Abstract
AIM: To assess the effects of neoadjuvant chemoradiotherapy (CRT) on the presence of extracapsular lymph node involvement (LNI) and its prognostic value in patients with resected esophageal cancer.
METHODS: Two hundred and ninety-eight patients with advanced esophageal cancer underwent esophagectomy between 1997 and 2006. One hundred and ninety patients (63.8%) were treated with neoadjuvant CRT prior to resection. A total of 986 metastatic LNs were examined. Survival of the patients was analyzed according to intra- and extra-capsular LNI.
RESULTS: Five-year survival rate was 22.5% for the entire patient population. Patients with extracapsular LNI had a 5-year survival rate of 16.7%, which was comparable to the 15.8% in patients with infiltrated nodes of the celiac trunk (pM1lymph). In contrast to patients treated with surgery alone, neoadjuvant therapy resulted in significantly (P = 0.001) more patients with pN0/M0 (51.6% vs 25.0%). In 17.6% of the patients with surgery alone vs 16.8% with neoadjuvant CRT, extracapsular LNI was detected. Neoadjuvant therapy does not reduce the occurrence of extracapsular LNI.
CONCLUSION: Extracapsular LNI is an independent negative prognostic factor not influenced by neoadjuvant CRT. In a revised staging system for esophageal cancer, extracapsular LNI should be considered.
Keywords: Esophageal cancer, Neoadjuvant therapy, Chemotherapy, Radiotherapy, Adenocarcinoma, Squamous cell carcinoma, Lymph node metastasis, Extracapsular lymph node involvement, Prognosis
INTRODUCTION
Surgery remains the treatment of choice for most localized esophageal cancers. However, despite complete tumor resection and extensive lymphadenectomy, systemic and local recurrence is common, and the 5-year survival rate is 15%-39%[1]. There is increasing evidence to include combined neoadjuvant chemoradiotherapy (CRT) as an alternative to surgical resection alone, to improve survival for locoregional esophageal cancer. The aim of combining neoadjuvant chemotherapy and radiotherapy is to reduce the tumor size and maximize local control. A meta-analysis from Gebski et al[2] that included data from 10 neoadjuvant CRT trials of 1209 patients has shown an absolute overall survival benefit of 13% when compared with surgery alone. This result prompted some investigators to consider neoadjuvant treatment as the standard of care in esophageal cancer[3].
Regarding prognostic factors for esophageal cancer treated with neoadjuvant CRT, histomorphological tumor regression and the extent of lymphatic dissemination are among the most important predictors for survival in gastrointestinal malignancies[4,5]. More recently, attention has been paid to the prognostic value of extracapsular lymph node involvement (LNI) in patients with gastrointestinal malignancies. Extracapsular LNI is the extension of cancer cells through the nodal capsule into the perinodal fatty tissue: a common phenomenon in gastrointestinal cancer patients[6]. Its presence identifies a subgroup of patients with a significantly worse long-term survival.
However, the effects of neoadjuvant therapy on the presence of extracapsular LNI and its prognostic relevance are unclear because to date, most studies have not included patients undergoing such therapy.
The aim of this study was to assess the prevalence as well as the prognostic impact of extracapsular LNI in patients with resected esophageal cancer who were treated with neoadjuvant CRT.
MATERIALS AND METHODS
Patients
Four hundred and thirty-six patients with esophageal cancer were treated in the Department of Surgery between January 1, 1997 and December 31, 2006. Two hundred and ninety-eight patients had locally advanced tumors with cT3-4. The histological distribution was 155 (52.0%) squamous cell carcinoma and 143 (48%) adenocarcinoma. One hundred and ninety of these patients were treated with neoadjuvant CRT prior to resection. The reasons for primary surgical resection were exclusion criteria for chemoradiation, such as age, comorbidity, and lack of patient consent. Relevant patient characteristics are summarized in Table 1. Written informed consent was obtained from all patients in this study.
Table 1.
