Abstract
Cell expansion and metastasis are considered hallmarks of tumour progression. Therefore, efforts have been made to develop novel anti-cancer drugs that inhibit both the proliferation and the motility of tumour cells. Synthetic alkylphospholipids, compounds with aliphatic side chains that are ether linked to a glycerol backbone, are structurally derived from platelet-activating factor and represent a new class of drugs with anti-proliferative properties in tumour cells. These compounds do not interfere with the DNA or mitotic spindle apparatus of the cell. Instead, they are incorporated into cell membranes, where they accumulate and interfere with lipid metabolism and lipid-dependent signalling pathways. Recently, it has been shown that the most commonly studied alkylphospholipids inhibit proliferation by inducing apoptosis in malignant cells while leaving normal cells unaffected. This review focuses on a novel group of synthetic alkylphospholipids, the glycosidated phospholipids, which contain carbohydrates or carbohydrate-related molecules at the sn-2 position of the glycerol backbone. Members of this subfamily also exhibit anti-proliferative capacity and modulate the cell adhesion, differentiation, and migration of tumour cells. Among this group, Ino-C2-PAF shows the highest efficacy and low cytotoxicity. Apart from its anti-proliferative effect, Ino-C2-PAF strongly reduces cell motility via its inhibitory effect on the phosphorylation of the cytosolic tyrosine kinases FAK and Src. Signalling pathways under the control of the FAK/Src complex are normally required for both migration and proliferation and play a prominent role in tumour progression. We intend to highlight the potential of glycosidated phospholipids, especially Ino-C2-PAF, as a promising new group of drugs for the treatment of hyperproliferative and migration-based skin diseases.
Keywords: glycosidated phospholipids analogues, anti-tumour lipids, growth inhibition, cell matrix adhesion, migration apoptosis
Introduction
The development of anti-tumour drugs remains the most significant hurdle that modern medicine has to overcome. The first attempts to repress tumours through the use of chemotherapeutic tools were performed in the early 1940s. Later, researchers discovered and synthesized a pool of chemotherapeutic drugs (e.g. mercaptopurine, fluorouracil, vincristine and cisplatin) that displayed the following similar features: (i) inhibition of the tumour growth and proliferation as a consequence of the inhibition of RNA and DNA synthesis, (ii) inhibition of cell division via blockade of microtubule polymerization, and (iii) induction of apoptosis.
However, the impact of conventional chemotherapeutic agents affected not only tumour tissues, but also rapidly dividing cells of healthy organs (e.g. bone marrow, gastrointestinal epithelial cells and hair follicles). Furthermore, other organs, like the heart, liver and kidney, were also observed to be damaged. One of the major obstacles of the anti-cancer therapy was represented by the multi-drug resistance (MDR), whose mechanisms include accelerated drug efflux, drug inactivation, alterations in drug targeting and evasion of apoptosis (Wong and Goodin, 2009). Therefore, it was necessary to develop novel strategies to overcome these severe problems.
After more than a half century of cancer research, it is evident that new anti-tumour drugs should be metabolically stable, well adsorbed after oral administration, and accompanied by low toxicity at biologically effective doses while producing limited effects on the bone marrow and intestinal epithelium.
Development of anti-tumour lipids
In the early 1960s, it was observed that the generation of lysolecithin (2-lysophosphatidylcholine, LPC) by phospholipase A2 induced the phagocytic activity of peritoneal macrophages in vitro and in vivo (Munder et al., 1969; Munder and Modolell, 1973). Since LPC is not stable and becomes biologically inactivated either by the action of acyltransferase into lecithin (phosphatidylcholine, PC) or by lysophospholipase into glycerophosphocholine (Mulder and van Deenen, 1965), subsequent efforts have been made to synthesize metabolically stable LPC analogues for clinical research and trials. Some resultant synthetic phospholipid analogues not only worked as effective immune modulators (Munder et al., 1979), but also possessed selective anti-neoplastic activities in vitro and in vivo (Andreesen et al., 1978; 1979; Tarnowski et al., 1978; Modolell et al., 1979; Munder, 1982). Until now, compounds like edelfosine, miltefosine and perifosine have been tested for their anti-tumour activity in clinical phase I and phase II trials for a variety of tumours. Furthermore, miltefosine was the first anti-tumour lipid to be used clinically for the treatment of cutaneous metastases of mammary carcinomas (Unger and Eibl, 1988; Unger et al., 1990; Clive et al., 1999). Finally, alkylphospholipids represent a group of compounds that are attractive for use in combination with radiotherapy, since they enhance radiation-induced apoptosis. Encouraging results have been obtained with these compounds, primarily in the treatment of leukaemic malignancies (Vink et al., 2007).
