Abstract
AIM: To investigate genes around the locus D4S2964 affected by loss of heterozygosity (LOH) and their clinical implications.
METHODS: Four hundred and forty single nucleotide polymorphisms (SNPs) located at 49 genes around D4S2964 were selected from the National Center for Biotechnology Information website for the SNPs microarray fabrication. LOH of SNPs markers in 112 cases of hepatocellular carcinoma (HCC) tissues and paired adjacent liver tissues were investigated by the SNPs microarray. The correlation between allelic losses with clinicopathological features and overall survival was analyzed.
RESULTS: A fine map of LOH of SNPs in genes around D4S2964 was plotted. The average frequency of LOH in genes was 0.39. A correlation between cirrhosis and the FAL index (fractional allelic loss) was found (P = 0.0202). Larger tumor size was found to be significantly associated with LOH in genes ADP-ribosyltransferase 3 (ART3), nucleoporin 54 kDa (NUP54), scavenger receptor class B, member 2 (SCARB2) and coiled-coil domain containing 158 (CCDC158) (P = 0.043, P = 0.019, P = 0.001, P = 0.037, respectively). Kaplan-Meier analysis showed that patients with LOH in ARD1 homolog B (ARD1B) and septin 11 (SEPT11) had a significantly lower survival rate than those with retention (P = 0.021 and P = 0.004, respectively). A Cox regression model suggested that LOH in ARD1B and SEPT11, respectively, were predictors of the overall survival in HCC (P = 0.006 and P = 0.026, respectively).
CONCLUSION: LOH in genes around D4S2964 may play an important role in HCC development and progression. LOH in ARD1B and SEPT11 could serve as novel prognostic predictors in HCC patients.
Keywords: ARD1 homolog B, Hepatocellular carcinoma, Loss of heterozygosity, Septin 11, Single nucleotide polymorphism
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most prevalent cancers and the third leading cause of cancer mortality worldwide[1]. It has been ranked the second leading cause of cancer death in China since the 1990s[2]. HCC incidence and mortality rates continue to increase, particularly among middle-aged black, Hispanic, and white men in the United States[3]. The main causes of HCC are hepatitis B and C virus (HBV and HCV) infection, dietary exposure to aflatoxin B1 and high-level alcohol consumption[4]. Although many risk factors are known, the molecular mechanisms of carcinogenesis and progression of HCC have not been well elucidated.
Loss of heterozygosity (LOH) at microsatellite markers has been reported in nearly all types of human cancers. LOH regions found in a significant portion of tumors are thought to embody tumor suppressor genes (TSG). However, hundreds of LOH regions remain to be explored for the presence of TSGs. LOH in HCC is frequently detected in many chromosome regions, including 1p, 4q, 8p, 9p, 10q, 11p, 13q, 16q, 17p and 22q[5-10]. Allelic losses in some of the regions were correlated to clinicopathologic features and HCC progression[11]. An association between LOH and poor prognosis was also implicated in some studies[9,12].
In our previous study, we performed a genome-wide LOH analysis in HCC with 382 microsatellite (MS) markers, and found that LOH frequency of D4S2964 located at 4q21 in HCC was as high as 50% and correlated with HBV infection[5]. A high frequency of LOH in HCC at the D4S2964 locus was also observed by Nishimura et al[13]. The high frequency of LOH at D4S2964 was only found in HCC. Those results strongly suggest the presence of TSGs specifically for human HCC in this region. Because of the low density of the microsatellite markers, it is very difficult to use these markers for TSG identification. In the present study, we took advantage of the National Center for Biotechnology Information (NCBI) single nucleotide polymorphism (SNP) database and designed a novel strategy to gain deeper insight into the D4S2964 locus and to determine genes that may be involved in hepatocarcinogenesis. We included all 49 genes located within 4 Mb upstream and downstream of the D4S2964 locus, and selected 440 SNPs from the 49 genes with an average of 9 SNPs in each gene from the NCBI website. With the microarray consisting of probes for all the 440 SNPs, we detected LOH in 112 HCC cases paired with non-cancerous liver tissues and analyzed the correlation between the LOH affected genes and clinical features. Our study provided important clues for the identification of candidate TSGs in the LOH affected region and their clinical implication.
MATERIALS AND METHODS
Patients and tissue specimens
All 112 HCC patients, 94 males and 18 females with an age range of 13 to 72 years (median, 45.5 years), underwent surgical resection at the Department of Hepatobiliary Oncology, Sun Yat-sen University Cancer Center between 2004 and 2007. All of the tumors removed from these patients were confirmed as HCC by pathological examination. None of the patients had received any other therapies such as chemoembolization or chemotherapy before surgery. The follow-up time for these patients ranged from 1 to 58 mo with a median follow-up time of 36 mo. Most (96 of 112, 85.7%) of the patients were HBsAg positive. This study was approved by the Committee for the Conduct of Human Research of the Sun Yat-Sen University Cancer Center. Informed consent was obtained from each patient.
