Abstract
Central melanocortins (MC) evoke potent but transient anorectic responses with tachyphylaxis developing within days. We hypothesized that intermittent therapy using the MC analog, melanotan II (MTII), would minimize the tachyphylaxis and enhance the long-term efficacy of MTII treatment. F344/BN rats were infused with MTII or vehicle into the lateral ventricle by mini pump for 14 days. Half the MTII-infused rats were then given vehicle (MTII-On/Off), while the remaining received fresh MTII (MTII-On) for 10 days. Finally, pumps in both groups were replaced with ones containing fresh MTII for an additional 6 days. The first MTII application induced a 30% food reduction that attenuated within 5 days. Reapplication of MTII in MTII-On/Off rats, after the off period, invoked a new and equally robust anorectic response while continuation of MTII supplement in the MTII-On group did not change food intake from the control level. Body weights decreased similarly in both MTII groups at termination (day 30). Hypothalamic MC3 receptor, AgRP, and POMC expressions were unchanged, but MC4 receptor expression was diminished by 25%, and adiposity reduced by 80% in both MTII groups. Acetyl-CoA carboxylase 1 phosphorylation was elevated in perirenal fat by over 10 fold with either MTII treatment. In conclusion, intermittent MTII treatment preserves anorectic responses but does not prevent tachyphylaxis, whereas constant MTII application blunts further food response after the initial tachyphylaxis. Either form of MTII administration results in significant weight and adiposity reductions, involving perhaps fatty acid oxidation within specific adipose tissues.
Keywords: Melanocortin Activation, MTII, ACC1, Fatty Acid Synthesis, Tachyphylaxis
1. Introduction
The central melanocortin (MC) system is a therapeutic target for treating obesity [4, 14, 17-19, 28]. The principle central melanocortin, alpha-melanocyte simulating hormone (α-MSH), is among a family of bioactive peptides cleaved from a common precursor, pro-opiomelanocortin (POMC) [7]. Alpha-MSH binds to and activates the melanocortin-3 and melanocortin-4 receptors (MC3R and MC4R), the two major central MC receptors involved in homeostatic regulation of food intake and energy expenditure [7]. MC3R and MC4R lie downstream of leptin receptor activation [7, 25], and central administration of α-MSH or its synthetic analog, melanotan II (MTII), evoke leptin-like anorectic responses that mediate body fat and weight loss in various rodent models of obesity associated with leptin resistance [8, 10, 18, 23, 28]. Although, activation of the MC system is able to bypass leptin resistance, following either α-MSH or MTII treatment, there is a rapid attenuation of the suppression in food intake occurring within days, a phenomenon generally described as tachyphylaxis [5, 8, 11, 18, 23, 28]. This tachyphylaxis probably involves down-regulation of MC3Rs and MC4Rs as well as other compensatory mechanisms that could potentially limit the effectiveness of melanocortin agonists in treating chronic obesity [18]. We hypothesize that intermittent central MTII application will minimize this tachyphylaxis and thus, be more effective in reducing weight and adiposity than continuous central MTII infusion. To test this postulate, we infused MTII into the left lateral ventricle in the brain either continuously or intermittently in lean F344×BN male rats over a 30-day period, and compared selected physiological and biochemical parameters in these animals to those in the vehicle-treated rats.
2. Materials and methods
2.1 Reagents
MTII was purchased from Phoenix Pharmaceuticals, Inc. (Burlingame, CA 94010), and dissolved in the artificial cerebral spinal fluid (ACSF) to a final concentration of 80mM prior to use.
2.2 Animals
Three-month-old male F344 × Brown Norway rats were purchased from Harlan Sprague-Dawley (Indianapolis, IN). Upon arrival, rats were examined and remained in quarantine for one week. Animals were cared for in accordance with the principles of the Guide to the Care and Use of Experimental Animals. Rats were housed individually with a 12:12 h light:dark cycle (07:00 to 19:00 hr), and had free access to water and a standard chow diet.
