Abstract
Adenoviral-mediated overexpression of the intracellular superoxide (O2•−) scavenging enzyme copper/zinc superoxide dismutase (CuZnSOD) in the brain attenuates central angiotensin II (AngII)-induced cardiovascular responses. However, the therapeutic potential for adenoviral vectors is weakened by toxicity and the inability of adenoviral vectors to target the brain following peripheral administration. Therefore, we developed a non-viral delivery system in which CuZnSOD protein is electrostatically bound to a synthetic poly(ethyleneimine)-poly(ethyleneglycol) (PEI-PEG) polymer to form a polyion complex (CuZnSOD nanozyme). We hypothesized that PEI-PEG polymer increases transport of functional CuZnSOD to neurons, which inhibits AngII intra-neuronal signaling. The AngII-induced increase in O2•−, as measured by dihydroethidium fluorescence and electron paramagnetic resonance spectroscopy, was significantly inhibited in CuZnSOD nanozyme-treated neurons compared to free CuZnSOD- and non-treated neurons. CuZnSOD nanozyme also attenuated the AngII-induced inhibition of K+ current in neurons. Intracarotid injection of CuZnSOD nanozyme into rabbits significantly inhibited the pressor response of intracerebroventricular-delivered AngII; however, intracarotid injection of free CuZnSOD or PEI-PEG polymer alone failed to inhibit this response. Importantly, neither the PEI-PEG polymer alone nor the CuZnSOD nanozyme induced neuronal toxicity. These findings indicate that CuZnSOD nanozyme inhibits AngII intra-neuronal signaling in vitro and in vivo.
Keywords: brain, superoxide dismutase, nanotechnology, drug delivery, copolymer, potassium current
1. Introduction
Dysregulation of brain angiotensinergic systems is implicated in a number of cardiovascular diseases, including hypertension and heart failure [1,2]. Recent investigations have identified NADPH oxidase and mitochondrial-derived reactive oxygen species (ROS), particularly superoxide (O2•−), as key signaling intermediates in angiotensin (AngII) intra-neuronal signaling [3–7]. In particular, overexpressing the intracellular O2•− scavenging enzyme copper/zinc superoxide dismutase via adenoviral-mediated gene transfer (AdCuZnSOD) in the brain significantly inhibits the acute pressor response to centrally administered AngII [8]. Furthermore, intracerebroventricular (ICV) administration of AdCuZnSOD, which results in the robust and localized expression of CuZnSOD in the subfornical organ (SFO), a blood-brain barrier (BBB)-deficient brain region, markedly attenuates the development of hypertension induced by chronic systemic infusion of AngII [9]. In cultured neurons, AdCuZnSOD infection inhibits the AngII-induced increase in O2•− and the AngII-mediated increase in intracellular calcium concentration [8,10]. Together, these studies indicate that AngII intra-neuronal signaling involves an increase in O2•− and that overexpressing CuZnSOD in the brain may serve as a therapeutic approach for cardiovascular diseases associated with aberrant AngII signaling in the central nervous system (CNS).
Although adenoviral vectors are an excellent experimental tool to deliver genes of interest (e.g. CuZnSOD) to cultured neurons and to specific cardiovascular control brain nuclei (e.g. SFO), enthusiasm for adenoviral-mediated therapeutics is dampened by the potential for an inflammatory response, toxicity, and the inability of viral vectors to target the CNS following peripheral administration [11–13]. In addition, adenoviral-mediated delivery is complicated by the low frequency of viral integration and the susceptibility of the integrated vector sequences to rearrange [12]. Alternatively, nanotechnology-driven delivery has received great interest in recent years because of its many favorable characteristics. Nanomedicines consist of nanocarriers, such as liposomes or polymers, in the dimensions of 1 to 100 nanometers that entrap, encapsulate, or bind to therapeutic agents, including proteins, DNA, or drugs [14]. Nanotechnolgy can help these potential therapies penetrate physiological barriers, prolong circulation by avoiding renal clearance, reduce the frequency of administration by releasing drugs in a sustained manner, and minimize systemic adverse effects by delivering drugs preferentially to target tissues [15].
In the current study, we selected a cationic block copolymer, poly(ethyleneimine)-poly(ethyleneglycol) (PEI-PEG) as an alternative to adenoviral-mediated delivery of CuZnSOD. Previously, Batrakova et al. demonstrated that bone-marrow-derived monocytes deliver PEI-PEG protected catalase to the CNS of a Parkinson’s disease mouse model [16]. In addition, Vinogradov et al. reported that a PEI-PEG nanogel protected oligonucleotides from enzymatic degradation and resulted in significant accumulation in the CNS [17]. Given the former success of the PEI-PEG polymer delivery system, we hypothesized that PEI-PEG encapsulated CuZnSOD (i.e. CuZnSOD nanozyme) can enter neurons, inhibit AngII intra-neuronal signaling, and attenuate the central AngII-induced pressor response. Herein, using a neuronal cell line (CATH.a neurons) and in vivo rabbit experiments, we report an increase of functional CuZnSOD protein in neurons following treatment with CuZnSOD nanozyme.
2. Materials and Methods
2.1. Reagents
RPMI-1640 culture medium and penicillin-streptomycin were purchased from Gibco/Invitrogen, Grand Island, NY. Normal horse serum (NHS) was purchased from American Type Culture Collection (ATCC, Manassas, VA). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals, Lawrenceville, GA. Angiotensin II (AngII), N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate sodium salt (dbcAMP), polyethylenimine (PEI) (2 kDa, branched, 50% aq. solution), CuZnSOD protein, PEG-SOD, rhodamine B isothiocyanate, Triton X-100, hypoxanthine, xanthine oxidase, hydrogen peroxide (H2O2), 4-acetamidophenol (AAP), horseradish peroxidase (HRP), diethylenetriaminepentaacetic acid (DTPA), catalase protein, iron (II) heptahydrate (FeSO4), dimethylthiourea (DMTU), and tetrodotoxin (TTX) were purchased from Sigma-Aldrich, St. Louis, MO. Methoxypolyethylene glycol epoxy (Me-PEG-epoxy) was purchased from Shearwater Polymer Inc., Huntsville, AL. Dihydroethidium (DHE) was purchased from Invitrogen. 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH), EPR buffer, diethyldithiocarbamic acid sodium salt (DETC), and deferoxamine were purchased from Noxygen Science Transfer and Diagnostics, Elzach, Germany.
2.2. CATH.a Neuronal Cell Culture
Mouse catecholaminergic CATH.a neurons were purchased from ATCC and cultured in RPMI-1640 medium supplemented with 8% NHS, 4% FBS, and 1% penicillin-streptomycin. Cultures were maintained in a humidified incubator at 37°C and 5% CO2. CATH.a neurons were differentiated by adding 1 mM of dbcAMP to the culture medium every other day for 6–8 days. Neurons were serum starved 24 hr before experimentation. Notably, previous studies have identified CATH.a neurons as a reliable cell culture model for investigating AngII intra-neuronal signaling [18–20].
2.3. Animals
New Zealand White rabbits weighing 2.9 – 4.4 kg were used for in vivo experiments. Rabbits were housed in individual cages under controlled temperature and humidity with a 12 hr dark-light cycle and were fed standard rabbit diet with water available ad libitum. These experiments were reviewed and approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee and conform to the Guidelines for the Care and Use of Experimental Animals of the American Physiological Society and the National Institutes of Health.
