Abstract
AIM: To analyze the value of computed tomography (CT) volume measurements for evaluation of the survival rate of unresectable hepatocellular carcinoma (HCC) patients after transcatheter arterial chemoembolization (TACE).
METHODS: One hundred and sixty-six unresectable HCC patients after TACE were involved in this retrospective study. Hepatic CT scan was performed for all patients before and 4 wk to 2 mo after TACE to define the morphologic features of HCC including its largest diameter, volume, product of the greatest axial dimension, tumor to liver volume ratio (TTLVR), and tumor shrinkage ratio. Clinical variables used in the study included gender, age, pattern of tumor growth, number of lesions, Child-Pugh classification of liver function, repeated TACE times, pre- or post-treatment α-fetoprotein (AFP) level, portal vein cancerous thrombus, tumor metastasis, degree of lipiodol retention within the tumor, and percutaneous ethanol injection. The correlation between survival time and clinical variables of patients or lesions was analyzed by combining morphologic features with the corresponding clinical and general data as input. A Cox proportional hazard model was used to analyze prognostic factors. The Kaplan-Meier method was used to calculate the cumulative survival time. Influence of the parameters on prognosis was analyzed by the log-rank test.
RESULTS: The overall 6, 12, 24, 36 and 60 mo cumulative survival rates were 78.92%, 49.85%, 23.82%, 15.60% and 8.92%, respectively. The median survival time was 12 mo. Univariate and multivariate analysis showed that only 4 parameters were the independent prognostic factors including TTLVR (χ2 = 14.328, P < 0.001), portal vein cancerous thrombus (χ2 = 5.643, P = 0.018), repeated TACE times (χ2 = 8.746, P = 0.003), and post-treatment serum AFP level (χ2 = 5.416, P = 0.020). When the TTLVR value was less than 70%, the survival time was inversely correlated with the TTLVR value.
CONCLUSION: CT volume measurement technique can predict the prognosis of unresectable HCC patients after TACE.
Keywords: Hepatocellular carcinoma, Chemoembolization, Tumor volume, Computed tomography, Prognosis
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignancies in Africa and Asia, especially in China and Japan. The tumor is often already large in size when it is diagnosed, and the prognosis of HCC patients, especially those with unresectable lesions tends to be poor. The median survival time of untreated HCC patients is less than 6 mo from its diagnosis[1,2]. The treatment of HCC is very challenging. The outcome of resection and liver transplantation is good. However, they are only suitable for less than 15% of patients. Although many treatment modalities for HCC are available, transcatheter arterial chemoembolization (TACE) has been shown to be the most effective procedure for unresectable HCC[3]. Patients receiving TACE often demonstrate a remarkable embolization effect and consequently have a higher cumulative survival rate[4].
Advances in computed tomography (CT) technology can quantify volumetric tumor burden due to its volumetric data acquisition and image processing[5-8]. Compared with uni- and bi-dimensional measurement techniques, volumetric quantification can result in a different assessment result[9-11].
The largest tumor diameter, product of the greatest axial dimension, and several volume measurements show a congruence with the actual volume when the measured lesion is a sphere, an ellipsoid, or a cube. However, these measurements may have significant differences if the tumor has an irregular shape[12,13]. Three-dimensional (3D) measurements have advantages for the assessment of irregular masses, because they compensate for actual tumor shape rather than assume it to be a sphere, an ellipsoid, or a cube[6,14]. Spiral CT theoretically offers advantages in terms of accuracy and reproducibility over 3D measurements[14,15]. In follow-up studies, 3D measurements enable the evaluation of changes in the diameter of a neoplasm, and offer a more complete evaluation of changes in tumor size over time.
It has been reported that TACE in combination with cytostatic drugs (doxorubicin, cisplatin, mitomycin C) for treatment of HCC patients can reduce vital tumor tissue and prolong survival time[1,3,4,16]. When an indication for palliative TACE is established, strict guidelines based on the condition of patients and tumor stage must be followed. In this study, we retrospectively reviewed the morphologic CT data about lesions in 166 unresectable HCC patients after TACE. The correlation between morphological variables and survival rate was also intensively investigated.
MATERIALS AND METHODS
Clinical data and variables
Segmental lipiodol-TACE was performed for 166 HCC patients (143 men, 23 women) at the age of 51 ± 12 years (range: 21-78 years) in February 1999 to August 2006. HCC was diagnosed based either on percutaneous liver needle biopsy (112 patients) or on imaging and clinical examination (54 patients). This retrospective study was approved by our institution’s research ethics board. Informed consent was obtained from all patients. Cirrhosis was classified according to the Child-Pugh system. Child-Pugh grades A and B were found in 148 and 18 patients, respectively.
The morphologic features of HCC, including its largest diameter, product of the greatest axial dimensions, tumor volume (TV), tumor to liver volume ratio (TTLVR) and tumor shrinkage ratio (TSR) post-treatment, were evaluated for their correlation with treatment outcome. In addition, other study variables influencing treatment outcome were analyzed, including gender, age, pattern of tumor growth, number of lesions, Child-Pugh classification of liver function, repeated TACE times, pre-treatment or post-treatment serum α-fetoprotein (AFP) levels, portal vein cancerous thrombus, tumor metastasis, degree of lipiodol retention within the tumor, and percutaneous ethanol injection (PEI). A total of 17 variables were analyzed in the study.