2.3 Central MTII infusion
Rats were anesthetized with xylazine, 8 mg/kg, s.c. and 5 min later, 90 mg/kg ketamine, i.p. until a stable surgical plane of anesthesia was reached. The analgesic, Buprenorphine (0.05mg/kg) was injected s.c. prior to surgical operation and again 6 to 12 hours post surgery. MTII (1nmol/rat/day) or ACSF was introduced through a brain infusion cannula placed stereotaxically into the lateral ventricle (1.3 mm posterior to bregma, 1.9 mm lateral to the midsaggital suture and to a depth of 3.5 mm) at a rate of 0.5ul/hr. The cannula was connected to a subcutaneous osmotic mini pump through a catheter. All animals received ACSF for the first 10 days of surgical recovery phase after cannula implantation. Afterwards, the rats were first infused with MTII or vehicle for 14 days (pump 1). Then, pumps in half the MTII-infused rats were replaced with those containing vehicle (MTII-On/Off), while the remaining were replaced with those containing fresh MTII (MTII-On) for 10 days (pump 2). Finally, pumps in both groups were replaced with new ones containing fresh MTII for an additional 6 days (pump 3). The above scheme depicts the details of the experiment.
2.4 Tissue harvesting and preparation
Rats were killed by cervical dislocation under 85mg/kg pentobarbital anesthetic. Blood samples were collected by heart puncture, and serum was harvested by a 10-min centrifugation in serum separator tubes. The circulatory system was perfused with 20 ml of cold saline, and brown adipose tissue (BAT), perirenal and retroperitoneal white adipose tissues (PWAT and RTWAT), and hypothalamus were excised. The hypothalamus was removed by making an incision medial to piriform lobes, caudal to the optic chiasm and anterior to the cerebral crus to a depth of 2-3 mm [22]. The hypothalami were sonicated briefly in 0.3 ml 10mM Tris-HCL, pH 6.8, 2% SDS, and 0.08 μg/ml okadaic acid in the presence of the protease inhibitors, 1mM PMSF, 0.1mM benzamidine, and 2μM leupeptin. An aliquot of 100ul homogenate was removed and frozen for RNA isolation. The remaining homogenate was immediately boiled and stored frozen at -80°C for Western analysis. Protein concentrations were determined using the DC protein assay kit (Bio-Rad, Hercules, CA). BAT and WAT samples were filtered through a 0.45micron syringe filter (Whatman, Clifton, NJ) to remove lipid particles prior to protein measurements.
2.5 Western analysis
Uncoupling protein 1 (UCP1) in BAT homogenates was measured with anti-human UCP1 antibody (Linco Research, St. Charles, MO) [15]. To determine the phosphorylated acetyl-CoA carboxylase 1 (P-ACC1) and total ACC1 in PWAT, the protein homogenate (20 - 50 μg) were assessed with either a monoclonal antibody specific to the P-ACC (Upstate Cell Signaling Solutions, Lake Placid, NY) or a Streptavidin-HRP-conjugated antibody (at 1:10,000 dilution) specific for the biotin-associated total ACC1 (PIERCE Biotechnology, Rockford, IL), and visualized by enhanced chemiluminescent detection (ECL-plus, Amersham Pharmacia Biotech, Piscataway, NJ).
2.6 RT-PCR
Expression levels of POMC, AgRP, MC3R and MC4R in the hypothalamus were identified by relative quantitative RT-PCR using the QuantumRNA 18S Internal Standards kit (Ambion, Austin, TX, USA) [15, 16, 18]. First-strand cDNA was generated from 1.5ug RNA in a 20ul volume using random primers containing 200 units of M-MLV reverse transcriptase (Life Technologies). RT-PCR was performed to co-amplify both sample signals and internal 18S standards by multiplexing specific primers, 18S primers, and competimers. The primer sequences employed for measuring each specific message are: MC3R; sense, 5′-AGCAACCGGAGTGGCAGT-3′, anti-sense, 5′-GGCCACGATCAAGG-AGAG-3′; MC4R; sense, 5′-AGTCTCTGGGGAAGGGGCA-3′, anti-sense, 5′-CAACTGA-TGATGATCCCGAC-3′; POMC; sense, 5′-GCTTGCAAACTCGACCTCTC-3′, anti-sense, 5′-CTTGATGATGGC-GTTCTTGA-3′; AgRP; sense, 5′-AGGGCATCAGAAGGCCTGA-CCAGG-3′, anti-sense, 5′-CTTGAAGAAGCGGCAGTAGCACGT-3′. Linearity for amplicons was determined to be 18 to 32 cycles for MC3R, 24-44 for MC4R, 20-40 for POMC and 22-28 for AgRP. The PCR products were electrophoresed on acrylamide gel and stained with SYBR green (Molecular Probes, Eugene, OR). Gels were scanned using a STORM fluorescent scanner and data analyzed using ImageQuant (Molecular Dynamics). The relative values of particular RNA transcript were derived from dividing the signal obtained for corresponding amplicon by that for 18S amplicon.
