Abstract
In hemostasis, the serine protease inhibitor (serpin) plasminogen activator inhibitor-1 (PAI-1) functions to stabilize clots via inhibition of tissue plasminogen activator (tPA) with subsequent inhibition of fibrinolysis. In tissues, PAI-1 functions to inhibit extracellular matrix degradation via inhibition of urokinase plasminogen activator (uPA). Elevated levels of PAI-1 in the vasculature and in tissues have long been known to be associated with thrombosis and fibrosis, respectively. However, there is emerging evidence that PAI-1 may participate in the pathophysiology of a number of diseases such as atherosclerosis, restenosis, and cancer. In many of these disease states, the canonical view of PAI-1 as an inhibitor of tPA and uPA cannot fully account for a mechanism whereby PAI-1 contributes to the disease. In these cases, one must consider recent data, which indicates PAI-1 can directly promote pro-proliferative and anti-apoptotic signaling in a variety of cell types. Given the wide variety of inflammatory, hormonal, and metabolic signals that increase PAI-1 expression, it is important to consider mechanisms by which PAI-1 can directly participate in disease etiology.
Keywords: Plasminogen activator inhibitor-1, urokinase, tissue plasminogen activator, inflammation, proliferation, and apoptosis
Introduction
Traditional paradigms for PAI-1 in fibrinolysis and tissue remodeling
Within the intravascular space, the primary role for the serpin (serine protease inhibitor) PAI-1 is to regulate fibrinolysis to stabilize hemostatic plug formation. When bound to fibrin in a clot, the serine protease tissue plasminogen activator (tPA) activates plasminogen to its active form of plasmin, which subsequently degrades fibrin [1]. During thrombus formation, tPA is inhibited by PAI-1 released from platelets [2], thereby limiting further plasminogen activation and fibrinolysis.
The primary role of PAI-1 in the extravascular space is to regulate matrix remodeling via inhibition of the urokinase plasminogen activator (uPA) [3]. uPA bound to cells expressing its cognate receptor uPAR, can catalyze the pericellular conversion of plasminogen to plasmin, which can subsequently cleave and/or activate numerous proteins such as gelatinase, fibronectin, laminin, and latent forms of collagenases including MMP-1 to lead to matrix degradation. As with tPA, PAI-1 forms a 1:1 complex with uPA and renders the protease inactive, thereby inhibiting pericellular proteolysis.
PAI-1 has also been implicated in inhibiting adhesion of cells to extracellular matrix proteins, although the precise mechanism remains debated. uPAR has been shown to associate with multiple different integrin subunits and it has been suggested that uPAR can act as an integrin ligand to promote both cell adhesion to various matrix proteins [4], as well as cell to cell adhesion [5]. Given these findings, it has been suggested that PAI-1 may promote de-adhesion from various substrata via destruction of integrins [6]. Alternatively, is has been suggested that PAI-1 can promote de-adhesion specifically for the extracellular matrix protein vitronectin (VN). PAI-1 and uPAR/uPA complexes compete for binding to VN [7], and binding of PAI-1 to uPA dissociates uPAR from vitronectin. Endocytosis of the uPAR/uPA/PAI-1 ternary complex by the low-density lipoprotein-like receptor 1 (LRP-1) then promotes de-adhesion of cells from VN [8].
Regulation of PAI-1 expression in the intravascular and extravascular space
During the initiation of thrombus formation, release of PAI-1 by platelets represents the most likely primary source of PAI-1 [2]. However, multiple cell types are capable of producing PAI-1 in response to various inflammatory cytokines. The multiplicity of potential sources of PAI-1 as a response factor has implications for PAI-1 function in both physiological and pathophysiological conditions.
As a recognized acute phase reactant, PAI-1 levels in plasma increase quickly in response to vessel injury and a heightened inflammatory state [9]. Like C-reactive protein (CRP) and fibrinogen, PAI-1 levels in plasma have been shown to increase in response both to acute trauma such as local tissue injury [10] and to chronic inflammatory states such as cardiovascular disease [11] and insulin resistance [12]. In mouse models, this increase has been attributed to increased synthesis of PAI-1 by the liver in response to inflammatory cytokines IL-1β [10], IL-6 [13], and tumor necrosis factor-α (TNFα) [14]. Under physiological conditions, acute increases in the plasma concentration of PAI-1 in response to inflammatory cytokines could be viewed as a mechanism to stabilize thrombus formation by inhibiting tPA-mediated plasminogen activation. However, under pathological conditions such as atherosclerosis, sustained elevated levels of PAI-1 could promote thrombosis.