Criteria for inclusion or exclusion of TACE
TACE was performed for HCC patients to control their local tumor growth and prolong their survival time. All patients undergoing TACE had contraindications for liver resection judged by a multidisciplinary team composed of hepatic surgeons, radiologists, hepatologists, and oncologists. The inclusion criteria for unresectable HCC include disease multifocality, early vascular invasion, decompensated liver disease, or poor performance status. To simplify the study, those whose main trunk of the portal vein was completely obstructed by a tumor thrombus or those who underwent surgical resection after TACE, were excluded from the study.
TACE procedures
Transcatheter hepatic segmental arterial chemoembolization using lipiodol mixed with anticancer drugs, including 40-60 mg adriamycin, 50-100 mg cisplatin (CDDP) or 10-20 mg mitomycin C (MMC) and a mixture of 2-30 mL iodized oil (lipiodol, André Guerbet, Aulnay-sous-bois, France), was carried out followed by injection of gelfoam particles. The mixture of lipiodol and anticancer drugs was super selectively infused into hepatic arteries supplying the tumor-bearing segment or subsegment until the lipiodol was densely accumulated in the tumor and tumor-bearing area. The arteries were then embolized with gelatin sponge particles. The volume of injected lipiodol in each patient was 2-30 mL depending on the tumor size, vascularity, and fluoroscopic findings[4].
Tumor markers and CT/MR images were detected during the follow-up after TACE. TACE procedure was repeated if any residual tumor was detected after 4 wk. TACE was performed 1-7 times (1 time for 60, 2 times for 57, and ≥ 3 times for 49 patients) in the study, and the mean repetition frequency was 2.12 times. Of the 166 patients, 9 were treated with PEI after TACE.
CT scanning and measurement techniques
CT scanning was performed for patients using a helix-spiral CT scanner (Somatom Plus 4, Siemens, Germany) before treatment. Pre- and post-contrast enhanced images of the liver of all patients were obtained. Contrast medium (Omnipaque, 300 mgI/mL, Schering, Germany) was administered by intravenous bolus injection into a cubital vein during the arterial phase (volume = 100 mL, flow rate = 2-4 mL/s, scan delay time = 25 s), portal venous phase (scan delay time = 60 s) or venous phase (scan delay time = 90 s)[16,17]. Biphasic and triphasic CT scans were performed for 76 and 90 patients, respectively.
CT scan and tumor measurements were performed using three measurement techniques during follow-up. Uni-dimensional measurement (Figures 1A and 2A) and product of the greatest axial dimension (Figures 1B and 2B) were obtained as previously described[2]. TV was measured by manually delineating (with a computer mouse) its contours on all sections, followed by calculating the lesion area with computer software (Figures 1C and 2C) as previously described[7,8,16,18]. Nonenhanced (Figure 2D) and contrast-enhanced CT (Figure 1D) were performed again within 4 wk to 2 mo (44 ± 8 d) after TACE during the follow-up. Volumetric CT evaluation included analysis of TV, liver volume, TTLVR, and TSR. The TTLVR value was less than 25% (n = 74), less than 50% (n = 48), less than 70% (n = 38), and 70% or over (n = 8)[15], respectively. The TSR value was greater than 50% reduction in TV (n = 33), greater than 25% reduction in TV (n = 64), 0%-25% reduction in TV (n = 27), and disease progression (n = 42), respectively, after treatment. The retention of lipiodol within tumor tissue was classified into four types according to the lipiodol accumulation pattern observed after lipiodol-TACE[19]: type I = homogeneous (n = 38), type II = defective (defect is found in lipiodol accumulation in the main tumor, n = 44), type III = inhomogeneous (inhomogeneous lipiodol accumulation was observed in the main tumor, n = 53), and type IV = only slight accumulation (n = 31).
Figure 1.
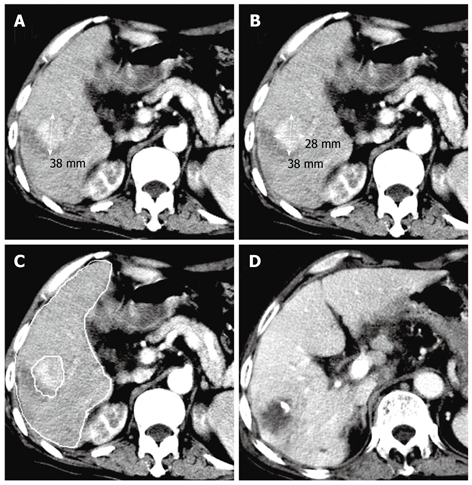
Transverse computed tomography (CT) scans showing a mononodular pattern of hepatocellular carcinoma (HCC) in the right posterior lobe of liver and an observed survival time of 33 mo in a 76-year-old man with a tumor volume of 55.64 cm3 and a tumor to liver volume ratio (TTLVR) of 5.16%. A and B: Arterial phase contrast-enhanced CT scan showing a high density nodule in the right lobe of liver before the fist transcatheter arterial chemoembolization (TACE) procedure, while one-dimensional measurement showing the largest tumor diameter (3.8 cm), and two-dimensional measurement showing the tumor product of the greatest axial dimension (10.64 cm2); C: Contrast-enhanced CT scan showing hepatic and lesion volumetric measurements before the TACE procedure; D: Follow-up CT scan displaying complete tumor necrosis 44 d after the first TACE procedure.