2.7 Statistical analysis
Results are presented as means ± SE. Repeated measures ANOVA was used for analyses of body weight and food intake. When the main effect was significant, the Bonferroni post-hoc test was applied to determine individual group differences between means. A value of p < 0.05 was considered significant. When data analysis only involved two groups, a student t-test was used to perform statistics.
3. Results
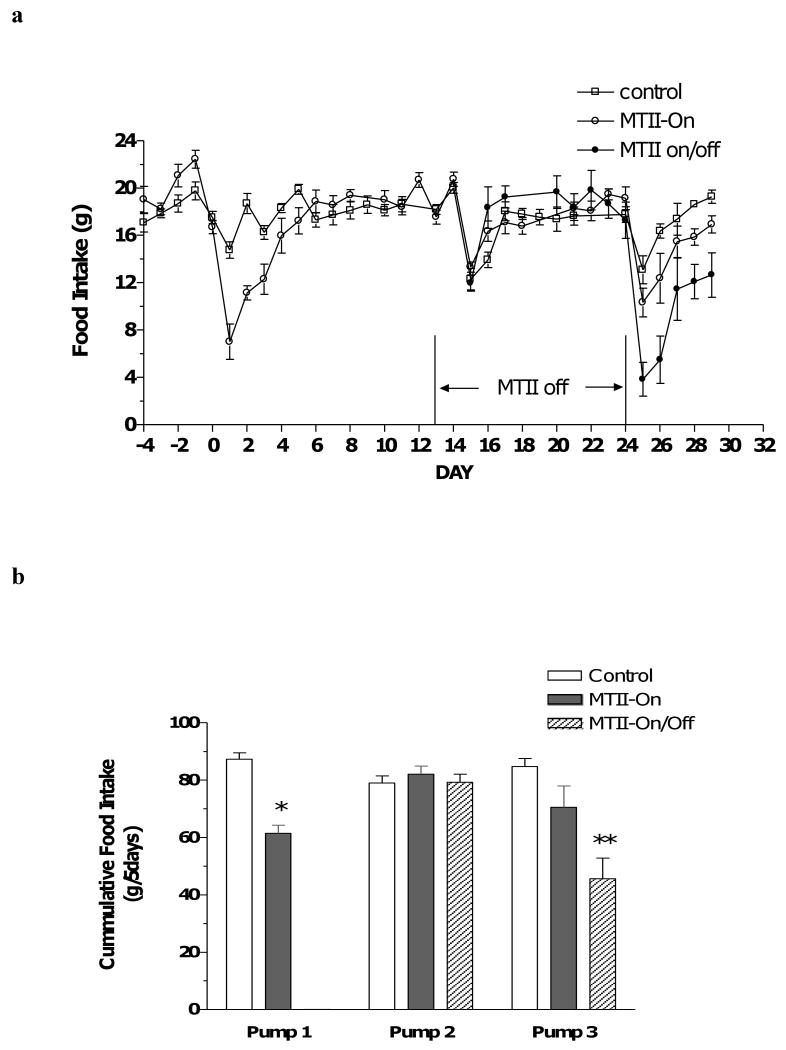
MTII evoked a strong anorectic response lasting 5 days upon first application (Fig. 1a, day 1-5). Following new pump replacement on day 14, the groups refreshed with ACSF (Control and MTII-On/Off) exhibited a transient reduction in food intake that rebounded quickly in two days, typical of the response following the surgery of pump replacement (Fig. 1a, day 15-17). The MTII-On group, after replenishment with fresh MTII, displayed a similar surgical effect but was devoid of any MTII-induced anorectic response observed with the initial introduction of MTII (Fig. 1a, days 15-17). During the third infusion period, the control rats, refreshed with ACSF, again exhibited a transient, surgery-related anorexia. The rats replenished with fresh MTII in the MTII-On group had a similar decrease in food intake as the controls, appeared also due to the surgical effect. On the other hand, the renewed MTII application in the MTII-On/Off rats suppressed food consumption significantly until the termination of the experiment (Fig. 1a, day 25-29). The calculated 5-day cumulative food intake for each infusion period is presented in Fig. 1b. The MTII application during the first pump infusion period induced a nearly 30% reduction in cumulative food intake (Fig. 1b, pump 1, solid bar), but this effect waned with persistent MTII treatment (Fig 1b, pump 2 and pump 3, solid bar). When switched to ACSF infusion, cumulative food intake in the MTII-On/Off group reverted to the level of the control group (Fig. 1b, pump 2, hatched bar). During the final infusion period, after refreshed with MTII again, the continuously infused MTII rats consumed a comparable amount of food as the control animals (Fig. 1b, pump 3, solid bar versus open bar), whereas cumulative food intake of the MTII-On/Off rats replenished with MTII decreased significantly compared to either the control or MTII group (Fig. 1b, pump 3, hatched bar versus either open or solid bar).
