Abstract
AIM: To investigate the prognostic impact of metastatic lymph node ratio (rN) on gastric cancer after curative distal gastrectomy.
METHODS: A total of 634 gastric cancer patients who underwent curative resection (R0) of lymph nodes at distal gastrectomy in 1995-2004. Correlations between positive nodes and retrieved nodes, between rN and retrieved nodes, and between rN and negative lymph node (LN) count were analyzed respectively. Prognostic factors were identified by univariate and multivariate analyses. Staging accuracy of the pN category (5th UICC/TNM system) and the rN category was compared according to the survival rates of patients. A linear regression model was used to identify the relation between rN and 5-year survival rate of the patients.
RESULTS: The number of dissected LNs was related with metastatic LNs but not related with rN. Cox regression analysis showed that depth of invasion, pN and rN category were the independent predictors of survival (P < 0.05). There was a significant difference in survival between LN stages classified by the rN category or by the pN category (P < 0.05). However, no significant difference was found in survival rate between LN stages classified by the pN category or by the rN category (P > 0.05). Linear regression model showed a significant linear correlation between rN and the 5-year survival rate of gastric cancer patients (β = 0.862, P < 0.001). Pearson’s correlation test revealed that negative LN count was negatively correlated with rN (P < 0.001).
CONCLUSION: rN category is a better prognostic tool than the 5th UICC pN category for gastric cancer patients after curative distal gastrectomy. Increased negative LN count can reduce rN and improve the survival rate of gastric cancer patients.
Keywords: Stomach neoplasm, Gastrectomy, Lymphadenectomy, Metastatic lymph node ratio, Prognosis
INTRODUCTION
Gastric cancer, a common malignancy worldwide, is the second most common cause of cancer-related death[1]. Despite recent advances in multimodality treatment and targeted therapy, complete resection remains the only curative treatment modality. Lymph node metastasis is one of the most important prognostic factors for gastric cancer[2-4], and accurate categorization of lymph node metastasis or optimization of pN category is critical for decision making of its subsequent therapies after surgery. Compared with total gastrectomy, distal gastrectomy is one of the most important operative procedures for gastric cancer, which can decrease postoperative morbidity and mortality and improve the postoperative life quality and nutrition condition of gastric cancer patients[5-8]. Although there are some studies on the prognostic significance of rN in gastric cancer[9-11], few relevant researches are available on gastric cancer after curative distal gastrectomy. Therefore, the aim of this retrospective study was to evaluate the prognostic value of metastatic lymph node ratio (rN) for gastric cancer after curative distal gastrectomy.
MATERIALS AND METHODS
Between January 1995 and November 2004, 1289 patients diagnosed as primary gastric cancer were treated with curative resection at Department of Oncology, Fujian Medical University Union Hospital (Fuzhou, China). Of the 1289 patients, 709 underwent distal gastrectomy, 75 were excluded from the study for T4 lesions in order to avoid data heterogeneity due to more extensive resections and higher positive margins. Finally, 634 patients were enrolled in this study. Curative surgery was defined as complete tumor removal with no macroscopic residual tumor, no invasion of carcinoma cells in the margin, and no evidence of distant metastasis. Clinical and histopathologic data about each patient were classified according to the Japanese Classification of Gastric Carcinoma (JCGC)[12]. Lymph nodes were meticulously dissected from the en bloc specimens, and the dissected lymph nodes were classified by surgeons who reviewed the excised specimens after surgery based on the JCGC. Based on the 5th edition of UICC/TNM system[13], pN category was defined as pN0 with no metastatic lymph nodes, pN1 with 1-6 metastatic lymph nodes, pN2 with 7-15 metastatic lymph nodes, and pN3 with > 15 metastatic lymph nodes, respectively. rN intervals were determined using the best cut-off approach and the survival rate of patients (log-rank statistic) was considered a dependent variable. The best-fit cut-off values of rN intervals were rN0: 0%, rN1: 1%-20%, rN2: 21%-50%, and rN3: > 50%, respectively. The patients were followed up by trained investigators through e-mail, telephone, visiting patients or recording the consultations of patients at the outpatient clinic. The survival time was the time when diagnosis was made to the last contact, or to the date of death, or to the date when the survival information was collected. All surviving patients were followed up for more than 5 years. The median follow-up time of the entire cohort was 62.0 mo (range 1-172 mo). A total of 591 patients were followed up, accounting for 93.2%.
