresponding sham value +p<0.05 compared with corresponding within group normotension value #p<0.05 05 compared with corresponding FPI alone value & p<0.05 compared with corresponding female value.
2.5 SNP aggravates sex dependent reductions in the autoregulatory index (ARI) after FPI
Calculated ARI was unchanged during hypotension prior to FPI, but was reduced during hypotension after FPI in both male and female piglets (Fig. 7). However, the magnitude of the decrease in ARI was greater in male than female pigs (Fig. 7). SNP aggravated reductions in ARI during severe hypotension after FPI in the male and had no effect on decreased ARI in the female after FPI (Fig. 7).
Figure 7.
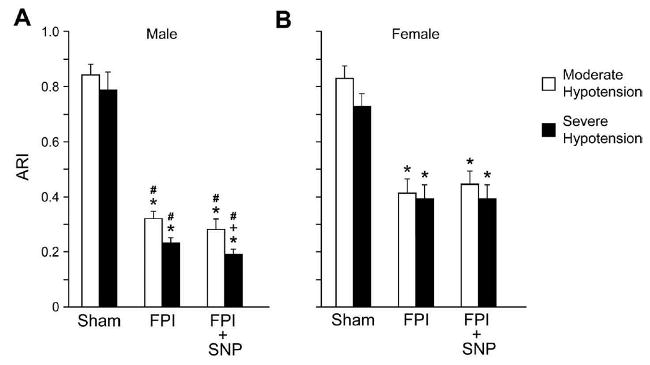
Autoregulatory index (ARI) values for moderate and severe hypotension in sham, FPI and FPI + SNP (0.1 mg/kg iv) treated male and female pigs, n= 6. *p<0.05 compared with corresponding sham value, +p<0.05 compared wih corresponding FPI vehicle value, #p<0.05 compared with corresponding female value.
2.6 FPI produces phosphorylation of CSF ERK MAPK which is aggravated by SNP in a sex dependent manner
FPI produced a marked phosphorylation (activation) of CSF ERK MAPK at 1 h and 4h post injury (Fig. 8). ERK MAPK phoshorylation induced by FPI was significantly greater in male versus female pigs (Fig. 8). SNP (0.1 mg/kg iv) treatment either 30 min prior to or 30 min after FPI aggravated ERK MAPK phosphorylation in the male, but had no effect on ERK MAPK in the female (Fig. 8). U 0126 (1 mg/kg iv) blocked ERK MAPK upregulation after FPI (Fig. 8).
Figure 8.
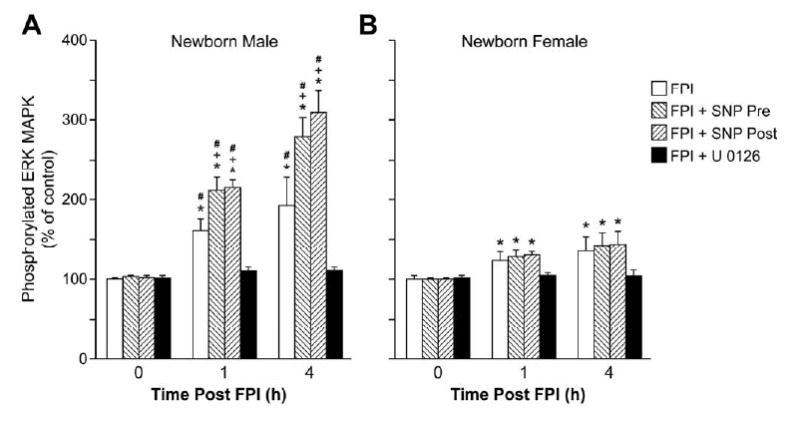
Phosphorylation of ERK MAPK in CSF prior to FPI (0 time), as a function of time after FPI (1,4h) in pigs treated with vehicle (FPI), SNP (0.1 mg/kg iv) prior to or after FP, or U 0126 (1 mg/kg iv) + FPI, n=6. Data expressed as percent of control by ELISA determination of phospho ERK MAPK and total ERK MAPK isoforms and subsequent normalization to total form. A: male, B: female. *p<0.05 compared with corresponding 0 time value +p<0.05 compared with corresponding vehicle treated value #p<0.05 compared with corresponding female value.