Figure 1.
a, daily food intake in Control (open square), MTII-On (open circle) and MTII-On/Off (closed circle) rats following ACSF or MTII infusion. b, cumulative food intake in Control (n=6, open bar), MTII-On (n=5, solid bar) and MTII-On/Off (n=5, hatched bar) rats for 5 days following initiation of ACSF or MTII infusion. Food consumption differs by t-test between MTII-On and Control (pump 1, * p < 0.001), or by one-way ANOVA and post hoc analysis between MTII-On/Off and Control and between MTII-On/Off and MTII-On groups (pump3, ** p < 0.01).
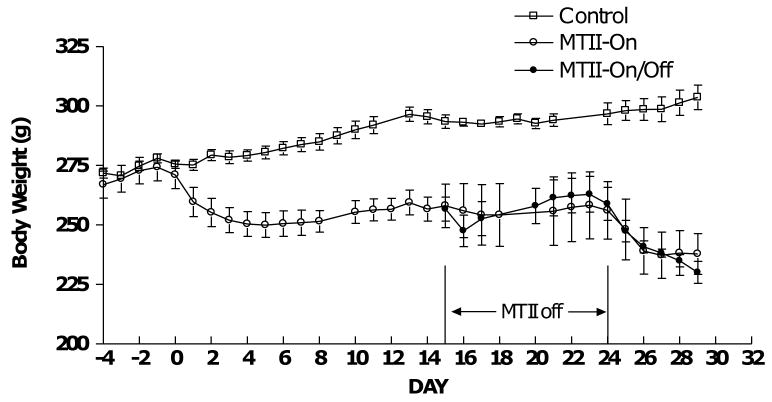
MTII caused a significant weight loss during the first infusion period (Fig. 2, days 1–14). The body weights remained relatively stable during the second infusion period in both the MTII-On and MTII-On/Off rats, but declined again in the third infusion period after replenishment with MTII beginning at day 25 (Fig. 2, days 15-30).
Figure 2.
Body mass in Control (n=6, open square), MTII-On (n=5, open circle) and MTII-On/Off (n=5, closed circle) rats following ACSF or MTII infusion. Body mass differs between MTII and control rats (p < 0.0001 by one-way ANOVA) beginning on day 3 and ending on day 29 in both the MTII-On and MTII-On/Off rats.
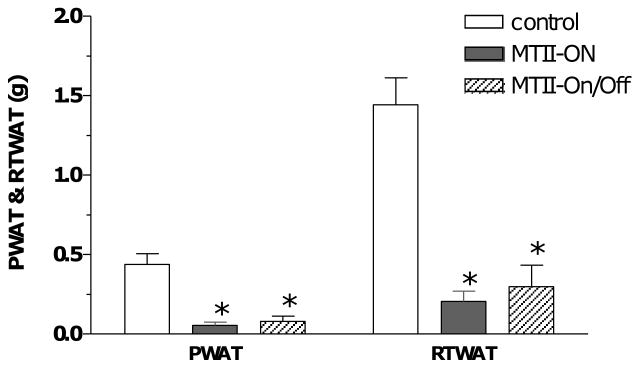
The animals responded similarly to either intermittent or continuous MTII treatment with respect to specific fat depot loss at the end of the experiment. Both the perirenal and retroperitoneal white adipose tissues (PWAT or RTWAT) were diminished by greater than 80% in the MTII-On and MTII-On/Off group (Fig. 3). The PWAT and RTWAT were completely depleted in several of the MTII-On and MTII-On/Off rats (data not shown).
Figure 3.
End point PWAT and RTWAT tissue mass. P < 0.0001 by one-way ANOVA with MTII treatment. By Post Hoc analysis, MTII-On animals had significantly less PWAT or RTWAT than the Controls (* p < 0.001), as did the MTII-On/Off animals versus the Controls (* p < 0.001). There was no statistical difference in adiposity between MTII-On versus MTII-On/Off groups. Note, PWAT was completely depleted in two out of five animals in the MTII-On group and one out of the five animals in the MTII-On/Off group.