Statistical analysis
Pearson’s correlation coefficient (r) was used to study the relations between positive nodes and retrieved nodes, between rN and retrieved nodes, and between rN and negative LN count. Survival rate was calculated with the Kaplan-Meier method. Cox proportional hazard model was used in multivariate analysis, with a backward elimination model for all covariates. A linear regression model was established to correlate rN with survival based on Kaplan-Meier 5-year survival estimates at each rN interval, using 5% of rN interval as an independent variable. P < 0.05 was considered statistically significant. All statistical analyses were performed using the statistical package for social science (SPSS) version 13.0 for Windows.
RESULTS
Clinicopathological parameters of patients
The clinicopathological parameters of patients are summarized in Table 1. There were 467 males and 167 females at the age of 22-87 years (56.1 ± 12.1 years). The tumor diameter was 4.3 ± 1.7 cm. Gastric cancer was found at the lower third (L), lower and middle third (LM), and middle third (M) of stomach in 521, 41, and 71 patients, respectively. Based on the Japanese Classification of Gastric Carcinoma[12], 118 were at stage pT1, 174 at stage pT2, and 342 at pT3, respectively. As far as the histological grades were concerned, 140 were differentiated and 494 were undifferentiated. Lymph node metastasis was observed in 439 patients (69.2%). Based on the 5th UICC/TNM pN category[13], 195 patients (30.8%) were classified as pN0, 232 (36.6%) as pN1, 156 (24.6%) as pN2 and 51 (8.0%) as pN3, respectively. Using the rN category, 195 patients (30.8%) were classified as rN0, 172 (27.1%) as rN1, 156 (24.6%) as rN2, and 111 (17.5%) as rN3, respectively. The median of total LN number was 23 (range 5-61, mean 23.1 ± 8.6) in each patient.
Table 1.
Clinical parameters of patients included in this study n (%)
| Characteristics | Patients (n = 634) |
| Gender | |
| Male | 467 (73.6) |
| Female | 167 (26.4) |
| Age (yr) | 56.1 ± 12.1 |
| Tumor size (cm) | 4.3 ± 1.7 |
| Tumor location | |
| Lower | 521 (80.8) |
| Middle | 71 (11.2) |
| Lower and middle | 42 (8.0) |
| Digestive tract construction | |
| Billroth I | 469 (74.0) |
| Billroth II | 148 (22.3) |
| Roux-en-Y | 17 (3.7) |
| Pathology | |
| Differentiated | 140 (22.1) |
| Undifferentiated | 494 (77.9) |
| Depth of invasion | |
| pT1 | 118 (18.6) |
| pT2 | 174 (27.4) |
| pT3 | 342 (54.0) |
| pN category | |
| pN0 | 195 (30.8) |
| pN1 | 232 (36.6) |
| pN2 | 156 (24.6) |
| pN3 | 51 (8.0) |
| rN category | |
| rN0 | 195 (30.8) |
| rN1 | 172 (27.1) |
| rN2 | 156 (24.6) |
| rN3 | 111 (17.5) |
| Number of resected LNs | 23.1 ± 8.6 |
Correlation between LN metastasis and retrieved nodes
Pearson’s correlation test showed that the number of metastatic lymph nodes was significantly related with that of retrieved nodes (r = 0.252, P < 0.001, Figure 1A), but the rN was not related with the number of retrieved nodes (r = -0.075, P > 0.05, Figure 1B).
Figure 1.