In contrast to fast up-regulation of PAI-1 in plasma as an acute phase reactant, there are also mechanisms by which PAI-1 levels in the intravascular space may be up-regulated in a more sustained fashion via endothelial cell production. Similar to hepatocytes, cultured endothelial cells have been shown to increase PAI-1 production in response to the inflammatory cytokines IL-1 [15] and TNF-α [16]. PAI-1 synthesis by endothelial cells has also been shown to increase as a consequence of hypoxia [17], the generation of reactive oxygen species [18], and shear stress [19]. Alternatively, increased PAI-1 production by endothelial cells has also been associated with senescence, a process that increases with age [20].
Extravascularly, regulation of PAI-1 expression involves multiple cell types. In fibroblasts, PAI-1 synthesis is increased in response to TGF-β [21] and IL-6 [22]. Perhaps the most important source of PAI-1 in tissues is adipocytes. Mature adipocytes express relatively high basal levels of PAI-I in culture [23]. Despite high basal expression, adipocytes can increase PAI-1 synthesis in response to many cytokines and hormones such as TNF-α, TGF-β, and insulin [24]. Finally, macrophages represent another source of PAI-1 in the extravascular space. Activation of monocytes with endotoxin increases PAI-1 expression and histological studies indicate that tissue macrophages in artheromatous plaques express PAI-1 [25].
Given the diversity of cellular sources and multitude of inflammatory signals which promote PAI-1 expression, it is not surprising that elevated PAI-1 levels in serum and tissue have been observed in a variety of pathological conditions. One might suggest elevated PAI-1 levels could be merely a marker of inflammation. However, multiple studies have shown that PAI-1 participates directly in the pathophysiology of a number of diseases. In some cases, the traditional paradigms for the function of PAI-1 can fully explain its role in pathology. Alternatively, in other disease states, the traditional paradigms for the function of PAI-1 are not sufficient to understand its participatory role. Thus, new data are emerging that strongly suggests PAI-1 has novel functions far beyond its ability to inhibit tPA and uPA.
Pathologic consequences of PAI-1 expression explained by traditional paradigms
PAI-1 in thrombosis
As an inhibitor of plasminogen activation and fibrin degradation, it is logical that elevated PAI-1 levels in serum would lead to thrombosis. With regard for thrombosis in the coronary arteries, elevated PAI-1 levels have been documented in the serum of survivors of a myocardial infarction and patients that have recurrent myocardial infarctions [26]. In an experimental model, transgenic mice that express a stable form of human PAI-1 develop spontaneous coronary thrombi [27]. Elevated levels of PAI-1, especially in the elderly, are thought to be associated with both venous and arterial thrombosis [28].
PAI-1 in fibrosis
In tissues, increased expression of PAI-1 has been associated with multiple forms of fibrosis including glomerulosclerosis [29], liver fibrosis [30], and pulmonary fibrosis [31]. While there are competing schools of thought on the role of PAI-1 in promoting fibrosis, all utilize the traditional paradigm for PAI-1 function. First, it is thought that elevated PAI-1 expression decreases tPA/uPA activity leading to increased fibrin deposition at the site of a vessel injury. Due to the increased fibrin deposition, more cells infiltrate the wound, leading to increased collagen deposition. In an alternative model, it has been suggested elevated PAI-1 promotes de-adhesion, allowing for more cells to infiltrate. Finally, it has also been suggested that the primary consequence of elevated PAI-1 is actually decreased collagenase activity downstream of reduced uPA and MMP activation. In this model PAI-1 directly promotes fibrosis by inhibiting collagen degradation [32].
In contrast to observations linking PAI-1 to thrombosis and fibrotic disease, the role of PAI-1 in other pathological conditions are not explained by traditional paradigms, which focus solely on the protease inhibitor activity of PAI-1. More recent studies elucidating the ability of PAI-1 to alter cell signaling is providing insight to explain how PAI-1 can contribute to other disease states.
Pathologic consequences of PAI-1 expression not explained by traditional paradigms
PAI-1 in vascular disease
PAI-1 has a well-documented association with the development of vascular disease. Multiple studies have demonstrated the presence of excess PAI-1 in atherosclerotic plaques [33] [34] [25]. Furthermore, PAI-1 deposition in the vascular wall [35], and atherosclerotic plaques [36], is elevated in patients with type II diabetes. This is not surprising given that elevated PAI-1 expression has been directly linked to hyperinsulinemia as well as glucose/lipid imbalance [37].