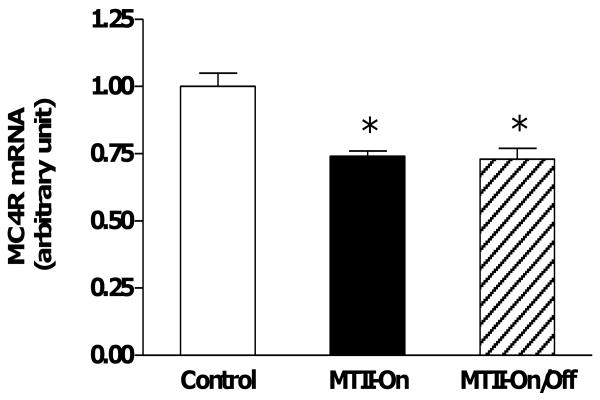
Neither hypothalamic expressions of AgRP, POMC, and MC3R, nor BAT UCP1 protein levels were altered by either MTII supplement regimen (Table 1). However, the mRNA levels of hypothalamic MC4R were significantly reduced by 23% following either the intermittent or continuous MTII treatment (Fig. 4).
Table 1. BAT UCP1 and Hypothalamic Expressions of POMC, AgRP and MC3R.
Data are means (n = 5-6 rats/group) ±SE. There are no statistical differences in BAT
| Control | MTII-On | MTII-On/Off | |
|---|---|---|---|
| BAT UCP1 (units/BAT) | 72.0 ± 8.3 | 64.4 ± 7.4 | 69.3 ± 7.9 |
| POMC mRNA (arbitrary units) | 0.89 ± 0.02 | 0.83 ± 0.02 | 0.84 ± 0.03 |
| AgRP mRNA (arbitrary units) | 1.27 ± 0.05 | 1.26 ± 0.05 | 1.51 ± 0.35 |
| MC3R mRNA (arbitrary units) | 0.68 ± 0.03 | 0.65 ± 0.02 | 0.57 ± 0.03 |
UCP1 protein levels, and hypothalamic expressions of POMC, AgRP and MC3R among all three groups by one-way ANOVA.
Figure 4.
MC4 receptor expression in the hypothalamus. p = 0.0003 by one-way ANOVA with MTII treatment. By Post Hoc analysis, hypothalamic MC4R expression was decreased significantly in both the MTII-On (n=5) and MTII-On/Off (n=5) animals as compared to the Controls (n=6) (* p < 0.01).
Inactivation of Acetyl-CoA carboxylase (ACC) by phosphorylation is one indicator of potentially diminished fatty acid synthesis and/or augmented fatty acid oxidation [12]. We examined phosphorylation of ACC1 at ser-79 at the termination of the experiment (day 30) in PWAT. Both the continuous and intermittent MTII treatments produced a substantial increase in the degree of phosphorylation of ACC1 without affecting the total ACC1 levels in perirenal white adipose tissue (Fig. 5a and b). A similar increase in P-ACC1 was also observed in RTWAT (data not shown).
Figure 5.
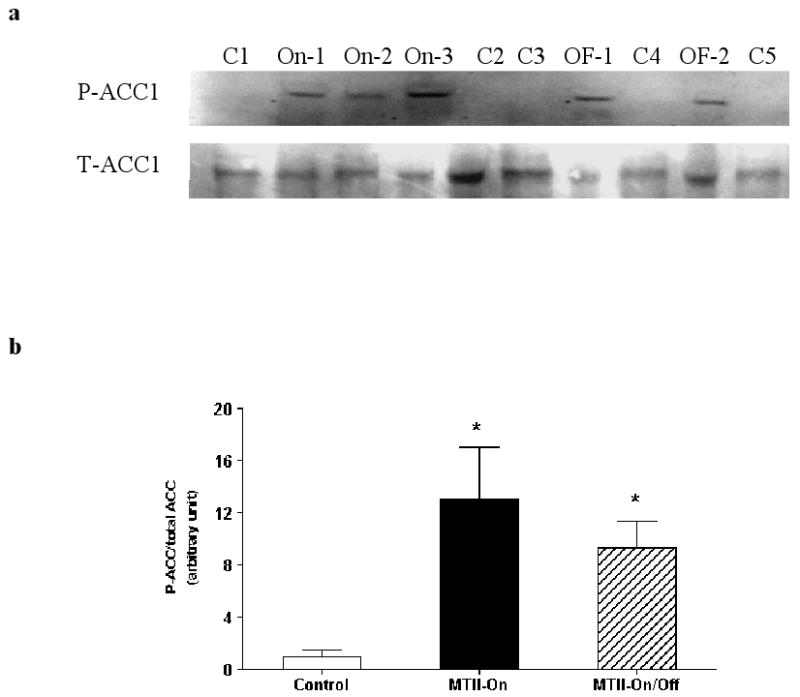
a, Representative Western blot of phosphorylation of ACC1 (P-ACC1) and total ACC1 (T-ACC1) in PWAT of Control (C1-C5), MTII-On (On-1, On-2 and On-3) and MTII-On/Off (OF-1 and OF-2) samples. b, Degree of ACC1 phosphorylation in PWAT expressed as a ratio of P-ACC1/total ACC1. p = 0.0037 with MTII treatment by one-way ANOVA. By post hoc analysis, P-ACC1 levels were significantly elevated in the MTII-On (p < 0.01) and MTII-On/Off (p < 0.05) groups relative to the Controls.