2.4. Synthesis of PEI-PEG Polymer
The copolymer was synthesized by conjugation of PEI and Me-PEG-epoxy, as we previously reported [16]. Me-PEG-epoxy, 10.4 kDa (Mw), Mw/Mn = 1.02 (lot No. ZF-028-01) was custom synthesized by Shearwater Polymers. Briefly, Me-PEG-epoxy water solution was added to 5% PEI in water and incubated overnight at room temperature. To filter free PEI and low molecular weight residuals, the conjugates were dialyzed in SpectraPor membrane tubes with molecular weight cutoff 6000–8000 Da against water (replaced twice for 48 hr) and then concentrated in vacuo. For the final purification, the conjugate was dissolved in 20 mL of pure methanol and then added dropwise to 400 mL of ether. The precipitate was centrifuged (400×g, 5 min), washed twice with ether, and dried in a desiccator. Detailed characterization of the PEI-PEG was performed by spectrophotometry and mass spectrometry as we previously described [16].
2.5. Preparation of CuZnSOD Nanozyme
CuZnSOD nanozyme was prepared by mixing purified PEI-PEG polymer and CuZnSOD protein at a ratio of 18.4 mg PEI-PEG to 1 mg CuZnSOD protein in 1 mL of media (pH 7.4). The mixture was incubated for 30 min to allow for formation of complexes. CuZnSOD protein and PEI-PEG self-assemble into polyion complexes with a PEG corona and a PEI core electrostatically bound to CuZnSOD through the positively charged amine groups of the polymer and the negatively charged carboxyl groups of the protein. In all experiments, we used 400 U/mL of CuZnSOD nanozyme, free CuZnSOD protein, or PEG-SOD.
2.6. Labeling CuZnSOD with Rhodamine and Neuronal Uptake
Neuronal uptake of CuZnSOD nanozyme was measured by labeling CuZnSOD protein with fluorescent rhodamine B isothiocyanate. CuZnSOD protein (1 mg) in 1 ml of reaction buffer (100 mM carbonate buffer, pH 9.5, 300 mL of 0.1 M Na2CO3 + 700 mL of 0.1 M NaHCO3) was incubated with 0.5 mg rhodamine B isothiocyanate (in 100 μl DMSO) for 2 hr at room temperature. To remove free rhodamine, the solution was purified through an Illustra NAP-10 column (GE Healthcare Fischer). CATH.a neurons were treated with rhodamine-labeled CuZnSOD protein or CuZnSOD nanozyme for 1, 3 or 6 hr. Fluorescent images were captured with a Zeiss LSM 510 Meta Confocal Microscope.
2.7. CuZnSOD Immunofluorescence
Neuronal uptake of CuZnSOD nanozyme was confirmed with immunofluorescence staining. CATH.a neurons were treated with free CuZnSOD protein, PEG-SOD, or CuZnSOD nanozyme in media for 3 hr. Neurons were rinsed with 0.1 M phosphate buffer (PB) and fixed with 4% paraformaldehyde for 30 min at room temperature. After rinsing with 0.1 M PB, neurons were incubated with blocking solution (0.1 M PB + 10% NHS + 0.3% Triton-X) for 1 hr at room temperature. Neurons were then incubated overnight at 4 °C with anti-CuZnSOD primary antibody (Upstate/Millipore) diluted (1:500) in 0.1 M PB containing 2% NHS and 0.3% Triton-X. The primary antibody was removed and neurons were rinsed with 0.1 M PB. Finally, neurons were incubated for 2 hr in the dark with an Alexa Fluor 488 secondary antibody (Invitrogen) diluted (1:500) in 0.1 M PB containing 2% NHS. Fluorescent images were captured with confocal microscopy. All confocal microscope parameters were equal between images. Fluorescence intensity per cell was quantified using the Zeiss LSM 510 analysis software.
2.8. Dihydroethidium Fluorescence
Levels of O2•− were detected using DHE, a fluorogenic probe that is converted to 2-hydroxyethidium in a O2•−-dependent reaction or converted to ethidium in a non-specific ROS reaction [21]. CATH.a neurons pretreated (3 hr) with CuZnSOD protein, CuZnSOD nanozyme, or vehicle (control) were loaded with DHE (5 μM) for 30 min at 37°C. Fluorescence confocal microscopy images of CATH.a neurons were taken before and after AngII (100 nM, 20 min) stimulation using a 405 nm excitation wavelength. Notably, as previously characterized and described by Robinson and colleagues [21], the 405 nm excitation wavelength selectively detects 2-hydroxyethidium, the O2•−-specific product of DHE.
2.9. Electron Paramagnetic Resonance Spectroscopy
The specificity of CuZnSOD nanozyme to scavenge O2•− was tested with electron paramagnetic resonance (EPR) spectroscopy in a cell-free system. Superoxide was generated by hypoxanthine (HX) and xanthine oxidase (XO). Samples were prepared with 200 μM of the CMH spin probe, 25 μM HX, and 10 mU/mL XO in 100 μL of EPR buffer (pH 7.4). EPR buffer is a Krebs-HEPES buffer consisting of (in mM): 99 NaCl, 4.69 KCl, 2.5 CaCl2, 1.2 MgSO4, 25 NaHCO3, 1.03 KH2PO4, 5.6 D-glucose, 20 HEPES and supplemented with the metal chelators DETC (5 μM) and deferoxamine (25 μM). For the experimental groups, 400 U/ml of free CuZnSOD protein, CuZnSOD nanozyme, or the equivalent amount of PEI-PEG polymer alone was added prior to the HX and XO. Samples were incubated at room temperature for 5 min and 50 μL of the sample was loaded into a glass capillary tube, which was then inserted into the capillary holder of a Bruker e-scan EPR spectrometer.
The ability of CuZnSOD nanozyme to scavenge hydrogen peroxide (H2O2) was tested in samples with 200 μM CMH and 10 μM H2O2 in 100 μL of a supplemented EPR buffer called, KDD+ buffer (pH 7.4), as previously described [22]. KDD+ is the EPR buffer, as described above, supplemented with 1 mM AAP, 1 U/mg HRP, and 200 μM DTPA. In the different experimental groups, 400 U/ml of catalase protein, CuZnSOD protein, CuZnSOD nanozyme, or the equivalent amount of PEI-PEG polymer alone was added prior to the H2O2. Catalase, a well-known H2O2 scavenger, was used to corroborate the fidelity of this assay. Samples were incubated at room temperature for 5 min and then EPR spectra were obtained.
In the last set of cell-free experiments, the ability of CuZnSOD nanozyme to scavenge hydroxyl radical (OH•) was tested in samples with 200 μM CMH, 10 μM FeSO4, 100 μM H2O2, in EPR buffer (pH 7.4). Through Fenton chemistry, H2O2 oxidizes iron (II) yielding iron (III) and OH•. In the various experimental groups, 400 U/ml of CuZnSOD protein, CuZnSOD nanozyme, the equivalent amount of PEI-PEG polymer, or 10 mM DMTU, a well-known OH• scavenger, was added prior to the H2O2 and FeSO4. Similar to the O2•− and H2O2 experiments, samples were incubated at room temperature for 5 min then inserted into Bruker e-scan EPR spectrometer.