It has been suggested that the presence of PAI-1 in atherosclerotic plaques may directly contribute to atherogenesis primarily via inhibition of MMP activation with subsequent decreased activation of TGFβ [38], which promotes smooth muscle cell proliferation, and decreased processing of cholesterol aggregates [26]. However, there are other mechanisms by which PAI-1 may promote the development of atherosclerosis that reach beyond the traditional paradigms of tPA/uPA inhibition. Of note, PAI-1 has shown to directly promote migration, inhibit apoptosis [39], and promote proliferation [40] of smooth muscle cells. These studies elucidate that PAI-1 has unique capabilities to promote cell signaling. With regard to apoptosis and proliferation, these studies show purified PAI-1 inhibits caspase-3 activity in vitro [39], and promotes cell proliferation via activation of the NFκB and ERK signaling [40]. Given the importance of smooth muscle cell proliferation in the development of atherosclerotic lesions, it is important to consider the potential role of PAI-1 directly mediating intracellular signaling events in vascular disease.
Another interesting potential mechanism of PAI-1 mediated signaling in vascular disease involves macrophages recruitment. In in vitro studies, PAI-1 in combination with tPA and LRP-1 (the co-receptor for clearance of tPA-PAI-1 and uPA-PAI-1 complexes), has been shown coordinate Mac-1 dependent macrophage migration [41]. While the authors of these studies suggest PAI-1 mediated migration of macrophages is a function of the de-adhesive functions of PAI-1, it is likely to involve more complicated signaling events in light of data which shows the LRP-1 co-receptor acts as a motogenic receptor for PAI-1, and can activate Jak/Stat signaling when it binds PAI-1 [42].
PAI-1 in the tumor microenvironment
The tumor microenvironment represents a heterogeneous mixture of cell types, many of which can produce and respond to PAI-1, including the tumor cells themselves. Considering the well documented role of the immune system and inflammation in cancer and the tight linkage between inflammation and elevated PAI-1, one might hypothesize that PAI-1 could play a direct role in the pathophysiology of cancer. This hypothesis should garner close investigation given that elevated PAI-1 levels in the tumor microenvironment correlates to poor prognosis in multiple cancers including breast [43], ovarian [44], pulmonary adenocarcinoma [45], and neuroblastoma [46]. The commonly observed elevation of PAI-1 in cancer presents a difficult paradox. Given the well-documented association of matrix degradation with tumor viability and metastasis, one would expect that PAI-1, as an inhibitor of uPA, would represent a positive prognostic indicator. However, insights into the novel functions of PAI-1 as a cell signaling mediator may help explain why PAI-1 is often a poor prognostic indicator.
Most directly, PAI-1 produced by adipocytes, fibroblasts, or macrophages in response to various cytokines in the tumor microenvironment can promote angiogenesis, which is crucial to tumor viability and dissemination. While not without controversy, PAI-1 is generally accepted to promote angiogenesis in the tumor microenvironment. Utilizing PAI-1−/− mice, it has been shown PAI-1 promotes angiogenesis in the tumor microenvironment at low concentrations but inhibits angiogenesis at extremely high concentrations [47]. The precise mechanism by which PAI-1 promotes angiogenesis has remained largely elusive, although it has been suggested that the ability of PAI-1 to promote angiogenesis is dependent on it protease inhibitor activity, not vitronectin binding [48]. This finding would suggest PAI-1 does not promote angiogenesis by promoting de-adhesion of endothelial cells from matrix proteins. The most complete study analyzing angiogenesis suggests that PAI-1 inhibits pro-apoptotic cell signaling by inhibiting plasmin mediated cleavage of Fas-ligand on the surface of endothelial cells [49]. This represents a novel mechanism for how PAI-1 prevents apoptosis of endothelial cells and therefore promotes angiogenesis.
Another mechanism whereby PAI-1 could function to promote cancer dissemination comes from a novel finding with senescence in fibroblasts. PAI-1 has long been known as a marker of senescence in multiple cell types [50] [51]. However, it has been shown that PAI-1 is not only a marker of senescence in fibroblasts, but that it is a critical downstream target of p53 in the induction of senescence, through a PI3K-dependent pathway [52]. If PAI-1 can participate directly in the induction of replicative senescence, it is possible that an excess of PAI-1 in the tumor microenvironment could induce the senescence of fibroblasts, leading to decreased deposition of matrix.
Outside of its influence on endothelial cells and fibroblasts in the tumor microenvironment, emerging data suggests PAI-1 has direct influence over pro-proliferative and anti-apoptotic signaling in tumor cells themselves. While mouse models designed to examine the effect of PAI-1 on tumor development have yielded often-conflicting data [53], in vitro examination of various cancer cell lines indicates that PAI-1 has a positive influence on cancer cell growth and survival. While limited, this data may provide the most direct link to explain why elevated PAI-1 levels are associated with poor prognosis in cancer.