Primary liver carcinoma and matched non-tumor liver tissues were obtained from 112 patients when they underwent hepatectomy. Both tumors and corresponding adjacent non-tumor tissues (not less than 2 cm away from the HCC) were sampled. The fresh tissues were immediately immersed in RNAlater (Ambion, Inc., USA) after surgical resection, stored at 4°C overnight to allow thorough penetration of the RNase inhibitor, and then frozen at -80°C until use. After total RNA extraction using TRIzol reagent (Invitrogen, USA), DNA in the interphase and phenol phase from the initial homogenate was isolated according to the manufacturer’s instructions.
SNP genotyping by microarray
Forty-nine genes located within approximately 8 Mb (from 77 to 85 Mb on the long arm of chromosome 4) and flanking the D4S2964 locus were included in the study. A total of 440 SNP markers with a heterozygosity greater than 0.10 were selected from the NCBI SNP database (http://www.ncbi.nlm.nih.gov/snp). The SNPs were located either within or 5 kb upstream/downstream of the 49 corresponding genes. On average, 9 SNPs (2-14) were selected for each gene. The average distance between adjacent markers for each gene was 7.8 kb and ranged from 0.2 to 111 kb. The polymorphic sites of the SNPs were all transition variations (A/G-C/T) to facilitate microarray analysis by two fluorescent colors.
The high-throughput SNP microarray genotyping system described by Wang et al[14] was used with minor modifications. The microarray was printed using a SmartArray™-136 printer and scanned by a LuxScan™-10K Scanner (both devices are products of CapitalBio Inc., Beijing, China). Genotype call for each SNP was determined by a computer program called “AccuTyping2b”[15] based on two color signal intensities. If the log ratio of the two color (Cy5 and Cy3) intensities for a SNP was between -3.3 and 3.3, the SNP genotype was considered as heterozygous (C/T). If the log ratio was greater than 3.3 or less than -3.3, the genotype was assigned as homozygous, C/C and T/T, respectively.
LOH analysis and DNA sequencing validation
In the present study, LOH was defined as the SNP that was homozygous in tumor tissue and heterozygous in the corresponding non-tumor tissue. If any one of the SNPs in a gene had LOH, the gene was assigned as LOH. The FAL index (fractional allelic loss) for individual cases was defined as the ratio of the number of SNPs showing LOH to the total number of informative SNPs in a tumor patient; it reflects the degree of LOH of the tumor[16].
In order to confirm the genotypes and LOH of SNPs, DNA sequences containing these SNPs were amplified separately using the same condition as described above for multiplex PCR, and the PCR products were sent to Invitrogen (Guangzhou, China) for DNA sequencing.
Statistical analysis
The χ2-test, Fisher’s exact test and Mann-Whitney U test were used to analyze the correlations between clinicopathological features and the FAL index or LOH index. The overall survival curves were estimated using the Kaplan-Meier analysis. The log-rank test was used to evaluate the statistical significance of the differences. The prognostic significance of each clinicopathologic characteristic was determined using univariate Cox regression analysis. Parameters that were significantly related to survival rate in the univariate analysis were entered into the multivariate analysis. With a Cox proportional hazard model, multivariate analysis was carried out to identify the prognostic significance of clinicopathologic variables. A forward stepwise selection with the likelihood ratio criterion or an enter mode was used to identify the variables in the Cox model whenever appropriate. The SPSS version 15.0 software package and Graph-Pad Prism were used for statistical analysis.
RESULTS
SNP genotyping by microarray and validation by DNA sequencing
A total of 13 322 SNP genotypes were determined as informative (heterozygous) in non-tumor liver tissues. The heterozygosity rate was 0.27 [13322/(440*112)]. Typical microarray images for SNP genotyping of the tumor and corresponding non-tumor tissue in one patient are shown in Figure 1A and B. Twenty-five informative genotypes for six SNPs, rs17352824, rs2280101, rs3733329, rs6828114, rs6845080, and rs9307811 in 10 pairs of tumor/non-tumor tissues were validated by the sequence analysis. Typical results are shown in Figure 1C and D. The concordant rate of SNP genotypes from microarray and DNA sequencing was 96% (24/25). The inconsistent genotype was rs6845080 in a non-tumor tissue, in which the genotype by microarray was determined as C/T heterozygote, whereas that from DNA sequencing was C/C homozygote.
Figure 1.
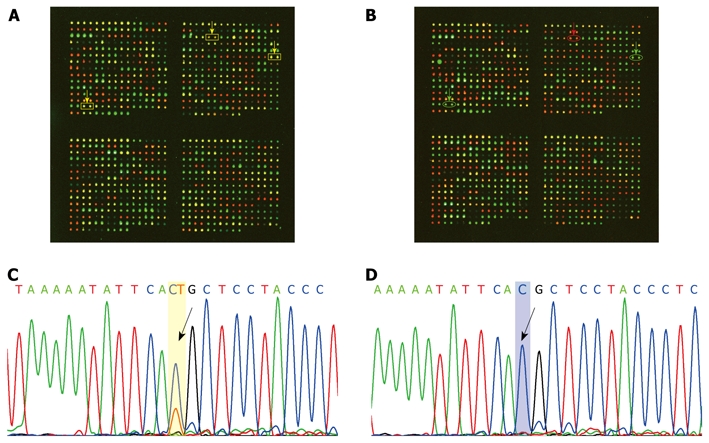
Representative case showing loss of heterozygosity. A: Informative normal adjacent tissue in SNP array. Yellow arrow shows the SNP loci with heterozygous genotype; B: Corresponding tumor tissue in SNP array. Red (or green) arrow shows the corresponding SNP loci with homozygous genotype; C: Informative normal adjacent tissue in DNA sequencing. Arrow shows the SNP loci with heterozygous genotype; D: Corresponding tumor tissue in DNA sequencing. Arrow shows the corresponding SNP loci with homozygous genotype.