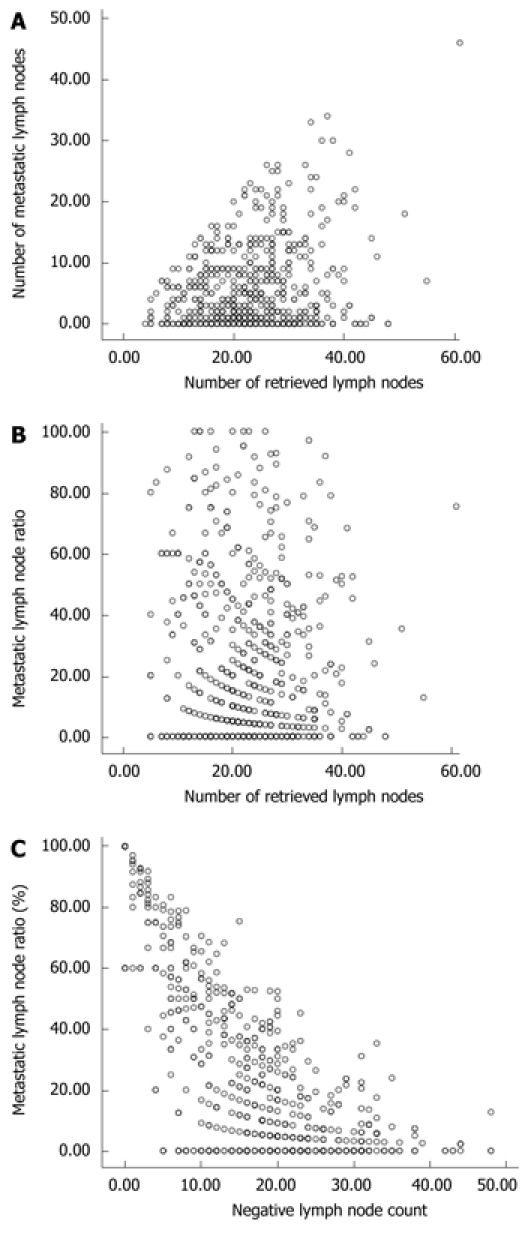
Pearson’s correlation tests. A: A significant correlation between the number of lymph node metastases and retrieved lymph nodes; B: No significant correlation between the metastatic lymph node ratio and the number of retrieved lymph nodes; C: A significant correlation between rN and the negative lymph node count.
Univariate and multivariate survival analysis
The 5-year survival rate of the entire cohort was 57.6%. The clinicopathological variables tested in univariate analysis are shown in Table 2. The factors influencing the 5-year survival rate were tumor diameter (P = 0.036), pathology types (P = 0.003), depth of invasion (P < 0.001), pN category (P < 0.001) and rN category (P < 0.001). The covariates of gender (P = 0.991), age (P = 0.423), tumor location (P = 0.058) and digestive tract construction (P = 0.064) had no significant influence on the survival rate. Multiple survival rates were calculated with the Cox’s proportional hazard regression model. In order to confirm the influence of rN, the prognostic factors including tumor diameter, pathology types, depth of invasion and pN category except for rN category in univariate analysis were analyzed by stepwise regression. As a result, two independent prognostic parameters, namely depth of invasion (P < 0.001) and pN category (P < 0.001), were obtained. When rN category was included in the Cox’s regression, the two independent prognostic parameters were also obtained. The risk ratios and their 95% confident interval are listed in Table 3.
Table 2.