Figure 2.
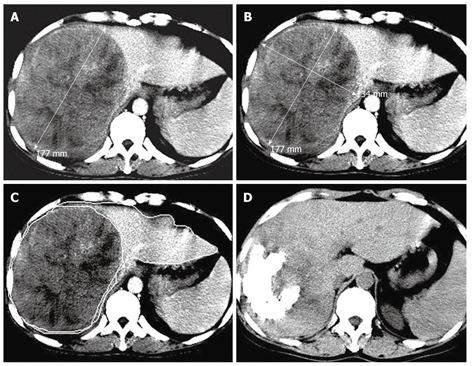
Transverse CT scans showing a massive pattern of HCC in the right lobe of a 46-year-old man with a tumor volume of 1936.70 cm3, a TTLVR of 67.52%, and a survival time of 6 mo. A and B: Arterial phase contrast- enhanced CT scan showing a high density tumor in the right lobe of liver before the first TACE procedure, while one-dimensional measurement showing the largest tumor diameter (17.7 cm) and two-dimensional measurement showing the tumor product of the greatest axial dimension (237.18 cm2); C: Contrast-enhanced CT scan showing hepatic lesion volumetric measurements before TACE; D: Nonenhanced CT follow-up scan showing lipiodol retention of grade III and a reduced tumor size 60 d after the first TACE procedure.
Statistical analysis
Statistical analysis was performed using SPSS11.5. Survival time from the commencement of treatment was analyzed using the Kaplan-Meier method. The influence of parameters on prognosis was evaluated by log-rank test. The influence of clinical variables on prognosis was evaluated by univariate analysis. When the result was statistically significant, multivariate regression analysis according to the Cox proportional hazard model was used to analyze the factors influencing the prognosis to avoid any confounding interaction between them. The largest tumor diameter, product of the greatest axial dimensions, and TV/TTLVR represented the 1D, 2D, and 3D measurements, respectively. Correlations between tumor size, each of the three different measurements, and survival time were analyzed using the Spearman coefficient of correlation. P < 0.05 was considered statistically significant.
RESULTS
General features
The formed mass was divided into nodular type (n = 39), and massive type (n = 127) on the basis of imaging examination[20]. Multiple lesions were identified in 31 patients and a mono-nodular pattern was detected in 135 patients. Portal vein cancerous thrombus was classified into four subgroups according to location: bilateral or trunk (n = 20), right branch (n = 20), left branch (n = 6), and no cancerous thrombus (n = 119). The serum AFP levels (< 20 ng/mL) were normal in 100 patients and elevated in 66 patients (≥ 20 ng/mL) before TACE. The patients with elevated AFP levels were monitored in 4 wk to 2 mo after TACE. The AFP level was decreased in 38 patients and increased in 28 patients after TACE. Tumor metastasis was divided into intrahepatic metastasis (n = 24), extrahepatic metastasis (n = 6), lymphoid node metastasis (n = 13), and no metastasis (n = 123).
Measurement data
The mean liver volume was 1621.39 ± 637.83 cm3 (range: 637.38-3514.73 cm3). The TV was 6 to 2432 cm3 with a mean of 590.13 ± 608.08 cm3 and a median of 384.26 cm3. The TTLVR was 30.54% ± 21.69% (range: 0.44%-85%) with a median of 26.62%. The largest tumor diameter was 2.0-22 cm with a mean diameter of 9.47 ± 4.00 cm and a median diameter of 9.1 cm. The tumor product of the greatest axial dimension was 2.64-264 cm2 with a mean of 79.76 ± 56.46 cm2 and a median of 65.85 cm2.
Cox proportional hazard model analysis
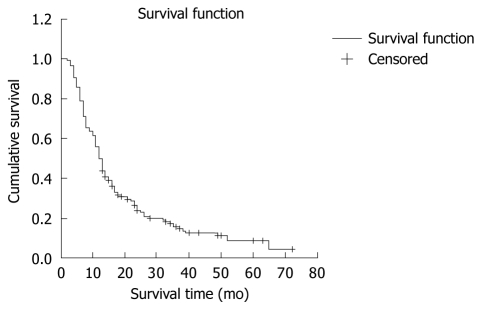
The survival time was analyzed using the Kaplan-Meier method. The overall results obtained by log-rank test are shown in Figure 3. The 6 mo-, 1-, 2-, 3-, and 5-year cumulative survival rates of patients after TACE were 78.92%, 49.85%, 23.82%, 15.60% and 8.92%, respectively. The median and mean survival time was 12 ± 1 mo and 20 ± 2 mo, respectively.
Figure 3.
Cumulative survival time of 166 HCC patients after TACE. The 6 mo- 1-, 2-, 3-, and 5-year cumulative survival rates were 78.92%, 49.85%, 23.82%, 15.60% and 8.92%, respectively, in HCC patients after TACE.
Univariate analysis showed that 13 parameters were the significant prognostic factors, including pattern of tumor growth, Child-Pugh’s classification of liver function, repeated TACE times, post-treatment AFP level, portal vein cancerous thrombus, tumor metastasis, largest tumor diameter, tumor product of the greatest axial dimension, TV, TTLVR, TSR, degree of lipiodol retention within the tumor, and PEI (Table 1). Since these variables are related to each other, multivariate Cox regression analysis was performed using the significant variables identified in the univariate analysis model. Finally, 4 variables including TTLVR, portal vein cancerous thrombus, repeated TACE times and post-treatment AFP level that entered the model could not be removed (Table 2).