EPR spectroscopy was also used to measure O2•−-levels in CATH.a neurons stimulated with AngII (100 nM). Neurons pretreated (3 hr) with free CuZnSOD protein, PEG-SOD, CuZnSOD nanozyme, or left untreated (control) were incubated (1 hr at 37°C) with CMH (200 μM), a cell permeable, O2•−-sensitive spin probe [23,24] in EPR buffer (pH 7.4). After CMH incubation, neurons were collected in a cell suspension, stimulated with AngII, and 50 μL of the cell suspension was placed into the Bruker e-scan EPR spectrometer. The following EPR settings were used for all experiments: field sweep width, 60.0 G; microwave frequency, 9.75 kHz; microwave power, 21.90 mW; modulation amplitude, 2.37 G; conversion time, 10.24 ms; time constant, 40.96 ms. Levels of O2•− were normalized to the number of neurons in each sample.
2.10. Electrophysiological Recordings
Using a Warner PC-505B patch-clamp amplifier (Warner Instrument Corp) and the whole cell configuration of the patch-clamp technique, potassium current (IKv) was recorded from untreated CATH.a neurons and neurons pretreated (3 hr) with free CuZnSOD protein, PEG-SOD, SOD nanozyme, or PEI-PEG polymer alone. The patch pipette resistance ranged from 3–5 MΩ when filled with (in mM): 130 KCl, 2 MgCl2, 0.25 CaCl2, 5 EGTA, 1 Mg-ATP, 0.1 Tris-GTP, 10 HEPES, and 8 glucose, pH 7.2. The extracellular bath solution included (in mM): 137 NaCl, 5.4 KCl, 1.35 CaCl2, 2 MgCl2, 0.3 NaH2PO4, 10 HEPES, 10 sucrose, pH 7.4. Na+ and Ca2+ channels were blocked by TTX (0.5 μM) and CdCl2 (0.3 mM), respectively. Current traces were sampled at 10 kHz and filtered at 5 kHz. Holding potential was −80 mV. Current-voltage (I–V) relations were elicited by test potential over the range of −80 mV to +80 mV with 400 ms duration in 10 mV increments (5 s between steps). Resulting data were acquired and analyzed with P-clamp 8.1 software (Axon Instruments). Recordings were performed at 22–24°C. The effect of AngII (100 nM) on current density (pA/pF) was tested by superfusing neurons with the peptide for 5 min and repeating the voltage pulse regimen. Cell membrane capacitance (Cm) was determined by integrating the capacity current evoked by a voltage step of 5 mV and dividing the resulting charge by the voltage step.
2.11. Toxicity Assay
CATH.a neuronal toxicity was measured with the Cell Toxicity Kit-8 assay (Dojindo Technologies) according to the manufacturer’s directions. To obtain a baseline measurement of viable cells in each culture, neurons were incubated with the CCK-8 solution (1:10 in serum-free media) prior to SOD treatment. Levels of the colored formazan product, which are indicative of the number of live cells, were determined by measuring the absorbance at 450 nm. Neurons were then incubated with free CuZnSOD protein, PEG-SOD, CuZnSOD nanozyme, or PEI-PEG polymer alone for 3 hr and neuron viability assessed 24 and 48 hr thereafter. Percent viability was calculated by normalizing post to pretreatment (baseline) absorbance values obtained from each culture and viability of non-treated control neurons was set at 100%.
2.12. Recording Arterial Pressure with Radiotelemetry
A radiotelemetry device (model TA11PA-C40, Physiotel, Data Sciences International) was implanted for the measurement of arterial pressure in conscious rabbits. A central groin region incision was made and the radiotelemetry device was secured subcutaneously. The sensing catheter was inserted into the left femoral artery against blood flow. The signals received by the device were processed and digitized as radiofrequency data, which were recorded and stored in a computer with the Dataquest IV system (Data Sciences International). The measured parameters included arterial blood pressure (AP), mean arterial pressure (MAP), and heart rate (HR). These parameters were displayed and stored in a PowerLab system (AD Instruments).
2.13. Intracerebroventricular Cannulation
The skull was exposed through an incision on the midline of the scalp. After bregma was identified, a 19-gauge stainless steel intracerebroventricular (ICV) cannula was implanted into the right lateral cerebral ventricle, 3.5 mm lateral to the bregma and 6 mm below the cerebral surface, and fixed tightly to the skull with super-glue adhesive and dental cement. The position of the cannula in the lateral cerebral ventricle was confirmed by the staining of all four ventricles after injection of 0.1 ml of Evans blue dye at the end of experiments.
2.14. ICV Injection of AngII and Intracarotid Injection of CuZnSOD nanozyme
Rabbits were placed in a quiet, dimly lit room and while they stood in a Plexiglas box AP, MAP, and HR values were recorded. After the animal had adjusted to the environment and all hemodynamics were stable, AngII (1, 10, and 100 ng in 100 μL aCSF) was ICV injected via the ICV cannula. ICV AngII injections were performed one day pre-intracarotid (i.c.) injection or 1, 2, 3, and 7 days post i.c. injection of CuZnSOD protein, CuZnSOD nanozyme or PEI-PEG polymer alone (400 U/ml in 1 ml of sterile saline). For i.c. injection, these agents were administered through a 30 g needle connected to a 1 ml syringe that was inserted into the right carotid artery after isolating the artery through a midline incision in the neck.
2.15. Statistical Analysis
All data are expressed as mean ± SEM. For the in vitro neuronal cell culture experiments, data were analyzed by one-way ANOVA followed by the Newman-Keuls post-test for multiple comparisons. For the in vivo rabbit experiments, data were analyzed by a two-way ANOVA followed by the Newman–Keuls post-test. Differences before and after ICV injection of AngII in each group were analyzed with a paired t-test. P-value less than 0.05 was considered statistically significant. Statistical analyses were performed using Prism (GraphPad Software, Inc).
3. Results
3.1. Specificity of CuZnSOD nanozyme
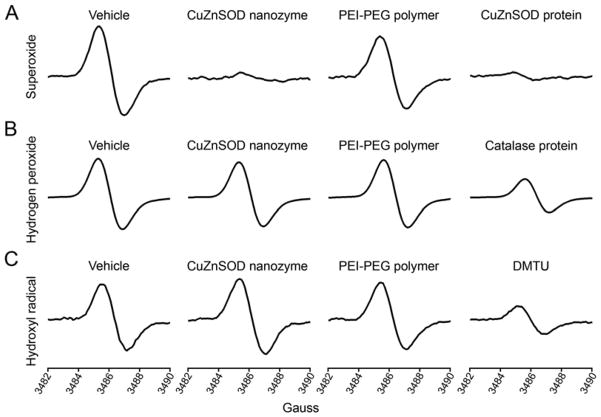
Using a cell-free system and EPR spectroscopy, we tested the specificity of CuZnSOD nanozyme; that is, we investigated the ability of CuZnSOD nanozyme to scavenge O2•−, H2O2, and OH•. As shown in the representative EPR spectra, HX/XO-generated O2•− was virtually abolished by free CuZnSOD protein and CuZnSOD nanozyme, but not by polymer alone (Figure 1A). Conversely, CuZnSOD nanozyme failed to decrease H2O2 levels, while the well-known H2O2 scavenger, catalase, did significantly reduce H2O2 levels (Figure 1B). In addition, CuZnSOD nanozyme did not decrease levels of OH• (generated by FeSO2 + H2O2), whereas DMTU, a commonly used OH• scavenger, did significantly decrease OH• levels (Figure 1C). These data indicate that CuZnSOD nanozyme is capable of and is specific for scavenging O2•−.
Figure 1. CuZnSOD nanozyme specifically scavenges O2•−.
