Abstract
Cardiovascular disease (CVD) remains the single leading cause of death in both men and women. A large proportion of the population with CVD will die with a diagnosis of congestive heart failure (CHF). It is becoming increasingly recognized that sex differences exist in the etiology, development, and outcome of CHF. For example, compared to male counterparts, women that present with CHF are typically older and have systolic cardiac function that is not impaired. Despite a growing body of literature addressing the underlying mechanisms of sex dimorphisms in cardiac disease, there remain significant inconsistencies reported in these studies. Given that the development of CHF results from the complex integration of genetic and nongenetic cues, it is not surprising that the elucidation and subsequent identification of molecular mechanisms remains unclear. In this review, key aspects of sex differences in CVD and CHF will be highlighted with an emphasis on some of the unanswered questions regarding these differences. The contention is presented that it becomes critical to reference cellular mechanisms within the context of each sex to better understand these sex dimorphisms.
1. Introduction
Risk assessment for cardiovascular disease (CVD) begins with a close examination of genetic modifiers (age, sex, family history) and nongenetic environmental modifiers (smoking, alcohol, diet). The prevailing thought among contemporary investigators is that the severity of CVD depends on contributions from both genetic and non-genetic factors. Of the genetic factors, much attention has been paid to biological sex or gender as a potent modifier of cardiovascular health. (It is generally accepted that biologic sex is defined as being chromosomally male or female while gender is a function of biologic sex, culture, behavior, and environment. For simplicity, we have decided to use the term sex in this review.)Although the vast majority of clinical and laboratory studies have been carried out in males, there is a growing body of literature directly addressing sex-specific differences in cardiovascular disease and outcomes. Premenopausal women consistently have a better prognosis than men in response to hypertension, aortic stenosis, myocardial infarction (MI), and hypertrophic cardiomyopathies [1–3]. The hearts of women with these disorders maintain adequate or elevated cardiac function whereas men typically demonstrate increased chamber dilation and wall thinning, both of which contribute to the observed poor contractility [4, 5]. The same is also true for congestive heart failure (CHF); women have better survival than men even when adjusted for severity of cardiac function [6, 7] and the long-term prognosis is better for women than for men [8, 9].
Because of this sex difference, estrogen has been proposed as a major cardioprotective agent in premenopausal women. However, a recent study showed that hormone replacement therapy (HRT) in postmenopausal women increased their CVD risk [10] forcing reconsideration of estrogen as being cardioprotective. Moreover, it seems unlikely that the male/female dimorphisms in CVD can be attributed to a single factor such as estrogen. This review will not explicitly discuss the impact of estrogen on cardiovascular health and disease as we have addressed this previously [11].
Nevertheless, estrogen is positioned to play a unique role in CVD since estrogen can respond to environmental, non-genetic cues and subsequently impact genetic expression [11]. Consequently, difficulty arises when attempting to understand how environmental factors, such as blood lipid profiles, impact CVD in men and women. For example, although statin therapy reduces cardiovascular events in both men and women equally, women do not have the same reductions in mortality and stroke as their male counterparts [12]. To further complicate matters, plasma triglycerides are better predictors of cardiovascular risk in women, whereas LDL-cholesterol concentration is a stronger predictor in men [13–16]. However, this discrepancy disappears in older, postmenopausal (estrogen-free) women where LDL levels exceed those in men and become better correlated with cardiovascular risk [17, 18].
Consequently, elucidating the cellular and molecular mechanisms of cardiac disease progression and how it differs between the sexes becomes tantamount to the discovery of clinical treatment strategies. Despite an increasing knowledge regarding the sex dimorphisms in the pathophysiology of cardiac disease, which we have extensively reviewed previously [11], many inconsistencies remain regarding the identification of these differences. More importantly, as will be discussed below, interpretation of these mechanisms explicitly depends on context, that is, how these underlying mechanisms act within each sex. In this review, we will focus on these inconsistencies in sex-specific differences in cardiac disease development. Considering that CHF is characterized by progressive impairments in cardiac function and contractility and that pharmacological manipulation of cardiac contractility is the predominant therapeutic strategy, there will be a particular emphasis on detailing the underlying contractile function in a sex specific manner.
2. Heart Failure in Women
This review is not intended to be a comprehensive or clinical exposition on the etiology, diagnosis, and treatment of CHF in women; other reports are available for this information (see [19]). Nevertheless, a few key facts require highlighting. Of all-cause mortality in women, CVD ranks as the highest [20]. Over one-third of CVD deaths in women are due to CHF. Interestingly, the Rotterdam Study shows an increasing incidence and prevalence of CHF for both men and women with age; yet, the rate in men always exceeds that in women independent of age [21, 22]. In the same study, no differences between men and women were found in heart failure prognosis [22]. Another publication, citing a self-reported heart failure survey administered by the National Center for Health Statistics in 1999, reports a greater prevalence of heart failure in men than women ages 65–74 but that after age 74, this difference significantly diminishes [23]. On the other hand, a recent study reports that idiopathic dilated cardiomyopathy is a much deadlier disease in women than in men [24].
In most cases, measures of cardiac contractility are valuable prognostic indicators in patients with severe CHF [25, 26]. Data suggest that the risk of death increases with worsening left ventricular (LV) systolic function [27]. Yet, when broken down by sex, the data are less conspicuous. In general, both men and women show increased mortality associated with impaired LV function [27]. However, more women than men with CHF present with preserved, not impaired, LV systolic function [28, 29]. It seems logical to assert that the increased survival in women over men is due to this elevated contractile function. However, more recent studies argue that there is a lack of survival benefit for patients with preserved LV function as measured by ejection fraction over patients with impaired systolic function [30, 31]. The suggestion is that the sex dimorphism in CHF survival may not necessarily be due to differences in CHF presentation but, instead, a fundamental difference in the ability of the female heart to tolerate the physiological changes associated with CHF (for review see [19]).
These apparent inconsistent reports underscore the difficulty in interpreting the CHF mortality literature and any sexual dimorphisms that may be revealed by these studies. A potentially major source of error contributing to these discrepancies lies in the definition of CHF. CHF is an end-result of many CVD etiologies and it is unclear whether there are sex differences in the etiology of CHF [19, 32]. Similarly, the clinical definition of CHF with preserved LV systolic function (which presents in over 50% of the patient population [33]) may be misleading. These patients typically experience underlying diastolic dysfunction, which, according to the studies cited above, may lead to similar mortality rates as those patients with impaired LV function.
The presentation of diastolic dysfunction with preserved systolic LV function is a clinically relevant phenotype [33, 34]. Impairments in LV relaxation, distensibility, and stiffness are rooted in the cardiac cell and, thus, have underlying cellular mechanisms [35–37]. The findings that more women than men have CHF with normal LV function indicate that females do not demonstrate a similar risk to diastolic dysfunction as their male counterparts. To date, there are no studies directly examining the mechanism of sex dimorphisms in diastolic dysfunction and CHF.
Since treatment strategies are typically not sex specific, the extent and intensity of the medical intervention could also affect the outcome of these studies [38, 39]. For example, when the attainment of recommended LDL cholesterol levels is the goal, even if appropriate LDL cholesterol levels are reached, there is no clear evidence that men and women benefit equally [39, 40]. The findings with LDL cholesterol may also reflect fundamental sex differences in the hydrolysis of LDL and substrate delivery to cardiomyocytes as elaborated below. Furthermore, studies attempting to delineate the differential impact of preserved or impaired LV systolic function and CHF survival struggle to maintain consistency with the above parameters. Apart from investigating the underlying causes of the apparent sex differences in CHF, investigators are challenged to find appropriate models to study the impact of sex on the development, response, and progression of heart disease.
3. Sex Differences in Cardiac Function
Considering that cardiac pathophysiology is unique among men and women, it is reasonable to assume that basal cardiac physiology is similarly unique among the sexes. Unfortunately, the data supporting this assertion vary from study to study and are not clear. Whether examining humans or rodents, the anatomical arrangement of the heart and peripheral cardiovascular system is identical between males and females. However, the size of the female heart is smaller than age- and race-matched men [41]. This appears to be consistent among species; the larger the animal, the larger the heart. If cardiac physiology is proportionate to organism size, it is not surprising to find that the difference is eliminated when heart size is adjusted for body mass. We have examined cardiac morphometrics in mice detailing similar findings [42, 43].