Univariate analysis of variables for gastric cancer patients after curative distal gastrectomy
| Parameters | n | 5-yr survival (%) | χ2 | P |
| Gender | < 0.001 | 0.991 | ||
| Male | 467 | 58.1 | ||
| Female | 167 | 56.3 | ||
| Age (yr) | 0.641 | 0.423 | ||
| < 60 | 370 | 58.9 | ||
| ≥ 60 | 264 | 56.0 | ||
| Tumor size (cm) | 4.409 | 0.036 | ||
| ≤ 4 | 343 | 61.0 | ||
| > 4 | 291 | 53.6 | ||
| Tumor location | 5.693 | 0.058 | ||
| Lower | 521 | 57.7 | ||
| Middle | 71 | 63.7 | ||
| Lower and Middle | 42 | 45.4 | ||
| Digestive tract construction | 5.508 | 0.064 | ||
| Billroth I | 469 | 60.7 | ||
| Billroth II | 148 | 48.5 | ||
| Roux-en-Y | 17 | 51.0 | ||
| Pathology | 9.135 | 0.003 | ||
| Differentiated | 140 | 68.1 | ||
| Undifferentiated | 494 | 54.6 | ||
| Depth of invasion | 70.466 | 0.000 | ||
| pT1 | 118 | 90.6 | ||
| pT2 | 174 | 64.4 | ||
| pT3 | 342 | 42.9 | ||
| pN category | 142.279 | 0.000 | ||
| pN0 | 195 | 83.3 | ||
| pN1 | 232 | 59.3 | ||
| pN2 | 156 | 33.9 | ||
| pN3 | 51 | 20.0 | ||
| rN category | 217.494 | 0.000 | ||
| rN0 | 195 | 83.3 | ||
| rN1 | 172 | 68.6 | ||
| rN2 | 156 | 40.7 | ||
| rN3 | 111 | 17.2 |
Table 3.
Multiple stepwise regression analysis with the Cox proportional hazards model
| Parameters | β | SE | Wald | P | RR | 95% CI |
| rN excluded | ||||||
| Tumor size | -0.025 | 0.117 | 0.045 | 0.832 | 0.976 | 0.776-1.227 |
| Pathology | 0.000 | 0.161 | 0.000 | 0.999 | 1.000 | 0.730-1.370 |
| Depth of invasion | 27.144 | 0.000 | ||||
| pT2 vs pT1 | 0.956 | 0.259 | 13.59 | 0.000 | 2.601 | 1.565-4.325 |
| pT3 vs pT1 | 1.253 | 0.247 | 25.668 | 0.000 | 3.500 | 2.156-5.683 |
| pN category | 20.572 | 0.000 | ||||
| pN1 vs pN0 | 0.556 | 0.188 | 8.754 | 0.003 | 1.734 | 1.206-2.519 |
| pN2 vs pN0 | 0.829 | 0.203 | 16.673 | 0.000 | 2.291 | 1.539-3.410 |
| pN3 vs pN0 | 1.02 | 0.248 | 16.916 | 0.000 | 2.772 | 1.705-4.507 |
| rN included | ||||||
| Tumor size | -0.033 | 0.117 | 0.078 | 0.779 | 0.968 | 0.769-1.218 |
| Pathology | 0.011 | 0.161 | 0.005 | 0.945 | 1.011 | 0.738-1.385 |
| Depth of invasion | 24.388 | 0.000 | ||||
| pT2 vs pT1 | 0.908 | 0.263 | 11.974 | 0.001 | 2.48 | 1.483-4.149 |
| pT3 vs pT1 | 1.206 | 0.252 | 22.827 | 0.000 | 3.34 | 2.036-5.477 |
| rN category | 17.927 | 0.000 | ||||
| rN1 vs rN0 | 0.502 | 0.199 | 6.349 | 0.012 | 1.651 | 1.118-2.440 |
| rN2 vs rN0 | 0.842 | 0.205 | 16.928 | 0.000 | 2.322 | 1.554-3.468 |
| rN3 vs rN0 | 0.867 | 0.234 | 13.758 | 0.000 | 2.380 | 1.505-3.763 |
Comparison of survival rates between subsets of patients in either LN classification
The subgroups of patients, defined by the pN category, were discriminated with rN category. A significant difference was observed in survival rates between LN stages classified by the rN category or by the pN category (P < 0.05, Table 4), whereas no significant difference was found in survival rates between LN stages classified by the pN category or by the rN category (Table 5).
Table 4.