A) Representative EPR spectra in a cell-free system in which superoxide (O2•−) was generated by HX (25 μM) and XO (mU/mL) and detected with the CMH spin probe. Experimental samples were treated with vehicle, CuZnSOD nanozyme (400 U/mL), PEI-PEG polymer alone, or free CuZnSOD protein (400 U/mL). B). Hydrogen peroxide (H2O2)-dependent EPR spectra (see Methods for details) obtained from cell-free samples treated with vehicle, CuZnSOD nanozyme (400 U/mL), PEI-PEG polymer alone, or the H2O2 scavenger catalase (400 U/mL). C) EPR spectra from cell-free samples in which hydroxyl radical (OH•) was generated by FeSO4 (10 μM) and H2O2 (100 μM). Experimental samples were treated with vehicle, CuZnSOD nanozyme (400 U/mL), PEI-PEG polymer alone, or the OH• scavenger DMTU (10 mM).
3.2. Neuronal uptake of CuZnSOD nanozyme
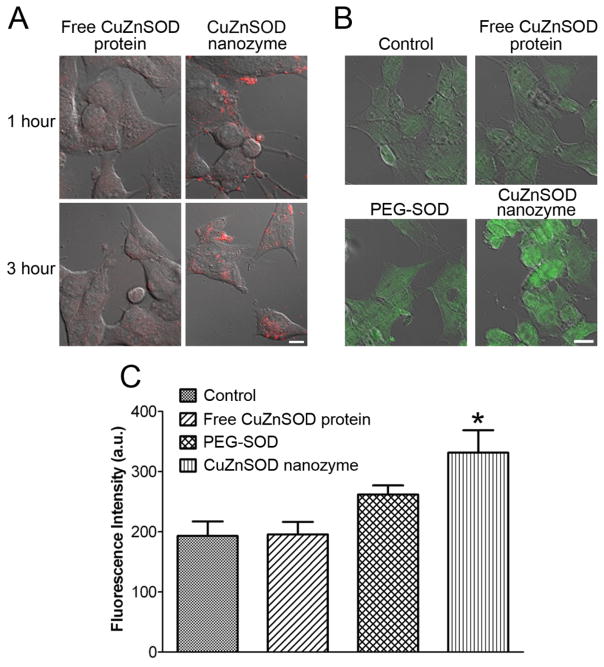
To evaluate transport of CuZnSOD nanozyme into CATH.a neurons, cells were incubated with rhodamine-labeled free CuZnSOD protein or CuZnSOD nanozyme for 1, 3, or 6 hours and neuronal uptake was assessed by capturing rhodamine fluorescence. As shown in the representative confocal microscopy images (Figure 2A), negligible rhodamine fluorescence was detected in free CuZnSOD-treated neurons. In contrast, after 1 hour of CuZnSOD nanozyme treatment, robust rhodamine fluorescence was observed near the cell membrane, and within 3 hours rhodamine fluorescence was detected intracellularly (Figure 2A). Similar fluorescence was observed in CATH.a neurons treated with CuZnSOD nanozyme for 6 hours (data not shown).
Figure 2. CuZnSOD nanozyme penetrates CATH.a neuronal cell membrane to increase intracellular levels of CuZnSOD protein.
(A) Representative confocal microscopy images showing rhodamine fluorescence in CATH.a neurons following 1 or 3 hours of incubation with rhodamine-labeled free CuZnSOD protein or CuZnSOD nanozyme (400 U/mL). (B) Representative confocal microscopy images showing CuZnSOD immunofluorescence staining in control CATH.a neurons and neurons treated (3 hours) with free CuZnSOD protein, PEG-SOD, or CuZnSOD nanozyme (400 U/mL). (C) Summary data (n=4 separate cultures per group) of CuZnSOD immunofluorescence in control CATH.a neurons (n=96 neurons) and neurons treated (3 hours) with free CuZnSOD protein (n=81 neurons), PEG-SOD (n=75 neurons) or CuZnSOD nanozyme (n=66 neurons). *p<0.05 vs. control and free CuZnSOD. a.u.= arbitrary units. Magnification bar in (A) and (B) equals 10 μm.
The increased neuronal uptake of CuZnSOD nanozyme was confirmed with immunofluorescence staining for CuZnSOD (Figure 2B, 2C). Confocal microscopy images show an increase in the levels of CuZnSOD in CATH.a neurons treated with CuZnSOD nanozyme for 3 hours compared to untreated (control), free CuZnSOD-, or PEG-SOD-treated neurons (Figure 2B). Quantitation of the fluorescence intensity per cell revealed a significant increase (p<0.05) in CuZnSOD levels in CuZnSOD nanozyme-treated neurons (n=66 neurons on 4 coverslips) compared to non-treated control (n=96 neurons on 4 coverslips), free CuZnSOD- (n=81 neurons on 4 coverslips), and PEG-SOD- (n=75 neurons on 4 coverslips) treated neurons (Figure 2C). Together, these data indicate that CuZnSOD nanozyme is transported into cultured neurons.
3.3. Effect of CuZnSOD nanozyme on intra-neuronal O2•−
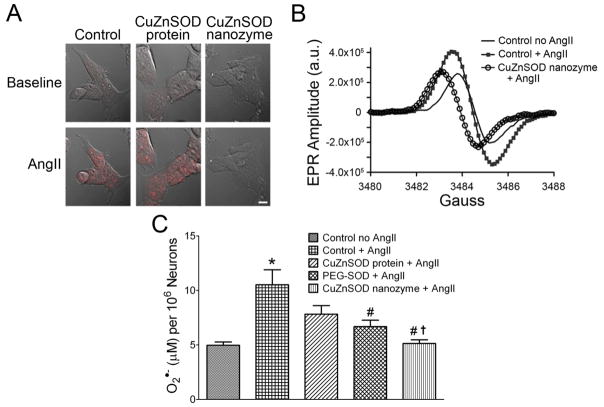
Although rhodamine-labeling and immunofluorescence staining clearly revealed neuronal uptake of CuZnSOD nanozyme, these techniques do not indicate if the CuZnSOD protein delivered is biologically functional. To address this issue, we investigated the ability of CuZnSOD nanozyme to scavenge intra-neuronal O2•− levels following AngII stimulation, a response that has been well-characterized in numerous cell types including neurons [3–7]. CATH.a neurons left untreated (control) or pretreated with free CuZnSOD or CuZnSOD nanozyme were loaded with DHE, the O2•−-sensitive fluorogenic probe. Baseline DHE fluorescence was virtually undetectable in all groups. As previously reported [8,25], AngII increased DHE fluorescence in control neurons (Figure 3A), thus indicating an increase in intracellular O2•−. AngII induced a similar increase in DHE fluorescence in free CuZnSOD-treated neurons, but this response was virtually abolished in CuZnSOD nanozyme-treated neurons (Figure 3A). Quantitation of the DHE fluorescence intensity (mean fluorescent intensities per cell post-AngII stimulation: 141.3 ± 14.6, 190.1 ± 21.3, and 32.71 ± 8.4 fluorescence arbitrary units for control, free CuZnSOD, and CuZnSOD nanozyme-treated neurons, respectively) revealed that CuZnSOD significantly attenuated AngII-induced increase in DHE fluorescence (p<0.05 vs. control and free CuZnSOD protein-treated neurons). It should be noted that we detected DHE fluorescence using a 405 nm excitation wavelength, which, as previously reported [21], excites the O2•−-specific 2-hydroxyethidium product of DHE.
Figure 3. CuZnSOD nanozyme inhibits the AngII-induced increase in O2•− levels in CATH.a neurons.