Demographic data of 298 patients with advanced esophageal cancer n (%)
| Variables | Surgery alone | NeoadjuvantCRT | Significance |
| Patients | 108 | 190 | |
| Sex | NS | ||
| Male | 86 (80.0) | 151 (79.4) | |
| Female | 22 (20.0) | 39 (20.6) | |
| Age (yr) | 63.6 | 60.1 | P < 0.01 |
| < 50 | 11 (10.2) | 38 (20.0) | |
| 50-70 | 65 (60.2) | 124 (65.3) | |
| > 70 | 32 (29.6) | 18 (14.7) | |
| Histological subtype | 0.871 | ||
| Squamous cell carcinoma | 55 (51.0) | 100 (52.6) | |
| Adenocarcinoma | 53 (49.0) | 90 (47.4) | |
| No. of resected LNs (median, range) | 30 (3-74) | 27 (4-55) | P = 0.007 |
| pN category | P < 0.001 | ||
| pN0 | 27 (25.0) | 98 (51.6) | |
| pN1 | 81 (75.0) | 92 (48.5) | |
| LN ratio | P < 0.001 | ||
| pN0 | 27 (25.0) | 98 (51.6) | |
| LN ratio < 0.2 | 52 (48.1) | 69 (36.3) | |
| LN ratio ≥ 0.2 | 29 (26.9) | 23 (12.1) | |
| pM1 organ | 11 (10.1) | 8 (4.2) | NS |
| R category | NS | ||
| R0 | 98 (92.6) | 179 (94.2) | |
| R1/R2 | 10 (7.4) | 11 (5.8) | |
| Response after neoadjuvant therapy | |||
| Minor response | - | 112 (59.0) | |
| Major response | - | 78 (41.0) |
LN: Lymph node; CRT: Chemoradiotherapy; NS: Not significant.
Staging
TNM staging was performed according to the criteria of the International Union Against Cancer[7]. Clinical staging was based on results from barium swallow examination, endoscopic ultrasound, and computed tomography (CT) of the chest and abdomen (4-mm sections). Endoscopy and endoscopic ultrasound were performed by two experienced examiners for all patients.
Surgical resection and CRT regimen
Surgical treatment of choice was subtotal en bloc esophagectomy using a right transthoracic approach including two-field lymphadenectomy of mediastinal and abdominal LNs. The specimens were removed en bloc including the LNs. To ensure primary tumor integrity, the LNs were dissected partially in the operating theater and partially by pathologists according to a standardized protocol. The examined LNs were documented according to the sixth edition of the TNM classification[7]. The median number of examined LNs was 28 (4-74).
Standard reconstruction for patients receiving transthoracic esophagectomy was done by stomach interposition with high intrathoracic esophagogastrostomy[8,9].
One hundred and ninety patients received preoperative CRT according to a standardized protocol, which is described in detail elsewhere[5]. Locally advanced tumors (cT3-4) were included unless documented systemic metastases or bronchoscopically proven invasion of the tracheobronchial tree was present. Patients with cT2 tumors were offered this treatment protocol when CT showed a tumor mass compatible with a T3 category, while endoscopic ultrasound showed complete invasion of the muscularis propria without clear invasion of the adventitia (so-called near T3 categories). Cisplatin (20 mg/m2 per day) was administered as a short-term infusion on days 1-5 and 5-fluorouracil (1000 mg/m2 per day) as a continuous infusion over 24 h on days 1-5. Radiotherapy was administered by linear accelerators with 10-15-MV photons. Radiotherapy was simulated to encompass the tumor volume with 5-cm cephalo-caudal margins and 2-cm radial margins, and treatment ports were designed to include enlarged regional nodes based on CT evaluation and endoscopic ultrasound. Radiation was delivered in daily fractions of 1.8 Gy with a total dose of 36 Gy using a multiple field technique. Surgical resection was performed 4-5 wk following completion of CRT, after clinical restaging. If there was evidence for tumor progression, patients underwent definitive CRT without surgery.
Histopathology
Histopathological examination of all resected specimens consisted of thorough evaluations of tumor stage, residual tumor (R) category, grading, and number of examined and involved LNs. The specimens were fixed in 5% formaldehyde and embedded in paraffin. The LNs were counted and a series of sections from each node was selected and stained with hematoxylin and eosin, as well as periodic acid-Schiff. All dissected LNs were microscopically analyzed for metastatic disease.
Extracapsular LNI was defined as metastatic cancer extending through the nodal capsule into the perinodal fatty tissue. Examination of the LNs was performed by two experienced pathologists (S.E.B. and U.D.). Deposits of metastatic cancer cells without a recognizable LN were considered as extracapsular LNI, unless these deposits were associated with perineural and/or had vessel involvement. In cases of desmoplastic reactions resulting in difficulty in identifying the preexisting LN capsule, an imaginary line representing the original capsule was drawn to facilitate this interpretation (see also Lagarde et al[10]).