2.7 Blood chemistry
Blood chemistry values for pCO2, pO2, and pH were collected before and after all experiments. There were no statistically significant differences between sham control, FPI, and FPI drug treated animals. The intensity of the insult (1.9 ± 0.1 atm) was uniform among all animal groups.
3. Discussion
Several principal new findings emerged from this study. First, SNP, administered 30 min post injury, attenuated reductions in pial artery diameter and cerebral hypoperfusion associated with FPI, making this a translationally relevant therapeutic intervention. SNP further caused a reduction in elevated ICP following FPI. However, clinicians believe that SNP increases ICP due to cerebrovasodilation. Nonetheless, it was observed that intracarotid SNP failed to increase CBF in baboons (12). Therefore, any increase in ICP may be indirect and due to reflex increase in cerebral blood volume due to autoregulatory vasodilation. In the present study, SNP induced a reduction in elevated ICP following FPI, which was associated with an increase in CPP. However, this clinically beneficial action was mitigated by a key second finding which relates to the observation that SNP aggravated reductions in pial artery diameter and CBF during combined hypotension and FPI, thereby contributing to impaired cerebral autoregulation. The latter SNP induced impairment occurred to a greater extent in the male compared to the female. We were rather surprised that administration of a vasodilator would make hemodynamic outcome worse after a combined insult of FPI and hypotension in general, and in the male in particular, until the role of mechanism was considered. A third major finding, then, relates to the observation that SNP aggravated ERK MAPK upregulation after FPI in the male, but not the female. It is speculated that the higher ICP associated with SNP administration after FPI and hemorrhagic hypotension in the male could be due to the aggravated ERK MAPK upregulation causing more edema (and hence a higher ICP). However, SNP had no effect on cerebral autoregulation in sham control animals. Therefore, NO appears to have 2 functions in the setting of brain injury, the one to promote vasodilation via stimulation of cGMP, the other being to upregulate ERK MAPK through an as yet uncharacterized mechanism. ERK MAPK upregulation had been previously shown to contribute to hypoperfusion and impaired cerebral autoregulation in a sex dependent manner after FPI in the pig (3,4).
The Lund Concept is an approach to the treatment of TBI which is based on basic physiological principles regarding brain volume and cerebral perfusion regulation (11). This therapy has two major goals: 1. To reduce or prevent an increase in ICP and 2. To improve cerebral perfusion (11). In the latter context, administration of a cerebrovasodilator such as SNP can be interpreted as improving cerebral hemodynamics after FPI in fulfillment of both reducing ICP and increasing CPP. However, it may be simplistic to view treatment of TBI in purely hemodynamic terms. Adrenomedullin, another cerebrovasodilator, improves cerebral hemodynamics and cerebral autoregulation during both normotension and hypotension after FPI (4,5). Adrenomedullin, in contrast to SNP, blocks ERK MAPK upregulation post injury (4). These data support the concept that consideration towards both cerebral hemodynamic and signaling events may be important in fostering improved outcome after TBI. Fulfillment of the Lund Concept principles alone may be insufficient to achieve beneficial cerebral hemodynamic outcome after TBI.
TBI can cause flow-metabolism uncoupling, resulting in cerebral ischemia (CBF less than cerebral metabolic demand) or cerebral hyperemia (CBF in excess of cerebral metabolism) (6,16). Reductions in CBF observed to occur early after TBI produces cerebral ischemia in clinical studies in adults (8). Decreased CBF and cerebral metabolism following TBI may not be problematic, however, if there is compensatory increased oxygen extraction (9). Following pediatric TBI, cerebral hypoperfusion appears to be the dominant derangement and is often associated with poor outcome (1,8,18). However, after severe TBI in children, CBF may actually be normal or high (17) and result in cerebral hyperemia and hemorrhage.