Comparison of survival rates for patients classified by rN category or by pN category
| N stage | n |
rN1 |
rN2 |
rN3 |
P | |||
| n | OS (%) | n | OS (%) | n | OS (%) | |||
| pN1 | 232 | 169 | 68.6 | 54 | 36.3 | 9 | 22.2 | 0.000 |
| pN2 | 156 | 3 | 66.7 | 95 | 40.8 | 58 | 20.4 | 0.007 |
| pN3 | 51 | NA | NA | 7 | 57.4 | 44 | 11.6 | 0.012 |
OS: Overall 5-year survival rate; NA: Not applicable.
Table 5.
Comparison of survival rates for patients classified by pN category or by rN category
| N stage | n |
pN1 |
pN2 |
pN3 |
P | |||
| n | OS (%) | n | OS (%) | n | OS (%) | |||
| rN1 | 147 | 169 | 68.6 | 3 | 66.7 | NA | NA | 0.638 |
| rN2 | 156 | 54 | 36.3 | 95 | 40.8 | 7 | 57.4 | 0.188 |
| rN3 | 51 | 9 | 22.2 | 58 | 20.4 | 44 | 11.6 | 0.257 |
Projected rN impact on overall survival rate
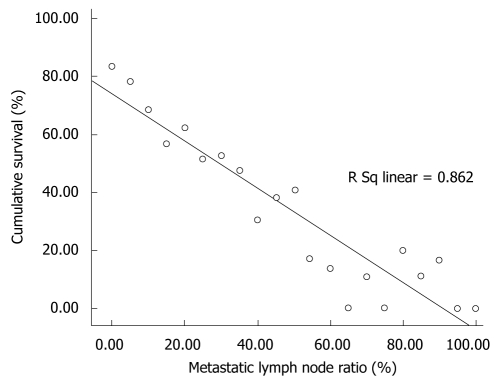
The linear regression model demonstrated that the regression equation of assumed linearity was y = -0.814x + 74.4 (β = 0.862, P < 0.001). The regression line of this calculated rN effect on the 5-year survival rate is shown in Figure 2. The hypothetical baseline 5-year survival rate (based on the y-intercept, i.e. 0% rN) was 74.4%. When rN was increased 10%, the calculated 5-year survival rate was decreased by 8.14%.
Figure 2.
Regression line of rN impact on the 5-year survival rate.
Correlation analysis between rN and negative LN count
Pearson’s correlation test showed that the negative LN count was negatively correlated with rN (r = 0.684, P < 0.001, Figure 1C).
DISCUSSION
Accurate staging of gastric cancer is of great importance in clinical practice, especially when adjuvant treatment is considered. Metastasis to lymph nodes is considered the main prognostic factor for gastric cancer patients who undergo radical resection. In 1997, the International Union against Cancer (UICC) proposed that the category of lymph node involvement should be classified according to the number of metastatic lymph nodes. It has been reported that the category based on the number of metastatic lymph nodes is more sensitive than that based on the location of metastatic lymph nodes in prognostic evaluation of gastric cancer[14-16]. However, the UICC/TNM classification (5th edition) only recommended that 15 or more lymph nodes should be examined for the accurate staging of gastric cancer. From the statistical viewpoint, the number of metastatic lymph nodes increases in proportion to the number of dissected nodes, suggesting that pN category can be influenced by the extent of lymphadenectomy. In addition, the pN category may be changed by adding or reducing one positive lymph node, suggesting that gastric cancer classified as N1 after limited lymph node dissection may be classified as N2 or N3 after extensive lymphadenectomy. This phenomenon is the so-called stage migration[17,18]. Sun et al[19] reviewed 2159 gastric cancer patients who underwent gastrectomy with a curative intent, and found that there is a closed linear correlation between the number of retrieved LNs and the number of metastatic nodes, while there is no correlation between rN and the number of retrieved nodes, which is consistent with the results of our present study, suggesting that patients with identical rN, even with a different number of detected metastatic nodes, will have a similar outcome, and that rN category is superior to the 5th UICC/TNM classification in terms of minimizing stage migration.