It has been suggested that male and female hearts begin with an equivalent number of cardiac myocytes and, therefore, the difference in cardiac size, although proportional to body mass, is due to larger myocyte size in males compared to females [44–46]. Considering that males are typically larger in body mass, the corresponding increase in myocyte size is most likely a result of differences in physiological demand. However, since prevailing systemic pressures are equivalent among men and women, males and females utilize unique mechanisms to maintain homeostasis as discussed by Huxley (2007) [47].
Differences in cardiac myocyte size translate into differences in ventricular chamber dimensions. Looking at a study population (57 years of age on average) free of clinically overt cardiovascular disease, all volumetric parameters measured by magnetic resonance imaging (MRI) are elevated in males, even when adjusted for subject height or body surface area [48, 49]. In these same studies, no differences in LV functional parameters are evident although these data are not always consistent [47–49].
In a more invasive approach employed by Shioura et al. (2008) [50] male and female mice were instrumented with a conductance catheter able to accurately and simultaneously measure LV pressure and volume. The authors demonstrate similar baseline hemodynamic parameters in male and female mice. More importantly, this is the first study that shows no significant differences between male and female hearts using the end-systolic pressure-volume relationship (ESPVR) to index ventricular contractility. The ESPVR provides a measure of ventricular contractile state that is independent of heart rate and, more importantly, loading conditions within the physiological range [51–53]. In addition, a measure of diastolic function as indexed by the end-diastolic pressure-volume relationship (EDPVR) can be derived from these studies [50]. Using these parameters of ventricular function, these investigators evaluated male and female mice at different timepoints following an MI. Both male and female mice demonstrate an immediate deterioration in function following MI. Ten weeks post MI, female hearts improve contractility to baseline levels as measured by the ESPVR while male hearts do not [50]. Female mice show a similar attenuation in volumetric parameters. Interestingly, only female hearts display diastolic dysfunction but only at an early timepoint.
Interestingly, the authors document similar baseline volumetric parameters in both male and female hearts using this technique. Considering that male hearts are typically larger than female hearts under normal physiological conditions, one would predict a sex dimorphism in end-systolic and end-diastolic volumes as indicated in the human MRI studies. Although this most likely would not alter fundamental contractile characteristics, it does point out the limitations of these studies regarding absolute volume calculations [54]. Nevertheless, many noninvasive techniques such as MRI or echocardiography harbor more significant limitations when measuring cardiac contractility [55, 56]. Using the pressure-volume technique, investigators will be able to monitor cardiac function and contractility with and without disease and/or disease interventions in the appropriate context; that is, within the same sex. Thus, this paper represents a significant advancement and establishes the benchmark for detecting sex dimorphisms in cardiac function under normal and diseased states. More importantly, it predicts a cellular mechanism underlying changes in contractile function detailed below.
4. Cardiac Cellular Dynamics and Contractility
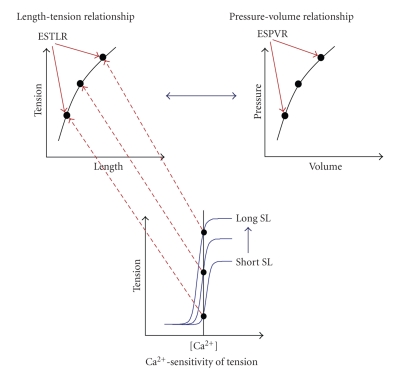
Despite the technical limitations, the use of the ESPVR and the EDPVR, and resultant measures from this approach, provides an important link to cardiac cellular dynamics. When addressing sex dimorphisms in cardiac physiology and pathophysiology, it becomes necessary to integrate intact ventricular function (systolic and diastolic) with myofilament function, the ultimate determinant of cellular dynamics. The ability to maintain contractile force at a given cytosolic Ca2+ concentration is in part determined by the sensitivity of the myofilaments to Ca2+ with the output of tension development as the endpoint. At the cellular level, this type of analysis provides an index of cardiac contractility and is directly related to contractility as indexed by the ESPVR (Figure 1). The reason is that we and others have proposed that a fundamental association exists between myofilament Ca2+-sensitivity, sarcomere length and tension of the cardiac cell and pressure and volume in the intact ventricle. In this context, the relationship between length and tension (end-systolic tension-length relationship; ESTLR) of isolated cardiac muscle preparations determines the shape of the pressure-volume relationship at end-systole (ESPVR) of the intact ventricle (Figure 1) [57–59].
Figure 1.
Relationship between Ca2+-sensitivity, length, and tension to the pressure-volume relationship. The sensitivity of cardiac myofilaments to Ca2+ indicates the contractile state of the cardiac muscle cell. The relationship between Ca2+ and tension is illustrated in the bottom panel labeled Ca2+-sensitivity of tension. There exists a unique Ca2+-sensitivity of tension relationship at each length (SL: sarcomere length) and these relationships can be used to create the Length-tension relationship (upper left panel) as indicated by the arrows. The Length-tension relationship can alternatively be called the end-systolic tension-length relationship (ESTLR) and is the underlying cellular mechanism of the end-systolic pressure-volume relationship (ESPVR; upper right panel).
However, the literature examining sex dimorphisms in myofilament function is equivocal. Some studies illustrate that isolated cardiac muscle preparation from female hearts responds with greater tension than males at a given amount of Ca2+ indicative of enhanced Ca2+-sensitivity [60, 61]. Other studies document the exact opposite results; female hearts are less sensitive to Ca2+; that is, isolated cardiac muscle preparations respond with lower tension than males at a given level of Ca2+ [62, 63]. We have similarly found a reduced Ca2+-sensitivity in the hearts of female mice compared to males (unpublished observations). In support of reduced myofilament Ca2+-sensitivity in females, the loss of estrogen by ovariectomy increases the sensitivity of the myofilaments to Ca2+ [64–66]. Interestingly, loss of testosterone in males reduces Ca2+-sensitivity, which can be reversed by testosterone replacement [67]. Using a similar approach, another study shows no differences in Ca2+-sensitivity between male and female cardiac trabeculae [68]. In this same study, female trabeculae fail to generate equivalent levels of force with increasing stimulating frequencies compared to males. This effect is exaggerated at longer lengths and at higher extracellular [Ca2+].
The reasons for these inconsistencies are multifold. Ca2+-responsiveness of isolated cardiac muscle was determined using either chemically permeabilized preparations or cardiac muscle preparations with membranes intact. Chemical permeabilization removes membrane-bound organelles that include the Ca2+-handling machinery. Therefore, it is not surprising that differences in Ca2+-sensitivities are observed since sex dimorphisms exist in excitation-contraction coupling, which remains intact in nonpermeabilized preparations [69–71]. Additionally, 2 critical confounding factors are differences in (1) species (rats, mice, or cats) and (2) ages of the animals used in the studies described above. Although the impact of species on myofilament function is rather apparent, age-dependent changes highlight how signaling components within the cardiac myocyte can alter myocellular function. Similarly, the impact of estrogen and/or testosterone predicts a potential sex-dimorphism in intracellular cardiac signaling. This becomes especially important in the context of sex, cardiac disease, and disease progression.
There are numerous signaling mediators in the cardiac cell, many of which target myofilament proteins and are involved in excitation-contraction coupling, growth signaling, and other processes involved in cellular remodeling as occured with cardiac disease progression [72–74]. Predicting a priori how sex impacts these signaling pathways, which, in turn, impacts contractile properties and cellular remodeling to disease, becomes problematic. Moreover, it is not known how sex, and potentially sex hormones, influences these signaling pathways in the short- and long-term. Consequently, an integrated approach to understanding how signaling pathways modulate both cellular remodeling and contractile dynamics in a sex-specific manner is required of future investigations, again pointing to the context in which these studies must be performed and interpreted.