Determination of the autoregulatory index under conditions of combined FPI and hypotension in the piglet in this study provided several key pieces of information. First, ICP was increased after FPI more in the male than the female at an equivalent insult level. Values of ICP during moderate and severe hypotension after FPI were also greater in the male than the female. Second, while SNP decreased the peak ICP in both sexes, it blunted the elevation of ICP after FPI during both normotension and hypotension more in the male than the female. Third, CBF was decreased more in the male than in the female after FPI. An ARI of 1 by definition designates perfect autoregulation, while a value of 0 designates absent autoregulation. Calculated ARI was unchanged during hypotension prior to FPI, indicating intact cerebral autoregulation. ARI was reduced during hypotension after FPI in both male and female piglets, though the magnitude of the decreased ARI was greater in the male than the female. SNP did not help to prevent reductions in ARI in both male and female piglets. However, SNP actually made ARI worse during severe hypotension after FPI in males but not females. The above observations point to salient sex dependent differences in the hemodynamic effects of an equivalent insult level and how administration of a cerebrovasodilator may have markedly different effects on post brain injury cerebral hemodynamics as a function of sex. These data suggest that reduction of ICP by SNP may be a mechanism whereby administration of a cerebrovasodilator post brain injury may improve CPP, and thereby cerebral hemodynamics post insult. Importantly, changes in ARI were paralleled by that observed with determination of CBF by radiolabeled microspheres. These data suggest that ARI may be a useful indicator of cerebral hemodynamic status in brain injured patients. The translational significance of this cannot be underestimated, given the correlation between ARI and Glasgow Coma Scale observed in pediatric brain injured patients (10,19).
Quantification of ERK MAPK in CSF appears to parallel changes in brain parenchyma under TBI conditions (3,4). The latter studies suggest endothelial cells and neurons as cellular sites of origin for ERK MAPK detected in CSF (3,4). The present experimental design, however, does not allow for conclusions to be drawn relative to cellular site of origin for the present results.
In conclusion, results of the present study suggest the role for sex dependent mechanisms in cerebral autoregulation after pediatric TBI. These data also indicate that despite improved cerebral hemodynamics after FPI during normotension, elevation of CPP via decreased ICP with cerebrovasodilator administration does not restore CBF or autoregulation during hemorrhagic hypotension after FPI. These data suggest that therapies directed at a purely hemodynamic increase in CPP will fail to improve outcome during combined TBI and hemorrhagic hypotension.
4. Experimental Procedure
4.1 Closed cranial window and brain injury procedures
Male and female newborn pigs (1-5 days old, 1.0-1.4 Kg) were studied. All protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Animals were sedated with isoflurane (1-2 MAC). Anesthesia was maintained with a-chloralose (30-50 mg/ kg. supplemented with 5 mg / kg/h i.v.). A catheter was inserted into a femoral artery to monitor blood pressure and to sample blood gas tensions and pH. Drugs to maintain anesthesia were administered through a second catheter placed in a femoral vein. The trachea was cannulated, and the animals were ventilated with room air. A heating pad was used to maintain the animals at 37° - 39° C, monitored rectally.
A cranial window was placed in the parietal skull of these anesthetized animals. This window consisted of three parts: a stainless steel ring, a circular glass coverslip, and three ports consisting of 17-gauge hypodermic needles attached to three precut holes in the stainless steel ring. For placement, the dura was cut and retracted over the cut bone edge. The cranial window was placed in the opening and cemented in place with dental acrylic. The volume under the window was filled with a solution, similar to CSF, of the following composition (in mM): 3.0 KCl, 1.5 MgCl2, 1.5 CaCl2, 132 NaCl, 6.6 urea, 3.7 dextrose, and 24.6 NaHCO3. This artificial CSF was warmed to 37° C and had the following chemistry: pH 7.33, pCO2 46 mm Hg, and pO2 43 mm Hg, which was similar to that of endogenous CSF. Pial arterial vessel diameter was measured with a microscope, a camera, a video output screen and a video microscaler.