LOH detection in the 112 HCC cases
Among the 440 SNPs selected in the study, 22 were shown to be non-informative in all cases, and were excluded from the data analysis. Of the 13 322 informative genotypes, 2553 (19.2%) showed LOH in tumor tissues. The results from LOH analysis for the 418 SNPs in the 49 genes in the 112 HCC patients are summarized in Figure 2. The FAL values for the 112 cases ranged from 0 to 0.769. All genes had at least one SNP affected by LOH in at least two patients. The fraction of the 112 cases with at least one SNP affected by LOH for each gene ranged from 0.227 (the FGF5 gene) to 0.643 (the PPEF2 gene) with an average of 0.382. However, for some genes, such as CXCL10 and CXCL11, the number of HCC patients with informative SNPs was very small, and therefore the fractions may have fluctuated in a rather wide range. The genes statistically shown to be associated with clinical features had > 30 informative cases.
Figure 2.

Summary of LOH analysis results. Case numbers are indicated in the right column. Gene names are listed in the bottom row, in the order of physical position from 77 Mb to 84 Mb (left to right). Gray boxes: Retention; White boxes: Noninformative; Black boxes: LOH.
Association between clinicopathological features and LOH
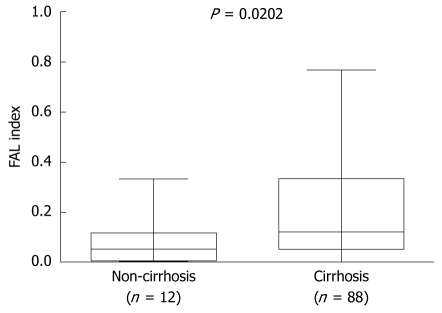
In order to elucidate the possible clinical significance of sample FAL indices, the 112 cases were divided into two groups according to the median FAL value and a χ2 analysis was performed. Results showed a significant correlation between FAL and cirrhosis but not with gender, age, serum α-fetoprotein (AFP) concentration, HBsAg status, tumor stage, tumor size, or recurrence (Table 1). The Mann-Whitney U test also revealed that HCCs with cirrhosis had significantly higher FAL indices than those without cirrhosis (P = 0.020, Figure 3).
Table 1.
Clinicopathologic correlation of FAL index around D4S2964
| Parameter |
FAL index |
||
| Low | High | P value | |
| Sex | |||
| Female | 9 | 9 | 1.000 |
| Male | 47 | 47 | |
| Serum HBsAg | |||
| Negative | 9 | 7 | 0.589 |
| Positive | 47 | 49 | |
| Cirrhosis | |||
| Absent | 10 | 2 | 0.021 |
| Present | 42 | 46 | |
| Tumor size (cm) | |||
| ≤ 5 | 12 | 13 | 0.820 |
| > 5 | 44 | 43 | |
| Tumor stage | |||
| I and II | 44 | 41 | 0.608 |
| III and IV | 11 | 13 | |
| Recurrence | |||
| No | 39 | 36 | 0.547 |
| Yes | 17 | 20 | |
FAL: Fractional allelic loss.
Figure 3.
Correlation between FAL index (fractional allelic loss) and tumor size. The box plot shows the median, quartile, and extreme values; the lines across the boxes indicate median FAL index.
Furthermore, we analyzed the correlation between LOH in genes and clinical features, including serum AFP concentration, HBsAg status, HBeAg status, tumor size, tumor stage, cirrhosis, recurrence and metastasis. The results are summarized in Table 2. Larger tumor size was found to be significantly associated with LOH in genes ADP-ribosyltransferase 3 (ART3), nucleoporin 54 kDa (NUP54), scavenger receptor class B, member 2 (SCARB2) and coiled-coil domain containing 158 (CCDC158) (P = 0.043, P = 0.019, P = 0.001, P = 0.037, respectively, Table 2), which are tandemly located. LOH in two genes, septin 11 (SEPT11) and chemokine (C-X-C motif) ligand 13 (CXCL13) was detected more frequently in HCC patients who were HBeAg-negative than in those who were HBeAg-positive (P = 0.041, P = 0.024, respectively, Table 2). However, LOH in cyclin I (CCNI) and SEC31 homolog A (SEC31A) were also more frequently detected in HBsAg-positive HCC patients than in HBsAg-negative patients (P = 0.037, P = 0.013 respectively, Table 2). The patients with LOH in ARD1 homolog B (ARD1B) showed a higher serum HBV-DNA level and a higher recurrence rate than those not affected by LOH in the gene (P = 0.049, P = 0.046, respectively, Table 2). LOH in genes helicase, POLQ-like (HELQ) and 1-acylglycerol-3-phosphate O-acyltransferase 9 (AGPAT9) was also found to be related with cirrhosis status (P = 0.006, P = 0.033, respectively, Table 2).
Table 2.