(A) Representative confocal microscopy images showing DHE fluorescence in non-treated control, free CuZnSOD protein-, and CuZnSOD nanozyme-treated CATH.a neurons before (baseline) and after AngII (100 nM, 20 min) stimulation. Magnification bar equals 10 μm. (B) Representative CMH-EPR spectra obtained from control CATH.a neurons, AngII (100 nM) stimulated neurons, and CuZnSOD nanozyme-treated neurons stimulated with AngII. a.u. = arbitrary units. (C) Summary EPR spectroscopy data showing O2•− levels in CATH.a neurons with and without AngII, and in CATH.a neurons incubated with CuZnSOD protein, PEG-SOD, or CuZnSOD nanozyme followed by AngII stimulation (n=9). *p<0.05 vs. control no AngII; #p<0.05 vs. control + AngII; †p<0.05 vs. free CuZnSOD protein + AngII.
To confirm and support our DHE results, we used EPR spectroscopy to measure O2•− levels in CATH.a neurons pretreated with free CuZnSOD protein, PEG-SOD, or CuZnSOD nanozyme. Control neurons were left untreated. Using the O2•−-sensitive, cell permeable CMH spin probe, we detected an increase in the EPR amplitude of control neurons stimulated with AngII compared to non-treated control neurons (Figure 3B). CuZnSOD nanozyme decreased the AngII-induced EPR amplitude to near control levels (Figure 3B). To quantify the EPR spectra, EPR amplitude arbitrary units were fit to a standard curve obtained from known amounts of a stable nitroxide radical. AngII significantly increased O2•− levels in control neurons and this response was markedly attenuated in PEG-SOD and CuZnSOD nanozyme-treated CATH.a neurons, but not free CuZnSOD-treated neurons (Figure 3C). Together, these data indicate that CuZnSOD nanozyme is able to scavenge intracellular O2•−; thus, demonstrating that the CuZnSOD protein delivered to neurons by CuZnSOD nanozyme is indeed biologically functional.
3.4. Effect of CuZnSOD nanozyme on neuronal K+ current
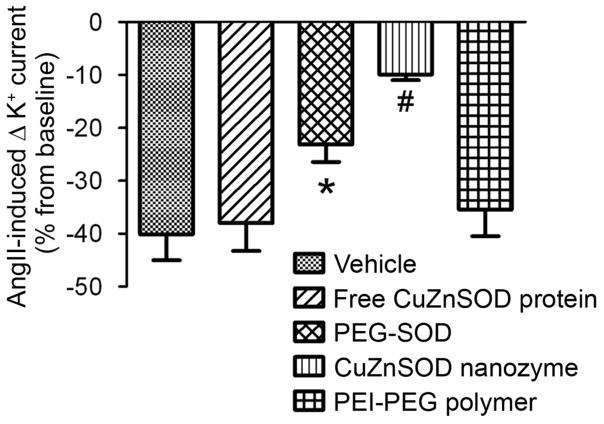
In addition to inhibiting the AngII-induced increase in O2•−, we sought to test the ability of CuZnSOD nanozyme to inhibit physiological responses of AngII. To do so, we performed electrophysiological experiments and studied the well-known AngII-induced inhibition of neuronal voltage-gated K+ current (IKv) [19,26,27]. As shown in Figure 4, superfusion of AngII significantly inhibits outward IKv (40 ± 5% inhibition; p<0.05 vs. baseline). CuZnSOD nanozyme attenuated the AngII-induced inhibition of IKv (10 ± 1% inhibition, p<0.05 vs. control; Figure 4B). Similarly, PEG-SOD attenuated the IKv inhibition (23 ± 3% inhibition, p<0.05 vs. control). However, AngII-induced inhibition of IKv was not attenuated in neurons treated with free CuZnSOD protein or PEI-PEG polymer alone (38 ± 5% and 35 ± 5% inhibition, respectively, p>0.05 vs. control; Figure 4B). These data demonstrate that CuZnSOD nanozyme inhibits a physiological response of AngII in cultured neurons.
Figure 4. AngII-induced inhibition of neuronal K+ current is attenuated by CuZnSOD nanozyme.
AngII (100nM)-induced inhibition of K+ current (% from baseline) in control (n=13), free CuZnSOD protein (n=7), PEG-SOD (n=10), CuZnSOD nanozyme (n=10), and PEI-PEG polymer (n=6)-treated CATH.a neurons evoked by a voltage step from −80 mV to +80 mV. *p<0.05 vs. vehicle; #p<0.05 vs. vehicle, free CuZnSOD, and PEI-PEG.
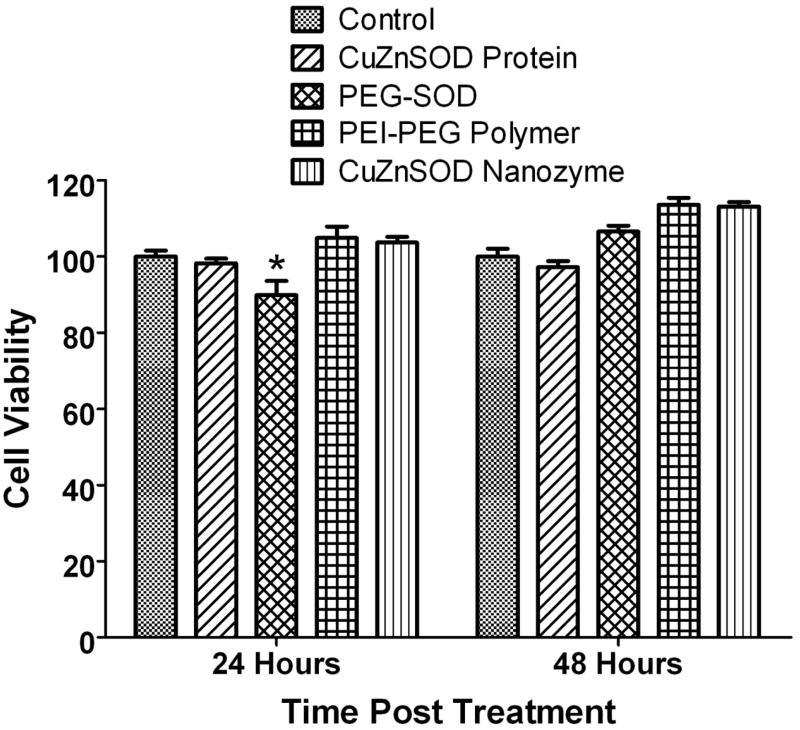
3.5. Viability of CuZnSOD nanozyme-treated neurons
It is imperative that a delivery system is safe and does not have cytotoxic effects. Thus, we evaluated the potential cytotoxicity of CuZnSOD nanozyme. CATH.a neurons were treated with free CuZnSOD, PEG-SOD, CuZnSOD nanozyme, or PEI-PEG polymer alone for 3 hours and changes in cell viability were assessed at 24 and 48 hours post incubation. As shown in Figure 5, neurons treated with PEI-PEG polymer alone or CuZnSOD nanozyme showed similar viability as compared to non-treated control neurons. PEG-SOD induced modest, but significant toxicity 24 hours post incubation (Figure 5). These data indicate that the PEI-PEG polymer and CuZnSOD nanozyme are safe and non-toxic to cultured neurons, at least at the concentration and time periods we tested.
Figure 5. CuZnSOD nanozyme is non-toxic to neurons.