CBF was measured in the cerebral cortex using radioactively labeled microspheres. Briefly, a known amount of radioactivity in 15-μm microspheres (300,000-800,000 spheres) was injected into the left ventricle and the injection line flushed with 1 ml of saline. Withdrawal of reference blood samples was begun 15s before microsphere injection and continued for 2 min after the injection. The reference withdrawal rate was 1.03 ml/min. After each experiment, the pig was sacrificed and the brain removed and weighed. CBF was determined by counting cerebral cortex brain tissue samples in a gamma counter. The energy from each nuclide was separated by differential spectroscopy. Aliquots of the actual microsphere solutions injected were used for overlap calculations. The count in each milliliter per minute of blood flow was determined by dividing the counts in the reference withdrawal by the rate of reference withdrawal. Thus blood flow can be calculated as Q = C × R × CR-1, where Q is brain blood flow (in ml/min), C is counts per minute (cpm) in the tissue sample, R is the rate of withdrawal of reference blood sample (in ml/min), and CR is the total counts in the reference blood sample. CBF so determined reflect flow to the cerebral cortex both ipsilateral and contralateral to the injury site.
The method used to induce brain FPI has been described previously (3-5,20). A device designed by the Medical College of Virginia was used. A small opening was made in the parietal skull contralateral to the cranial window. A metal shaft was sealed into the opening on top of intact dura. This shaft was connected to the transducer housing, which was in turn connected to the fluid percussion device. The device itself consisted of an acrylic plastic cylindrical reservoir 60 cm long, 4.5 cm in diameter, and 0.5 cm thick. One end of the device was connected to the transducer housing, whereas the other end had an acrylic plastic piston mounted on O-rings. The exposed end of the piston was covered with a rubber pad. The entire system was filled with 0.9 % saline. The percussion device was supported by two brackets mounted on a platform. FPI was induced by striking the piston with a 4.8 kg pendulum. The intensity of the injury (usually 1.9-2.3 atm. with a constant duration of 19-23 ms) was controlled by varying the height from which the pendulum was allowed to fall. The pressure pulse of the injury was recorded on a storage oscilloscope triggered photoelectrically by the fall of the pendulum. The amplitude of the pressure pulse was used to determine the intensity of the injury.
Protocol
Two types of pial vessels, small arteries (resting diameter, 120-160 μm) and arterioles (resting diameter, 50-70 μm) were examined to determine whether segmental differences in the effects of FPI could be identified. Typically, 2-3 ml of artificial CSF were flushed through the window over a 30s period, and excess CSF was allowed to run off through one of the needle ports. For sample collection, 300 μl of the total cranial window volume of 500 μl was collected by slowly infusing artificial CSF into one side of the window and allowing the CSF to drip freely into a collection tube on the opposite side.
Six experimental groups were studied (all n=6): (1) sham control, (2) FPI, vehicle pre-treated, (3) FPI pre-treated with SNP (0.1 mg/kg iv), (4) FPI post-treated with vehicle), (5) FPI post-treated with SNP. Hypotension was induced by the rapid withdrawal of either 5-8 or 10-15 ml blood/Kg to induce moderate or severe hypotension (decreases in mean arterial blood pressure of 25 and 45%, respectively) (4). Such decreases in blood pressure were maintained constant for 10 min by titration of additional blood withdrawal or blood reinfusion (4). The vehicle for all agents was 0.9% saline. In sham control animals, responses to hypotension (moderate, severe) and papaverine (10-8, 10-6 M) were obtained initially and then again 1h later in the presence of the agent vehicle. In drug treated FPI animals, SNP (0.1 mg/kg iv) was administered 30 min before or 30 min after FPI and the responses to hypotension and papaverine obtained at 1h after injury. The order of agonist administration was randomized within animal drug treatment groups.