General structure of anti-tumour lipids
The chemical structures of most currently used anti-tumour lipids (ATLs) can be divided into two main classes: (i) alkylphospholipids (APL) and (ii) alkylphosphocholines (APC). APLs are compounds with aliphatic side-chains that are ether linked to a glycerol backbone and are structurally derived from the platelet-activating factor (PAF; Figure 1), which is a naturally occurring phospholipid and a mediator of platelet aggregation and inflammation (Chignard et al., 1979; Prescott et al., 1990; Snyder, 1995; Wolf et al., 2006; Edwards and Constantinescu, 2009). The prototype of this class is 1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine (Et-18-OCH3, edelfosine, Figure 1), which presents an 18-C long alkyl-chain at the sn-1 position and a methoxy group at the sn-2 position of the glycerol backbone. In contrast, the glycerol backbone in APCs is absent. These molecules consist of a simple 16-C long-chain alcohol conjugated to the phosphocholine head group. Miltefosine (hexadecylphosphocholine, HePC, Figure 1) represents the prototype of this class. Another well-studied and promising new alkylphospholipid is perifosine (D-21266, octadecyl-(1,1-dimethyl-piperidino-4-yl)-phosphate), in which the choline moiety of miltefosine is replaced by a heterocyclic piperidine group (Figure 1).
Figure 1.
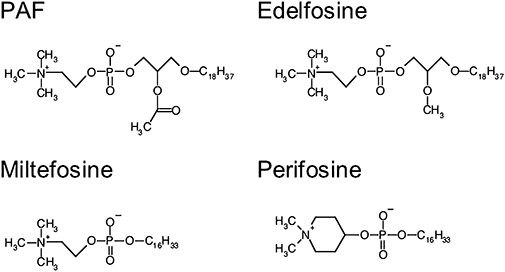
Structure of platelet-activating factor (PAF) and the common antitumour lipids edelfosine, miltefosine and perifosine.
Mechanism of action of common anti-tumour lipids
In the following sections, we will briefly illustrate the most relevant findings regarding the influence of the known ATLs (edelfosine, perifosine and miltefosine) on cellular morphology and signal transduction to provide an impression of options offered by anti-tumour lipids in general. For a more detailed review of the mechanisms of action of these anti-cancer alkylphospholipids, we recommend the reviews written by Gajate and Mollinedo (2002) and by van Blitterswijk and Verheij (2008).
Uptake/absorption of anti-tumour lipids
In contrast to commonly used cisplatin or vincristine, APLs do not interfere with the DNA or mitotic spindle apparatus of the cell; instead, they are incorporated into cell membranes, where they accumulate and interfere with a wide variety of key enzymes (Unger et al., 1992). At high concentrations, ATLs exert a detergent-like effect and cause cell lysis. In fact, the ordered plasma membrane bilayer is destroyed after the formation of micellar clusters. Pores with a size of about 1.5 µM, which represent up to 13% of the total membrane surface, are responsible for the lytic effect of ATLs (Tertoolen et al., 1988; Noseda et al., 1989). However, cell lines like fibroblasts, neutrophil granulocytes, glia cells and bone marrow precursor cells seem to be unaffected by the action of phospholipid analogues (Fleer et al., 1990).
In addition to this lytic action, it has been proposed that phospholipid analogues accumulate in cellular membranes via other mechanisms. Absorption in the outer layer of the membrane (Van blitterswijk et al., 1987; Kelley et al., 1993; Wiese et al., 2000) and subsequent uptake of ether lipids through passive diffusion has been discussed (Kelley et al., 1993; Van der Veer et al., 1993). Internalization of ATLs during membrane renewal has been proposed as an alternative mechanism (Bratton et al., 1992; Fleer et al., 1992), but a process catalysed by phosphatidylinositol transfer proteins is also possible (Wirtz, 1991). Recent studies suggest that the cellular uptake of APLs (e.g. edelfosine and perifosine) is dependent on the integrity of lipid rafts (Heczková and Slotte, 2006; Van der Luit et al., 2007; Ausili et al., 2008).
Furthemore, the similarity between the platelet-activating factor (PAF) and the ATLs might be relevant for the effects of these compounds. PAF is able to induce the programmed cell death in erythrocytes, called eryptosis, which is characterized by cell shrinkage, membrane blebbing and cell membrane phospholipd scrambling. The production of prostaglandin E2 in eryptosis results in the activation of cation channels and Ca2+ entry and/or release of PAF. The subsequent activation of sphingomyelinase and formation of ceramide stimulate scrambling of the cell membrane and consequently induce cell death (Lang et al., 2005; Föller et al., 2008).
Thus, a similar mechanism was presented for ATL as well. Treatment of leukemic and transformed cells with perifosine and miltefosine, respectively, leads to increased cellular ceramide content, mostly accompamied by ROS production, and causes apoptotic cell death (Wieder et al., 1998; Rahmani et al., 2005).