The ratio of the number of involved to examined regional LNs was termed the lymph node ratio. According to the sixth edition of the TNM classification for tumors of the lower esophagus, metastasis in the celiac lymph node group (LNG 9) is classified as M1a, and in other non-regional locations, as M1b[7]. After neoadjuvant therapy, the pathological assessment is difficult because of potential tumor regression. For this reason, such classification is identified with the prefix “y” to indicate that it does not have the same reliability as the pTNM classification after surgery alone[11].
Statistical analysis
Beginning in 1997, data were collected prospectively according to a standardized protocol. Analysis of the data was performed retrospectively. The median, with the lower quartile and upper quartile, was used for descriptive statistics. χ2 statistics were calculated for factor frequencies, with a significance level of P < 0.05.
The median follow-up time for the study patients was calculated using the time between surgical procedure and the end of follow-up for censored data; for deceased patients until December 31, 2007[4]. The median follow-up time was 4.1 years (range: 0.5-10.5 years). All living patients had follow-up of > 1 year.
Kaplan-Meier plots were used to describe survival distribution[12]. The log-rank test was used to evaluate survival differences[13]. For multiple comparisons, the Holm-Sidak method was used. In addition, 95% CI for the different survival curves was calculated. Postoperative mortality was included in the calculation of prognosis. The 30-d postoperative mortality was 3.4%. The multivariate analysis of survival used Cox regression analysis to identify independent prognostic variables. The level of significance was set at P < 0.05.
All statistical analyses were performed using SPSS for Windows version 15.0. For graphic presentation of the results, Sigma-Plot version 8.0 was used.
RESULTS
Two hundred and ninety-eight patients with locally advanced esophageal cancer were included in this study. The median age of the patients was 61.0 years (range: 22-82 years). Neoadjuvant CRT was administered to 190 of the 298 patients (63.8%). The demographics and the established prognostic factors for the patients are shown in Tables 1 and 2.
Table 2.
Univariate survival analysis of 298 patients with advanced esophageal cancer
| Variables | P |
Surgery alone |
Neoadjuvant CRT |
||
| n | 5-yr survival (%) | n | 5-yr survival (%) | ||
| Patients | NS | 108 | 20.4 | 190 | 25.6 |
| Sex | |||||
| Male | NS | 86 | 22.0 | 151 | 24.4 |
| Female | NS | 22 | 13.8 | 39 | 32.6 |
| Age (yr) | |||||
| < 50 | NS | 11 | 58.3 | 38 | 33.7 |
| 50-70 | 0.015 | 65 | 15.8 | 124 | 24.7 |
| > 70 | NS | 32 | 15.1 | 18 | 0.0 |
| Histological subtype | |||||
| Squamous cell carcinoma | NS | 55 | 31.3 | 100 | 19.8 |
| Adenocarcinoma | 0.030 | 53 | 16.9 | 90 | 35.4 |
| pN category | |||||
| pN0 | NS | 27 | 36.5 | 98 | 41.6 |
| pN1 | NS | 81 | 15.4 | 92 | 12.3 |
| LN ratio | |||||
| pN0 | NS | 27 | 36.5 | 98 | 41.6 |
| LN ratio < 0.2 | NS | 52 | 23.7 | 69 | 10.3 |
| LN ratio ≥ 0.2 | NS | 29 | 0.0 | 23 | 0.0 |
| pM1 organ | NS | 11 | 0.0 | 8 | 0.0 |
| R category | |||||
| R0 | NS | 98 | 22.1 | 179 | 26.4 |
| R1/R2 | NS | 10 | 0.0 | 11 | 0.0 |
| Response after neoadjuvant therapy | |||||
| Minor response | - | 112 | 11.4 | ||
| Major response | - | 78 | 42.4 | ||
A total of 8376 LNs (median 28; range: 4-74) were resected. All positive LNs (n = 986) were reexamined for the presence of extracapsular LNI. Tumor growth beyond the LN capsule was detected in a total of 351 lymph nodes.
Age, sex and differentiation grade were comparable between the patients with and without extracapsular LNI. The number of LNs with extracapsular involvement was significantly (P < 0.001) correlated with the number of positive nodes (data not shown). Extracapsular LNI was seen more often when the numbers of resected and identified nodes, the numbers of positive nodes, and the lymph node ratios were higher.