Representative clinicopathologic correlation of LOH in different genes around D4S2964
| Parameter |
LOH |
||
| Absent | Present | P value | |
| Tumor size (cm) | ART3a | ||
| ≤ 5 | 16 | 3 | 0.043 |
| > 5 | 39 | 27 | |
| Tumor size (cm) | NUP54 | ||
| ≤ 5 | 15 | 6 | 0.019 |
| > 5 | 30 | 41 | |
| Tumor size (cm) | SCARB2 | ||
| ≤ 5 | 18 | 3 | 0.001 |
| > 5 | 33 | 39 | |
| Tumor size (cm) | CCDC158 | ||
| ≤ 5 | 11 | 3 | 0.037 |
| > 5 | 19 | 22 | |
| Serum HBeAg | SEPT11 | ||
| Negative | 50 | 32 | 0.041 |
| Positive | 4 | 9 | |
| Serum HBsAg | CCNI | ||
| Negative | 11 | 1 | 0.037 |
| Positive | 48 | 31 | |
| Serum HBeAg | CXCL13 | ||
| Negative | 50 | 37 | 0.024 |
| Positive | 2 | 8 | |
| Recurrence | |||
| No | 40 | 26 | 0.044 |
| Yes | 12 | 19 | |
| Tumor stage | ANXA3 | ||
| I and II | 37 | 29 | 0.014 |
| III and IV | 15 | 2 | |
| Serum HBV-DNA | ARD1B | ||
| Negative | 24 | 5 | 0.049 |
| Positive | 14 | 10 | |
| Recurrence | |||
| No | 32 | 9 | 0.046 |
| Yes | 7 | 7 | |
| Serum HBsAg | SEC31A | ||
| Negative | 4 | 10 | 0.013 |
| Positive | 47 | 26 | |
| Metastasis | |||
| No | 40 | 34 | 0.039 |
| Yes | 11 | 2 | |
| Cirrhosis | HELQ | ||
| Absent | 8 | 0 | 0.006 |
| Present | 32 | 34 | |
| Cirrhosis | AGPAT9 | ||
| Absent | 7 | 3 | 0.033 |
| Present | 27 | 50 | |
Genes are listed in order of the most centromeric (top) to the most telomeric (bottom). LOH: Loss of heterozygosity; HBV: Hepatitis B virus; ART3: ADP-ribosyltransferase 3; NUP54: Nucleoporin 54 kDa; SCARB2: Scavenger receptor class B, member 2; CCDC158: Coiled-coil domain containing 158; SEPT11: Septin 11; CCNI: Cyclin I; CXCL13: Chemokine (C-X-C motif) ligand 13; ANXA3: Annexin A3; SEC31A: SEC31 homolog A; HELQ: Helicase, POLQ-like; AGPAT9: 1-acylglycerol-3-phosphate O-acyltransferase 9.
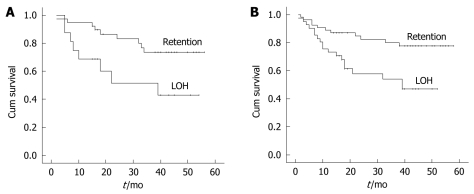
Correlation between LOH in genes ARD1B and SEPT11 and survival rate of HCC patients
Using the Kaplan-Meier curve, we showed that LOH in genes ARD1B and SEPT11 was a significant prognostic factor for poor overall survival rate in HCC patients. Patients with LOH in genes ARD1B and SEPT11 had a significantly lower survival rate than those without LOH in these genes (P = 0.021, P = 0.004, respectively, Figure 4).
Figure 4.
The association of loss of heterozygosity (LOH) in gene ARD1 homolog B (ARD1B) and septin 11 (SEPT11) with the postoperative survival of patients. A: Kaplan-Meier overall survival plot based on LOH in ARD1B (log-rank test, P = 0.021); B: Kaplan-Meier overall survival plot based on LOH in SEPT11 (log-rank test, P = 0.004).
Univariate and multivariate analyses of prognostic variables in HCC patients
Univariate and multivariate Cox regression models were performed to identify the variables of potential prognostic significance in all patients with HCC. The difference in predicting the prognosis was assessed by examining the ratio hazard and P value for each variable. Univariate Cox regression analysis showed that LOH in ARD1B and/or SEPT11 was significantly associated with overall survival rate (Table 3). Univariate Cox regression analysis also indicated that clinical variables including tumor size, recurrence, number of nodules and serum AFP were significantly associated with overall survival rate. Multivariate analysis with an enter mode was performed for LOH in ARD1B or in SEPT11, tumor size, recurrence, number of nodules, and serum AFP with overall survival rate. Results showed that LOH in ARD1B or in SEPT11 and number of nodules were predictors of the overall survival rate in HCC patients (P = 0.006, P = 0.001; and P = 0.026, P = 0.005, respectively, Table 4). LOH in ARD1B, LOH in SEPT11, tumor size, recurrence, number of nodules, and serum AFP altogether were analyzed with multivariate Cox regression model using a forward stepwise selection method with the likelihood ratio criterion, only LOH in ARD1B and number of nodules were shown to be prognostic predictors of HCC (P = 0.007, P = 0.004, respectively, Table 4).
Table 3.