4. Discussion
Central melanocortin α-MSH is one endogenous regulator of energy balance, and its synthetic analog MTII produces robust reductions in food consumption and body weight in lean and obese animals [10, 18, 23, 28]. Typically, tachyphylaxis develops within a week following MTII administration with regards to both the anorectic and energy expenditure responses, potentially limiting the long-term efficacy of MTII as therapeutic modality for reversing obesity [5, 11, 18, 28].
This study was conducted to test our postulate that intermittent MTII therapy would minimize the tachyphylaxis and increase the efficacy of the treatment over that of continuous MTII infusion. After the initial tachyphylaxis to the anorexic response developed within a week following the first application of MTII, the intermittent approach evoked equally strong anorectic response during the second round of MTII application following a withdrawal period. This finding suggests a pliable central melanocortin system capable of rapid desensitization to melanocortin activation and resensitization after the withdrawal of the active compound. The continuous MTII supplement, however, failed to sustain the anorectic effect after the initial tachyphylaxis, which indicates a continued desensitization at least with respect to the anorexic response to constant MC activation.
Despite the obvious difference in energy intake responses between the intermittent and continuous MTII treatments, both regimens produced parallel reductions in body fat and weight in the end. One explanation for this observation could be that the multiple peripheral pump replacements (minor surgeries) and MTII administration prompted an interaction between surgery-related stress and MTII. The neuroendocrine milieu induced by the surgical manipulation may render the rats more sensitive to MTII or the continuous MTII presence may subject the animals to unusual vulnerability to the surgical manipulation. The impressive weight loss in the MTII-On rats during the pump 3 phase in the absence of a significant anorectic response seems to concur with these speculations. New experimentation with less surgical complication by using a gentler anesthesia procedure for example, will be required to further evaluate if the intermittent and continuous MTII administrations ultimately produce similar or different physiological and metabolic outcomes. It is unlikely the renewed body weight response in the MTII-On rats upon the third pump change is due to replacement of stale drug with fresh compound (the MTII was residing in pump 2 for 14 days). In fact, the stability of the MTII at 37°C for at least 28 days has been documented in literature [11].
Elevation in both hypothalamic NPY and AgRP expression levels was reported following 8-day peripheral MTII administration in lean or diet-induced obese mice [2]. In our case, the food consumption in all animals was recovering either from the surgical impact or MTII effect by day 30. This may explain the similar neuropeptide POMC and AgRP expression levels observed in the hypothalamus of all three groups. A more quantitative technique such as real-time PCR rather than the relative-quantitative PCR method used in this study could help to evaluate these neuropeptides more accurately. Alternatively, we found a significant reduction in the hypothalamic MC4R expression but not MC3R with both continuous and intermittent MTII treatments. This evidence is consistent with a ligand-induced receptor down-regulation, and confirms our previous findings [18]. Whether or not the reduction in MC4R expression specifically mediates the MTII-induced tachyphylaxis requires further investigation.