To analyze the effect of neoadjuvant CRT on the prevalence of extracapsular LNI, patients with and without neoadjuvant therapy were compared. 190 patients received neoadjuvant therapy, and 108 underwent surgery alone (Tables 2 and 3).
Table 3.
Influence of neoadjuvant CRT on extent of LN metastases and extracapsular LNI n (%)
| T3/4 Category only(n = 298) |
Surgery alone |
Neoadjuvant CRT |
Surgery vs neoadjuvant CRT |
|||
| Total | pN1 caps (+) | Total | pN1 caps (+) | Total | pN1 caps (+) | |
| Total | 108 (100.0) | 45 (41.7) | 190 (100.0) | 43 (22.6) | P < 0.001 | |
| pN0M0 | 27 (25.0) | - | 98 (51.6) | - | P < 0.001 | |
| pN1M0 caps (-) | 28 (27.8) | - | 38 (20.0) | - | NS | |
| pN1M0 caps (+) | 19 (17.6) | 19 (100) | 32 (16.8) | 32 (100.0) | NS | |
| pM1 lymph | 23 (21.6) | 18 (78.3) | 14 (7.4) | 8 (57.1) | P < 0.001 | NS |
| pM1 organ | 11 (10.1) | 8 (72.7) | 8 (4.2) | 3 (37.5) | NS | NS |
LNI: Lymph node involvement; Caps (-): Without extracapsular LNI; Caps (+): With extracapsular LNI.
The 5-year survival rate was 22.5% for the entire patient population. LN-negative patients had a 5-year survival rate of 34%. Intracapsular LNI decreased the 5-year survival rate to 20%. Detection of extracapsular LNI resulted in a 5-year survival rate of 7% (P < 0.001). The resection plane (R0 resection vs R1/2 resection) also proved to be an independent prognostic parameter (P < 0.001) in univariate analysis.
In the group with the neoadjuvant regimen, we observed a decrease in distant metastases (pM1 lymph + pM1 organ). Neoadjuvant therapy also resulted in a significant (P = 0.001) increase of patients with pN0/M0 (51.6%), compared with surgery alone (25%) (Table 3). After neoadjuvant therapy, there was also a survival benefit for patients with negative LNs. However, this was not significant compared to surgery alone (Table 2).
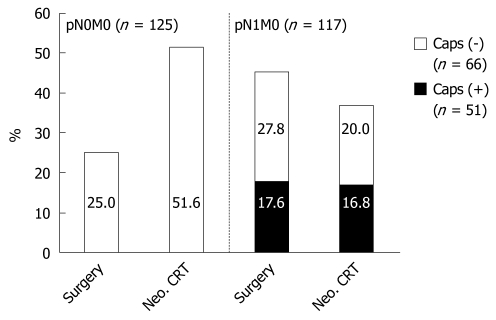
Neoadjuvant therapy did not reduce the occurrence of extracapsular LNI. In 19/108 patients (17.6%) with surgery alone vs 32/190 patients (16.8%) with neoadjuvant CRT, extracapsular LNI was detected (Table 3, Figure 1).
Figure 1.
Extent of lymph node (LN) metastases and extracapsular LN involvement (LNI), comparing surgery alone with neoadjuvant chemoradiotherapy (CRT) and surgery. pN0M0: Neoadjuvant CRT resulted in an increase of patients with pN0/M0 compared to surgery alone (P = 0.001); pN1M0: Neoadjuvant CRT did not reduce extracapsular LNI. Caps (-): Without extracapsular LNI; Caps (+): With extracapsular LNI; Neo.: Neoadjuvant.
All LNs with extracapsular involvement were within the radiation field. We did not detect a difference between patients treated with neoadjuvant CRT and those who were resected primarily.
Univariate and multivariate analysis (Table 4) revealed that pN, pM category, extracapsular LNI, and radicality of the resection plane were all significant prognostic parameters for esophageal cancer.
Table 4.