Univariate analyses of individual parameters for correlations with overall survival rate: Cox proportional hazards model
| Parameter | Hazard ratio | 95% CI | P value |
| LOH in ARD1B | 2.915 | 1.119-7.594 | 0.029 |
| LOH in SEPT11 | 2.856 | 1.354-6.023 | 0.006 |
| Sex | 1.327 | 0.518-3.398 | 0.556 |
| Tumor grade | 1.693 | 0.948-3.023 | 0.075 |
| Serum HBsAg | 0.902 | 0.377-2.158 | 0.817 |
| Serum AFP | 3.319 | 1.295-8.504 | 0.012 |
| Tumor size | 4.266 | 1.31-13.888 | 0.016 |
| No. of nodules | 3.143 | 1.627-6.073 | 0.001 |
| Cirrhosis | 0.937 | 0.329-2.673 | 0.903 |
| Metastasis | 1.736 | 0.764-3.946 | 0.188 |
| Recurrence | 1.992 | 1.049-3.783 | 0.035 |
Table 4.
Multivariate analyses of individual parameters for correlations with overall survival rate: Cox proportional hazards model
| Parameter |
ARD1B |
SEPT11 |
ARD1B and SEPT11a |
||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| LOH in ARD1B | 4.615 | 1.538-13.851 | 0.006 | 4.866 | 1.528-15.493 | 0.007 | |||
| LOH in SEPT11 | 2.393 | 1.107-5.170 | 0.026 | 0.533 | |||||
| Serum AFP | 1.781 | 0.468-6.774 | 0.397 | 2.166 | 0.748-6.274 | 0.154 | 0.208 | ||
| Tumor size | 2.146 | 0.459-10.033 | 0.332 | 3.316 | 1.001-10.984 | 0.050 | 0.393 | ||
| No. of nodules | 6.173 | 2.072-18.394 | 0.001 | 3.002 | 1.401-6.434 | 0.005 | 5.579 | 1.738-17.904 | 0.004 |
| Recurrence | 0.900 | 0.305-2.659 | 0.849 | 1.707 | 0.829-3.516 | 0.147 | 0.859 | ||
Forward LR method was used. HR: Hazard radio.
DISCUSSION
Allelic losses on chromosome 4q have been reported in several human cancers, including esophageal adenocarcinoma, cervical cancer, head and neck squamous cell carcinoma, oral squamous cell carcinomas, malignant mesothelioma, small-cell lung cancer and HCC[17-27]. However, allelic losses on 4q occurred more frequently in HCC than in all other cancers and was found to be correlated with elevated α-fetoprotein production and p53 gene mutations[17,26]. Many important genes encoding albumin, alcohol dehydrogenase (ADH3), fibrinogen and UDP-glucuronyltransferase, which are predominantly expressed in the liver, are located on chromosome 4q. Genes on the long arm of this chromosome including protein tyrosine phosphatase, non-receptor type 13 (PTPN13), nucleosome assembly protein 1-like 5 (NAP1L5), and PR domain containing 5 (PRDM5), were documented as potential TSGs[28-30]. However, a large number of loci with high LOH frequency found in HCC have not been explored. Therefore, it is postulated that chromosome 4q should contain other unknown TSGs.
In the present study, based on our previous study and those by other researchers[5,13], we detected LOH in 49 genes around the D4S2964 locus by a custom microarray consisting of 440 SNPs. The average distance between these SNPs in the genes was 7.8 kb. To our knowledge, this is the first time LOH has been detected in such a way. The high-density markers in genes would display more detailed and smaller LOH regions in single genes, and compensate for the weakness of SNP markers with much less informativeness than microsatellite markers.
Then we analyzed the correlations between allelic losses and clinicopathological features. The results showed that HCC patients with cirrhosis had significantly higher FAL indices (Table 1, Figure 3) and higher LOH fraction in HELQ and AGPAT9 (Table 2) than those without cirrhosis. These results showed that patients with or without cirrhosis can be discriminated by the FAL index and LOH in genes HELQ and AGPAT9. Cirrhosis is a definite, established risk factor for HCC. In patients with cirrhosis, cirrhotic livers with high liver cell proliferative activity have a higher risk of developing cancer[31]. Higher LOH on several microsatellite markers on chromosome 16 and chromosome 17 was reported to be related to cirrhosis status in HCC patients[32]. In our previous study, LOH was found in non-cancerous liver tissues with cirrhosis caused by chronic viral hepatitis B (data not published). Therefore, some LOH may be involved in hepatocarcinogenesis. Furthermore, the analysis revealed a correlation between larger tumor size and LOH in genes ART3, NUP54, SCARB2 and CCDC158 (Table 2). Interestingly, these genes are located in a cluster in the same chromosomal region. Therefore, further analysis of this region may lead to identification of new TSGs. The data also suggested that LOH in genes SEPT11, CXCL13, CCNI, SEC31A and ARD1B were correlated with HBV infectious status (Table 2). HBV infection in humans has been recognized as one of the main risk factors for liver cirrhosis and HCC. However, the molecular mechanism underlying HBV infection is still unclear. Our results may provide insight into the understanding of HBV-related HCC.