The remarkable adiposity loss at termination with either MTII treatment regimen cannot be fully explained by only a transient reduction in energy intake. Besides suppressing food intake, central administration of α-MSH or MTII is also known to increase energy expenditure by stimulating CNS sympathetic outflow [20, 24, 27]. The mRNA expression of MC4R, upon which α-MSH or MTII acts, has been identified in many brain regions implicated in lipid mobilization/metabolism, including the hypothalamic paraventricular nucleus, arcuate nucleus and dorsomedial nuclei [13, 26]. Conceivably, the melanocortins can stimulate fat oxidation via a direct central modulation of the sympathetic outflow to white adipose tissue through the MC4R. In the current study, we examined phosphorylation of acetyl-CoA carboxylase 1 at Ser-79 in perirenal fat depot. Enriched in liver, adipose and lactating mammary tissues, the activity of ACC1 is finely regulated by hormone-dependent phosphorylation and dephosphorylation [3, 12] and is the rate-limiting step for intracellular fatty acid synthesis [3, 12]. An increase in P-ACC1 suppresses ACC1 activity, leading to decreased fatty acid synthesis. We found P-ACC1 was highly elevated in both the PWAT and RTWAT for the two MTII-infused groups compared to that in the control rats. These data are indicative of a suppression of lipid synthesis in certain white adipose tissues and provide one potential mechanism underlying the MTII-mediated dramatic fat reduction. Other information in literature suggests that the central melanocortin system could enhance insulin sensitivity independent of energy intake at the level of white adipose tissue, liver and possibly muscle in rodents [1, 9, 21]. Therefore, MTII may decrease adiposity via broad periphery insulin action separate from any MTII-related impact on energy balance, even though this possibility was not examined in our experiment. Previous reports note that MTII administration in rodents elevates brown fat thermogenesis assessed as an increase in BAT UCP1 expression [6, 18, 27]. We did not detect an enhancement in UCP1 protein levels in BAT in either of the MTII groups. This result may be related to the long-term nature of this study.
In conclusion, we demonstrate that intermittent MTII application successfully preserves MTII's anorectic effect, but fails to reduce or avoid tachyphylaxis during periods when MTII is present. The continuous MTII treatment, as expected, induces an initial tachyphylaxis that persists throughout the treatment period. Both MTII application paradigms result in a remarkable and sustained weight and fat loss. The potent fat dissolution in response to MTII seems to at least involve enhanced lipid metabolism in specific fat depots.
Acknowledgments
This work was supported by the Medical Research Service of the Department of Veterans Affairs and NIH grants AG20985 and AG026159.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banno R, Arima H, Hayashi M, Goto M, Watanabe M, Sato I, Ozaki N, Nagasaki H, Oiso Y. Central administration of melanocortin agonist increased insulin sensitivity in diet-induced obese rats. FEBS Lett. 2007;581(6):1131–1136. doi: 10.1016/j.febslet.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Bluher S, Ziotopoulou M, Bullen JW, Jr, Moschos SJ, Ungsunan L, Kokkotou E, Maratos-Flier E, Mantzoros CS. Responsiveness to peripherally administered melanocortins in lean and obese mice. Diabetes. 2004;53(1):82–90. doi: 10.2337/diabetes.53.1.82. [DOI] [PubMed] [Google Scholar]
- 3.Brownsey RW, Boone AN, Elliott JE, Kulpa JE, Lee WM. Regulation of acetyl-CoA carboxylase. Biochem Soc Trans. 2006;34(Pt 2):223–227. doi: 10.1042/BST20060223. [DOI] [PubMed] [Google Scholar]
- 4.Butler AA, Cone RD. The melanocortin receptors: lessons from knockout models. Neuropeptides. 2002;36(2-3):77–84. doi: 10.1054/npep.2002.0890. [DOI] [PubMed] [Google Scholar]
- 5.Cabeza de Vaca S, Hao J, Afroz T, Krahne LL, Carr KD. Feeding, body weight, and sensitivity to non-ingestive reward stimuli during and after 12-day continuous central infusions of melanocortin receptor ligands. Peptides. 2005;26(11):2314–2321. doi: 10.1016/j.peptides.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 6.Cettour-Rose P, Rohner-Jeanrenaud F. The leptin-like effects of 3-d peripheral administration of a melanocortin agonist are more marked in genetically obese Zucker (fa/fa) than in lean rats. Endocrinology. 2002;143(6):2277–2283. doi: 10.1210/endo.143.6.8871. [DOI] [PubMed] [Google Scholar]