Summary data showing viability of non-treated CATH.a neurons or neurons treated with free CuZnSOD protein, PEG-SOD, PEI-PEG polymer alone, or CuZnSOD nanozyme for 3 hours (n=8–10). Changes in cell viability were assessed 24 and 48 after treatment. In all SOD-treated groups, 400 U/mL of CuZnSOD was used. *p<0.05 vs. control (non-treated) neurons.
3.6. In vivo effect of CuZnSOD nanozyme on AngII-induced pressor response
Our ultimate goal is to develop a system for the delivery of CuZnSOD protein to the brain following peripheral administration. Here, we sought to initiate these studies by recording the well-known pressor response induced by AngII injected directly into the brains (ICV) of rabbits that had previously received a peripheral (i.c.) injection of CuZnSOD nanozyme. ICV injection of AngII into conscious rabbits evoked a dose-dependent increase in MAP (Figure 6A). This response was significantly attenuated for up to 3 days after the rabbits received a single i.c. injection of CuZnSOD nanozyme (Figure 6A). In contrast, i.c. injection of free CuZnSOD protein or the PEI-PEG polymer alone failed to inhibit the ICV AngII-induced pressor response. (Figure 6B, 6C). There was no significant change in HR after AngII injection (data not shown).
Figure 6. Intracarotid injection of CuZnSOD nanozyme inhibits the central AngII-induced pressor response in rabbits.
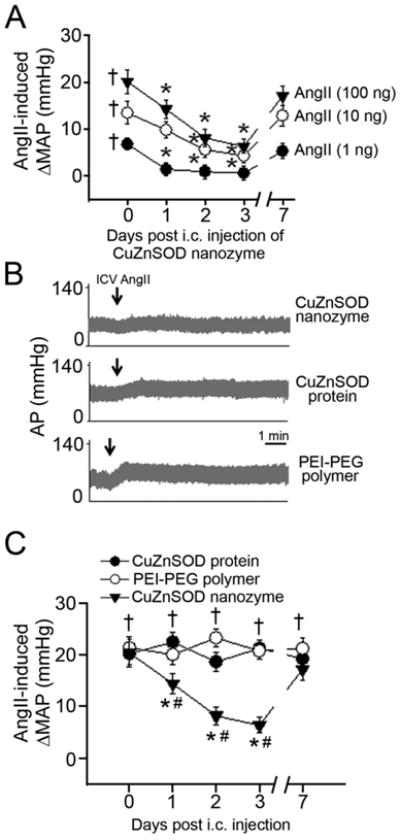
(A) Mean data (n=7 rabbits/group) showing the AngII-induced dose-dependent change in mean arterial pressure (ΔMAP) following ICV administration of AngII in rabbits that received an intracarotid injection of CuZnSOD nanozyme (1 ml at 400 U/mL) 1, 2, 3, or 7 days earlier. † p<0.05 vs. baseline (pre-AngII); *p<0.05 vs. day 0 (i.e. before CuZnSOD nanozyme intracarotid injection). (B) Representative original recording of arterial pressure (AP) before and after ICV injection of AngII (100 ng) 3 days after intracarotid injection of CuZnSOD nanozyme, CuZnSOD protein (400 U/mL), or the PEI-PEG polymer alone. Arrows indicate ICV injection of AngII. (C) Mean data showing the ΔMAP following ICV injection of AngII (100 ng) in rabbits that received an intracarotid injection of CuZnSOD protein (n=5), CuZnSOD nanozyme (n =7), or the PEI-PEG polymer alone (n=5) 1, 2, 3, and 7 days earlier. †p<0.05 vs. baseline (pre-AngII); *p<0.05 vs day 0; #p<0.05 vs. CuZnSOD protein and PEI-PEG polymer.
4. Discussion
Previous studies have shown that adenoviral-mediated delivery of the intracellular O2•− scavenger CuZnSOD to the brain attenuates the changes in blood pressure, heart rate, and drinking elicited by central AngII administration [8]. In addition, adenoviral- mediated overexpression of CuZnSOD in cultured neurons inhibits AngII intra-neuronal signaling [5,8,10]. In the present study, we report that CuZnSOD nanozyme similarly inhibits AngII-dependent signaling. Furthermore, we provide evidence that peripheral administration (i.c. injection) of CuZnSOD nanozyme inhibits the central AngII-induced pressor response in rabbits.
Adenoviral-mediated gene transfer of CuZnSOD has been extensively used in experimental animal and cell culture models to increase the intracellular levels of this critical antioxidant. In a systemic AngII infused mouse model of neurogenic hypertension, ICV administration of AdCuZnSOD led to robust and localized expression of CuZnSOD in the SFO, which resulted in a significant reduction of O2•− levels in this BBB-deficient brain region [9]. Furthermore, the development of hypertension in this model was significantly attenuated by overexpressing CuZnSOD in the SFO [9]. In a separate study, adenoviral-mediated gene transfer of CuZnSOD into the rostral ventrolateral medulla (RVLM) promoted a long-lasting (approximately 14 days) reduction of mean arterial pressure in spontaneously hypertensive rats [28]. In animal models of heart failure, administration of AdCuZnSOD to the paraventricular nucleus (PVN) or SFO decreased neuronal activity and sympathetic output while improving cardiac function and survival [29–31]. Collectively, these studies indicate that overexpressing CuZnSOD in the brain may be a therapeutic approach for neuro-cardiovascular diseases, such as hypertension and heart failure.
Although adenoviral vectors have proven to be a useful tool to experimentally overexpress CuZnSOD in the brain of animal models, the potential for translating adenoviral gene therapy to the clinical setting is limited. Adenovirus has been shown to cause inflammation and toxicity [12]. In addition, peripheral administration of adenovirus results in the rapid and virtually exclusive uptake in the liver, thus limiting its ability to target the CNS [13]. Furthermore, it has been demonstrated that CuZnSOD protein has a short half-life in circulation (about 9 minutes) and poor permeability across the plasma membrane [3,33], thus, greatly limiting its ability to inhibit central AngII signaling. The circulation time of CuZnSOD protein can be increased by modification with polyethylene glycol (PEG) and previous studies have shown that centrally administered PEG-SOD inhibits brain angiotensinergic signaling [34–36]. However, PEGylation decreases the permeability of CuZnSOD protein across the brain microvessels; thus, peripheral administration of PEG-SOD fails to increase brain SOD activity [37–39]. Therefore, new approaches for the delivery of potentially therapeutic proteins, such as CuZnSOD, to the brain must be developed.
Previous studies have shown that numerous nanocarries can deliver their cargo to cells in vitro and in vivo by increasing cell membrane permeability, improving the solubility of drugs, prolonging circulation, and minimizing systemic adverse effects with localized delivery [1,15,40]. In the current study, we examined the ability of PEI-PEG block copolymer to transport CuZnSOD protein to CNS neurons. The so-called CuZnSOD nanozyme, formed by the electrostatic encapsulation of CuZnSOD protein with the cationic block PEI-PEG copolymer, effectively delivered functional CuZnSOD protein to cultured neurons. As a result, the AngII-induced increase in intracellular O2•− levels and the AngII-induced inhibition of IKv were significantly attenuated in CATH.a neurons treated with CuZnSOD nanozyme. In contrast, treating neurons with the same amount of free CuZnSOD protein (400 U/mL) failed to inhibit these AngII responses. We also examined the ability of the commercially available PEG-SOD to inhibit AngII intra-neuronal signaling. Previous studies have used PEG-SOD to increase SOD levels in neurons and have shown that centrally administered PEG-SOD inhibits brain angiotensinergic signaling [34–36]. Interestingly, we observed only a modest increase in CuZnSOD expression in PEG-SOD treated CATH.a neurons. Nevertheless, PEG-SOD treatment did significantly inhibit the AngII-induced increase in intra-neuronal O2•− levels and the AngII-induced decrease in neuronal IKv. However, CuZnSOD nanozyme was slightly more effective in inhibiting these AngII responses. We interpret this to mean that compared to PEG-SOD CuZnSOD nanozyme is more efficient in delivering active CuZnSOD protein to cultured neurons. We speculate that formation of a polyelectrolyte complex between positively charged PEI block and negatively charged groups of the protein results in a decrease of total negative charge of the obtained nanoparticle and therefore, improved adsorption and transport of CuZnSOD nanozyme across the cell membrane. Furthermore, incorporation of the enzyme into the PEI-PEG polymer might improve stability and preserve enzymatic activity of SOD inside the cells.