Most importantly, we found that LOH in genes ARD1B and SEPT11 were significantly correlated with poor overall survival in HCC patients (Figure 4). Univariate and multivariate Cox regression models further revealed that LOH in genes ARD1B and SEPT11 were significant prognostic factors for poor overall survival. However, when LOH in ARD1B, LOH in SEPT11, and clinical features altogether were analyzed by a multivariate Cox regression model, only LOH in ARD1B, not LOH in SEPT11, and the number of nodules were prognostic predictors of HCC (Table 4).
The ARD1B gene encodes a human protein with 81% sequence identity to ARD1 homolog A (ARD1A), which encodes the human protein N-α-acetyltransferase. Compared to lower organisms which only have one ARD, ARD1B potentially complements the functions of ARD1A, adding more flexibility and complexity to protein N-α-acetylation in human cells[33]. Although the biological function of ARD1B in cancer remains unclear, it is recognized that the N-terminal acetylation of proteins influences multiple processes such as the cell cycle, heat-shock resistance, mating, sporulation, and telomeric silencing. However, RNAi-mediated knockdown of ARD1A and N (α)-acetyltransferase 15, NatA auxiliary subunit (NATH) induced apoptosis in human cell lines[34]. Immunohistochemical analysis showed that ARD1A protein was expressed extensively in several cancer tissues including glandular carcinoma and squamous cancer[35]. It is proposed that ARD1A or the ARD1A-NATH complex could be drug targets in cancer treatment[36]. Homologous to ARD1A, ARD1B is probably a functional protein in cancer development and/or progression. Although further study of ARD1B in cancer is necessary, our data suggest that LOH in ARD1B is a prognostic predictor of HCC and ARD1B is a potential TSG.
COMMENTS
Background
High frequency of loss of heterozygosity (LOH) at locus D4S2964 was previously reported in hepatocellular carcinoma (HCC) by this research group and was confirmed by another group in Japan. The aim of this study was to investigate genes around the locus D4S2964 affected by LOH using a customized single nucleotide polymorphisms (SNP) microarray and their clinical implications.
Research frontiers
In HCC and nearly all other cancers, there are still a lot of important LOH regions which need to be explored with regard to what genes are involved in carcinogenesis and their biological and clinical implications. In general, LOH regions are thought to embody tumor suppressor genes (TSG), which are able to inhibit the development and progression of cancer.
Innovations and breakthroughs
This study reported LOH in genes at the D4S2964 locus that are proposed to be involved in the development and progression of HCC. With the SNP microarray, the authors showed that LOH in ARD1 homolog B (ARD1B) is an independent prognostic predictor of HCC and LOH in other genes correlated with tumor size, cirrhosis, hepatitis B virus infection, recurrence and metastasis.
Applications
These results provide novel and valuable information on LOH at the D4S2964 locus in HCC and provide clues for identifying TSGs related to HCC. In addition, this study proposes a new strategy to gain deeper insight into LOH regions existing in various cancers.
Terminology
Loss of heterozygosity (LOH): In a heterozygote, the loss of one of the two alleles at one or more loci in a cell lineage or cancer cell population due to chromosome loss, deletion, or mitotic cross-over.
Peer review
The study has been well conducted, including a reasonable series of paired samples from patients with HCC. Experimental procedures and statistics are correct. The results reported provide novel and valuable information that may contribute to our understanding and management of HCC.
Acknowledgments
The authors are grateful to Mr. Shajo Kunnath for his comments on this paper.
Footnotes
Supported by National Natural Science Foundation of China, No. 30772491 to Wang HY; and partially supported by Research Fund of State Key Laboratory of Oncology in South China to Wang HY
Peer reviewer: Fernando J Corrales, Associate Professor of Biochemistry, Division of Hepatology and Gene Therapy, Proteomics Laboratory, CIMA, University of Navarra, Avd. Pío XII, 55, Pamplona 31008, Spain
S- Editor Tian L L- Editor Webster JR E- Editor Lin YP
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.He J, Gu D, Wu X, Reynolds K, Duan X, Yao C, Wang J, Chen CS, Chen J, Wildman RP, et al. Major causes of death among men and women in China. N Engl J Med. 2005;353:1124–1134. doi: 10.1056/NEJMsa050467. [DOI] [PubMed] [Google Scholar]
