Abstract
The intracellular, cytosolic, delivery of quantum dots is an important goal for cellular imaging. Recently, a hydrophobic anion, pyrenebutyrate had been proposed to serve as a delivery agent for cationic quantum dots as characterized by confocal microscopy. Using an extracellular quantum dot quencher, QSY-21, as an alternative to confocal microscopy, we demonstrate that quantum dots remain on the cell surface and do not cross the plasma membrane following pyrenebutyrate treatment, a result that is confirmed with transmission electron microscopy. Pyrenebutyrate leads to increased cellular binding of quantum dots rather than intracellular delivery. These results characterize the use of QSY-21 as a quantum dot quencher and highlight the importance of the use of complementary techniques when using confocal microscopy.
Keywords: fluorescence microscopy, biophysical chemistry, live cell imaging, quantum dots, pyrenebutyrate
The intracellular delivery of quantum dots (QDs), nanometer-diameter semiconductor particles, is of great interest for the cellular imaging community as QDs offer the possibility of a bright, photostable probe of intracellular dynamics.1–5 A key goal for the intracellular delivery of QDs, as well as other nanoparticles and therapeutic materials, is cytosolic access, as compared to retention in endocytic vesicles.6,7 In the case of cellular imaging, cytosolic access is necessary to label intracellular proteins and organelles. For therapeutic applications such as nucleic acid delivery, cytosolic access is a necessary first step for siRNA activity or nuclear entry of DNA.8–11 Cytosolic delivery is challenging as the cargo must traverse the plasma membrane or be released from an endocytic vesicle. Based on the goal of cytosolic delivery, T. Takeuchi et al. developed a method for the delivery of peptides and proteins to the cytosol of multiple living cells simultaneously.12 This method is noteworthy as it overcame two significant difficulties associated with prior delivery methods; retention in endocytic vesicles and permeabilization of the plasma membrane.13–16 The delivery method is straightforward requiring a cationic peptide, polyarginine, in combination with a hydrophobic counterion to induce a direct translocation across the plasma membrane, bypassing the barrier of the endocytic vesicles and without permeabilizing the plasma membrane.12,17–20
The use of pyrenebutyrate for cytosolic delivery was recently applied to polyarginine-functionalized QDs (PA-QDs).21 Unlike the peptides and proteins that had been successfully delivered using this method, the PA-QDs had a relatively large hydrodynamic diameter of 32 nm.22 Confocal microscopy images suggested that incubation of cells with the hydrophobic anion pyrenebutyrate (1-pyrenebutyric acid) led to rapid cytosolic delivery of the PA-QDs. While confocal microscopy, which uses an aperture to discriminate against out of focus light, thereby imaging a thin slice of an intact cell, has been used successfully to characterize the cellular internalization of QDs,23–25 it differs from a method such as TEM which uses a physically sectioned slice of a cell for imaging.26,27 The use of confocal microscopy to confirm internalization leaves open the possibility that a bright fluorescent signal from the cell surface may appear to be within the cell.
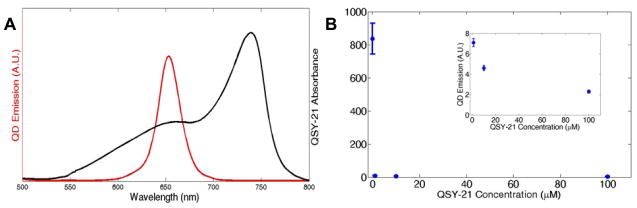
To test the possibility that pyrenebutyrate led to increased extracellular binding of the PA-QDs, rather than internalization, we used an extracellular quencher as an alternative approach to confocal microscopy. QSY-21 (Invitrogen, Cat. Q-20132) absorbs broadly from 550 nm to 775 nm (Figure 1a), but is not fluorescent, thereby serving as an efficient quencher for QDs with an emission centered at 655 nm. The optimal concentration of QSY-21 for quenching QDs (655 nm emission, QTracker, Invitrogen) was determined using a fluorometer (RF-5301, Shimadzu) to measure the emission of QDs (4 nM) in a solution of phosphate buffered saline (PBS) with increasing concentrations of QSY-21 (Figure 1b). Concentrations of QSY-21 as low as 10 μM resulted in a significant quenching of the QD signal.