Multivariate analysis of prognostic factors for patients with neoadjuvant CRT
| Variable | HR | 95% CI | P |
| Age | 1.00 | 0.99-1.03 | 0.376 |
| Sex | 0.353 | ||
| Male | 1 (ref) | ||
| Female | 0.77 | 0.44-1.34 | |
| pN/pM category | 0.000 | ||
| pN0/pM0 | 1 (ref) | ||
| pN1 (-)/pM0 | 1.89 | 1.10-3.22 | 0.021 |
| pN1 (+)/pM0 | 2.70 | 1.57-4.64 | 0.000 |
| pM1 lymph | 2.92 | 1.49-5.74 | 0.002 |
| pM1 organ | 3.92 | 1.76-8.72 | 0.001 |
| Histology | 0.145 | ||
| Squamous cell carcinoma | 1 (ref) | ||
| Adenocarcinoma | 0.73 | 0.48-1.11 | |
| Response | 0.010 | ||
| Minor response | 1 (ref) | ||
| Major response | 0.56 | 0.36-0.87 |
pN1 (-): pN1 category without extracapsular LNI; pN1 (+): pN1 category with extracapsular LNI.
DISCUSSION
The presence and extent of lymphatic dissemination are among the most important predictors for survival in gastrointestinal malignancies[14,15]. More recently, attention has been paid to the presence of extracapsular LNI, which identifies a subgroup of patients with significantly worse long-term survival[6,10,16,17]. For breast cancer, extracapsular spread is such an important factor that it has been appended to the TNM classification as a specific subcategory[18].
In a recently published systematic review on the significance of extracapsular LNI in gastrointestinal malignancies, Wind and coauthors have identified seven papers that discuss the impact of extracapsular LNI in esophageal cancer[6,10,17,19-23]. In general, these studies applied variable inclusion and exclusion criteria with respect to the type and stage of tumors and the use of (neo)adjuvant therapies. Only three studies from Tachikawa et al[23], Lerut et al[17], and Lagarde et al[10] have provided some detailed information on the prognostic value of the number of LNs with extracapsular involvement.
Due to the heterogeneity of the design of the existing studies, the effect of preoperative CRT on the presence and extent of extracapsular LNI is unclear.
In the present study, we demonstrated that extracapsular LNI was an independent prognostic parameter for esophageal cancer. Lerut et al[17], and Lagarde et al[10] have analyzed relatively uniform groups of patients with adenocarcinoma of the esophagus and have found a similar result. Also, for patients with squamous cell carcinoma, Tachikawa et al[23] have concluded that prognosis is significantly worse when extracapsular disease is confirmed. However, Tachikawa et al[23] analyzed a non-homogeneous cohort of patients with squamous cell carcinoma, treated with surgery and different chemotherapeutic regimens or no adjuvant therapy.
The present study comprised a consecutive series of 298 patients with adenocarcinoma and squamous cell carcinoma of the esophagus. There was a significant correlation between the number of positive nodes resected and the number with extracapsular involvement. The greater the number of positive LNs, the higher the number with extracapsular involvement. These results underline the findings of Lerut et al[17] and Lagarde et al[10], that extracapsular LNI is an independent prognostic parameter for esophageal cancer.
To explain the aggressiveness of tumors with extracapsular LNI, investigators have discussed the ability of cancer cells not only to spread into an LN, but also to invade through the node capsule in an immunologically hostile environment[24,25]. The prognostic relevance of this extracapsular spread is heightened by the fact that patients with only one positive node have significantly worse survival rates if tumor involvement extends beyond the LN capsule[10]. As a result of this, several authors have proposed designing a new or revised staging system for esophageal cancer that includes the presence and extent of extracapsular LNI as an additional parameter[15,26]. For breast cancer, the occurrence of extracapsular LNI is such an important factor that it has been added to the TNM classification as a specified subcategory[18].
There is increasing evidence to support neoadjuvant CRT for treatment of esophageal cancer. A meta-analysis published by Gebski et al[2] has shown significant survival benefits from preoperative CRT for patients with esophageal cancer. However, until now, the effects of such a neoadjuvant regimen on the presence of extracapsular LNI in esophageal cancer have not been analyzed.
The present study is believed to be the first, in which a relatively uniform group of patients with esophageal carcinoma was evaluated to determine the extent to which neoadjuvant CRT influences the presence of extracapsular LNI. We compared the data from patients receiving neoadjuvant therapy with those from patients treated with primary esophagectomy. This comparison showed some selection bias. However, although patients who were excluded from neoadjuvant CRT might have been older or have had greater comorbidity, the preoperative staging for both groups was the same.