One such study demonstrated that both the PC-3 prostate cancer cell line and the HL-60 promyelocytic cell line demonstrated decreased apoptosis in the presence of PAI-1 when treated with apoptosis inducing agents camptothecin or etoposide [54]. Similarly, our laboratory has found that stable expression of wild type PAI-1, but not an inactive mutant of PAI-1, enhances the recovery of MDA-MB-435 cancer cells after exposure to apoptosis inducing agent paclitaxel [55], and enhances motility and adhesion via alteration of the integrin profile at the cell surface [56]. Fibrosarcoma cell lines derived from PAI-1−/− mice were significantly more sensitive to apoptotic stimulus etoposide than counterpart fibrosarcoma cell lines from wild type mice [57].
More recent data reveals how PAI-1 may influence cancer cell viability via direct alteration of cell signaling. An expanded analysis of the murine fibrosarcoma cells discussed above revealed that PAI-1 deficient fibrosarcomas have reduced Akt signaling and reintroduction of PAI-1 expression in deficient cells results in an increase in Akt signaling [58]. Our laboratory has found that stable knockdown of PAI-1 expression in MDA-MB-231 breast cancer cells results in decreased Akt activation (Gramling, M.W. and F.C. Church, unpublished data). Besides regulation of anti-apoptotic Akt signaling, PAI-1 has also been shown to activate pro-proliferative MAPK signaling. In MCF-7 breast cancer cells, PAI-1 promotes sustained phosphorylation of ERK1/2 via a mechanism which is dependent on its interaction with uPA as well as the activity of uPAR and the LRP-1 co-receptor [59]. This study further showed that the presence of PAI-1 allowed uPA to act as a mitogen for the breast cancer cells [59].
Conclusion
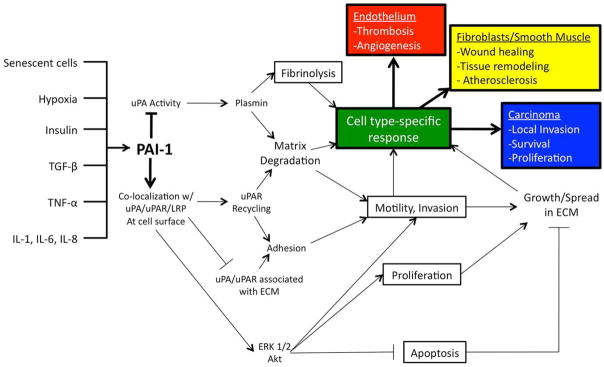
Given that PAI-1 expression is elevated both the intra- and extra-vascular space by such a wide variety of inflammatory, hormonal, and metabolic signals, it has potential to influence many physiological and pathophysiological conditions. The pleiotropic effects of PAI-1 are diagrammed in Figure 1. From thrombosis and fibrosis to atherosclerosis and cancer, PAI-1 directly contributes to the etiology of disease via mechanisms explained by traditional paradigms of its function and by new mechanisms explained by its emerging role in altering cell signaling.
Figure 1.
PAI-1 has been implicated in modulating a variety of physiological and pathophysiological processes. PAI-1 expression in the intra- and extra-vascular space is increased in response to many inflammatory, hormonal, and metabolic signals. By modulating protease activity, adhesion, and altering signaling in multiple cell types, PAI-1 can contribute to many normal and disease processes.
Acknowledgments
Thomas and Carolyn Royster Fellowship and Robert H. Wagner Scholarship (to M.W.G.), R21 AG031068 from the National Institutes of Health (to F.C.C.), and BCTR0503475 and BCTR45206 from the Susan G. Komen Breast Cancer Foundation (to F.C.C.).