To illustrate this contention and the complexity of how sex can modify signaling at multiple steps, we can use β-adrenoceptor stimulation as the model. During a cardiac insult such as MI, posttranslational modification of myofilament proteins and the subsequent impact on Ca2+-sensitivity becomes a key mechanism of short- and long-term remodeling within the cardiac cell [75–77]. Short-term β-adrenoceptor stimulation immediately increases rates of relaxation and contraction in the myocardium [78, 79]. This is accomplished by enhancing Ca2+ cycling in the myocyte through phosphorylation of phospholamban [79–82], decreasing the sensitivity of the myofilaments to Ca2+ [83, 84], and accelerating the rate-limiting steps in the crossbridge cycle [85, 86]. Thus, the integrated short-term response of β-adrenergic stimulation results from targeting Ca2+ cycling machinery, phospholamban, leading to enhanced reuptake of Ca2+ by the sarcoplasmic reticulum [79–82] and direct phosphorylation of contractile proteins, troponin I, and myosin binding protein C [87, 88] to alter contractile mechanics.
Yet, prolonged β-adrenergic stimulation induces pathological hypertrophy, cellular remodeling, and decreased myofilament protein phosphorylation that leads progressive deterioration of cardiac function [72, 73, 89, 90]. In a recent study that investigated the interaction of estrogen and β-adrenergic stimulation, concomitant exposure of neonatal ventricular myocytes with norepinephrine (β-adrenergic agonist) and estrogen mitigated the hypertrophy that occurs with norepinephrine treatment alone [91]. These data represent some of the first indications that hypertrophic, pathologic stimulation is directly affected by estrogen and may be blunted in the presence of estrogen.
Given this defined combinatorial integration of cellular signaling, the resultant impact on myofilament and intact ventricular function depends on the species, sex, and timepoint in disease progression of the animals used in the study. It is possible that sex dimorphisms exist at each step in the above signaling pathway leading to a more hastened worsening of cardiac pathophysiology in males as compared to females. Yet, the sex-dependent mechanism of the differential pathogenesis necessitates an integrative understanding from the cellular to the whole heart level.
Another example where this integrative approach may shed major insight can be illustrated in transgenic mice expressing a mutant myosin heavy chain transgene (R403Q) corresponding to a human mutation causing hypertrophic cardiomyopathy (HCM) [43, 92, 93]. We have studied sex dimorphisms in R403Q mice and characterized some of the cellular and molecular mechanisms behind the sex differences [43, 92]. These include a number of pathologic indicators such as fibrosis, induction of β-myosin heavy chain, inactivation of glycogen synthase kinase-3β, and activation of proapoptotic pathways in males but not females [43, 92, 94]. Males harboring the R403Q mutation show progressive deterioration in ventricular function associated with chamber dilation whereas females R403Q mice do not [92, 95, 96].
At the myofilament level, a recent study reports that demembranated (permeabilized) cardiac fibers from males develop more tension at a given [Ca2+] compared to females indicating that the contractile apparatus is more sensitive to Ca2+ in males [63]. Moreover, the R403Q mutation does not impact Ca2+-sensitive tension development in cardiac fibers when compared to controls from either sex. Using another R403Q transgenic model, we find that Ca2+-sensitivity is similarly greater in males compared to females and that the R403Q mutation does not impact Ca2+-sensitivity in males only (unpublished observations). However, cardiac muscle from female mice expressing the R403Q mutation is significantly more sensitive to Ca2+ than controls and both WT and R403Q cardiac fibers from males.
Again, the factors underlying the differences between our data and the previous report are the unique timepoints during disease progression at which the experiments were performed. In the comparable study by Palmer et al. (2008), measurements were performed in fibers taken from animals that were 10–20 weeks of age where mice expressing the R403Q mutation exhibit no observable HCM pathology [63, 93]. We performed our studies at 10–12 months of age, a timepoint where R403Q mice exhibit significant HCM pathology, functional deficits, and sex differences [92, 95, 96]. The implication from our data is that the increased Ca2+ sensitivity may provide sufficient contractile support in female R403Q hearts maintaining a compensated state, a characteristic not exhibited in males.
It is possible that as disease progresses, posttranslational changes occur to modify Ca2+-sensitivity and ultimately contribute to the phenotypic and functional differences between male and female R403Q mice. Interestingly, previous reports demonstrate that the R403Q mutation results in enhanced left-ventricular systolic function accompanied by compromised left-ventricular diastolic function at an early timepoint (4–6 months of age) [96–98]. Considering that females with clinically significant CHF typically present with diastolic dysfunction and preserved (nonimpaired) systolic function, this particular model may be useful to more fully elucidate the cellular mechanisms underlying sex dimorphisms in CHF presentation [28, 29]. As outlined above, an approach that integrates myofilament function, cellular signaling, and intact ventricular function at distinct timepoints during the progression of cardiac disease is needed. Once again, the context in which these studies are performed is critical.
5. Sex-Dependent Impact of Substrates on Cardiac Function
When discussing cardiac contractility and CHF, the impact of energy generation, utilization, and delivery on cardiac function cannot be ignored. Given the extreme energetic demands, cardiac muscle preferentially fulfills energy requirements through the oxidation of fatty acids rather than glucose. On a molar basis, fatty acid oxidation (FAO) provides the greatest yield of ATP compared to glycolytic or other substrates such as lactate [99]. However, during the early stages of pathological cardiac hypertrophy such as occured in HCM where cardiac function is not compromised, substrate preference in the heart switches to glucose while FAO either stays the same or decreases [100–102]. As compensated hypertrophy progresses to decompensated cardiac hypertrophy where severe ventricular dysfunction ensues, both glycolytic and FAO capacity diminish thereby decreasing the overall energy reserve in the heart [103–105]. At this point, cardiac energetic pathways no longer display the plasticity required for adaptation to a diseased state and contribute to the deterioration of the failing myocardium.
Since the major goal of metabolic pathways in the heart is to provide a sufficient supply of ATP for myocyte function, it is reasonable to predict that the molecular underpinnings of the metabolic derangements during cardiac disease or myocellular metabolism in general display sex differences. Clues as to these differences are revealed from studies using transgenic or knock-out mice targeting cellular mediators of fatty acid metabolism. Pharmacologic inhibition of carnitine palmitoyltransferase I (CPT I), a critical regulator in mitochondrial fatty acid import, is lethal in male mice null for peroxisome proliferator-activated receptor-α (PPAR-α) whereas only 25% of female PPAR-α-null mice die [106]. In mice overexpressing lipoprotein lipase (LPL) in cardiac and skeletal muscle but lacking global PPAR-α, over 50% of males die within 4 months of age while the remaining males do not survive past 11 months of age [107]. Females, on the other hand, survive for more than 12 months of age. Interestingly, the differences in plasma free fatty acid (FFA) levels as a result of the genetic manipulations cannot account for the extreme disparity in mortality [107]. In both sexes of double transgenics, circulating FFA is elevated while triglycerides are reduced. Therefore, the implication is that female hearts may be better adapted to utilize the circulating FFA as a substrate for myocardial energy utilization or that female hearts are protected against this elevation in circulating FFA than their male counterparts.
From the above studies, it appears that there are sex dimorphisms in the ability to utilize specific energy substrates. Yet, very little is known regarding any sex dimorphisms and the impact of substrates on contractile function. Consequently, even less is known about how this changes with cardiac disease progression. The delivery and utilization of substrates by cardiac muscle cells maintains a dynamic component such that the flux of these substrates undoubtedly plays an integral role in contractile dynamics. Again, any sex dimorphisms in substrate utilization necessitate a need to put these data in the context of each sex. Consequently, some key differences in lipid metabolism (providing about 95% of the substrate for cardiac cells) between the sexes need to be highlighted.