- 3.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen VT, Law MG, Dore GJ. Hepatitis B-related hepatocellular carcinoma: epidemiological characteristics and disease burden. J Viral Hepat. 2009;16:453–463. doi: 10.1111/j.1365-2893.2009.01117.x. [DOI] [PubMed] [Google Scholar]
- 5.Li SP, Wang HY, Li JQ, Zhang CQ, Feng QS, Huang P, Yu XJ, Huang LX, Liang QW, Zeng YX. Genome-wide analyses on loss of heterozygosity in hepatocellular carcinoma in Southern China. J Hepatol. 2001;34:840–849. doi: 10.1016/s0168-8278(01)00047-2. [DOI] [PubMed] [Google Scholar]
- 6.Okuno T, Ueda M, Tsuruyama T, Haga H, Takada Y, Maetani Y, Tamaki K, Manabe T, Tanaka K, Uemoto S. Loss of heterozygosity on 10q23 is involved in metastatic recurrence of hepatocellular carcinoma. Cancer Sci. 2009;100:520–528. doi: 10.1111/j.1349-7006.2008.01056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong CM, Lee JM, Lau TC, Fan ST, Ng IO. Clinicopathological significance of loss of heterozygosity on chromosome 13q in hepatocellular carcinoma. Clin Cancer Res. 2002;8:2266–2272. [PubMed] [Google Scholar]
- 8.Morimoto O, Nagano H, Sakon M, Fujiwara Y, Yamada T, Nakagawa H, Miyamoto A, Kondo M, Arai I, Yamamoto T, et al. Diagnosis of intrahepatic metastasis and multicentric carcinogenesis by microsatellite loss of heterozygosity in patients with multiple and recurrent hepatocellular carcinomas. J Hepatol. 2003;39:215–221. doi: 10.1016/s0168-8278(03)00233-2. [DOI] [PubMed] [Google Scholar]
- 9.Pang JZ, Qin LX, Ren N, Hei ZY, Ye QH, Jia WD, Sun BS, Lin GL, Liu DY, Liu YK, et al. Loss of heterozygosity at D8S298 is a predictor for long-term survival of patients with tumor-node-metastasis stage I of hepatocellular carcinoma. Clin Cancer Res. 2007;13:7363–7369. doi: 10.1158/1078-0432.CCR-07-0593. [DOI] [PubMed] [Google Scholar]
- 10.Chan KL, Lee JM, Guan XY, Fan ST, Ng IO. High-density allelotyping of chromosome 8p in hepatocellular carcinoma and clinicopathologic correlation. Cancer. 2002;94:3179–3185. doi: 10.1002/cncr.10612. [DOI] [PubMed] [Google Scholar]
- 11.Lee JM, Wong CM, Ng IO. Hepatitis B virus-associated multistep hepatocarcinogenesis: a stepwise increase in allelic alterations. Cancer Res. 2008;68:5988–5996. doi: 10.1158/0008-5472.CAN-08-0905. [DOI] [PubMed] [Google Scholar]
- 12.Kusano N, Okita K, Shirahashi H, Harada T, Shiraishi K, Oga A, Kawauchi S, Furuya T, Sasaki K. Chromosomal imbalances detected by comparative genomic hybridization are associated with outcome of patients with hepatocellular carcinoma. Cancer. 2002;94:746–751. doi: 10.1002/cncr.10254. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura T, Nishida N, Komeda T, Fukuda Y, Ikai I, Yamaoka Y, Nakao K. Genome-wide semiquantitative microsatellite analysis of human hepatocellular carcinoma: discrete mapping of smallest region of overlap of recurrent chromosomal gains and losses. Cancer Genet Cytogenet. 2006;167:57–65. doi: 10.1016/j.cancergencyto.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Wang HY, Luo M, Tereshchenko IV, Frikker DM, Cui X, Li JY, Hu G, Chu Y, Azaro MA, Lin Y, et al. A genotyping system capable of simultaneously analyzing >1000 single nucleotide polymorphisms in a haploid genome. Genome Res. 2005;15:276–283. doi: 10.1101/gr.2885205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu G, Wang HY, Greenawalt DM, Azaro MA, Luo M, Tereshchenko IV, Cui X, Yang Q, Gao R, Shen L, et al. AccuTyping: new algorithms for automated analysis of data from high-throughput genotyping with oligonucleotide microarrays. Nucleic Acids Res. 2006;34:e116. doi: 10.1093/nar/gkl601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roncalli M, Bianchi P, Grimaldi GC, Ricci D, Laghi L, Maggioni M, Opocher E, Borzio M, Coggi G. Fractional allelic loss in non-end-stage cirrhosis: correlations with hepatocellular carcinoma development during follow-up. Hepatology. 2000;31:846–850. doi: 10.1053/he.2000.5790. [DOI] [PubMed] [Google Scholar]
- 17.Rashid A, Wang JS, Qian GS, Lu BX, Hamilton SR, Groopman JD. Genetic alterations in hepatocellular carcinomas: association between loss of chromosome 4q and p53 gene mutations. Br J Cancer. 1999;80:59–66. doi: 10.1038/sj.bjc.6690321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou YH, Chung KC, Jeng LB, Chen TC, Liaw YF. Frequent allelic loss on chromosomes 4q and 16q associated with human hepatocellular carcinoma in Taiwan. Cancer Lett. 1998;123:1–6. doi: 10.1016/s0304-3835(97)00276-0. [DOI] [PubMed] [Google Scholar]