In accordance with earlier reports from our group, neoadjuvant therapy resulted in a significant increase of patients with pN0/M0 compared to those having surgery alone[5]. Mariette et al[27] also recently have reported a significant decrease of LN metastases after neoadjuvant CRT. Approximately 13% of patients develop tumor progression under neoadjuvant CRT[5]. To ease comparison between patients treated with surgery alone vs those receiving preoperative CRT, patients with progressive disease (i.e. pM1 lymph + pM1 organ) were excluded from our calculations of extracapsular LNI.
Regarding extracapsular LNI, neoadjuvant therapy did not reduce its occurrence. Extracapsular LNI was detected in 19 patients (17.6%) with surgery alone vs 32 (16.8%) with neoadjuvant CRT.
This observation demonstrates that extracapsular LNI reflects highly aggressive biological behavior of the primary tumor, which is not influenced by neoadjuvant CRT in a multimodal treatment setting. An issue that remains unresolved is the optimal radiation dosage for neoadjuvant CRT. In a review by Geh et al[28], a dose-response relationship between increasing radiotherapy, 5-FU and cisplatin doses, and pathological complete response (pCR) was reported. In contrast, increasing radiotherapy treatment time and increasing median age reduced the probability of pCR. However, low dosage of radiation reduced the risk of postoperative morbidity and mortality. Our study protocol, with 36 Gy, 5-FU and cisplatin is in the median range of published protocols[2,28]. The advantage of our current study is that all patients were treated with the same protocol regardless of histological tumor type, so that varying therapies could not affect the results.
Due to lack of evidence, the accuracy of the 6th UICC/TNM classification is suboptimal, especially when the extent of lymphadenectomy and the effects of neoadjuvant therapy are ignored[7,29]. There is no doubt that the ratio of positive LNs and the extent of extracapsular LNI have a significant impact on prognosis[10,17,27,30]. Nothing is known about the effects of neoadjuvant therapy on the extent and prognostic role of extracapsular LNI. By clarifying this issue, whether neoadjuvant treatment influences the spread of extracapsular LNI, the request for a revised staging system for esophageal cancer must be supported[29,31].
Extracapsular LNI identifies a subgroup of esophageal cancer patients with significantly worse long-term survival rates. The present study provides evidence to suggest that neoadjuvant CRT does not influence the occurrence of extracapsular LNI. This hypothesis has to be proven in a real prospective study.
Extracapsular LNI is an independent negative prognostic factor that reflects particularly aggressive biological behavior of tumors and has valuable prognostic potential. Therefore, in a revised staging system for esophageal cancer, extracapsular LNI should be taken into account as a negative prognostic factor.
COMMENTS
Background
The presence and extent of lymphatic dissemination are among the most important predictors for survival in gastrointestinal malignancies. Little is known about the effects of neoadjuvant chemoradiotherapy (CRT) on the presence of extracapsular lymph node involvement (LNI) and its prognostic value in patients with resected esophageal cancer. To clarify this issue might help to set up novel treatment strategies and lead to more individualized therapeutic strategies with better survival.
Research frontiers
In the present study, the authors describe, perhaps for the first time, the impact of neoadjuvant CRT on the presence of LNs with extracapsular tumor spread in esophageal cancer. The study demonstrates that extracapsular LNI is not influenced by neoadjuvant CRT.
Innovations and breakthroughs
The present study is believed to be the first in which a relatively uniform group of patients with esophageal cancer was evaluated to determine the extent to which neoadjuvant CRT influences the presence of extracapsular LNI.
Applications
Detection of extracapsular LNI might identify a subgroup of esophageal cancer patients with significantly worse long-term survival rates, who do not benefit from neoadjuvant CRT.
Terminology
Extracapsular LNI is defined as extension of cancer cells through the nodal capsule into the perinodal fatty tissue.
Peer review
This was an interesting study in a reasonably large population, with a novel aspect of the importance of extracapsular LNI in patients receiving neoadjuvant CRT for esophageal cancer.