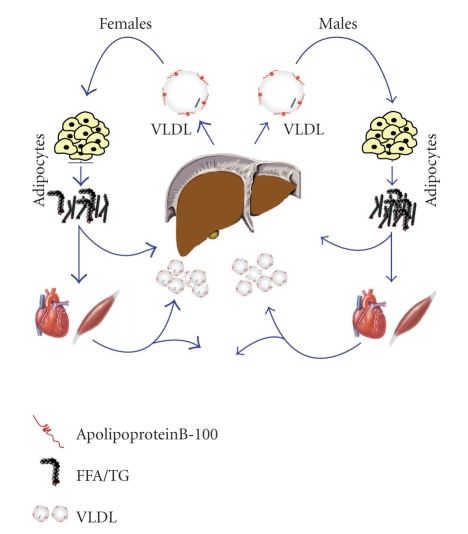
The delivery of these substrates is accomplished through the circulation, and the release of these substrates depends on metabolic mediators and tissues that include liver, adipose tissue, and muscle. For example, studies have shown that the basal rate of lipolysis as defined by the rate of appearance of plasma FFA is greater in women than in men [14, 108]. The released FFAs will either by oxidized for fuel or stored as TG. Therefore, VLDL-TG production depends on the availability of hepatic FFA and thus is a major determinant of VLDL-TG production [109]. The higher rate of FFA release is consistent with the finding that women demonstrate higher rates of VLDL production compared to men, which are unaltered by obesity like in men [15]. Interestingly, in the same study, the amount of plasma VLDL-TG between men and women was similar, suggesting that the rate of VLDL-TG clearance is greater in women when compared to men [15].
These sex differences in VLDL-TG dynamics were further investigated in a recent study by Magkos et al. (2007) [110]. In this study, the molar ratio between hepatic VLDL-TG and VLDL-apolipoproteinB-100 (VLDL-apoB-100, which provides the structural framework of the VLDL particle and permits the incorporation of TG, cholesterol and cholesterol esters, and other apolipoproteins before VLDL secretion occurs [111]) was determined in age-matched lean men and women [110]. The authors find a greater molar ratio of hepatic VLDL-TG and VLDL-apolipoproteinB-100 secretion rates in women compared to men. Because only one apolipoproteinB-100 molecule is found in a VLDL particle [112], the suggestion from these data is that the liver in women secretes larger VLDL particles containing more TG than those secreted by men [110]. A potential mechanism underlying these differences in VLDL-TG secretion and clearance rates between men and women begins with the observation that the lipolysis of TG that are in large, TG-rich lipoproteins is more efficient than lipolysis of TG in small, TG-poor particles [113]. Since the hydrolysis of lipoproteins including VLDL particles is largely mediated by lipoprotein lipase (LPL) in muscle and adipose tissue [114, 115], elevated LPL activity in females can further explain the differences in VLDL dynamics, which has been found in skeletal and adipose tissue [116, 117] (see Figure 2 for a summary). Interestingly, estrogen decreases LPL activity in plasma [118] and adipose tissue [119] in humans and it also inhibits LPL transcriptional activity [120].
Figure 2.
Schematic representation of VLDL and FFA flux. Women demonstrate higher rates of FFA release as a result of higher lipolytic rates when compared to men. This is consistent with elevated rates of VLDL production and clearance in women. In addition, the molar ratio between hepatic VLDL-TG and VLDL-apoB-100 was greater in women compared to men suggesting that women secrete larger, more TG-rich VLDL particles than men, which may contribute to some of the observed sex dimorphisms.
From the above discussion, it is apparent that there are sex differences in lipid metabolism and substrate flux through the circulation. There is, however, a lack of study directly examining how these sex dimorphisms impact cardiac function and potentially translate into a protection during cardiac disease and CHF. In general, when cardiac muscle is exposed to fatty acids, contractility decreases whether measured in isolated cardiac cells [121, 122] or the whole heart [123, 124]. Recent evidence suggests that this mechanism in intact cardiac cells is through augmentation of voltage-gated K+ current responsible for repolarization of the cardiac cell [122]. Interestingly, demembranated cardiac muscle strips from rainbow trout fed a diet high in omega-3 fatty acid are less sensitive to Ca2+ than those from trout fed a diet low in omega-3 fatty acid [125]. In contrast to these results, permeabilized cardiac cells from mice with cardiac-specific overexpression of fatty acid transport protein 1, which leads to increased fatty acid uptake, accumulation, and usage, are more sensitive to Ca2+ than wild-type controls [121]. The inconsistencies in the latter phenomenon using permeabilized cardiac muscle may be explained by the differential impact of FFA on intracellular signaling pathways such as those including protein kinase A and protein kinase C [126–128].
When looking at cardiac disease and CHF, manipulation of FFA delivery is controversial. In the long-term, suppression of glycolytic oxidation by increasing FFA delivery decreases cardiac inotropy [129]. Consequently, lowering cardiac FAO by reducing circulating FFAs or inhibiting mediators of mitochondrial FFA has been suggested as a therapeutic measure to increase cardiac contractility for CHF (for review see [129]). However, this has been recently challenged [130, 131]. More importantly, given sex dimorphisms in lipid dynamics described above, there are no reports detailing whether this approach is effective in females. Considering that estrogen may inhibit LPL activity as detailed above [118–120], it has been hypothesized that estrogen provides protection against adipogensis and the clinical sequlae associated with increased adiposity. Therefore, females may exist at an inotropic state that is distinct from males due to this lack of FFA delivery in females because of this LPL inhibition and consequently decreased FFA delivery to myocardial cells.
This particular topic of substrate generation, utilization, and delivery and how it differs between the sexes perhaps best illustrates the need to understand the impact of lipids on the heart in the context of sex. For example, FFAs can impact myocardial contractile function and signaling. But, this effect will depend on the flux of substrate delivery and metabolism by the myocardium, which we know is different between males and females. Clearly, the dynamics of lipid metabolism and the delivery of these substrates to the heart will have a profound impact on cardiac function and, consequently, sex differences in cardiac disease development.
6. Conclusions
The difficulty that is presented to the contemporary scientific investigator is that differences between males and females exist. This difference is clinically relevant; yet there remain insufficient data to make global recommendations, especially when it comes to cardiac disease and CHF. The goal of this review was to highlight that, despite a growing body of literature describing sex dimorphisms in cardiac disease and some potential molecular mechanisms underlying these differences, there are lingering questions. For example, what is the relationship between diastolic dysfunction and CHF and how is this modified by sex? Furthermore, we must begin to define how comparisons between males and females are to be presented and whether it is appropriate to perform studies that directly compare males and females without providing how these underlying mechanisms operate in the proper sexual context. Despite implicit difficulties with the study of sex differences, significant advancements defining sex dimorphisms in CVD and CHF have been made.
Acknowledgment
This work was supported by a Mentored Research Scientist Development Award from the NIH awarded to J. P. Konhilas (K01 AR052840).