Table 1.
Results of univariate Cox regression analysis
| Parameter | B | χ2 | SE | P | Hazard ratio |
| Sex | 0.319 | 1.652 | 0.248 | 0.199 | 1.375 |
| Age | -0.006 | 0.706 | 0.007 | 0.401 | 0.994 |
| Child-Pugh classification | 0.546 | 5.189 | 0.240 | 0.023 | 1.727 |
| Pre-treatment level of AFP | 0.204 | 0.921 | 0.212 | 0.337 | 1.226 |
| Post-treatment level of AFP | -0.931 | 11.238 | 0.278 | 0.001 | 0.394 |
| Times of repeated TACE | -0.507 | 20.734 | 0.111 | < 0.001 | 0.602 |
| Number of lesions | -0.218 | 0.954 | 0.223 | 0.329 | 0.804 |
| Pattern of tumor growth | 0.743 | 16.507 | 0.183 | < 0.001 | 2.102 |
| Tumor largest diameter | 0.132 | 31.708 | 0.023 | < 0.001 | 1.141 |
| Tumor product of diameters | 0.009 | 30.972 | 0.002 | < 0.001 | 1.009 |
| TV | 0.001 | 38.448 | 0.000 | < 0.001 | 1.001 |
| TTLVR | 0.662 | 44.113 | 0.100 | < 0.001 | 1.938 |
| Portal vein cancerous thrombus | 0.304 | 16.869 | 0.074 | < 0.001 | 1.355 |
| Tumor metastasis | 0.287 | 10.160 | 0.090 | 0.001 | 1.333 |
| The degree of lipiodol retention | 0.336 | 15.208 | 0.086 | < 0.001 | 1.399 |
| TSR | 0.286 | 13.014 | 0.079 | < 0.001 | 1.331 |
| PEI | 0.739 | 3.120 | 0.418 | 0.077 | 2.094 |
B: Regression coefficient; AFP: α-fetoprotein; TACE: Transcatheter arterial chemoembolization; TV: Tumor volume; TTLVR: Tumor to liver volume ratio; TSR: Tumor shrinkage ratio; PEI: Percutaneous ethanol injection.
Table 2.
Enter factors of multivariable Cox regression analysis
| Parameter | B | χ2 | SE | P | Hazard ratio |
| Post-treatment level of AFP | -0.731 | 5.416 | 0.314 | 0.020 | 0.481 |
| Times of repeated TACE | -0.597 | 8.746 | 0.202 | 0.003 | 0.550 |
| TTLVR | 0.765 | 14.328 | 0.202 | < 0.001 | 2.150 |
| Portal vein cancerous thrombosis | 0.340 | 5.643 | 0.143 | 0.018 | 1.405 |
Method: Forward stepwise (likelihood ratio).
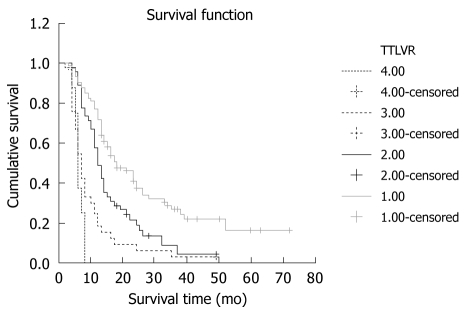
In this study, the survival time was inversely correlated with the TTLVR values. The higher the TTLVR value was, the shorter the survival rate would be. The mean and median survival time was 28 and 18 mo, respectively, in 74 patients with their TTLVR less than 25%. Those whose TTLVR was 25%-50% and 50%-70% had a shorter mean and median survival time. Patients whose TTLVR was ≥ 70% usually had a poor prognosis even after TACE. The survival time of 8 patients whose TTLVR was ≥ 70% was no more than 6 mo after TACE, indicating that TTLVR is a significant factor influencing the cumulative survival rate of HCC patients after TACE (χ2 = 14.328, P < 0.001). Furthermore, TTLVR is a risk factor affecting the outcome of treatment because of a positive B value. The mean, median and cumulative survival rates of patients are shown in Figure 4 and Table 3.
Figure 4.
Reverse correlation between survival time and TTLVR values. The TTLVR value was 0%-25%, 25%-50%, 50%-70%, and 70%, respectively.
Table 3.
Cumulative survival ratios of different TTLVR subgroups
| Subgroup |
Cumulative survival rate (%) |
Median survival (mo) | Mean survival (mo) | ||||
| 6 mo | 12 mo | 24 mo | 36 mo | 60 mo | |||
| 0%-25% | 90.54 | 77.03 | 37.68 | 26.56 | 16.30 | 18 | 28 |
| 25%-50% | 88.89 | 48.89 | 18.72 | 8.92 | 4.04 | 12 | 16 |
| 50%-70% | 54.55 | 18.18 | 6.06 | 3.03 | 0.00 | 7 | 10 |
| ≥ 70% | 37.50 | 0.00 | 0.00 | 0.00 | 0.00 | 6 | 6 |
Significant differences in survival were recognized for cumulative survival ratios of different TTLVR subgroups by log rank test: 0%-25% vs 25%-50% (χ2 = 9.1, P = 0.003), 0%-25% vs 50%-70% (χ2 = 33.71, P < 0.001), 0%-25% vs ≥ 70% (χ2 = 38.98, P < 0.001), 25%-50% vs 50%-70% (χ2 = 9.08, P = 0.003), 25%-50% vs ≥ 70% (χ2 = 22.91, P < 0.001), 50%-70% vs ≥ 70% (χ2 = 1.71, P = 0.19).