Footnotes
Supported by Department of General, Visceral and Cancer Surgery, Center for Integrated Oncology (CIO) Köln Bonn and the Hoff`sche Stiftung
Peer reviewer: Dr. Paul M Schneider, MD, Professor of Surgery, Department of Surgery, University Hospital Zurich, Raemistrasse 100, Zurich, 8091, Switzerland
S- Editor Wang YR L- Editor Kerr C E- Editor Zheng XM
References
- 1.Wilson M, Rosato EL, Chojnacki KA, Chervoneva I, Kairys JC, Cohn HE, Rosato FE Sr, Berger AC. Prognostic significance of lymph node metastases and ratio in esophageal cancer. J Surg Res. 2008;146:11–15. doi: 10.1016/j.jss.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8:226–234. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 4.Bollschweiler E. Benefits and limitations of Kaplan-Meier calculations of survival chance in cancer surgery. Langenbecks Arch Surg. 2003;388:239–244. doi: 10.1007/s00423-003-0410-6. [DOI] [PubMed] [Google Scholar]
- 5.Schneider PM, Baldus SE, Metzger R, Kocher M, Bongartz R, Bollschweiler E, Schaefer H, Thiele J, Dienes HP, Mueller RP, et al. Histomorphologic tumor regression and lymph node metastases determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer: implications for response classification. Ann Surg. 2005;242:684–692. doi: 10.1097/01.sla.0000186170.38348.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wind J, Lagarde SM, Ten Kate FJ, Ubbink DT, Bemelman WA, van Lanschot JJ. A systematic review on the significance of extracapsular lymph node involvement in gastrointestinal malignancies. Eur J Surg Oncol. 2007;33:401–408. doi: 10.1016/j.ejso.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Sobin LH, Wittekind C. TNM classification of malignant tumors. 6th ed. New Jersey: John Wiley & Sons; 2002. [Google Scholar]
- 8.Hölscher AH, Schneider PM, Gutschow C, Schröder W. Laparoscopic ischemic conditioning of the stomach for esophageal replacement. Ann Surg. 2007;245:241–246. doi: 10.1097/01.sla.0000245847.40779.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hölscher AH, Schröder W, Bollschweiler E, Beckurts KT, Schneider PM. [How safe is high intrathoracic esophagogastrostomy?] Chirurg. 2003;74:726–733. doi: 10.1007/s00104-003-0649-z. [DOI] [PubMed] [Google Scholar]
- 10.Lagarde SM, ten Kate FJ, de Boer DJ, Busch OR, Obertop H, van Lanschot JJ. Extracapsular lymph node involvement in node-positive patients with adenocarcinoma of the distal esophagus or gastroesophageal junction. Am J Surg Pathol. 2006;30:171–176. doi: 10.1097/01.pas.0000189182.92815.12. [DOI] [PubMed] [Google Scholar]
- 11.Brierley JD, Greene FL, Sobin LH, Wittekind C. The "y" symbol: an important classification tool for neoadjuvant cancer treatment. Cancer. 2006;106:2526–2527. doi: 10.1002/cncr.21887. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Asoc. 1958;53:457–481. [Google Scholar]
- 13.Pocock SJ, Clayton TC, Altman DG. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet. 2002;359:1686–1689. doi: 10.1016/S0140-6736(02)08594-X. [DOI] [PubMed] [Google Scholar]
- 14.Altorki N, Skinner D. Should en bloc esophagectomy be the standard of care for esophageal carcinoma? Ann Surg. 2001;234:581–587. doi: 10.1097/00000658-200111000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice TW, Blackstone EH, Rybicki LA, Adelstein DJ, Murthy SC, DeCamp MM, Goldblum JR. Refining esophageal cancer staging. J Thorac Cardiovasc Surg. 2003;125:1103–1113. doi: 10.1067/mtc.2003.170. [DOI] [PubMed] [Google Scholar]
- 16.Fleischmann A, Thalmann GN, Markwalder R, Studer UE. Prognostic implications of extracapsular extension of pelvic lymph node metastases in urothelial carcinoma of the bladder. Am J Surg Pathol. 2005;29:89–95. doi: 10.1097/01.pas.0000147396.08853.26. [DOI] [PubMed] [Google Scholar]
- 17.Lerut T, Coosemans W, Decker G, De Leyn P, Ectors N, Fieuws S, Moons J, Nafteux P, Van Raemdonck D. Extracapsular lymph node involvement is a negative prognostic factor in T3 adenocarcinoma of the distal esophagus and gastroesophageal junction. J Thorac Cardiovasc Surg. 2003;126:1121–1128. doi: 10.1016/s0022-5223(03)00941-3. [DOI] [PubMed] [Google Scholar]