Determination of Autoregulatory Index (ARI) via transcranial Doppler (TCD) ultrosonography
A Spencer Technologies PMD-100 TCD monitor was used to insonate the cerebral vessels prior to and after FPI in the absence and presence of induced hypotension and to measure the middle and posterior cerebral artery blood velocity (Vmca and Vpca) and calculate the autoregulatory index (ARI) (10). By definition, ARI = %Δ CVR / %Δ CPP where CVR ≈ CPP (mean arterial blood pressure, MAP-ICP) / Vcerebral blood vessel. ARI < 0.4 = impaired cerebral autoregulation, ARI > 0.4 infers intact cerebral autoregulation, ARI 0 = absent autoregulation and ARI 1.0 reflects perfect autoregulation. An Integra Camino monitor was used to measure ICP and ARI was calculated using CPP in all animals. We placed the TCD probe in the posterior portion of the cortex.
ELISA
Commercially available ELISA Kits were used to quantity CSF ERK MAPK (Assay Designs) concentration. Phosphorylated ERK MAPK enzyme values were normalized to total form and then expressed as percent of the control condition.
Statistical analysis
Pial artery diameter, CBF, and CSF ERK MAPK values were analyzed using 3-way ANOVA for repeated measures. If the value was significant, the data were then analyzed by Fishers protected least significant difference test. An α level of p<0.05 was considered significant in all statistical tests. Values are represented as mean ± SEM of the absolute value or as percentage changes from control value.
Acknowledgments
This research was funded by a grant from the National Institutes of Health, HD57355 (WMA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adelson PD, Clyde B, Kochanek PM, Wisniewski SR, Marion DW, Yonas H. Cerebrovascular response in infants and young children following severe traumatic brain injury: a preliminary report. Pediatr Neurosurg. 1997;26:200–207. doi: 10.1159/000121192. [DOI] [PubMed] [Google Scholar]
- 2.Alessandrini A, Namura S, Moskowitz MA, Bonventre JV. MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proc Nat Acad Sci. 1999;96:12866–12869. doi: 10.1073/pnas.96.22.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstead WM, Cines DB, Bdeir K, Bdeir Y, Stein SC, Higazi AAR. uPA modulates the age dependent effect of brain injury on cerebral hemodynamics through LRP and ERK MAPK. J Cereb Blood Flow Metab. 2009;29:524–533. doi: 10.1038/jcbfm.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstead WM, Kiessling JW, Bdeir K, Kofke WA, Vavilala MS. Adrenomedullin prevents sex dependent impairment of autoregulation during hypotension after piglet brain injury through inhibition of ERK MAPK upregulation. J Neurotrauma. 2010 doi: 10.1089/neu.2009.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstead WM, Vavilala MS. Adrenomedullin reduces gender dependent loss of hypotensive cerebrovasodilation after newborn brain injury through activation of ATP-dependent K channels. J Cereb Blood Flow Metab. 2007;27:1702–1709. doi: 10.1038/sj.jcbfm.9600473. [DOI] [PubMed] [Google Scholar]
- 6.Bruce DA, Alavi A, Bilaniuk L, Dolinskas C, Obrist W, Uzzell B. Diffuse cerebral swelling following head injuries in children; the syndrome of malignant brain edema. J Neurosurg. 1981;54:170–178. doi: 10.3171/jns.1981.54.2.0170. [DOI] [PubMed] [Google Scholar]