References
- 1.Villar AV, Llano M, Cobo M, et al. Gender differences of echocardiographic and gene expression patterns in human pressure overload left ventricular hypertrophy. Journal of Molecular and Cellular Cardiology. 2009;46(4):526–535. doi: 10.1016/j.yjmcc.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Villari B, Campbell SE, Schneider J, Vassalli G, Chiariello M, Hess OM. Sex-dependent differences in left ventricular function and structure in chronic pressure overload. European Heart Journal. 1995;16(10):1410–1419. doi: 10.1093/oxfordjournals.eurheartj.a060749. [DOI] [PubMed] [Google Scholar]
- 3.Dimitrow PP, Czarnecka D, Kawecka-Jaszcz K, Dubiel JS. The influence of age on gender-specific differences in the left ventricular cavity size and contractility in patients with hypertrophic cardiomyopathy. International Journal of Cardiology. 2003;88(1):11–16. doi: 10.1016/s0167-5273(02)00323-6. [DOI] [PubMed] [Google Scholar]
- 4.De Maria R, Gavazzi A, Recalcati F, Baroldi G, De Vita C, Camerini F. Comparison of clinical findings in idiopathic dilated cardiomyopathy in women versus men. American Journal of Cardiology. 1993;72(7):580–585. doi: 10.1016/0002-9149(93)90355-g. [DOI] [PubMed] [Google Scholar]
- 5.Douglas PS, Katz SE, Weinberg EO, Chen MH, Bishop SP, Lorell BH. Hypertrophic remodeling: gender differences in the early response to left ventricular pressure overload. Journal of the American College of Cardiology. 1998;32(4):1118–1125. doi: 10.1016/s0735-1097(98)00347-7. [DOI] [PubMed] [Google Scholar]
- 6.Deswal A, Bozkurt B. Comparison of morbidity in women versus men with heart failure and preserved ejection fraction. American Journal of Cardiology. 2006;97(8):1228–1231. doi: 10.1016/j.amjcard.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 7.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. Journal of the American College of Cardiology. 1992;20(2):301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 8.Martin CA, Thompson PL, Armstrong BK, Hobbs MS, de Klerk N. Long-term prognosis after recovery from myocardial infarction: a nine year follow-up of the Perth Coronary Register. Circulation. 1983;68(5):961–969. doi: 10.1161/01.cir.68.5.961. [DOI] [PubMed] [Google Scholar]
- 9.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. New England Journal of Medicine. 1999;341(4):217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 10.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial. Journal of the American Medical Association. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 11.Konhilas JP, Leinwand LA. The effects of biological sex and diet on the development of heart failure. Circulation. 2007;116(23):2747–2759. doi: 10.1161/CIRCULATIONAHA.106.672006. [DOI] [PubMed] [Google Scholar]
- 12.Dale KM, Coleman CI, Shah SA, Patel AA, Kluger J, White CM. Impact of gender on statin efficacy. Current Medical Research and Opinion. 2007;23(3):565–574. doi: 10.1185/030079906X167516. [DOI] [PubMed] [Google Scholar]
- 13.Knopp RH, Retzlaff BM. Saturated fat prevents coronary artery disease? An American paradox. American Journal of Clinical Nutrition. 2004;80(5):1102–1103. doi: 10.1093/ajcn/80.5.1102. [DOI] [PubMed] [Google Scholar]
- 14.Mittendorfer B. Sexual dimorphism in human lipid metabolism. Journal of Nutrition. 2005;135(4):681–686. doi: 10.1093/jn/135.4.681. [DOI] [PubMed] [Google Scholar]
- 15.Mittendorfer B, Patterson BW, Klein S. Effect of sex and obesity on basal VLDL-triacylglycerol kinetics. American Journal of Clinical Nutrition. 2003;77(3):573–579. doi: 10.1093/ajcn/77.3.573. [DOI] [PubMed] [Google Scholar]
- 16.Cullen P. Evidence that triglycerides are an independent coronary heart disease risk factor. American Journal of Cardiology. 2000;86(9):943–949. doi: 10.1016/s0002-9149(00)01127-9. [DOI] [PubMed] [Google Scholar]
- 17.LaRosa JC. Lipids and cardiovascular disease: do the findings and therapy apply equally to men and women? Women’s Health Issues. 1992;2(2):102–113. doi: 10.1016/s1049-3867(05)80278-6. [DOI] [PubMed] [Google Scholar]
- 18.Bush TL, Fried LP, Barrett-Connor E. Cholesterol, lipoproteins, and coronary heart disease in women. Clinical Chemistry. 1988;34(8):B60–B70. [PubMed] [Google Scholar]
- 19.Hsich EM, Piña IL. Heart failure in women. A need for prospective data. Journal of the American College of Cardiology. 2009;54(6):491–498. doi: 10.1016/j.jacc.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 21.Bleumink GS, Knetsch AM, Sturkenboom MCJM, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure—the Rotterdam Study. European Heart Journal. 2004;25(18):1614–1619. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 22.Hofman A, Grobbee DE, de Jong PTVM, van den Ouweland FA. Determinants of disease and disability in the elderly: the Rotterdam Elderly Study. European Journal of Epidemiology. 1991;7(4):403–422. doi: 10.1007/BF00145007. [DOI] [PubMed] [Google Scholar]
- 23.Ni H. Prevalence of self-reported heart failure among US adults: results from the 1999 National Health Interview Survey. American Heart Journal. 2003;146(1):121–128. doi: 10.1016/S0002-8703(02)94800-3. [DOI] [PubMed] [Google Scholar]
- 24.Mohan SB, Parker M, Wehbi M, Douglass P. Idiopathic dilated cardiomyopathy: a common but mystifying cause of heart failure. Cleveland Clinic Journal of Medicine. 2002;69(6):481–487. doi: 10.3949/ccjm.69.6.481. [DOI] [PubMed] [Google Scholar]
- 25.Ciampi Q, Villari B. Role of echocardiography in diagnosis and risk stratification in heart failure with left ventricular systolic dysfunction. Cardiovascular Ultrasound. 2007;5, article 34 doi: 10.1186/1476-7120-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marmor A, Schneeweiss A. Prognostic value of noninvasively obtained left ventricular contractile reserve in patients with severe heart failure. Journal of the American College of Cardiology. 1997;29(2):422–428. doi: 10.1016/s0735-1097(96)00493-7. [DOI] [PubMed] [Google Scholar]
- 27.Alla F, Al-Hindi AY, Lee CR, Schwartz TA, Patterson JH, Adams KF., Jr. Relation of sex to morbidity and mortality in patients with heart failure and reduced or preserved left ventricular ejection fraction. American Heart Journal. 2007;153(6):1074–1080. doi: 10.1016/j.ahj.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. Journal of the American Medical Association. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 29.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. Journal of the American College of Cardiology. 1999;33(7):1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 30.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. New England Journal of Medicine. 2006;355(3):260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 31.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. New England Journal of Medicine. 2006;355(3):251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 32.Cowie MR, Mosterd A, Wood DA, et al. The epidemiology of heart failure. European Heart Journal. 1997;18(2):208–225. doi: 10.1093/oxfordjournals.eurheartj.a015223. [DOI] [PubMed] [Google Scholar]
- 33.Tschöpe C, Westermann D. Heart failure with normal ejection fraction. Pathophysiology, diagnosis, and treatment. Herz. 2009;34(2):89–96. doi: 10.1007/s00059-009-3197-6. [DOI] [PubMed] [Google Scholar]
- 34.Aurigemma GP. Diastolic heart failure—a common and lethal condition by any name. New England Journal of Medicine. 2006;355(3):308–310. doi: 10.1056/NEJMe068128. [DOI] [PubMed] [Google Scholar]
- 35.Borbély A, Falcao-Pires I, van Heerebeek L, et al. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circulation Research. 2009;104(6):780–786. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 36.Borbély A, Papp Z, Édes I, Paulus WJ. Molecular determinants of heart failure with normal left ventricular ejection fraction. Pharmacological Reports. 2009;61(1):139–145. doi: 10.1016/s1734-1140(09)70016-7. [DOI] [PubMed] [Google Scholar]