Abbreviations used
- PAI-1
plasminogen activator inhibitor-1
- uPA
urokinase plasminogen activator
- tPA
tissue plasminogen activator
- MMP
matrix metalloprotease
- uPAR
urokinase plasminogen activator receptor
- VN
vitronectin
- LRP-1
low density lipoprotein-like receptor-1
- CRP
C-reactive protein
- IL
interleukin
- TNFα
tumor necrosis factor alpha
- TGFβ
transforming growth factor beta
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- ERK
extracellular signal-regulated kinase
- MAPK
mitogen activated protein kinase
Footnotes
Disclosure of Conflict of Interests- The authors state that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cesarman-Maus G, Hajjar KA. Molecular mechanisms of fibrinolysis. Br J Haematol. 2005;129:307–321. doi: 10.1111/j.1365-2141.2005.05444.x. [DOI] [PubMed] [Google Scholar]
- 2.Brogren H, Karlsson L, Andersson M, Wang L, Erlinge D, Jern S. Platelets synthesize large amounts of active plasminogen activator inhibitor 1. Blood. 2004;104:3943–3948. doi: 10.1182/blood-2004-04-1439. [DOI] [PubMed] [Google Scholar]
- 3.Collen D. The plasminogen (fibrinolytic) system. Thromb Haemost. 1999;82:259–270. [PubMed] [Google Scholar]
- 4.van der Pluijm G, Sijmons B, Vloedgraven H, van der Bent C, Drijfhout JW, Verheijen J, Quax P, Karperien M, Papapolis S, Lowik C. Urokinase-receptor/integrin complexes are functionally involved in adhesion and progression of human breast cancer in vivo. Am J Pathol. 2001;159:971–982. doi: 10.1016/S0002-9440(10)61773-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarui T, Mazar AP, Cines DB, Takada Y. Urokinase-type plasminogen activator receptor (cd87) is a ligand for integrins and mediates cell-cell interaction. J Biol Chem. 2001;276:3983–3990. doi: 10.1074/jbc.M008220200. [DOI] [PubMed] [Google Scholar]
- 6.Czekay R, Aertgeerts K, Curriden SA, Loskutoff DJ. Plasminogen activator inhibitor-1 detaches cells from extracellular matrices by inactivating integrins. J Cell Biol. 2003;160:781–791. doi: 10.1083/jcb.200208117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng G, Curriden SA, Wang S, Rosenberg S, Loskutoff DJ. Is plasminogen activator inhibitor-1 the molecular switch that governs urokinase receptor-mediated cell adhesion and release? J. Cell Biol. 1996;134:1563–1571. doi: 10.1083/jcb.134.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodenburg KW, Kjoller L, Petersen HH, Andreasen PA. Binding of urokinase-type plasminogen activator-plasminogen activator inhibitor-1 complex to the endocytosis receptors alpha2-macroglobulin receptor/low-density lipoprotein receptor-related protein and very-low-density lipoprotein receptor involves basic residues in the inhibitor. Biochem J. 1998;329 (Pt 1):55–63. doi: 10.1042/bj3290055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 10.Seki T, Healy AM, Fletcher DS, Noguchi T, Gelehrter TD. Il-1beta mediates induction of hepatic type 1 plasminogen activator inhibitor in response to local tissue injury. Am J Physiol. 1999;277:G801–809. doi: 10.1152/ajpgi.1999.277.4.G801. [DOI] [PubMed] [Google Scholar]
- 11.Juhan-Vague I, Thompson SG, Jespersen J. Involvement of the hemostatic system in the insulin resistance syndrome. a study of 1500 patients with angina pectoris. the ecat angina pectoris study group. Arterioscler Thromb. 1993;13:1865–1873. doi: 10.1161/01.atv.13.12.1865. [DOI] [PubMed] [Google Scholar]
- 12.Festa A, D’Agostino R, Mykkänen L, Tracy RP, Zaccaro DJ, Hales CN, Haffner SM. Relative contribution of insulin and its precursors to fibrinogen and pai-1 in a large population with different states of glucose tolerance. the insulin resistance atherosclerosis study (iras) Arterioscler Thromb Vasc Biol. 1999;19:562–568. doi: 10.1161/01.atv.19.3.562. [DOI] [PubMed] [Google Scholar]
- 13.Fattori E, Cappelletti M, Costa P, Sellito C, Cantoni L, Carelli M, Faggioni R, Fantuzzi G, Ghezzi P, Poli V. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994;180:1243–1250. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alessi M, Bastelica D, Mavri A, Morange P, Berthet B, Grino M, Juhan-Vague I. Plasma pai-1 levels are more strongly related to liver steatosis than to adipose tissue accumulation. Arterioscler Thromb Vasc Biol. 2003;23:1262–1268. doi: 10.1161/01.ATV.0000077401.36885.BB. [DOI] [PubMed] [Google Scholar]