Univariate analysis showed that the influence of tumor size measured with different methods including TV, TTLVR, largest tumor diameter, and tumor product of the greatest axial dimensions was significant on prognosis of HCC patients and their correlations are listed in Table 4.
Table 4.
Correlations between largest tumor diameter, tumor product of the greatest axial dimension, TV, and TTLVR
| Parameter | Tumor largest diameter | Tumor product of diameters | TV |
| TTLVR | 0.836 | 0.842 | 0.897 |
| Tumor largest diameter | 0.976 | 0.916 | |
| Tumor product of diameters | 0.928 |
Largest tumor diameter vs TTLVR (r = 0.836, P < 0.001), tumor product of the greatest axial dimension vs TTLVR (r = 0.842, P < 0.001), TV vs TTLVR (r = 0.897, P < 0.001), largest tumor diameter vs tumor product of the greatest axial dimension (r = 0.976, P < 0.001), largest tumor diameter vs TV (r = 0.916, P < 0.001), tumor product of the greatest axial dimension vs TV (r = 0.928, P < 0.001).
DISCUSSION
It was reported that the median survival time of untreated HCC patients is less than 6 mo from its diagnosis[2]. However, it was 12 mo after TACE in our study, indicating that TACE is an effective treatment modality for HCC, a finding in agreement with the reported data[4].
Selection of optimal CT tumor measurement methods is still a challenge in treatment of HCC patients. Tumor size is widely considered a good indication for choosing an appropriate treatment modality for HCC patients, although it is difficult to accurately determine the lesion volume. In 1979, the World Health Organization (WHO) established the guidelines for tumor response to treatment. According to the WHO guidelines, the maximum diameter in the transverse plane and the largest perpendicular diameter of a tumor, measured on the same image, are multiplied to yield a cross product[9]. In 1994, the European Organization for Research and Treatment in Oncology, the National Cancer Institute of the United States, and the National Cancer Institute of Canada Clinical Trials Group reviewed the tumor measurement techniques in view of the advances in imaging technologies. The revised response evaluation criteria in solid tumors guidelines advocate that uni-dimensional measurement (largest diameter in the transverse plane) can be used alone to quantify tumor burden[6].
Tumor diameter, cross-sectional area or TV can be used to measure the size of tumors with a perfect sphere shape for the classification of their responses to TACE or other treatments. Unfortunately, since most tumors have an irregular geometry the size of tumors estimated with each parameter is usually significant different[21]. Prasad et al[9] showed that 37 patients with secondary liver tumors responsed to treatment with uni-dimensional and bi-dimensional techniques. However, tumor burden measured with TV gave a different response to treatment compared with uni-dimensional or bi-dimensional technique in a considerable proportion of patients, suggesting that methods to obtain directly TV are very important in treatment of HCC with an irregular geometry.
Advances in medical imaging have provided new means to assess tumor with volumetric imaging technologies such as helical CT and magnetic resonance imaging, both of which allow physicians to accurately quantify tumor burden and to efficiently evaluate the outcomes of treatment[5,9,16,22,23]. In our study, 3D measurements of HCC were made in patients with HCC using helical CT images. It has been shown that CT measurements of TV can provide a more sensitive assessment of tumor change than qualitative evaluation using linear methods[24,25]. Moreover, changes in TV may be correlated with the prognosis of HCC patients[25,26].
To our knowledge, only one study[16] is available on the survival time of HCC patients after TACE. However, no studies are available on the correlation between tumor size measurements of unresectable HCC and survival time of HCC patients after TACE. In this study, we investigated not only the correlation between any variable and survival time, but also the correlation between multiple variables and survival data with confounding interaction effects taken into account.
In this study, univariate Cox regression analysis showed that there was an almost complete agreement between treatment response and TTLVR, TV, largest tumor diameter and tumor product of the greatest axial dimensions in 166 HCC patients after TACE (P < 0.001). However, multivariable Cox regression analysis demonstrated that TTLVR was the only significant factor for the prognosis of HCC patients after TACE, suggesting that TTLVR may be more important for evaluating the prognosis of HCC patients than either a simple volumetric measurement or a linear measurement after TACE. Moreover, a volumetric measurement would not only overcome the difficulties in estimating the size of lesions that are confluent and irregular in shape, but also permit the measurement of overall tumor burden in an organ. Kim et al[27] have shown the significance of TTLVR for prognosis of HCC patients compared with other treatment modalities. Using a multivariate Cox hazard model, they investigated the prognostic factors for 119 HCC patients after liver transplantation, and showed that the TV ratio greater than 10% can be used as an independent prognostic factor.