Figure 1.
Use of QSY-21 to quench QD emission. (a) The absorption of QSY-21 in PBS (black) is well-overlapped with the emission of 655 nm QDs (red). The QDs were excited at 458 nm. (b) Increasing concentrations of QSY-21 quench the 4 nM QD signal. QDs were excited at 450 nm. Error bars show the standard deviation of three samples. At high QSY-21 concentrations the error bars are smaller than the data point. The inset shows an expanded view of 10 μM–100 μM QSY-21.
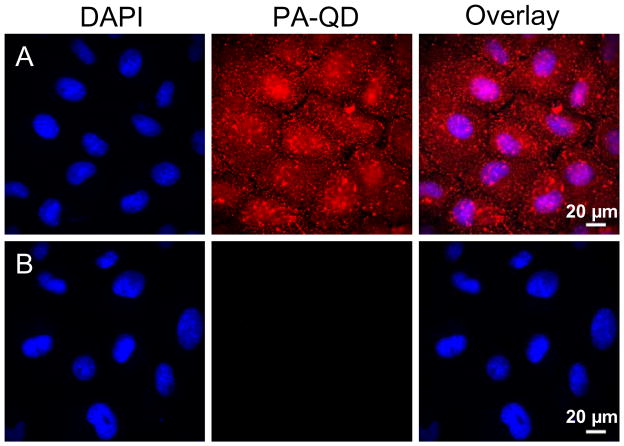
Based on these solution phase measurements, 100 μM was chosen as the optimal QSY-21 concentration to measure the cellular internalization of PA-QDs. Using the same protocols as reported previously,21 QDs were complexed to a nine-unit polyarginine (PA) peptide through a biotin-streptavidin linkage. BS-C-1 monkey kidney cells were incubated with PBS supplemented with 4 μM pyrenebutyrate (Sigma-Aldrich, Cat. 257354) for 2 min at 37°C. PA-QDs were added to the cells at a concentration of 4 nM and incubated at 37°C for four min before washing with PBS. Cells were imaged immediately, without fixation, with an epi-fluorescence microscope (Olympus IX71, 40X, 0.65 N.A. objective). The PA-QDs result in a bright signal dispersed throughout the cell (Figure 2a). The addition of 100 μM QSY-21 resulted in a significant reduction of the QD fluorescence (Figure 2b).
Figure 2.
Epi-fluorescence microscopy was used to image cells after incubation with 4 nM PA-QDs (red) in the presence of 4 μM pyrenebutyrate at 37°C. DAPI (blue) was used as a nuclear stain. (a) Following a wash with PBS, a strong QD signal (red) is visible. (b) Following the addition of QSY-21, the QD signal (red) is no longer visible.
These images strongly suggest that the PA-QDs remain on the cell surface where they are quenched by QSY-21. There are two concerns with this interpretation. First, it is possible that QSY-21 moves across the plasma membrane, thereby quenching internally. This is especially important to consider as pyrenebutyrate has been used previously as a delivery agent.12 While pyrenebutyrate remaining in solution is removed from the cells before the addition of QSY-21, residual pyrenebutyrate remaining in the cell could aid delivery of QSY-21. Second, pyrenebutyrate could quench across the plasma membrane. While quenching formally requires molecular-level contact, energy transfer across the relatively short distance of the plasma membrane could lead to a similar reduction in QD emission.