- 37.Radke MH, Peng J, Wu Y, et al. Targeted deletion of titin N2B region leads to diastolic dysfunction and cardiac atrophy. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(9):3444–3449. doi: 10.1073/pnas.0608543104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castanho VS, Oliveira LS, Pinheiro HP, Oliveira HC, de Faria EC. Sex differences in risk factors for coronary heart disease: a study in a Brazilian population. BMC Public Health. 2001;1(1, article 3) doi: 10.1186/1471-2458-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Z, Otvos JD, Lamon-Fava S, et al. Men and women differ in lipoprotein response to dietary saturated fat and cholesterol restriction. Journal of Nutrition. 2003;133(11):3428–3433. doi: 10.1093/jn/133.11.3428. [DOI] [PubMed] [Google Scholar]
- 40.Hayward RA, Hofer TP, Vijan S. Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Annals of Internal Medicine. 2006;145(7):520–530. doi: 10.7326/0003-4819-145-7-200610030-00010. [DOI] [PubMed] [Google Scholar]
- 41.Vasan RS, Larson MG, Levy D, Evans JC, Benjamin EJ. Distribution and categorization of echocardiographic measurements in relation to reference limits: the Framingham Heart Study: formulation of a height- and sex-specific classification and its prospective validation. Circulation. 1997;96(6):1863–1873. doi: 10.1161/01.cir.96.6.1863. [DOI] [PubMed] [Google Scholar]
- 42.Konhilas JP, Maass AH, Luckey SW, Stauffer BL, Olson EN, Leinwand LA. Sex modifies exercise and cardiac adaptation in mice. American Journal of Physiology. 2004;287(6):H2768–H2776. doi: 10.1152/ajpheart.00292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stauffer BL, Konhilas JP, Luczak ED, Leinwand LA. Soy diet worsens heart disease in mice. Journal of Clinical Investigation. 2006;116(1):209–216. doi: 10.1172/JCI24676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luczak ED, Leinwand LA. Sex-based cardiac physiology. Annual Review of Physiology. 2009;71:1–18. doi: 10.1146/annurev.physiol.010908.163156. [DOI] [PubMed] [Google Scholar]
- 45.de Simone G, Devereux RB, Daniels SR, Meyer RA. Gender differences in left ventricular growth. Hypertension. 1995;26(6):979–983. doi: 10.1161/01.hyp.26.6.979. [DOI] [PubMed] [Google Scholar]
- 46.Olivetti G, Giordano G, Corradi D, et al. Gender differences and aging: effects on the human heart. Journal of the American College of Cardiology. 1995;26(4):1068–1079. doi: 10.1016/0735-1097(95)00282-8. [DOI] [PubMed] [Google Scholar]
- 47.Huxley VH. Sex and the cardiovascular system: the intriguing tale of how women and men regulate cardiovascular function differently. American Journal of Physiology. 2007;31(1):17–22. doi: 10.1152/advan.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cain PA, Ahl R, Hedstrom E, et al. Age and gender specific normal values of left ventricular mass, volume and function for gradient echo magnetic resonance imaging: a cross sectional study. BMC Medical Imaging. 2009;9, article 2 doi: 10.1186/1471-2342-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salton CJ, Chuang ML, O’Donnell CJ, et al. Gender differences and normal left ventricular anatomy in an adult population free of hypertension: a cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. Journal of the American College of Cardiology. 2002;39(6):1055–1060. doi: 10.1016/s0735-1097(02)01712-6. [DOI] [PubMed] [Google Scholar]
- 50.Shioura KM, Geenen DL, Goldspink PH. Sex-related changes in cardiac function following myocardial infarction in mice. American Journal of Physiology. 2008;295(2):R528–R534. doi: 10.1152/ajpregu.90342.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suga H. Left ventricular time-varying pressure-volume ratio in systole as an index of myocardial inotropism. Japanese Heart Journal. 1971;12(2):153–160. doi: 10.1536/ihj.12.153. [DOI] [PubMed] [Google Scholar]
- 52.Sagawa K, Maughan L, Suga H, Sunagawa K. Cardiac Contraction and the Pressure-Volume Relationship. New York, NY, USA: Oxford University Press; 1988. [Google Scholar]
- 53.Georgakopoulos D, Kass DA. Minimal force-frequency modulation of inotropy and relaxation of in situ murine heart. Journal of Physiology. 2001;534(2):535–545. doi: 10.1111/j.1469-7793.2001.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lankford EB, Kass DA, Maughan WL, Shoukas AA. Does volume catheter parallel conductance vary during a cardiac cycle? American Journal of Physiology. 1990;258(6):H1933–H1942. doi: 10.1152/ajpheart.1990.258.6.H1933. [DOI] [PubMed] [Google Scholar]
- 55.Tomita M, Spinale FG, Crawford FA, Zile MR. Changes in left ventricular volume, mass, and function during the development and regression of supraventricular tachycardia-induced cardiomyopathy. Disparity between recovery of systolic versus diastolic function. Circulation. 1991;83(2):635–644. doi: 10.1161/01.cir.83.2.635. [DOI] [PubMed] [Google Scholar]
- 56.Kato R, Yokota M, Ishihara H, Sobue T. Correlation between left ventricular contractility and relaxation in patients with idiopathic dilated cardiomyopathy. Clinical Cardiology. 1996;19(5):413–418. doi: 10.1002/clc.4960190516. [DOI] [PubMed] [Google Scholar]
- 57.Dobesh DP, Konhilas JP, de Tombe PP. Cooperative activation in cardiac muscle: impact of sarcomere length. American Journal of Physiology. 2002;282(3):H1055–H1062. doi: 10.1152/ajpheart.00667.2001. [DOI] [PubMed] [Google Scholar]
- 58.Kentish JC, ter Keurs HEDJ, Ricciardi L, Bucx JJ, Noble MI. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circulation Research. 1986;58(6):755–768. doi: 10.1161/01.res.58.6.755. [DOI] [PubMed] [Google Scholar]
- 59.Burkhoff D, Sugiura S, Yue DT, Sagawa K. Contractility-dependent curvilinearity of end-systolic pressure-volume relations. American Journal of Physiology. 1987;252(6):H1218–H1227. doi: 10.1152/ajpheart.1987.252.6.H1218. [DOI] [PubMed] [Google Scholar]
- 60.Schwertz DW, Beck JM, Kowalski JM, Ross JD. Sex differences in the response of rat heart ventricle to calcium. Biological Research for Nursing. 2004;5(4):286–298. doi: 10.1177/1099800403262615. [DOI] [PubMed] [Google Scholar]
- 61.Wang SN, Wyeth RP, Kennedy RH. Effects of gender on the sensitivity of rat cardiac muscle to extracellular Ca2+ European Journal of Pharmacology. 1998;361(1):73–77. doi: 10.1016/s0014-2999(98)00736-5. [DOI] [PubMed] [Google Scholar]
- 62.Curl CL, Delbridge LMD, Wendt IR. Sex differences in cardiac muscle responsiveness to Ca2+ and L-type Ca2+ channel modulation. European Journal of Pharmacology. 2008;586(1–3):288–292. doi: 10.1016/j.ejphar.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 63.Palmer BM, Wang Y, Teekakirikul P, et al. Myofilament mechanical performance is enhanced by R403Q myosin in mouse myocardium independent of sex. American Journal of Physiology. 2008;294(4):H1939–H1947. doi: 10.1152/ajpheart.00644.2007. [DOI] [PubMed] [Google Scholar]
- 64.Thawornkaiwong A, Pantharanontaga J, Wattanapermpool J. Hypersensitivity of myofilament response to Ca2+ in association with maladaptation of estrogen-deficient heart under diabetes complication. American Journal of Physiology. 2007;292(2):R844–R851. doi: 10.1152/ajpregu.00365.2006. [DOI] [PubMed] [Google Scholar]
- 65.Wattanapermpool J, Reiser PJ. Differential effects of ovariectomy on calcium activation of cardiac and soleus myofilaments. American Journal of Physiology. 1999;277(2):H467–H473. doi: 10.1152/ajpheart.1999.277.2.H467. [DOI] [PubMed] [Google Scholar]
- 66.Wattanapermpool J. Increase in calcium responsiveness of cardiac myofilament activation in ovariectomized rats. Life Sciences. 1998;63(11):955–964. doi: 10.1016/s0024-3205(98)00353-1. [DOI] [PubMed] [Google Scholar]
- 67.Curl CL, Delbridge LMD, Canny BJ, Wendt IR. Testosterone modulates cardiomyocyte Ca2+ handling and contractile function. Physiological Research. 2009;58(2):293–297. doi: 10.33549/physiolres.931460. [DOI] [PubMed] [Google Scholar]