Considering our in vitro data were obtained from the catecholaminergic CATH.a neuronal cell line, additional studies using primary neurons isolated from cardiovascular control brain regions, such as the SFO, PVN, and/or RVLM are needed to confirm that CuZnSOD nanozyme delivers functional CuZnSOD protein to central neurons. It should be noted that we selected the CATH.a neurons as a neuronal cell culture for these initial studies because previous reports clearly demonstrate that the known AngII intra-neuronal signaling mechanisms in primary neurons isolated from the brain are also present in CATH.a neurons [18–20]. In particular, Sumners, Raizada and colleagues have elegantly shown that, through an AT1 receptor dependent mechanism, AngII inhibits IKv similarly in CATH.a neurons and primary neurons isolated from the hypothalamus and brain stem [18–20]. These previous studies convincingly identified CATH.a neurons as a useful neuronal cell model to study AngII intra-neuronal signaling. Nevertheless, as with all cell line-based studies, future studies must include the use of primary neurons to confirm our data collected from the CATH.a neurons.
Perhaps more important than repeating these studies with primary neurons, it is crucial that we examine the ability of CuZnSOD nanozyme to deliver CuZnSOD protein to CNS neurons in vivo. In the current study, we initiated these experiments by performing i.c. injections of CuZnSOD nanozyme and investigating the well-characterized pressor response to centrally administered AngII. We found that the AngII-induced pressor response following ICV administration was significantly attenuated 1, 2, and 3 days, but not 7 days after a single i.c. injection of CuZnSOD nanozyme. In contrast, i.c. injection of free CuZnSOD protein or the PEI-PEG polymer alone failed to inhibit this response. From the present study, it is unclear whether CuZnSOD nanozyme is delivering CuZnSOD protein across the BBB or if it is delivering CuZnSOD protein to neurons in BBB-deficient brain regions, such as the SFO. The SFO lies in the roof of the ventricular system and is easily accessible to AngII following ICV administration [41,42]. Thus, we speculate that i.c. injection of CuZnSOD nanozyme inhibits the pressor response of ICV-injected AngII because neurons in this important cardiovascular control brain region are taking up the CuZnSOD nanozyme. Future pharmacokinetic and distribution studies of CuZnSOD nanozyme following i.c. injection are required to determine the transport and location of CuZnSOD protein within the CNS.
In order for any delivery system to be clinically applicable, it must be safe. Importantly, in the CATH.a neuronal cell culture PEI-PEG polymer and CuZnSOD nanozyme did not induce toxicity. Furthermore, although the neurotoxicity of CuZnSOD nanozyme was not directly assessed in vivo, our data showing that the AngII-induced pressor response is restored to pretreatment levels 7 days after i.c. injection of CuZnSOD nanozyme (Figure 6) indicates that neurons in the cardiovascular control regions of the brain are still viable.
5. Conclusions
Herein we report that CuZnSOD nanozyme is taken up by neurons, resulting in the delivery of active CuZnSOD protein and inhibition of AngII intra-neuronal signaling. In conscious rabbits, intracarotid injection of CuZnSOD nanozyme inhibits the central AngII-induced pressor response for up to 3 days. These data suggest that CuZnSOD nanozyme is a safe alternative to adenoviral mediated delivery and may provide an improved therapeutic strategy for AngII-dependent neuro-cardiovascular diseases associated with increased O2•− levels in the brain, including hypertension and heart failure. In addition, establishing a CNS delivery system for antioxidant proteins like CuZnSOD could also be translated to other human diseases associated with oxidative stress in the brain, such as stroke, Parkinson’s disease, and Alzheimer’s disease.
Acknowledgments
This study was supported by National Institutes of Health grants P20RR021937 (M.C.Z. and A.V.K.) and NS057748 (E.V.B.). We would like to acknowledge the expert technical assistance of Jocelyn Jones. Additionally, we thank Janice A. Taylor and James R. Talaska of the Confocal Laser Scanning Microscope Core Facility at the University of Nebraska Medical Center for providing assistance with confocal microscopy and the Nebraska Research Initiative and the Eppley Cancer Center for their support of the Core Facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Veerasingham SJ, Raizada MK. Brain renin-angiotensin system dysfunction in hypertension: recent advances and perspectives. Br J Pharmacol. 2003;139:191–202. doi: 10.1038/sj.bjp.0705262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zucker IH. Novel mechanisms of sympathetic regulation in chronic heart failure. Hypertension. 2006;48(6):1005–11. doi: 10.1161/01.HYP.0000246614.47231.25. [DOI] [PubMed] [Google Scholar]
- 3.Chan SH, Hsu KS, Huang CC, Wang LL, Ou CC, Chan JY. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res. 2005;97(8):772–80. doi: 10.1161/01.RES.0000185804.79157.C0. [DOI] [PubMed] [Google Scholar]
- 4.Nozoe M, Hirooka Y, Koga Y, Araki S, Konno S, Kishi T, et al. Mitochondria-derived reactive oxygen species mediate sympathoexcitation induced by angiotensin II in the rostral ventrolateral medulla. J Hypertens. 2008;26(11):2176–84. doi: 10.1097/HJH.0b013e32830dd5d3. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, et al. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res. 2004;95(5):532–9. doi: 10.1161/01.RES.0000139957.22530.b9. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman MC, Davisson RL. Redox signaling in central neural regulation of cardiovascular function. Prog Biophys Mol Biol. 2004;84(2–3):125–49. doi: 10.1016/j.pbiomolbio.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Yin JX, Yang RF, Li S, Renshaw AO, Li Y, Schultz HD, et al. Mitochondria-Produced Superoxide Mediates Angiotensin II-Induced Inhibition of Neuronal Potassium Current. Am J Physiol Cell Physiol. 2010 doi: 10.1152/ajpcell.00313.2009. [Jan 20, Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, et al. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002;91(11):1038–45. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]
- 9.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95(2):210–6. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman MC, Sharma RV, Davisson RL. Superoxide mediates angiotensin II-induced influx of extracellular calcium in neural cells. Hypertension. 2005;45(4):717–23. doi: 10.1161/01.HYP.0000153463.22621.5e. [DOI] [PubMed] [Google Scholar]
- 11.Alemany R, Suzuki K, Curiel DT. Blood clearance rates of adenovirus type 5 in mice. J Gen Virol. 2000;81(Pt 11):2605–9. doi: 10.1099/0022-1317-81-11-2605. [DOI] [PubMed] [Google Scholar]
- 12.Costantini LC, Bakowska JC, Breakefield XO, Isacson O. Gene therapy in the CNS. Gene Ther. 2000;7(2):93–109. doi: 10.1038/sj.gt.3301119. [DOI] [PubMed] [Google Scholar]