- 19.Björkqvist AM, Tammilehto L, Anttila S, Mattson K, Knuutila S. Recurrent DNA copy number changes in 1q, 4q, 6q, 9p, 13q, 14q and 22q detected by comparative genomic hybridization in malignant mesothelioma. Br J Cancer. 1997;75:523–527. doi: 10.1038/bjc.1997.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pershouse MA, El-Naggar AK, Hurr K, Lin H, Yung WK, Steck PA. Deletion mapping of chromosome 4 in head and neck squamous cell carcinoma. Oncogene. 1997;14:369–373. doi: 10.1038/sj.onc.1200836. [DOI] [PubMed] [Google Scholar]
- 21.Hammoud ZT, Kaleem Z, Cooper JD, Sundaresan RS, Patterson GA, Goodfellow PJ. Allelotype analysis of esophageal adenocarcinomas: evidence for the involvement of sequences on the long arm of chromosome 4. Cancer Res. 1996;56:4499–4502. [PubMed] [Google Scholar]
- 22.Sherwood JB, Shivapurkar N, Lin WM, Ashfaq R, Miller DS, Gazdar AF, Muller CY. Chromosome 4 deletions are frequent in invasive cervical cancer and differ between histologic variants. Gynecol Oncol. 2000;79:90–96. doi: 10.1006/gyno.2000.5922. [DOI] [PubMed] [Google Scholar]
- 23.Lin SC, Chang MF, Chung MY, Chang CS, Kao SY, Liu CJ, Chang KW. Frequent microsatellite alterations of chromosome locus 4q13.1 in oral squamous cell carcinomas. J Oral Pathol Med. 2005;34:209–213. doi: 10.1111/j.1600-0714.2004.00296.x. [DOI] [PubMed] [Google Scholar]
- 24.Rumpel CA, Powell SM, Moskaluk CA. Mapping of genetic deletions on the long arm of chromosome 4 in human esophageal adenocarcinomas. Am J Pathol. 1999;154:1329–1334. doi: 10.1016/S0002-9440(10)65386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen I, Langreck H, Wolf G, Schwendel A, Psille R, Vogt P, Reichel MB, Ried T, Dietel M. Small-cell lung cancer is characterized by a high incidence of deletions on chromosomes 3p, 4q, 5q, 10q, 13q and 17p. Br J Cancer. 1997;75:79–86. doi: 10.1038/bjc.1997.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeh SH, Chen PJ, Lai MY, Chen DS. Allelic loss on chromosomes 4q and 16q in hepatocellular carcinoma: association with elevated alpha-fetoprotein production. Gastroenterology. 1996;110:184–192. doi: 10.1053/gast.1996.v110.pm8536855. [DOI] [PubMed] [Google Scholar]
- 27.Piao Z, Park C, Park JH, Kim H. Deletion mapping of chromosome 4q in hepatocellular carcinoma. Int J Cancer. 1998;79:356–360. doi: 10.1002/(sici)1097-0215(19980821)79:4<356::aid-ijc8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 28.Inazawa J, Ariyama T, Abe T, Druck T, Ohta M, Huebner K, Yanagisawa J, Reed JC, Sato T. PTPN13, a fas-associated protein tyrosine phosphatase, is located on the long arm of chromosome 4 at band q21.3. Genomics. 1996;31:240–242. doi: 10.1006/geno.1996.0039. [DOI] [PubMed] [Google Scholar]
- 29.Harada H, Nagai H, Ezura Y, Yokota T, Ohsawa I, Yamaguchi K, Ohue C, Tsuneizumi M, Mikami I, Terada Y, et al. Down-regulation of a novel gene, DRLM, in human liver malignancy from 4q22 that encodes a NAP-like protein. Gene. 2002;296:171–177. doi: 10.1016/s0378-1119(02)00855-7. [DOI] [PubMed] [Google Scholar]
- 30.Deng Q, Huang S. PRDM5 is silenced in human cancers and has growth suppressive activities. Oncogene. 2004;23:4903–4910. doi: 10.1038/sj.onc.1207615. [DOI] [PubMed] [Google Scholar]
- 31.Donato MF, Arosio E, Del Ninno E, Ronchi G, Lampertico P, Morabito A, Balestrieri MR, Colombo M. High rates of hepatocellular carcinoma in cirrhotic patients with high liver cell proliferative activity. Hepatology. 2001;34:523–528. doi: 10.1053/jhep.2001.26820. [DOI] [PubMed] [Google Scholar]
- 32.Zhang SH, Cong WM, Xian ZH, Wu MC. Clinicopathological significance of loss of heterozygosity and microsatellite instability in hepatocellular carcinoma in China. World J Gastroenterol. 2005;11:3034–3039. doi: 10.3748/wjg.v11.i20.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arnesen T, Betts MJ, Pendino F, Liberles DA, Anderson D, Caro J, Kong X, Varhaug JE, Lillehaug JR. Characterization of hARD2, a processed hARD1 gene duplicate, encoding a human protein N-alpha-acetyltransferase. BMC Biochem. 2006;7:13. doi: 10.1186/1471-2091-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnesen T, Gromyko D, Pendino F, Ryningen A, Varhaug JE, Lillehaug JR. Induction of apoptosis in human cells by RNAi-mediated knockdown of hARD1 and NATH, components of the protein N-alpha-acetyltransferase complex. Oncogene. 2006;25:4350–4360. doi: 10.1038/sj.onc.1209469. [DOI] [PubMed] [Google Scholar]
- 35.Yu M, Gong J, Ma M, Yang H, Lai J, Wu H, Li L, Li L, Tan D. Immunohistochemical analysis of human arrest-defective-1 expressed in cancers in vivo. Oncol Rep. 2009;21:909–915. doi: 10.3892/or_00000303. [DOI] [PubMed] [Google Scholar]
- 36.Arnesen T, Thompson PR, Varhaug JE, Lillehaug JR. The protein acetyltransferase ARD1: a novel cancer drug target? Curr Cancer Drug Targets. 2008;8:545–553. doi: 10.2174/156800908786241113. [DOI] [PubMed] [Google Scholar]