Fukuda et al[20] compared the pN category of the UICC/TNM in 1997 with a classification based on the metastatic lymph node ratio in patients who underwent R0 resection using the regression analysis, and identified that the metastatic lymph node ratio is the most important prognostic factor for gastric cancer. The results of univariable and multivariable analyses of potential prognostic factors in this study showed that the rN category was one of the independent prognostic parameters. Kunisaki et al[21] analyzed 1472 early gastric cancer patients undergone curative gastrectomy, and found that rN can identify subsets of patients with a significantly different survival rate within UICC/TNM pN1, pN2 and pN3 categories, suggesting that the outcome of patients with the same number of metastatic nodes, or with a larger number of retrieved LNs, is unfavorable. In the present study, however, a significant difference was observed in survival rates between lymph-node stages classified by the rN category or by the UICC pN category, but no significant difference was found in survival rates between lymph-node stages classified by the UICC pN category or by the rN category, indicating that the UICC/TNM classification can demonstrate stage migration and heterogeneous stratification for disease-specific survival. However, the metastatic lymph node ratio showed less stage migration and homogenous stratification in this study. The N category is currently defined by the number of positive nodes, but routine H&E staining may not exactly reflect the prognosis of lymph node metastasis due to lymph node micro-metastasis[22-25]. Therefore, the rN category may give more accurate prognostic information on nodal involvement than the current pN category of TNM, thus having a great impact on the selection of patients who may benefit from the adjuvant treatment.
A study from Italy[11] showed that the number of metastatic lymph nodes is significantly related with rN, and that the more the number of metastatic lymph nodes is, the higher the rN will be, indicating that gastric cancer patients with a higher rN have a greater risk of death after surgery and a shorter survival time. In this study, when the rN was increased 10%, the calculated 5-year survival rate was decreased by 8.14%, whereas when the rN was decreased 10%, the calculated 5-year survival rate was increased by 8.14%, suggesting that the long-term survival can be improved by reducing rN. Moreover, Person’s correlation test showed that rN was negatively correlated with the negative LN count (P < 0.001), indicating that removal of more negative lymph nodes can reduce rN.
In conclusion, increased negative LN count can reduce rN, thus improving the long-term survival of patients with gastric cancer after curative distal gastrectomy.
COMMENTS
Background
Distal gastrectomy is one of the most important operative procedures for gastric cancer. Although the staging system of gastric cancer has been refined step by step, staging techniques never stop updating. So far, few studies are available on the relative contribution of metastatic lymph node (LN) ratio to the prognostic evaluation of gastric cancer after curative distal gastrectomy.
Research frontiers
Some researches have shown that metastatic lymph node ratio (rN) is an excellent predictor for the survival rate of patients with colon cancer, pancreatic cancer, breast cancer and other carcinomas. Some related studies on gastric cancer have also found the potential effect of rN on prognostic evaluation, but no consensus on stratification cutoffs, especially lack of data on gastric cancer after curative distal gastrectomy.
Innovations and breakthroughs
The authors investigated the validity of metastatic LN ratio as a prognostic factor by retrospectively reviewing 634 gastric cancer patients who underwent curative resection (R0) with distal gastrectomy in 1995-2004. The relation between rN and survival was discovered.
Applications
Metastatic LN ratio can provide accurate information on LN metastasis and lymphoadenectomy for gastric cancer after curative distal gastrectomy. Moreover, the rN category is an important prognostic factor and increment of negative LN count can reduce rN, thus improving the survival rate of gastric cancer patients.
Peer review
This is an interesting study about the prognostic value of metastatic lymph node ratio (MLR) for 634 gastric cancer patients who underwent curative subtotal gastrectomies at a single institute. The study was well designed.
Acknowledgments
We thank the Follow-up Office established by the Department of Oncology, Fujian Medical University Union Hospital, Fuzhou, Fujian Province, China.