- 13.Descamps D, Benihoud K. Two key challenges for effective adenovirus-mediated liver gene therapy: innate immune responses and hepatocyte-specific transduction. Curr Gene Ther. 2009;9(2):115–27. doi: 10.2174/156652309787909544. [DOI] [PubMed] [Google Scholar]
- 14.Torchilin VP. In vitro and in vivo availability of liposomes. In: Kabanov AV, Felgner PL, Seymour LW, editors. Self-assembling complexes for gene delivery. 1997. pp. 278–9. [Google Scholar]
- 15.Gilmore JL, Yi X, Quan L, Kabanov AV. Novel nanomaterials for clinical neuroscience. J Neuroimmune Pharmacol. 2008;3(2):83–94. doi: 10.1007/s11481-007-9099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batrakova EV, Li S, Reynolds AD, Mosley RL, Bronich TK, Kabanov AV, et al. A macrophage-nanozyme delivery system for Parkinson’s disease. Bioconjug Chem. 2007;18(5):1498–506. doi: 10.1021/bc700184b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vinogradov SV, Batrakova EV, Kabanov AV. Nanogels for oligonucleotide delivery to the brain. Bioconjug Chem. 2004;15(1):50–60. doi: 10.1021/bc034164r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun C, Sumners C, Raizada MK. Chronotropic action of angiotensin II in neurons via protein kinase C and CaMKII. Hypertension. 2002;39(2 Pt 2):562–6. doi: 10.1161/hy0202.103057. [DOI] [PubMed] [Google Scholar]
- 19.Sun C, Du J, Raizada MK, Sumners C. Modulation of delayed rectifier potassium current by angiotensin II in CATH. a cells. Biochem Biophys Res Commun. 2003;310(3):710–4. doi: 10.1016/j.bbrc.2003.09.069. [DOI] [PubMed] [Google Scholar]
- 20.Zhu M, Gelband CH, Posner P, Sumners C. Angiotensin II decreases neuronal delayed rectifier potassium current: role of calcium/calmodulin-dependent protein kinase II. J Neurophysiol. 1999;82(3):1560–8. doi: 10.1152/jn.1999.82.3.1560. [DOI] [PubMed] [Google Scholar]
- 21.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci USA. 2006;103(41):15038–43. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45(9):1340–51. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49(4):717–27. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mariappan N, Elks CM, Fink B, Francis J. TNF-induced mitochondrial damage: a link between mitochondrial complex I activity and left ventricular dysfunction. Free Radic Biol Med. 2009;46(4):462–70. doi: 10.1016/j.freeradbiomed.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu D, Gao L, Roy SK, Cornish KG, Zucker IH. Role of oxidant stress on AT1 receptor expression in neurons of rabbits with heart failure and in cultured neurons. Circ Res. 2008;103(2):186–93. doi: 10.1161/CIRCRESAHA.108.179408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun C, Sellers KW, Sumners C, Raizada MK. NAD(P)H oxidase inhibition attenuates neuronal chronotropic actions of angiotensin II. Circ Res. 2005;96(6):659–66. doi: 10.1161/01.RES.0000161257.02571.4b. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson AV, Li Z. Whole cell patch recordings from forebrain slices demonstrate angiotensin II inhibits potassium currents in subfornical organ neurons. Regulatory Peptides. 1996;66(1–2):55–8. doi: 10.1016/0167-0115(96)00049-3. [DOI] [PubMed] [Google Scholar]
- 28.Chan SH, Tai MH, Li CY, Chan JY. Reduction in molecular synthesis or enzyme activity of superoxide dismutases and catalase contributes to oxidative stress and neurogenic hypertension in spontaneously hypertensive rats. Free Radic Biol Med. 2006;40(11):2028–39. doi: 10.1016/j.freeradbiomed.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 29.Gao L, Wang W, Liu D, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure. Circulation. 2007;115(24):3095–102. doi: 10.1161/CIRCULATIONAHA.106.677989. [DOI] [PubMed] [Google Scholar]
- 30.Lindley TE, Doobay MF, Sharma RV, Davisson RL. Superoxide is involved in the central nervous system activation and sympathoexcitation of myocardial infarction-induced heart failure. Circ Res. 2004;94(3):402–9. doi: 10.1161/01.RES.0000112964.40701.93. [DOI] [PubMed] [Google Scholar]
- 31.Lindley TE, Infanger DW, Rishniw M, Zhou Y, Doobay MF, Sharma RV, et al. Scavenging superoxide selectively in mouse forebrain is associated with improved cardiac function and survival following myocardial infarction. Am J Physiol Regul Integr Comp Physiol. 2009;296(1):R1–R8. doi: 10.1152/ajpregu.00078.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odlind B, Appelgren LE, Bayati A, Wolgast M. Tissue distribution of 125I-labelled bovine superoxide dismutase (SOD) in the rat. Pharmacol Toxicol. 1988;62(2):95–100. doi: 10.1111/j.1600-0773.1988.tb01853.x. [DOI] [PubMed] [Google Scholar]
- 33.Petkau A, Chelack WS, Kelly K, Barefoot C, Monasterski L. Tissue distribution of bovine 125I-superoxide dismutase in mice. Res Commun Chem Pathol Pharmacol. 1976;15(4):641–54. [PubMed] [Google Scholar]
- 34.Campese VM, Shaohua Y, Huiquin Z. Oxidative stress mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension. 2005;46(3):533–9. doi: 10.1161/01.HYP.0000179088.57586.26. [DOI] [PubMed] [Google Scholar]
- 35.Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, et al. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res. 2004;95(10):1019–26. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- 36.Zhong MK, Gao J, Zhang F, Xu B, Fan ZD, Wang W, et al. Reactive oxygen species in rostral ventrolateral medulla modulate cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf) 2009;197(4):297–304. doi: 10.1111/j.1748-1716.2009.02026.x. [DOI] [PubMed] [Google Scholar]
- 37.Beckman JS, Minor RL, Jr, White CW, Repine JE, Rosen GM, Freeman BA. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J Biol Chem. 1988;263(14):6884–92. [PubMed] [Google Scholar]
- 38.Veronese FM, Caliceti P, Schiavon O, Sergi M. Polyethylene glycol-superoxide dismutase, a conjugate in search of exploitation. Adv Drug Deliv Rev. 2002;54(4):587–606. doi: 10.1016/s0169-409x(02)00029-7. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida K, Burton GF, McKinney JS, Young H, Ellis EF. Brain and tissue distribution of polyethylene glycol-conjugated superoxide dismutase in rats. Stroke. 1992;23(6):865–9. doi: 10.1161/01.str.23.6.865. [DOI] [PubMed] [Google Scholar]
- 40.Medina C, Santos-Martinez MJ, Radomski A, Corrigan OI, Radomski MW. Nanoparticles: pharmacological and toxicological significance. Br J Pharmacol. 2007;150(5):552–8. doi: 10.1038/sj.bjp.0707130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganong WF. Circumventricular organs: definition and role in the regulation of endocrine and autonomic function. Clin Exp Pharmacol Physiol. 2000;27:422–7. doi: 10.1046/j.1440-1681.2000.03259.x. [DOI] [PubMed] [Google Scholar]
- 42.Mangiapane ML, Simpson JB. Subfornical organ: forebrain site of pressor and dipsogenic action of angiotensin II. Am J Physiol. 1980;239(5):R382–R389. doi: 10.1152/ajpregu.1980.239.5.R382. [DOI] [PubMed] [Google Scholar]