- 15.Emeis JJ, Kooistra T. Interleukin 1 and lipopolysaccharide induce an inhibitor of tissue-type plasminogen activator in vivo and in cultured endothelial cells. J Exp Med. 1986;163:1260–1266. doi: 10.1084/jem.163.5.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawdey M, Podor TJ, Loskutoff DJ. Regulation of type 1 plasminogen activator inhibitor gene expression in cultured bovine aortic endothelial cells. induction by transforming growth factor-beta, lipopolysaccharide, and tumor necrosis factor-alpha. J Biol Chem. 1989;264:10396–10401. [PubMed] [Google Scholar]
- 17.Gertler JP, Perry L, L’Italien G, Chung-Welch N, Cambria RP, Orkin R, Abbott WM. Ambient oxygen tension modulates endothelial fibrinolysis. J Vasc Surg. 1993;18:939–945. discussion 945–946. [PubMed] [Google Scholar]
- 18.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldber H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing sp1 glycosylation. Proc Natl Acad Sci US A. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergh N, Ulfhammer E, Glise K, Jern S, Karlsson L. Influence of tnf-alpha and biomechanical stress on endothelial anti- and prothrombotic genes. Biochem Biophys Res Commun. 2009;385:314–318. doi: 10.1016/j.bbrc.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto K, Takeshita K, Kojima T, Takamatsu J, Saito H. Aging and plasminogen activator inhibitor-1 (pai-1) regulation: implication in the pathogenesis of thrombotic disorders in the elderly. Cardiovasc Res. 2005;66:276–285. doi: 10.1016/j.cardiores.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Lund LR, Riccio A, Andreasen PA, Nielsen LS, Kristensen P, Laiho M, Saksela O, Blasi F, Danø K. Transforming growth factor-beta is a strong and fast acting positive regulator of the level of type-1 plasminogen activator inhibitor mrna in wi-38 human lung fibroblasts. EMBO J. 1987;6:1281–1286. doi: 10.1002/j.1460-2075.1987.tb02365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samad F, Bergtrom G, Amrani DL. Regulation of plasminogen activation by interleukin-6 in human lung fibroblasts. Biochim Biophys Acta. 1994;1221:307–314. doi: 10.1016/0167-4889(94)90255-0. [DOI] [PubMed] [Google Scholar]
- 23.Lundgren CH, Brown SL, Nordt TK, Sobel BE, Fujii S. Elaboration of type-1 plasminogen activator inhibitor from adipocytes. a potential pathogenetic link between obesity and cardiovascular disease. Circulation. 1996;93:106–110. doi: 10.1161/01.cir.93.1.106. [DOI] [PubMed] [Google Scholar]
- 24.Loskutoff DJ, Samad F. The adipocyte and hemostatic balance in obesity: studies of pai-1. Arterioscler Thromb Vasc Biol. 1998;18:1–6. doi: 10.1161/01.atv.18.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Tipping PG, Davenport P, Gallicchio M, Filonzi EL, Apostolopoulos J, Wojta J. Atheromatous plaque macrophages produce plasminogen activator inhibitor type-1 and stimulate its production by endothelial cells and vascular smooth muscle cells. Am J Pathol. 1993;143:875–885. [PMC free article] [PubMed] [Google Scholar]
- 26.Vaughan DE. Pai-1 and atherothrombosis. J Thromb Haemost. 2005;3:1879–1883. doi: 10.1111/j.1538-7836.2005.01420.x. [DOI] [PubMed] [Google Scholar]
- 27.Eren M, Painter CA, Atkinson JB, Declerck PJ, Vaughan DE. Age-dependent spontaneous coronary arterial thrombosis in transgenic mice that express a stable form of human plasminogen activator inhibitor-1. Circulation. 2002;106:491–496. doi: 10.1161/01.cir.0000023186.60090.fb. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto K, Takeshita K, Kojima T, Takamatsu J, Saito H. Aging and plasminogen activator inhibitor-1 (pai-1) regulation: implication in the pathogenesis of thrombotic disorders in the elderly. Cardiovasc Res. 2005;66:276–285. doi: 10.1016/j.cardiores.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Fogo AB. The role of angiotensin ii and plasminogen activator inhibitor-1 in progressive glomerulosclerosis. Am J Kidney Dis. 2000;35:179–188. doi: 10.1016/s0272-6386(00)70324-6. [DOI] [PubMed] [Google Scholar]