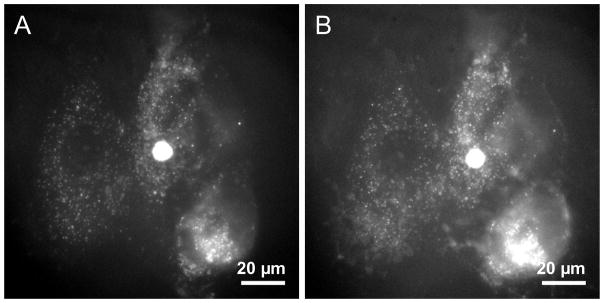
To test the possibility that QSY-21 entered the cells and quenched the QDs internally, cells were microinjected (Femtojet/Injectman, Eppendorf) with QDs. Cells were washed with PBS and imaged (Figure 3a) with an epi-fluorescent microscope (Olympus IX71, 60X, 1.20 N.A. objective). Cells were then incubated with pyrenebutyrate (4 μM) for 6 min, identical to the conditions described above, and imaged after the addition of QSY-21 (Figure 3b). No change in fluorescence was observed following the addition of QSY-21 suggesting that the quencher is not entering the cells.
Figure 3.
Epi-fluorescence images of cells microinjected with QDs. (a) QD signal (white) following microinjection. (b) The same cells were incubated for six min in PBS supplemented with 4 μM pyrenebutyrate, washed, and then imaged in the presence of 100 μM QSY-21.
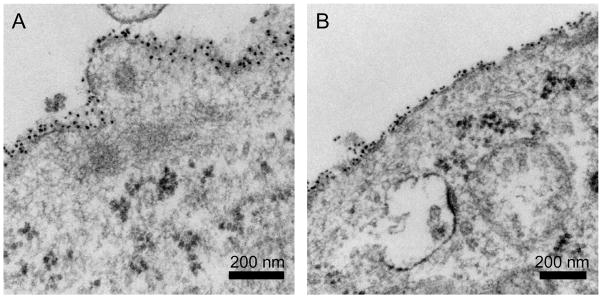
As further confirmation that the PA-QDs were not internalized following pyrenebutyrate treatment, we used TEM to image pyrenebutyrate-treated cells incubated with PA-QDs (Figure 4). Unlike confocal microscopy, which images an optical slice, preparation of cells for TEM includes physically slicing the cells into 70 nm-80 nm thin sections. Following the same incubation procedures as described for Figure 2, cells were imaged with TEM. Both control and pyrenebutyrate-treated cells show PA-QD binding to the cell surface. Cytosolic localization was not observed.
Figure 4.
TEM images of cells incubated with PA-QDs for 4 min. (a) Untreated cells. (b) Cells pretreated with 4 μM pyrenebutyrate for 2 min then incubated with PA-QDs and 4 μM pyrenebutyrate for 4 min. The dark circular structures within the cytosol are polyribosomes.
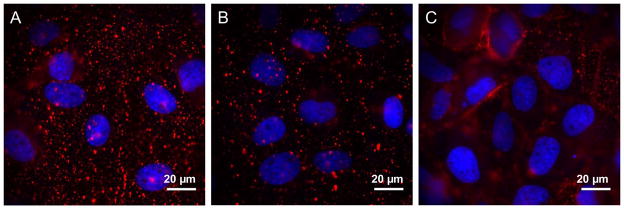
As reported previously,21 the increased QD signal does require both functionalization of the QD with PA and the incubation of the cells with pyrenebutyrate (Figure 5a). In the absence of pyrenebutyrate, PA-QDs have limited binding during the 4 min incubation period (Figure 5b). In the absence of PA, pyrenebutyrate does not lead to the binding of streptavidin-QDs (Figure 5c).
Figure 5.
Epi-fluorescence microscopy (Olympus IX71, 60X, 1.20 N.A. objective) was used to image cells following a 4 min incubation at 37°C. DAPI (blue) was used as a nuclear stain. (a) 4 nM PA-QDs (red) in the presence of 4 μM pyrenebutyrate (positive control). (b) 4 nM PA-QDs (red) in the absence of pyrenebutyrate (negative control). (c) 4 nM QDs (red), in the absence of PA, in the presence of 4 μM pyrenebutyrate (negative control).