- 7.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8(5):571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 8.Hansen MJ, Ball MJ, Morris MJ. Enhanced inhibitory feeding response to alpha-melanocyte stimulating hormone in the diet-induced obese rat. Brain Res. 2001;892(1):130–137. doi: 10.1016/s0006-8993(00)03246-7. [DOI] [PubMed] [Google Scholar]
- 9.Heijboer AC, van den Hoek AM, Pijl H, Voshol PJ, Havekes LM, Romijn JA, Corssmit EP. Intracerebroventricular administration of melanotan II increases insulin sensitivity of glucose disposal in mice. Diabetologia. 2005;48(8):1621–1626. doi: 10.1007/s00125-005-1838-8. [DOI] [PubMed] [Google Scholar]
- 10.Hwa JJ, Ghibaudi L, Gao J, Parker EM. Central melanocortin system modulates energy intake and expenditure of obese and lean Zucker rats. AmJPhysiol RegulIntegrCompPhysiol. 2001;281(2):R444–R451. doi: 10.1152/ajpregu.2001.281.2.R444. [DOI] [PubMed] [Google Scholar]
- 11.Jonsson L, Skarphedinsson JO, Skuladottir GV, Watanobe H, Schioth HB. Food conversion is transiently affected during 4-week chronic administration of melanocortin agonist and antagonist in rats. J Endocrinol. 2002;173(3):517–523. doi: 10.1677/joe.0.1730517. [DOI] [PubMed] [Google Scholar]
- 12.Kim KH. Regulation of mammalian acetyl-coenzyme A carboxylase. Annu Rev Nutr. 1997;17:77–99. doi: 10.1146/annurev.nutr.17.1.77. [DOI] [PubMed] [Google Scholar]
- 13.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457(3):213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 14.Lee M, K A, Chua SC, Obici S, Wardlaw SL. Transgenic MSH Overexpression Attenuates the Metabolic Effects of a High Fat Diet. Am J Physiol Endocrinol Metab. 2007 doi: 10.1152/ajpendo.00555.2006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Li G, Klein RL, Matheny M, King MA, Meyer EM, Scarpace PJ. Induction of uncoupling protein 1 by central interleukin-6 gene delivery is dependent on sympathetic innervation of brown adipose tissue and underlies one mechanism of body weight reduction in rats. Neuroscience. 2002;115(3):879–889. doi: 10.1016/s0306-4522(02)00447-5. [DOI] [PubMed] [Google Scholar]
- 16.Li G, Mobbs CV, Scarpace PJ. Central pro-opiomelanocortin gene delivery results in hypophagia, reduced visceral adiposity, and improved insulin sensitivity in genetically obese Zucker rats. Diabetes. 2003;52(8):1951–1957. doi: 10.2337/diabetes.52.8.1951. [DOI] [PubMed] [Google Scholar]
- 17.Li G, Zhang Y, Wilsey JT, Scarpace PJ. Hypothalamic pro-opiomelanocortin gene delivery ameliorates obesity and glucose intolerance in aged rats. Diabetologia. 2005;48(11):2376–2385. doi: 10.1007/s00125-005-1943-8. [DOI] [PubMed] [Google Scholar]
- 18.Li G, Zhang Y, Wilsey JT, Scarpace PJ. Unabated anorexic and enhanced thermogenic responses to melanotan II in diet-induced obese rats despite reduced melanocortin 3 and 4 receptor expression. J Endocrinol. 2004;182(1):123–132. doi: 10.1677/joe.0.1820123. [DOI] [PubMed] [Google Scholar]
- 19.Marks DL, Butler AA, Cone RD. Melanocortin pathway: animal models of obesity and disease. Ann Endocrinol (Paris) 2002;63(2 Pt 1):121–124. [PubMed] [Google Scholar]
- 20.Matsumura K, Tsuchihashi T, Abe I, Iida M. Central alpha-melanocyte-stimulating hormone acts at melanocortin-4 receptor to activate sympathetic nervous system in conscious rabbits. Brain Res. 2002;948(1-2):145–148. doi: 10.1016/s0006-8993(02)03045-7. [DOI] [PubMed] [Google Scholar]
- 21.Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L. Central melanocortin receptors regulate insulin action. J Clin Invest. 2001;108(7):1079–1085. doi: 10.1172/JCI12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd. San Diego: Academic Press; 1997. [Google Scholar]
- 23.Pierroz DD, Ziotopoulou M, Ungsunan L, Moschos S, Flier JS, Mantzoros CS. Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes. 2002;51(5):1337–1345. doi: 10.2337/diabetes.51.5.1337. [DOI] [PubMed] [Google Scholar]
- 24.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23(14):5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46(12):2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 26.Song CK, Jackson RM, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2005;289(5):R1467–1476. doi: 10.1152/ajpregu.00348.2005. [DOI] [PubMed] [Google Scholar]
- 27.Williams DL, Bowers RR, Bartness TJ, Kaplan JM, Grill HJ. Brainstem melanocortin 3/4 receptor stimulation increases uncoupling protein gene expression in brown fat. Endocrinology. 2003;144(11):4692–4697. doi: 10.1210/en.2003-0440. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Matheny M, Tumer N, Scarpace PJ. Aged-obese rats exhibit robust responses to a melanocortin agonist and antagonist despite leptin resistance. Neurobiol Aging. 2004;25(10):1349–1360. doi: 10.1016/j.neurobiolaging.2004.02.012. [DOI] [PubMed] [Google Scholar]