- 18.Hermanek P, Leale HRS. TNM classification of malignant tumors. Berlin: Springer; 1997. [Google Scholar]
- 19.D'Journo XB, Doddoli C, Michelet P, Loundou A, Trousse D, Giudicelli R, Fuentes PA, Thomas PA. Transthoracic esophagectomy for adenocarcinoma of the oesophagus: standard versus extended two-field mediastinal lymphadenectomy? Eur J Cardiothorac Surg. 2005;27:697–704. doi: 10.1016/j.ejcts.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Gatzinsky P, Berglin E, Dernevik L, Larsson I, William-Olsson G. Resectional operations and long-term results in carcinoma of the esophagus. J Thorac Cardiovasc Surg. 1985;89:71–76. [PubMed] [Google Scholar]
- 21.Nakano S, Baba M, Shimada M, Shirao K, Noguchi Y, Kusano C, Natsugoe S, Yoshinaka H, Fukumoto T, Aikou T. How the lymph node metastases toward cervico-upper mediastinal region affect the outcome of patients with carcinoma of the thoracic esophagus. Jpn J Clin Oncol. 1999;29:248–251. doi: 10.1093/jjco/29.5.248. [DOI] [PubMed] [Google Scholar]
- 22.Paraf F, Fléjou JF, Pignon JP, Fékété F, Potet F. Surgical pathology of adenocarcinoma arising in Barrett's esophagus. Analysis of 67 cases. Am J Surg Pathol. 1995;19:183–191. doi: 10.1097/00000478-199502000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Tachikawa D, Inada S, Kotoh T, Futami K, Arima S, Iwashita A. An evaluation of malignancy and prognostic factors based on mode of lymph node metastasis in esophageal carcinoma. Surg Today. 1999;29:1131–1135. doi: 10.1007/BF02482260. [DOI] [PubMed] [Google Scholar]
- 24.Lyons AJ, Bateman AC, Spedding A, Primrose JN, Mandel U. Oncofetal fibronectin and oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 2001;39:471–477. doi: 10.1054/bjom.2001.0702. [DOI] [PubMed] [Google Scholar]
- 25.Stitzenberg KB, Meyer AA, Stern SL, Cance WG, Calvo BF, Klauber-DeMore N, Kim HJ, Sansbury L, Ollila DW. Extracapsular extension of the sentinel lymph node metastasis: a predictor of nonsentinel node tumor burden. Ann Surg. 2003;237:607–612; discussion 612-613. doi: 10.1097/01.SLA.0000064361.12265.9A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tachibana M, Kinugasa S, Dhar DK, Yoshimura H, Shibakita M, Ohno S, Ueda S, Kubota H, Kohno H, Nagasue N. Dukes' classification as a useful staging system in resectable squamous cell carcinoma of the esophagus. Virchows Arch. 2001;438:350–356. doi: 10.1007/s004280000370. [DOI] [PubMed] [Google Scholar]
- 27.Mariette C, Piessen G, Briez N, Triboulet JP. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg. 2008;247:365–371. doi: 10.1097/SLA.0b013e31815aaadf. [DOI] [PubMed] [Google Scholar]
- 28.Geh JI, Bond SJ, Bentzen SM, Glynne-Jones R. Systematic overview of preoperative (neoadjuvant) chemoradiotherapy trials in oesophageal cancer: evidence of a radiation and chemotherapy dose response. Radiother Oncol. 2006;78:236–244. doi: 10.1016/j.radonc.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Rizk NP, Venkatraman E, Bains MS, Park B, Flores R, Tang L, Ilson DH, Minsky BD, Rusch VW. American Joint Committee on Cancer staging system does not accurately predict survival in patients receiving multimodality therapy for esophageal adenocarcinoma. J Clin Oncol. 2007;25:507–512. doi: 10.1200/JCO.2006.08.0101. [DOI] [PubMed] [Google Scholar]
- 30.Greenstein AJ, Litle VR, Swanson SJ, Divino CM, Packer S, Wisnivesky JP. Prognostic significance of the number of lymph node metastases in esophageal cancer. J Am Coll Surg. 2008;206:239–246. doi: 10.1016/j.jamcollsurg.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Bogoevski D, Onken F, Koenig A, Kaifi JT, Schurr P, Sauter G, Izbicki JR, Yekebas EF. Is it time for a new TNM classification in esophageal carcinoma? Ann Surg. 2008;247:633–641. doi: 10.1097/SLA.0b013e3181656d07. [DOI] [PubMed] [Google Scholar]