These results demonstrate that QSY-21 quenching of the PA-QDs occurs on the cell surface, with PA-QDs remaining bound to the plasma membrane rather than entering the cell. This leads to two conclusions. First, pyrenebutyrate in combination with PA leads to the increased binding, but not internalization, of PA-QDs (Figure 5). As smaller cargo, including GFP, has been successfully delivered to the cytosol of cells using pyrenebutyrate,12 it is likely that QDs are simply too large to take advantage of this delivery method. Second, this increase in binding creates a high level of fluorescence that cannot be distinguished from internalization with confocal microscopy. An example of such a false positive obtained with confocal microscopy is shown in the Supporting Information (Figure S1). While it is likely that this problem is more severe in very flat cells such as BS-C-1, it highlights the importance of the use of complementary techniques in combination with confocal microscopy.
Supplementary Material
Acknowledgments
This work was supported by an NIH Research Scholar Development Award to C.K.P and NIH R01-GM086195. The authors thank William H. Humphries IV and Craig J. Szymanski for assistance with fluorescence and TEM imaging, respectively. TEM assistance was provided by Hong Yi of the Robert P. Apkarian Integrated Electron Microscopy Core Facility of the Emory University School of Medicine.
Footnotes
Supporting Information Available: Detailed materials and methods. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Alivisatos AP, Gu WW, Larabell C. Quantum Dots as Cellular Probes. Annu Rev Biomed Eng. 2005;7:55–76. doi: 10.1146/annurev.bioeng.7.060804.100432. [DOI] [PubMed] [Google Scholar]
- 2.Jaiswal JK, Simon SM. Potentials and Pitfalls of Fluorescent Quantum Dots for Biological Imaging. Trends Cell Biol. 2004;14:497–504. doi: 10.1016/j.tcb.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Chan WCW, Nie SM. Quantum Dot Bioconjugates for Ultrasensitive Nonisotopic Detection. Science. 1998;281:2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 4.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum Dots for Live Cells, In Vivo Imaging, and Diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao XH, Yang LL, Petros JA, Marshal FF, Simons JW, Nie SM. In Vivo Molecular and Cellular Imaging with Quantum Dots. Curr Opin Biotech. 2005;16:63–72. doi: 10.1016/j.copbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Quantum Dot Bioconjugates for Imaging, Labelling and Sensing. Nat Mater. 2005;4:435–446. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- 7.Delehanty JB, Mattoussi H, Medintz IL. Delivering Quantum Dots into Cells: Strategies, Progress and Remaining Issues. Anal Bioanal Chem. 2009;393:1091–1105. doi: 10.1007/s00216-008-2410-4. [DOI] [PubMed] [Google Scholar]
- 8.Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. The Design, Synthesis, and Evaluation of Molecules That Enable or Enhance Cellular Uptake: Peptoid Molecular Transporters. Proc Natl Acad Sci US A. 2000;97:13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer R, Fotin-Mleczek M, Hufnagel H, Brock R. Break on through to the Other Side - Biophysics and Cell Biology Shed Light on Cell-Penetrating Peptides. ChemBioChem. 2005;6:2126–2142. doi: 10.1002/cbic.200500044. [DOI] [PubMed] [Google Scholar]
- 10.Duchardt F, Fotin-Mleczek M, Schwarz H, Fischer R, Brock R. A Comprehensive Model for the Cellular Uptake of Cationic Cell-Penetrating Peptides. Traffic. 2007;8:848–866. doi: 10.1111/j.1600-0854.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 11.Wadia JS, Dowdy SF. Transmembrane Delivery of Protein and Peptide Drugs by TAT-Mediated Transduction in the Treatment of Cancer. Adv Drug Deliver Rev. 2005;57:579–596. doi: 10.1016/j.addr.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi T, Kosuge M, Tadokoro A, Sugiura Y, Nishi M, Kawata M, Sakai N, Matile S, Futaki S. Direct and Rapid Cytosolic Delivery Using Cell-Penetrating Peptides Mediated by Pyrenebutyrate. ACS Chem Biol. 2006;1:299–303. doi: 10.1021/cb600127m. [DOI] [PubMed] [Google Scholar]