It is worth noting that some studies have shown that the survival time is not always associated with the size of tumors detected at the time of treatment with TACE[2,28]. In some cases, volumetric tumor measurement for evaluating therapeutic response can not show any advantages over uni-dimensional and bi-dimensional measurements[10,12]. To some extent, the difference is thought to be due to the fact that the patients in these studies had different clinical characteristics. For instance, Sohaib et al[10] studied only nodal masses, which tend to have regular ovoid structures. Furthermore, TTLVR was not investigated in such studies.
Our study also showed a strong correlation between survival time and frequency of repeated TACE, which is consistent with the findings in other studies[3,29,30]. The mean survival time of patients undergoing ≥ 2 times of TACE was significantly longer than that of those undergoing TACE once. The reason why repeated TACE can prolong the survival time of these patients is that additional embolization can make up for the incomplete effect of previous treatment, indicating that repeated TACE in treatment of HCC is very important for prolonging the survival time of patients[30].
It has been shown that portal vein cancerous thrombosis is associated with the survival time of HCC patients after TACE[26,30,31], which is consistent with the findings in our study. Portal vein cancerous thrombosis is an important factor for predicting survival time, as univariate or multivariable Cox regression analysis in our study has shown that it is statistically significant. Independent of initial values, a lower serum AFP level after TACE is another useful post-procedural predictor[29,32], which usually implies that a favorable tumor response occurs.
In conclusion, volumetric CT technique can be reasonably used to measure tumor burden in an organ. TTLVR is a significant prognostic factor influencing the survival time of HCC patients after TACE. Compared with uni-dimensional and bi-dimensional criteria for evaluating the response of unresectable HCC to TACE, the volumetric measurement technique gives favorable results.
However, there are some limitations in our study. First, some bias may have occurred in this retrospective study due to the lack of complete clinical data. Second, the TV measurement technique relies on a mostly manual operation. Tracing individual tumor margins with mouse is time consuming and the results may have some variations if performed by different observers[33]. Moreover, the software for accurate TV estimation must be installed and used[9]. In the future, advances in post-processing of images may allow for automatic lesion contouring and TV calculation, leaving the radiologist the simple task of refining the outlined tumor contours[12]. Using these techniques can further improve the reproducibility of volume measurements and allow precise measurement of tumor burden in an organ.
COMMENTS
Background
Hepatocellular carcinoma (HCC) is one of the most common malignancies in Asia and Africa. Transcatheter arterial chemoembolization (TACE) has been shown to be one of the most effective procedures for the treatment of unresectable HCC. Since quantification of tumor size with computed tomography (CT) is usually used to assess the prognosis of HCC patients after TACE, it is very important to measure the accurate tumor size. Many studies have shown that uni-dimensional or bi-dimensional measurements can be employed to evaluate the prognosis of HCC patients after TACE. However, it can not always achieve a satisfactory prognosis.
Research frontiers
Three-dimensional measurement has advantages over uni- and bi-dimensional measurements for the assessment of irregular masses. Spiral CT offers theoretical advantages in terms of the accuracy and reproducibility of three-dimensional measurements. Previous studies have shown that CT-based three-dimensional measurement provides a more sensitive assessment of tumor change than qualitative evaluation with linear methods. However, CT-based three-dimensional volumetric measurement for prognostic evaluation of unresectable HCC after TACE has not been systematically addressed. In this study, the authors demonstrate that a volumetric CT technique can predict the prognosis of unresectable HCC patients after TACE.
Innovations and breakthroughs
Previous studies evaluated the survival time of HCC patients after TACE on the basis of tumor to liver volume ratio (TTLVR) values, but multivariable Cox regression analysis was not done. In this study, the authors investigated not only the correlation between multiple variables and survival data, but also took the confounding interaction between these factors. Furthermore, this study showed that volumetric quantification might result in a more favorable assessment than uni-dimensional and bi-dimensional techniques.
Applications
The results of this study suggest that a volumetric CT technique can reasonably predict the prognosis of unresectable HCC patients after TACE.
Terminology
TTLVR: A measurement value for tumor burden in the liver. It may be a significant prognostic factor influencing the survival time of HCC patients after TACE.
Peer review
The authors studied the prognostic significance of CT volumetric measurements in HCC patients after TACE. This work states that simple response evaluation criteria in solid tumors and WHO criteria are insufficient for the adequate evaluation of HCC lesions after treatment. Furthermore, this study revealed that TTLVR might result in a more favorable assessment than uni-dimensional and bi-dimensional measurement techniques. The results are interesting and useful for readers.
Acknowledgments
The authors thank Professor Pang FM for his helpful advice on the statistical analysis.