- 68.Petre RE, Quaile MP, Rossman EI, et al. Sex-based differences in myocardial contractile reserve. American Journal of Physiology. 2007;292(2):R810–R818. doi: 10.1152/ajpregu.00377.2006. [DOI] [PubMed] [Google Scholar]
- 69.Dash R, Frank KF, Carr AN, Moravec CS, Kranias EG. Gender influences on sarcoplasmic reticulum Ca2+-handling in failing human myocardium. Journal of Molecular and Cellular Cardiology. 2001;33(7):1345–1353. doi: 10.1006/jmcc.2001.1394. [DOI] [PubMed] [Google Scholar]
- 70.Leblanc N, Chartier D, Gosselin H, Rouleau JL. Age and gender differences in excitation-contraction coupling of the rat ventricle. Journal of Physiology. 1998;511(2):533–548. doi: 10.1111/j.1469-7793.1998.533bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wasserstrom JA, Kapur S, Jones S, et al. Characteristics of intracellular Ca2+ cycling in intact rat heart: a comparison of sex differences. American Journal of Physiology. 2008;295(5):H1895–H1904. doi: 10.1152/ajpheart.00469.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamdani N, de Waard M, Messer AE, et al. Myofilament dysfunction in cardiac disease from mice to men. Journal of Muscle Research and Cell Motility. 2008;29(6–8):189–201. doi: 10.1007/s10974-008-9160-y. [DOI] [PubMed] [Google Scholar]
- 73.Hamdani N, Kooij V, van Dijk S, et al. Sarcomeric dysfunction in heart failure. Cardiovascular Research. 2008;77(4):649–658. doi: 10.1093/cvr/cvm079. [DOI] [PubMed] [Google Scholar]
- 74.Solaro RJ. Multiplex kinase signaling modifies cardiac function at the level of sarcomeric proteins. Journal of Biological Chemistry. 2008;283(40):26829–26833. doi: 10.1074/jbc.R800037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Day SM, Westfall MV, Metzger JM. Tuning cardiac performance in ischemic heart disease and failure by modulating myofilament function. Journal of Molecular Medicine. 2007;85(9):911–921. doi: 10.1007/s00109-007-0181-6. [DOI] [PubMed] [Google Scholar]
- 76.de Tombe PP, Solaro RJ. Integration of cardiac myofilament activity and regulation with pathways signaling hypertrophy and failure. Annals of Biomedical Engineering. 2000;28(8):991–1001. doi: 10.1114/1.1312189. [DOI] [PubMed] [Google Scholar]
- 77.Solaro RJ. Control mechanisms regulating contractile activity of cardiac myofilaments. In: Sperelakis N, editor. Physiology and Pathophysiology of the Heart. Boston, Mass, USA: Kluwer Academic Publishers; 1995. pp. 355–365. [Google Scholar]
- 78.Ross J, Jr., Sobel BE. Regulation of cardiac contraction. Annual Review of Physiology. 1972;34:47–90. doi: 10.1146/annurev.ph.34.030172.000403. [DOI] [PubMed] [Google Scholar]
- 79.Luo W, Grupp IL, Harrer J, et al. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of β-agonist stimulation. Circulation Research. 1994;75(3):401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- 80.Wolska BM, Stojanovic MO, Luo W, Kranias EG, Solaro RJ. Effect of ablation of phospholamban on dynamics of cardiac myocyte contraction and intracellular Ca2+ American Journal of Physiology. 1996;271(1):C391–C397. doi: 10.1152/ajpcell.1996.271.1.C391. [DOI] [PubMed] [Google Scholar]
- 81.Solaro RJ, Van Eyk J. Altered interactions among thin filament proteins modulate cardiac function. Journal of Molecular and Cellular Cardiology. 1996;28(2):217–230. doi: 10.1006/jmcc.1996.0021. [DOI] [PubMed] [Google Scholar]
- 82.Kranias EG. Regulation of calcium transport by protein phosphatase activity associated with cardiac sarcoplasmic reticulum. Journal of Biological Chemistry. 1985;260(20):11006–11010. [PubMed] [Google Scholar]
- 83.Janssen PML, de Tombe PP. Protein kinase A does not alter unloaded velocity of sarcomere shortening in skinned rat cardiac trabeculae. American Journal of Physiology. 1997;273(5):H2415–H2422. doi: 10.1152/ajpheart.1997.273.5.H2415. [DOI] [PubMed] [Google Scholar]
- 84.Konhilas JP, Wolska BM, Martin AF, Solaro RJ, de Tombe PP. PKA modulates length-dependent activation in murine myocardium. Biophysical Journal. 2000;78(1):p. 108A. [Google Scholar]
- 85.Kentish JC, McCloskey DT, Layland J, et al. Phosphorylation of troponin I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circulation Research. 2001;88(10):1059–1065. doi: 10.1161/hh1001.091640. [DOI] [PubMed] [Google Scholar]
- 86.Pi Y, Kemnitz KR, Zhang D, Kranias EG, Walker JW. Phosphorylation of troponin I controls cardiac twitch dynamics: evidence from phosphorylation site mutants expressed on a troponin I-null background in mice. Circulation Research. 2002;90(6):649–656. doi: 10.1161/01.res.0000014080.82861.5f. [DOI] [PubMed] [Google Scholar]
- 87.Garvey JL, Kranias EG, Solaro RJ. Phosphorylation of C-protein, troponin I and phospholamban in isolated rabbit hearts. Biochemical Journal. 1988;249(3):709–714. doi: 10.1042/bj2490709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Solaro RJ, Moir AJG, Perry SV. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature. 1976;262(5569):615–617. doi: 10.1038/262615a0. [DOI] [PubMed] [Google Scholar]
- 89.DiPaola NR, Sweet WE, Stull LB, Francis GS, Schomisch Moravec C. Beta-adrenergic receptors and calcium cycling proteins in non-failing, hypertrophied and failing human hearts: transition from hypertrophy to failure. Journal of Molecular and Cellular Cardiology. 2001;33(6):1283–1295. doi: 10.1006/jmcc.2001.1390. [DOI] [PubMed] [Google Scholar]
- 90.Bristow M. Antiadrenergic therapy of chronic heart failure: surprises and new opportunities. Circulation. 2003;107(8):1100–1102. doi: 10.1161/01.cir.0000054530.87613.36. [DOI] [PubMed] [Google Scholar]
- 91.Koshman YE, Piano MR, Russell B, Schwertz DW. Signaling responses after exposure to 5 alpha-dihydrotestosterone or 17 beta-estradiol in norepinephrine-induced hypertrophy of neonatal rat ventricular myocytes. Journal of Applied Physiology. 2010;108(3):686–-696. doi: 10.1152/japplphysiol.00994.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vikstrom KL, Factor SM, Leinwand LA. Mice expressing mutant myosin heavy chains are a model for familial hypertrophic cardiomyopathy. Molecular Medicine. 1996;2(5):556–567. [PMC free article] [PubMed] [Google Scholar]
- 93.Geisterfru-Lowrance AAT, Christe M, Conner DA, et al. A mouse model of familial hypertrophic cardiomyopathy. Science. 1996;272(5262):731–734. doi: 10.1126/science.272.5262.731. [DOI] [PubMed] [Google Scholar]
- 94.Konhilas JP, Watson PA, Maass A, et al. Exercise can prevent and reverse the severity of hypertrophic cardiomyopathy. Circulation Research. 2006;98(4):540–548. doi: 10.1161/01.RES.0000205766.97556.00. [DOI] [PubMed] [Google Scholar]
- 95.Freeman K, Colon-Rivera C, Olsson MC, et al. Progression from hypertrophic to dilated cardiomyopathy in mice that express a mutant myosin transgene. American Journal of Physiology. 2001;280(1):H151–H159. doi: 10.1152/ajpheart.2001.280.1.H151. [DOI] [PubMed] [Google Scholar]
- 96.Olsson MC, Palmer BM, Leinwand LA, Moore RL. Gender and aging in a transgenic mouse model of hypertrophic cardiomyopathy. American Journal of Physiology. 2001;280(3):H1136–H1144. doi: 10.1152/ajpheart.2001.280.3.H1136. [DOI] [PubMed] [Google Scholar]
- 97.Spindler M, Saupe KW, Christe ME, et al. Diastolic dysfunction and altered energetics in the αMHC(403/+) mouse model of familial hypertrophic cardiomyopathy. Journal of Clinical Investigation. 1998;101(8):1775–1783. doi: 10.1172/JCI1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Georgakopoulos D, Christe ME, Giewat M, Seidman CM, Seidman JG, Kass DA. The pathogenesis of familial hypertrophic cardiomyopathy: early and evolving effects from an α-cardiac myosin heavy chain missense mutation. Nature Medicine. 1999;5(3):327–330. doi: 10.1038/6549. [DOI] [PubMed] [Google Scholar]
- 99.Ingwall JS. ATP and the Heart. Boston, Mass, USA: Kluwer Academic Publishers; 2002. [Google Scholar]
- 100.Nascimben L, Ingwall JS, Lorell BH, et al. Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension. 2004;44(5):662–667. doi: 10.1161/01.HYP.0000144292.69599.0c. [DOI] [PubMed] [Google Scholar]