- 30.Bergheim I, Guo L, Davis MA, Duveau I, Arteel GE. Critical role of plasminogen activator inhibitor-1 in cholestatic liver injury and fibrosis. J Pharmacol Exp Ther. 2006;316:592–600. doi: 10.1124/jpet.105.095042. [DOI] [PubMed] [Google Scholar]
- 31.Gharaee-Kermani M, Hu B, Phan SH, Gyetko MR. The role of urokinase in idiopathic pulmonary fibrosis and implication for therapy. Expert Opin Investig Drugs. 2008;17:905–916. doi: 10.1517/13543784.17.6.905. [DOI] [PubMed] [Google Scholar]
- 32.Loskutoff DJ, Quigley JP. Pai-1, fibrosis, and the elusive provisional fibrin matrix. J Clin Invest. 2000;106:1441–1443. doi: 10.1172/JCI11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneiderman J, Sawdey MS, Keeton MR, Bordin GM, Bernstein EF, Dilley RB, Loskutoff DJ. Increased type 1 plasminogen activator inhibitor gene expression in atherosclerotic human arteries. Proc Natl Acad Sci US A. 1992;89:6998–7002. doi: 10.1073/pnas.89.15.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lupu F, Bergonzelli GE, Heim DA, Cousin E, Genton CY, Bachmann F, Kruithof EK. Localization and production of plasminogen activator inhibitor-1 in human healthy and atherosclerotic arteries. Arterioscler Thromb. 1993;13:1090–1100. doi: 10.1161/01.atv.13.7.1090. [DOI] [PubMed] [Google Scholar]
- 35.Pandolfi A, Cetrullo D, Polishuck R, Alberta MM, Calafiore A, Pellegrini G, Vitacollona E, Capani F, Consoli A. Plasminogen activator inhibitor type 1 is increased in the arterial wall of type ii diabetic subjects. Arterioscler Thromb Vasc Biol. 2001;21:1378–1382. doi: 10.1161/hq0801.093667. [DOI] [PubMed] [Google Scholar]
- 36.Sobel BE, Woodcock-Mitchell J, Schneider DJ, Holt RE, Marutsuka K, Gold H. Increased plasminogen activator inhibitor type 1 in coronary artery atherectomy specimens from type 2 diabetic compared with nondiabetic patients: a potential factor predisposing to thrombosis and its persistence. Circulation. 1998;97:2213–2221. doi: 10.1161/01.cir.97.22.2213. [DOI] [PubMed] [Google Scholar]
- 37.Calles-Escandon J, Mirza SA, Sobel BE, Schneider DJ. Induction of hyperinsulinemia combined with hyperglycemia and hypertriglyceridemia increases plasminogen activator inhibitor 1 in blood in normal human subjects. Diabetes. 1998;47:290–293. doi: 10.2337/diab.47.2.290. [DOI] [PubMed] [Google Scholar]
- 38.Lyons RM, Gentry LE, Purchio AF, Moses HL. Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J Cell Biol. 1990;110:1361–1367. doi: 10.1083/jcb.110.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Kelm RJ, Budd RC, Sobel BE, Schneider DJ. Inhibition of apoptosis and caspase-3 in vascular smooth muscle cells by plasminogen activator inhibitor type-1. J Cell Biochem. 2004;92:178–188. doi: 10.1002/jcb.20058. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Budd RC, Kelm RJ, Sobel BE, Schneider DJ. Augmentation of proliferation of vascular smooth muscle cells by plasminogen activator inhibitor type 1. Arterioscler Thromb Vasc Biol. 2006;26:1777–1783. doi: 10.1161/01.ATV.0000227514.50065.2a. [DOI] [PubMed] [Google Scholar]
- 41.Cao C, Lawrence DA, Li Y, Von Arnim CA, Herz J, Su EJ, Makarova A, Hyman BT, Strickland DK, Zhang L. Endocytic receptor lrp together with tpa and pai-1 coordinates mac-1-dependent macrophage migration. EMBO J. 2006;25:1860–1870. doi: 10.1038/sj.emboj.7601082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Degryse B, Neels JG, Czekay R, Aertgeerts K, Kamikubo Y, Loskutoff DJ. The low density lipoprotein receptor-related protein is a motogenic receptor for plasminogen activator inhibitor-1. J Biol Chem. 2004;279:22595–22604. doi: 10.1074/jbc.M313004200. [DOI] [PubMed] [Google Scholar]
- 43.Zemzoum I, Kates RE, Ross JS, Dettmar P, Dutta M, Henrichs C, Yurdseven S, Höfler H, Kiechle M, Schmitt M, Harbeck N. Invasion factors upa/pai-1 and her2 status provide independent and complementary information on patient outcome in node-negative breast cancer. J Clin Oncol. 2003;21:1022–1028. doi: 10.1200/JCO.2003.04.170. [DOI] [PubMed] [Google Scholar]