- 7.Coates BM, Vavilala MS, Mack CD, Muangman S, Suz P, Sharar SR, Bulger E, Lam AM. Influence of definition and location of hypotension on outcome following severe pediatric traumatic brain injury. Crit Care Med. 2005;33:2645–2650. doi: 10.1097/01.ccm.0000186417.19199.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coles JP. Regional ischemia after head injury. Curr Opin Crit Care. 2004;10:120–125. doi: 10.1097/00075198-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Diringer MN, Videen TO, Yundt K, Zazulia AR, Aiyagari V, Dacey RG, Jr, Grubb RL, Powers WJ. Regional cerebrovascular and metabolic effects of hyperventilation after severe traumatic brain injury. J Neurosurg. 2002;96:103–108. doi: 10.3171/jns.2002.96.1.0103. [DOI] [PubMed] [Google Scholar]
- 10.Freeman SS, Udomphorn Y, Armstead WM, Fisk DM, Vavilala MS. Young age as a risk factor for impaired cerebral autoregulation after moderate-severe pediatric brain injury. Anesthesiology. 2008;108:588–595. doi: 10.1097/ALN.0b013e31816725d7. [DOI] [PubMed] [Google Scholar]
- 11.Grande PO. The “Lund Concept” for the treatment of severe head trauma – physiological principles and clinical application. Intensive Care Med. 2006;32:1475–1484. doi: 10.1007/s00134-006-0294-3. [DOI] [PubMed] [Google Scholar]
- 12.Joshi S, Hartl R, Sun LS, Libow AD, Wang M, Pile-Spellman J, Young WL, Connolly ES, Hirshman CA. Despite in vitro increase in cyclic guanosine monophosphate concentrations, intracarotid nitroprusside fails to augment cerebral blood flow of Healthy baboons. Anesthesiology. 2003;98:412–419. doi: 10.1097/00000542-200302000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Laher I, Zhang JH. Protein kinase C and cerebral vasospasm. Journal of Cerebral Blood Flow & Metabolism. 2001;21:887–906. doi: 10.1097/00004647-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain brain injury among children in the United States: differences by race 1. J Head Trauma Rehabil. 2005;20:229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Newacheck PW, Inkelas M, Kim SE. Heath services use and health care expenditures for children with disabilities. Pediatrics. 2004;114:79–85. doi: 10.1542/peds.114.1.79. [DOI] [PubMed] [Google Scholar]
- 16.Richards HK, Simac S, Piechnik S, Pickard JD. Uncoupling of cerebral blood flow and metabolism after cerebral contusion in the rat. J Cereb Blood Flow Metab. 2001;21:779–781. doi: 10.1097/00004647-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Sharples PM, Stuart AG, Matthews DS, Aynsley-Green A, Eyre JA. Cerebral blood flow and metabolism in children with severe head injury. Part I: Relation to age, Glasgow coma score, outcome, intracranial pressure, and time after injury. J Neurol Neurosurg Psychiatry. 1995;58:145–152. doi: 10.1136/jnnp.58.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vavilala MS, Lee LA, Boddu K, Visco E, Newell DW, Zimmerman JJ, Lam AM. Cerebral autoregulation in pediatric traumatic brain injury. Pediatr Crit Care Med. 2004;5:257–263. doi: 10.1097/01.pcc.0000123545.69133.c3. [DOI] [PubMed] [Google Scholar]
- 19.Vavilala MS, Muangman SL, Tontisirin N, Fisk D, Roscigno C, Mitchell P, Kirkness C, Zimmerman JJ, Chesnut R, Lam AM. Imapired cerebral autoregulation and 6 month outcome in children with severe traumatic brain injury: preliminary findings. Dev Neurosci. 2006;28:348–353. doi: 10.1159/000094161. [DOI] [PubMed] [Google Scholar]
- 20.Wei EP, Dietrich WD, Povlishock JT, Navari RM, Kontos HA. Functional, morphological, and metabolic abnormalities of the cerebral microcirculation after concussive brain injury in cats. Circ Res. 1980;46:37–47. doi: 10.1161/01.res.46.1.37. [DOI] [PubMed] [Google Scholar]