Footnotes
Peer reviewers: Dr. Chikashi Shibata, Department of Surgery, Tohoku University, 1-1 Seiryo-machi, Aoba-ku, Sendai, 980-8574, Japan; Boris Kirshtein, MD, Department of Surgery “A”, Soroka Medical Center, Ben Gurion University of the Negev, POB 151, Beer Sheva, 84101, Israel; I-Rue Lai, Assistant professor, Department of Anatomy and Cell Biology, Medical College, National Taiwan University, 7, Chun-San S. Rd, Taipei 106, Taiwan, China
S- Editor Wang YR L- Editor Wang XL E- Editor Ma WH
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Wu CW, Hsieh MC, Lo SS, Tsay SH, Lui WY, P’eng FK. Relation of number of positive lymph nodes to the prognosis of patients with primary gastric adenocarcinoma. Gut. 1996;38:525–527. doi: 10.1136/gut.38.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li C, Kim S, Lai JF, Oh SJ, Hyung WJ, Choi WH, Choi SH, Noh SH. Solitary lymph node metastasis in gastric cancer. J Gastrointest Surg. 2008;12:550–554. doi: 10.1007/s11605-007-0285-x. [DOI] [PubMed] [Google Scholar]
- 4.Mishima Y, Hirayama R. The role of lymph node surgery in gastric cancer. World J Surg. 1987;11:406–411. doi: 10.1007/BF01655802. [DOI] [PubMed] [Google Scholar]
- 5.Davies J, Johnston D, Sue-Ling H, Young S, May J, Griffith J, Miller G, Martin I. Total or subtotal gastrectomy for gastric carcinoma? A study of quality of life. World J Surg. 1998;22:1048–1055. doi: 10.1007/s002689900515. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Cheong JH, Hyung WJ, Shen J, Choi SH, Noh SH. Predictors of long-term survival in pN3 gastric cancer patients. J Surg Oncol. 2004;88:9–13. doi: 10.1002/jso.20130. [DOI] [PubMed] [Google Scholar]
- 7.De Manzoni G, Verlato G, Roviello F, Di Leo A, Marrelli D, Morgagni P, Pasini F, Saragoni L, Tomezzoli A. Subtotal versus total gastrectomy for T3 adenocarcinoma of the antrum. Gastric Cancer. 2003;6:237–242. doi: 10.1007/s10120-003-0261-4. [DOI] [PubMed] [Google Scholar]
- 8.Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, Gennari L. Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg. 1999;230:170–178. doi: 10.1097/00000658-199908000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu J, Yang D, Wei F, Sui Y, Li H, Shao F, Li Y. The staging system of metastatic lymph node ratio in gastric cancer. Hepatogastroenterology. 2008;55:2287–2290. [PubMed] [Google Scholar]
- 10.Rodríguez Santiago JM, Muñoz E, Martí M, Quintana S, Veloso E, Marco C. Metastatic lymph node ratio as a prognostic factor in gastric cancer. Eur J Surg Oncol. 2005;31:59–66. doi: 10.1016/j.ejso.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Marchet A, Mocellin S, Ambrosi A, de Manzoni G, Di Leo A, Marrelli D, Roviello F, Morgagni P, Saragoni L, Natalini G, et al. The prognostic value of N-ratio in patients with gastric cancer: validation in a large, multicenter series. Eur J Surg Oncol. 2008;34:159–165. doi: 10.1016/j.ejso.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma-2nd English Edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 13.Sobin LH, Witeknd CN. TNM classification of malignant tumors. Internat-ional Union Cancer. 5th ed. New York: John Wiley & Sons; 1997. pp. 81–87. [Google Scholar]
- 14.Roder JD, Böttcher K, Busch R, Wittekind C, Hermanek P, Siewert JR. Classification of regional lymph node metastasis from gastric carcinoma. German Gastric Cancer Study Group. Cancer. 1998;82:621–631. [PubMed] [Google Scholar]