- 13.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In Vivo Imaging of Quantum Dots Encapsulated in Phospholipid Micelles. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 14.Derfus AM, Chan WCW, Bhatia SN. Intracellular Delivery of Quantum Dots for Live Cell Labeling and Organelle Tracking. Adv Mater. 2004;16:961–966. [Google Scholar]
- 15.Payne CK, Jones SA, Chen C, Zhuang XW. Internalization and Trafficking of Cell Surface Proteoglycans and Proteoglycan-Binding Ligands. Traffic. 2007;8:389–401. doi: 10.1111/j.1600-0854.2007.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nan XL, Sims PA, Chen P, Xie XS. Observation of Individual Microtubule Motor Steps in Living Cells with Endocytosed Quantum Dots. J Phys Chem B. 2005;109:24220–24224. doi: 10.1021/jp056360w. [DOI] [PubMed] [Google Scholar]
- 17.Sakai N, Matile S. Anion-Mediated Transfer of Polyarginine across Liquid and Bilayer Membranes. J Am Chem Soc. 2003;125:14348–14356. doi: 10.1021/ja037601l. [DOI] [PubMed] [Google Scholar]
- 18.Perret F, Nishihara M, Takeuchi T, Futaki S, Lazar AN, Coleman AW, Sakai N, Matile S. Anionic Fullerenes, Calixarenes, Coronenes, and Pyrenes as Activators of Oligo/Polyarginines in Model Membranes and Live Cells. J Am Chem Soc. 2005;127:1114–1115. doi: 10.1021/ja043633c. [DOI] [PubMed] [Google Scholar]
- 19.Nishihara M, Perret F, Takeuchi T, Futaki S, Lazar AN, Coleman AW, Sakai N, Matile S. Arginine Magic with New Counterions up the Sleeve. Org Biomol Chem. 2005;3:1659–1669. doi: 10.1039/b501472g. [DOI] [PubMed] [Google Scholar]
- 20.Futaki S. Membrane-Permeable Arginine-Rich Peptides and the Translocation Mechanisms. Adv Drug Deliver Rev. 2005;57:547–558. doi: 10.1016/j.addr.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Jablonski AE, Humphries WH, Payne CK. Pyrenebutyrate-Mediated Delivery of Quantum Dots across the Plasma Membrane of Living Cells. J Phys Chem B. 2009;113:405–408. doi: 10.1021/jp809956w. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Hess GT, Humphries WH, Fay NC, Payne CK. Cellular Binding, Motion, and Internalization of Synthetic Gene Delivery Polymers. Biochim Biophys Acta-Mol Cell Res. 2007;1773:1583–1588. doi: 10.1016/j.bbamcr.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delehanty JB, Medintz IL, Pons T, Brunel FM, Dawson PE, Mattoussi H. Self-Assembled Quantum Dot-Peptide Bioconjugates for Selective Intracellular Delivery. Bioconjugate Chem. 2006;17:920–927. doi: 10.1021/bc060044i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoo J, Kambara T, Gonda K, Higuchi H. Intracellular Imaging of Targeted Proteins Labeled with Quantum Dots. Exp Cell Res. 2008;314:3563–3569. doi: 10.1016/j.yexcr.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Wei YF, Jana NR, Tan SJ, Ying JY. Surface Coating Directed Cellular Delivery of Tat-Functionalized Quantum Dots. Bioconjugate Chem. 2009;20:1752–1758. doi: 10.1021/bc8003777. [DOI] [PubMed] [Google Scholar]
- 26.Lagerholm BC, Wang MM, Ernst LA, Ly DH, Liu HJ, Bruchez MP, Waggoner AS. Multicolor Coding of Cells with Cationic Peptide Coated Quantum Dots. Nano Lett. 2004;4:2019–2022. [Google Scholar]
- 27.Nisman R, Dellaire G, Ren Y, Li R, Bazett-Jones DP. Application of Quantum Dots as Probes for Correlative Fluorescence, Conventional, and Energy-Filtered Transmission Electron Microscopy. J Histochem Cytochem. 2004;52:13–18. doi: 10.1177/002215540405200102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.