- 101.Degens H, de Brouwer KFJ, Gilde AJ, et al. Cardiac fatty acid metabolism is preserved in the compensated hypertrophic rat heart. Basic Research in Cardiology. 2006;101(1):17–26. doi: 10.1007/s00395-005-0549-0. [DOI] [PubMed] [Google Scholar]
- 102.Barger PM, Brandt JM, Leone TC, Weinheimer CJ, Kelly DP. Deactivation of peroxisome proliferator-activated receptor-α during cardiac hypertrophic growth. Journal of Clinical Investigation. 2000;105(12):1723–1730. doi: 10.1172/JCI9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Osorio JC, Stanley WC, Linke A, et al. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-α in pacing-induced heart failure. Circulation. 2002;106(5):606–612. doi: 10.1161/01.cir.0000023531.22727.c1. [DOI] [PubMed] [Google Scholar]
- 104.Kalsi KK, Smolenski RT, Pritchard RD, Khaghani A, Seymour AML, Yacoub MH. Energetics and function of the failing human heart with dilated or hypertrophic cardiomyopathy. European Journal of Clinical Investigation. 1999;29(6):469–477. doi: 10.1046/j.1365-2362.1999.00468.x. [DOI] [PubMed] [Google Scholar]
- 105.Sack MN, Disch DL, Rockman HA, Kelly DP. A role for Sp and nuclear receptor transcription factors in a cardiac hypertrophic growth program. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(12):6438–6443. doi: 10.1073/pnas.94.12.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Djouadi F, Weinheimer CJ, Saffitz JE, et al. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor α-deficient mice. Journal of Clinical Investigation. 1998;102(6):1083–1091. doi: 10.1172/JCI3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nöhammer C, Brunner F, Wölkart G, et al. Myocardial dysfunction and male mortality in peroxisome proliferator-activated receptor alpha knockout mice overexpressing lipoprotein lipase in muscle. Laboratory Investigation. 2003;83(2):259–269. doi: 10.1097/01.lab.0000053916.61772.ca. [DOI] [PubMed] [Google Scholar]
- 108.Nielsen S, Guo Z, Albu JB, Klein S, O’Brien PC, Jensen MD. Energy expenditure, sex, and endogenous fuel availability in humans. Journal of Clinical Investigation. 2003;111(7):981–988. doi: 10.1172/JCI16253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lewis GF. Fatty acid regulation of very low density lipoprotein production. Current Opinion in Lipidology. 1997;8(3):146–153. doi: 10.1097/00041433-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 110.Magkos F, Patterson BW, Mohammed BS, Klein S, Mittendorfer B. Women produce fewer but triglyceride-richer very low-density lipoproteins than men. Journal of Clinical Endocrinology and Metabolism. 2007;92(4):1311–1318. doi: 10.1210/jc.2006-2215. [DOI] [PubMed] [Google Scholar]
- 111.Olofsson SO, Boren J. Apolipoprotein B: a clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. Journal of Internal Medicine. 2005;258(5):395–410. doi: 10.1111/j.1365-2796.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- 112.Elovson J, Chatterton JE, Bell GT, et al. Plasma very low density lipoproteins contain a single molecule of apolipoprotein B. Journal of Lipid Research. 1988;29(11):1461–1473. [PubMed] [Google Scholar]
- 113.Fisher RM, Coppack SW, Humphreys SM, Gibbons GF, Frayn KN. Human triacylglycerol-rich lipoprotein subfractions as substrates for lipoprotein lipase. Clinica Chimica Acta. 1995;236(1):7–17. doi: 10.1016/0009-8981(95)06032-3. [DOI] [PubMed] [Google Scholar]
- 114.Spriet LL. Regulation of skeletal muscle fat oxidation during exercise in humans. Medicine and Science in Sports and Exercise. 2002;34(9):1477–1484. doi: 10.1097/00005768-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 115.Farese RV, Jr., Yost TJ, Eckel RH. Tissue-specific regulation of lipoprotein lipase activity by insulin/glucose in normal-weight humans. Metabolism. 1991;40(2):214–216. doi: 10.1016/0026-0495(91)90178-y. [DOI] [PubMed] [Google Scholar]
- 116.Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB Journal. 1999;13(14):2051–2060. doi: 10.1096/fasebj.13.14.2051. [DOI] [PubMed] [Google Scholar]
- 117.Nikkilä EA, Taskinen MR, Rehunen S, Härkönen M. Lipoprotein lipase activity in adipose tissue and skeletal muscle of runners: relation to serum lipoproteins. Metabolism. 1978;27(11):1661–1667. doi: 10.1016/0026-0495(78)90288-3. [DOI] [PubMed] [Google Scholar]
- 118.Urabe M, Yamamoto T, Kashiwagi T, et al. Effect of estrogen replacement therapy on hepatic triglyceride lipase, lipoprotein lipase and lipids including apolipoprotein E in climacteric and elderly women. Endocrine Journal. 1996;43(6):737–742. doi: 10.1507/endocrj.43.737. [DOI] [PubMed] [Google Scholar]
- 119.Price TM, O’Brien SN, Welter BH, George R, Anandjiwala J, Kilgore M. Estrogen regulation of adipose tissue lipoprotein lipase—possible mechanism of body fat distribution. American Journal of Obstetrics and Gynecology. 1998;178(1):101–107. doi: 10.1016/s0002-9378(98)70634-9. [DOI] [PubMed] [Google Scholar]
- 120.Homma H, Kurachi H, Nishio Y, et al. Estrogen suppresses transcription of lipoprotein lipase gene. Existence of a unique estrogen response element on the lipoprotein lipase promoter. Journal of Biological Chemistry. 2000;275(15):11404–11411. doi: 10.1074/jbc.275.15.11404. [DOI] [PubMed] [Google Scholar]
- 121.Flagg TP, Cazorla O, Remedi MS, et al. Ca2+-independent alterations in diastolic sarcomere length and relaxation kinetics in a mouse model of lipotoxic diabetic cardiomyopathy. Circulation Research. 2009;104(1):95–103. doi: 10.1161/CIRCRESAHA.108.186809. [DOI] [PubMed] [Google Scholar]
- 122.Haim TE, Wang W, Flagg TP, et al. Palmitate attenuates myocardial contractility through augmentation of repolarizing Kv currents. Journal of Molecular and Cellular Cardiology. 2010;48(2):395–405. doi: 10.1016/j.yjmcc.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Finck BN, Lehman JJ, Leone TC, et al. The cardiac phenotype induced by PPARα overexpression mimics that caused by diabetes mellitus. Journal of Clinical Investigation. 2002;109(1):121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Finck BN, Han X, Courtois M, et al. A critical role for PPARα-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(3):1226–1231. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Paige JA, Liao R, Hajjar RJ, et al. Effect of a high omega-3 fatty acid diet on cardiac contractile performance in Oncorhynchus mykiss. Cardiovascular Research. 1996;31(2):249–262. [PubMed] [Google Scholar]
- 126.Huang X, Walker JW. Myofilament anchoring of protein kinase C-epsilon in cardiac myocytes. Journal of Cell Science. 2004;117(10):1971–1978. doi: 10.1242/jcs.01044. [DOI] [PubMed] [Google Scholar]
- 127.Pi Y, Walker JW. Diacylglycerol and fatty acids synergistically increase cardiomyocyte contraction via activation of PKC. American Journal of Physiology. 2000;279(1):H26–H34. doi: 10.1152/ajpheart.2000.279.1.H26. [DOI] [PubMed] [Google Scholar]
- 128.Szentandrássy N, Pérez-Bido MR, Alonzo E, Negretti N, O’neill SC. Protein kinase A is activated by the n-3 polyunsaturated fatty acid eicosapentaenoic acid in rat ventricular muscle. Journal of Physiology. 2007;582(1):349–358. doi: 10.1113/jphysiol.2007.132753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fragasso G. Inhibition of free fatty acids metabolism as a therapeutic target in patients with heart failure. International Journal of Clinical Practice. 2007;61(4):603–610. doi: 10.1111/j.1742-1241.2006.01280.x. [DOI] [PubMed] [Google Scholar]
- 130.Tuunanen H, Engblom E, Naum A, et al. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation. 2006;114(20):2130–2137. doi: 10.1161/CIRCULATIONAHA.106.645184. [DOI] [PubMed] [Google Scholar]
- 131.Tuunanen H, Engblom E, Naum A, et al. Decreased myocardial free fatty acid uptake in patients with idiopathic dilated cardiomyopathy: evidence of relationship with insulin resistance and left ventricular dysfunction. Journal of Cardiac Failure. 2006;12(8):644–652. doi: 10.1016/j.cardfail.2006.06.005. [DOI] [PubMed] [Google Scholar]