Footnotes
Peer reviewers: Itaru Endo, MD, PhD, Professor and Chairman, Department of Gastroenterological Surgery, Yokohama City University, Graduate School of Medicine, 3-9 Fukuura, Kanazawa-ku, Yokohama, 2360004, Japan; Patrick Veit-Haibach, MD, Department of Diagnostic and Interventional Radiology and Neuroradiology, University Hospital Essen, Hufelandstrasse 55, 45121 Essen, Germany
S- Editor Wang JL L- Editor Wang XL E- Editor Zheng XM
References
- 1.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.Taniguchi K, Nakata K, Kato Y, Sato Y, Hamasaki K, Tsuruta S, Nagataki S. Treatment of hepatocellular carcinoma with transcatheter arterial embolization. Analysis of prognostic factors. Cancer. 1994;73:1341–1345. doi: 10.1002/1097-0142(19940301)73:5<1341::aid-cncr2820730506>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 3.Lin DY, Liaw YF, Lee TY, Lai CM. Hepatic arterial embolization in patients with unresectable hepatocellular carcinoma--a randomized controlled trial. Gastroenterology. 1988;94:453–456. doi: 10.1016/0016-5085(88)90436-2. [DOI] [PubMed] [Google Scholar]
- 4.Uchida H, Ohishi H, Matsuo N, Nishimine K, Ohue S, Nishimura Y, Maeda M, Yoshioka T. Transcatheter hepatic segmental arterial embolization using lipiodol mixed with an anticancer drug and Gelfoam particles for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 1990;13:140–145. doi: 10.1007/BF02575465. [DOI] [PubMed] [Google Scholar]
- 5.Zeman RK, Fox SH, Silverman PM, Davros WJ, Carter LM, Griego D, Weltman DI, Ascher SM, Cooper CJ. Helical (spiral) CT of the abdomen. AJR Am J Roentgenol. 1993;160:719–725. doi: 10.2214/ajr.160.4.8456652. [DOI] [PubMed] [Google Scholar]
- 6.Jaffe CC. Measures of response: RECIST, WHO, and new alternatives. J Clin Oncol. 2006;24:3245–3251. doi: 10.1200/JCO.2006.06.5599. [DOI] [PubMed] [Google Scholar]
- 7.Preda L, Lovati E, Chiesa F, Ansarin M, Cattaneo L, Fasani R, Gandini S, Flor N, Cornalba G, Bellomi M. Measurement by multidetector CT scan of the volume of hypopharyngeal and laryngeal tumours: accuracy and reproducibility. Eur Radiol. 2007;17:2096–2102. doi: 10.1007/s00330-006-0573-y. [DOI] [PubMed] [Google Scholar]
- 8.Lemke AJ, Brinkmann MJ, Schott T, Niehues SM, Settmacher U, Neuhaus P, Felix R. Living donor right liver lobes: preoperative CT volumetric measurement for calculation of intraoperative weight and volume. Radiology. 2006;240:736–742. doi: 10.1148/radiol.2403042062. [DOI] [PubMed] [Google Scholar]
- 9.Prasad SR, Jhaveri KS, Saini S, Hahn PF, Halpern EF, Sumner JE. CT tumor measurement for therapeutic response assessment: comparison of unidimensional, bidimensional, and volumetric techniques initial observations. Radiology. 2002;225:416–419. doi: 10.1148/radiol.2252011604. [DOI] [PubMed] [Google Scholar]
- 10.Sohaib SA, Turner B, Hanson JA, Farquharson M, Oliver RT, Reznek RH. CT assessment of tumour response to treatment: comparison of linear, cross-sectional and volumetric measures of tumour size. Br J Radiol. 2000;73:1178–1184. doi: 10.1259/bjr.73.875.11144795. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J, Leng X, Dong N, Qi G, Du R. [Measurement of liver volume and its clinical significance in cirrhotic patients] Zhonghua Waike Zazhi. 1999;37:110–112. [PubMed] [Google Scholar]
- 12.Dachman AH, MacEneaney PM, Adedipe A, Carlin M, Schumm LP. Tumor size on computed tomography scans: is one measurement enough? Cancer. 2001;91:555–560. [PubMed] [Google Scholar]
- 13.Ettinger DS, Leichner PK, Siegelman SS, Fishman EK, Klein JL, Order SE. Computed tomography assisted volumetric analysis of primary liver tumor as a measure of response to therapy. Am J Clin Oncol. 1985;8:413–418. doi: 10.1097/00000421-198510000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Van Hoe L, Van Cutsem E, Vergote I, Baert AL, Bellon E, Dupont P, Marchal G. Size quantification of liver metastases in patients undergoing cancer treatment: reproducibility of one-, two-, and three-dimensional measurements determined with spiral CT. Radiology. 1997;202:671–675. doi: 10.1148/radiology.202.3.9051014. [DOI] [PubMed] [Google Scholar]
- 15.Arimoto T. Significance of computed tomography-measured volume in the prognosis of cervical carcinoma. Cancer. 1993;72:2383–2388. doi: 10.1002/1097-0142(19931015)72:8<2383::aid-cncr2820720815>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 16.Vogl TJ, Trapp M, Schroeder H, Mack M, Schuster A, Schmitt J, Neuhaus P, Felix R. Transarterial chemoembolization for hepatocellular carcinoma: volumetric and morphologic CT criteria for assessment of prognosis and therapeutic success-results from a liver transplantation center. Radiology. 2000;214:349–357. doi: 10.1148/radiology.214.2.r00fe06349. [DOI] [PubMed] [Google Scholar]