- 44.Ho C, Yuan C, Liu S. Diagnostic and prognostic values of plasma levels of fibrinolytic markers in ovarian cancer. Gynecologic Oncology. 1999;75:397–400. doi: 10.1006/gyno.1999.5610. [DOI] [PubMed] [Google Scholar]
- 45.Pedersen H, Grondahl-Hansen J, Francis D, Osterlind K, Hansen HH, Danø K, Brünner N. Urokinase and plasminogen activator inhibitor type 1 in pulmonary adenocarcinoma. Cancer Res. 1994;54:120–123. [PubMed] [Google Scholar]
- 46.Sugiura Y, Ma L, Sun B, Shimada H, Laug WE, Seeger RC, DeClerck YA. The plasminogen-plasminogen activator (pa) system in neuroblastoma: role of pa inhibitor-1 in metastasis. Cancer Res. 1999;59:1327–1336. [PubMed] [Google Scholar]
- 47.Devy L, Blacher S, Grignet-Debrus C, Bajou K, Masson V, Gerard RD, Gils A, Carmeliet G, Carmeliet P, Declerck PJ, Nöel A, Foidart JM. The pro- or antiangiogenic effect of plasminogen activator inhibitor 1 is dose dependent. FASEB J. 2002;16:147–154. doi: 10.1096/fj.01-0552com. [DOI] [PubMed] [Google Scholar]
- 48.Bajou K, Masson V, Gerard RD, Schmitt PM, Albert V, Praus M, Lund LR, Frandsen TL, Brunner N, Dano K, Fusenig NE, Weidle U, Carmeliet G, Loskutoff D, Collen D, Carmeliet P, Foidart JM, Noël A. The plasminogen activator inhibitor pai-1 controls in vivo tumor vascularization by interaction with proteases, not vitronectin. implications for antiangiogenic strategies. J Cell Biol. 2001;152:777–784. doi: 10.1083/jcb.152.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bajou K, Peng H, Laug WE, Maillard C, Noel A, Foidart JM, Martial JA, DeClerck YA. Plasminogen activator inhibitor-1 protects endothelial cells from fasl-mediated apoptosis. Cancer Cell. 2008;14:324–334. doi: 10.1016/j.ccr.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Comi P, Chiaramonte R, Maier JA. Senescence-dependent regulation of type 1 plasminogen activator inhibitor in human vascular endothelial cells. Exp Cell Res. 1995;219:304–308. doi: 10.1006/excr.1995.1232. [DOI] [PubMed] [Google Scholar]
- 51.Park C, Lee I, Kang WK. E2f-1 is a critical modulator of cellular senescence in human cancer. Int J Mol Med. 2006;17:715–720. [PubMed] [Google Scholar]
- 52.Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol. 2006;8:877–884. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balsara RD, Ploplis VA. Plasminogen activator inhibitor-1: the double-edged sword in apoptosis. Thromb Haemost. 2008;100:1029–1036. [PMC free article] [PubMed] [Google Scholar]
- 54.Kwaan HC, Wang J, Svoboda K, Declerck PJ. Plasminogen activator inhibitor 1 may promote tumour growth through inhibition of apoptosis. Br J Cancer. 2000;82:1702–1708. doi: 10.1054/bjoc.2000.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beaulieu LM, Whitley BR, Wiesner TF, Rehault SM, Palmieri D, Elkahloun AG, Church FC. Breast cancer and metabolic syndrome linked through the plasminogen activator inhibitor-1 cycle. Bioessays. 2007;29:1029–1038. doi: 10.1002/bies.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palmieri D, Lee JW, Juliano RL, Church FC. Plasminogen activator inhibitor-1 and -3 increase cell adhesion and motility of mda-mb-435 breast cancer cells. J Biol Chem. 2002;277:40950–40957. doi: 10.1074/jbc.M202333200. [DOI] [PubMed] [Google Scholar]
- 57.Rømer MU, Kirkebjerg Due A, Knud Larsen J, Hofland KF, Christensen IJ, Buhl-Jensen P, Almholt K, Lerberg Nielsen O, Brünner N, Lademann U. Indication of a role of plasminogen activator inhibitor type i in protecting murine fibrosarcoma cells against apoptosis. Thromb Haemost. 2005;94:859–866. [PubMed] [Google Scholar]
- 58.Rømer MU, Larsen L, Offenberg H, Brünner N, Lademann UA. Plasminogen activator inhibitor 1 protects fibrosarcoma cells from etoposide-induced apoptosis through activation of the pi3k/akt cell survival pathway. Neoplasia. 2008;10:1083–1091. doi: 10.1593/neo.08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Webb DJ, Thomas KS, Gonias SL. Plasminogen activator inhibitor 1 functions as a urokinase response modifier at the level of cell signaling and thereby promotes mcf-7 cell growth. J Cell Biol. 2001;152:741–752. doi: 10.1083/jcb.152.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]