- 15.Ichikura T, Tomimatsu S, Uefuji K, Kimura M, Uchida T, Morita D, Mochizuki H. Evaluation of the New American Joint Committee on Cancer/International Union against cancer classification of lymph node metastasis from gastric carcinoma in comparison with the Japanese classification. Cancer. 1999;86:553–558. doi: 10.1002/(sici)1097-0142(19990815)86:4<553::aid-cncr2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 16.Saito H, Fukumoto Y, Osaki T, Fukuda K, Tatebe S, Tsujitani S, Ikeguchi M. Prognostic significance of level and number of lymph node metastases in patients with gastric cancer. Ann Surg Oncol. 2007;14:1688–1693. doi: 10.1245/s10434-006-9314-3. [DOI] [PubMed] [Google Scholar]
- 17.de Manzoni G, Verlato G, Guglielmi A, Laterza E, Tomezzoli A, Pelosi G, Di Leo A, Cordiano C. Classification of lymph node metastases from carcinoma of the stomach: comparison of the old (1987) and new (1997) TNM systems. World J Surg. 1999;23:664–669. doi: 10.1007/pl00012365. [DOI] [PubMed] [Google Scholar]
- 18.Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, Morimoto T, Kato T, Kito T. The number of metastatic lymph nodes: a promising prognostic determinant for gastric carcinoma in the latest edition of the TNM classification. J Am Coll Surg. 1998;187:597–603. doi: 10.1016/s1072-7515(98)00229-4. [DOI] [PubMed] [Google Scholar]
- 19.Sun Z, Zhu GL, Lu C, Guo PT, Huang BJ, Li K, Xu Y, Li DM, Wang ZN, Xu HM. The impact of N-ratio in minimizing stage migration phenomenon in gastric cancer patients with insufficient number or level of lymph node retrieved: results from a Chinese mono-institutional study in 2159 patients. Ann Oncol. 2009;20:897–905. doi: 10.1093/annonc/mdn707. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda N, Sugiyama Y, Midorikawa A, Mushiake H. Prognostic significance of the metastatic lymph node ratio in gastric cancer patients. World J Surg. 2009;33:2378–2382. doi: 10.1007/s00268-009-0205-1. [DOI] [PubMed] [Google Scholar]
- 21.Kunisaki C, Makino H, Akiyama H, Otsuka Y, Ono HA, Kosaka T, Takagawa R, Nagahori Y, Takahashi M, Kito F, et al. Clinical significance of the metastatic lymph-node ratio in early gastric cancer. J Gastrointest Surg. 2008;12:542–549. doi: 10.1007/s11605-007-0239-3. [DOI] [PubMed] [Google Scholar]
- 22.Wu ZY, Li JH, Zhan WH, He YL, Wan J. Effect of lymph node micrometastases on prognosis of gastric carcinoma. World J Gastroenterol. 2007;13:4122–4125. doi: 10.3748/wjg.v13.i30.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee E, Chae Y, Kim I, Choi J, Yeom B, Leong AS. Prognostic relevance of immunohistochemically detected lymph node micrometastasis in patients with gastric carcinoma. Cancer. 2002;94:2867–2873. doi: 10.1002/cncr.10562. [DOI] [PubMed] [Google Scholar]
- 24.Morgagni P, Saragoni L, Scarpi E, Zattini PS, Zaccaroni A, Morgagni D, Bazzocchi F. Lymph node micrometastases in early gastric cancer and their impact on prognosis. World J Surg. 2003;27:558–561. doi: 10.1007/s00268-003-6797-y. [DOI] [PubMed] [Google Scholar]
- 25.Persiani R, Rausei S, Biondi A, Boccia S, Cananzi F, D’Ugo D. Ratio of metastatic lymph nodes: impact on staging and survival of gastric cancer. Eur J Surg Oncol. 2008;34:519–524. doi: 10.1016/j.ejso.2007.05.009. [DOI] [PubMed] [Google Scholar]