- 17.Kubota K, Ina H, Okada Y, Irie T. Growth rate of primary single hepatocellular carcinoma: determining optimal screening interval with contrast enhanced computed tomography. Dig Dis Sci. 2003;48:581–586. doi: 10.1023/a:1022505203786. [DOI] [PubMed] [Google Scholar]
- 18.Breiman RS, Beck JW, Korobkin M, Glenny R, Akwari OE, Heaston DK, Moore AV, Ram PC. Volume determinations using computed tomography. AJR Am J Roentgenol. 1982;138:329–333. doi: 10.2214/ajr.138.2.329. [DOI] [PubMed] [Google Scholar]
- 19.Nishimine K, Uchida H, Matsuo N, Sakaguchi H, Hirohashi S, Nishimura Y, Guo Q, Ohishi H, Nagano N, Yoshioka T. Segmental transarterial chemoembolization with Lipiodol mixed with anticancer drugs for nonresectable hepatocellular carcinoma: follow-up CT and therapeutic results. Cancer Chemother Pharmacol. 1994;33 Suppl:S60–S68. doi: 10.1007/BF00686670. [DOI] [PubMed] [Google Scholar]
- 20.Monden M, Sakon M, Gotoh M, Kanai T, Umeshita K, Wang KS, Sakurai M, Kuroda C, Okamura J, Mori T. Selection of therapeutic modalities for hepatocellular carcinoma in patients with multiple hepatic lesions. Cancer Chemother Pharmacol. 1992;31 Suppl:S38–S44. doi: 10.1007/BF00687103. [DOI] [PubMed] [Google Scholar]
- 21.Saini S. Radiologic measurement of tumor size in clinical trials: past, present, and future. AJR Am J Roentgenol. 2001;176:333–334. doi: 10.2214/ajr.176.2.1760333. [DOI] [PubMed] [Google Scholar]
- 22.Soutter WP, Hanoch J, D'Arcy T, Dina R, McIndoe GA, DeSouza NM. Pretreatment tumour volume measurement on high-resolution magnetic resonance imaging as a predictor of survival in cervical cancer. BJOG. 2004;111:741–747. doi: 10.1111/j.1471-0528.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- 23.Chong VF, Zhou JY, Khoo JB, Huang J, Lim TK. Nasopharyngeal carcinoma tumor volume measurement. Radiology. 2004;231:914–921. doi: 10.1148/radiol.2313030358. [DOI] [PubMed] [Google Scholar]
- 24.Friedman MA, Resser KJ, Marcus FS, Moss AA, Cann CE. How accurate are computed tomographic scans in assessment of changes in tumor size? Am J Med. 1983;75:193–198. doi: 10.1016/0002-9343(83)91190-7. [DOI] [PubMed] [Google Scholar]
- 25.Quivey JM, Castro JR, Chen GT, Moss A, Marks WM. Computerized tomography in the quantitative assessment of tumour response. Br J Cancer Suppl. 1980;4:30–34. [PMC free article] [PubMed] [Google Scholar]
- 26.Leung TK, Lee CM, Shen LK, Chen HC, Kuo YC, Chiou JF. Post-radiation survival time in hepatocellular carcinoma based on predictors for CT-determined, transarterial embolization and various other parameters. World J Gastroenterol. 2005;11:1697–1699. doi: 10.3748/wjg.v11.i11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim YS, Lim HK, Rhim H, Lee WJ, Joh JW, Park CK. Recurrence of hepatocellular carcinoma after liver transplantation: patterns and prognostic factors based on clinical and radiologic features. AJR Am J Roentgenol. 2007;189:352–358. doi: 10.2214/AJR.07.2088. [DOI] [PubMed] [Google Scholar]
- 28.O'Suilleabhain CB, Poon RT, Yong JL, Ooi GC, Tso WK, Fan ST. Factors predictive of 5-year survival after transarterial chemoembolization for inoperable hepatocellular carcinoma. Br J Surg. 2003;90:325–331. doi: 10.1002/bjs.4045. [DOI] [PubMed] [Google Scholar]
- 29.Farinati F, De Maria N, Marafin C, Herszènyi L, Del Prato S, Rinaldi M, Perini L, Cardin R, Naccarato R. Unresectable hepatocellular carcinoma in cirrhosis: survival, prognostic factors, and unexpected side effects after transcatheter arterial chemoembolization. Dig Dis Sci. 1996;41:2332–2339. doi: 10.1007/BF02100123. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda K, Kumada H, Saitoh S, Arase Y, Chayama K. Effect of repeated transcatheter arterial embolization on the survival time in patients with hepatocellular carcinoma. An analysis by the Cox proportional hazard model. Cancer. 1991;68:2150–2154. doi: 10.1002/1097-0142(19911115)68:10<2150::aid-cncr2820681011>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 31.Hatanaka Y, Yamashita Y, Takahashi M, Koga Y, Saito R, Nakashima K, Urata J, Miyao M. Unresectable hepatocellular carcinoma: analysis of prognostic factors in transcatheter management. Radiology. 1995;195:747–752. doi: 10.1148/radiology.195.3.7754005. [DOI] [PubMed] [Google Scholar]
- 32.Mondazzi L, Bottelli R, Brambilla G, Rampoldi A, Rezakovic I, Zavaglia C, Alberti A, Idèo G. Transarterial oily chemoembolization for the treatment of hepatocellular carcinoma: a multivariate analysis of prognostic factors. Hepatology. 1994;19:1115–1123. [PubMed] [Google Scholar]
- 33.Hopper KD, Kasales CJ, Van Slyke MA, Schwartz TA, TenHave TR, Jozefiak JA. Analysis of interobserver and intraobserver variability in CT tumor measurements. AJR Am J Roentgenol. 1996;167:851–854. doi: 10.2214/ajr.167.4.8819370. [DOI] [PubMed] [Google Scholar]