Influence of ATLs on proliferation, the cell cycle and apoptosis
Various signalling molecules that regulate proliferation and apoptosis have to be shown to be affected by ATLs. The biosynthesis of phosphatidylcholine (PC, Figure 2) is regarded as one of the main targets of ATLs. PC is the most abundant phospholipid in the eukaryotic plasma membrane, and its regulation has acquired new interest with the evidence that PC is involved in signal transduction processes (Daniel et al., 1986; Exton, 1990). Inhibition of its biosynthesis causes stress to cells, which is sufficient to stop cell growth and to induce apoptosis. The first studies with Madin–Darby canine kidney (MDCK) cells revealed that miltefosine stopped the incorporation of choline into phosphatidylcholine (Haase et al., 1991). Furthermore, it was demonstrated that miltefosine prevented the translocation of CTP:choline-phosphate cytidylyltransferase (CCT), which is the rate-limiting enzyme of PC biosynthesis, to the membrane (Geilen et al., 1992; Jimenez-lopez et al., 2002). Similarly, edelfosine is able to inhibit de novo PC biosynthesis at the CCT step, which results in cell cycle arrest at different stages (Boggs et al., 1995a,b; 1998; Baburina and Jackowski, 1998; Van der Luit et al., 2002).
Figure 2.
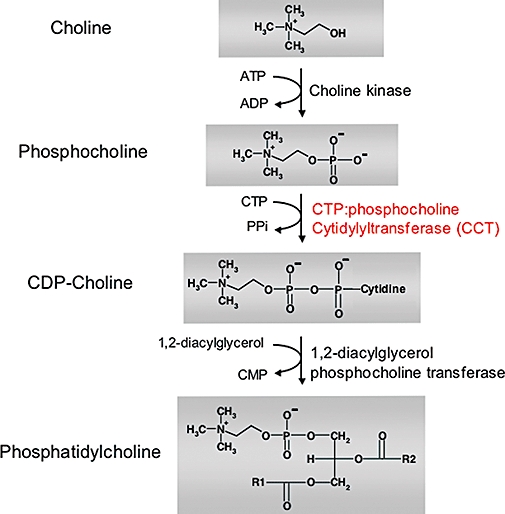
Biosynthesis of phosphatidylcholine.
In the early 1990s, edelfosine was demonstrated to trigger the apoptosis of tumour cells without affecting normal cells (Munder and Westphal, 1990; Diomede et al., 1993; Mollinedo et al., 1993; Houlihan et al., 1995). Mollinedo and coworkers proposed that the induction of apoptosis constitutes the critical step in the cytotoxic activity of edelfosine (Figure 3). They demonstrated that edelfosine affected the activity of anti-apoptotic proteins, such as Bcl-2 and Bcl-XL (Mollinedo et al., 1997). Moreover, the introduction of edelfosine into the cellular membrane induces (i) disruption of the mitochondrial transmembrane potential, (ii) DNA-fragmentation, (iii) cleavage of caspase-3 into p17 and PARP, and (iv) production of reactive oxygen species in human leukaemic T cell lines (e.g. Jurkat, Peer and HL-60 cells) (Cabaner et al., 1999; Gajate et al., 2000). Further studies demonstrated that the loss of mitochondrial membrane potential is caused by an alteration of the phosphocholine content of the mitochondrial membrane induced by edelfosine (Vrablic et al., 2001).
Figure 3.
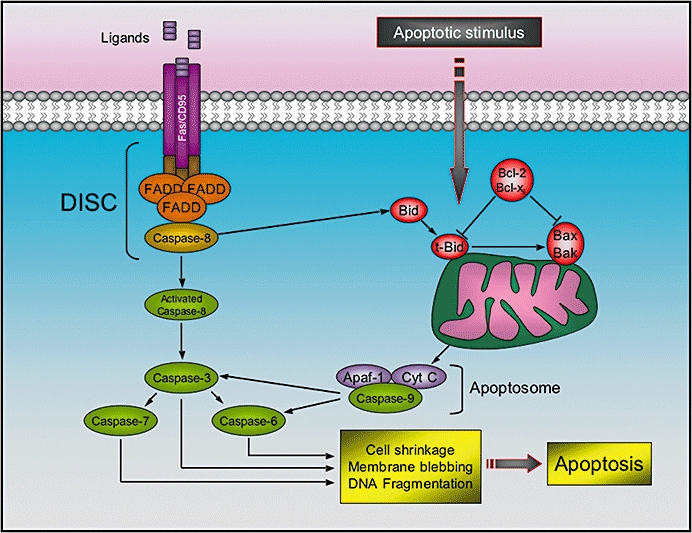
Intrinsic and extrinsic apoptotic pathways. The extrinsic pathway is initiated by binding of ligands (FasL, tumour necrosis factor, or other cytokines) to death receptors, which recruits the adaptor protein Fas-associated Death Domain (FADD), and, consequently, procaspase-8. The formation of the death-inducing-signaling-complex (DISC) results in cleavage and auto-activation of caspase-8. The activated caspase-8 can then proteolytically activate downstream caspases (particularly caspase-3), which induce cell shrinkage, membrane blebbing and DNA fragmentation. Apoptosis can also be triggered by various apoptotic stimuli, which results in the activation of the intrinsic pathway. Here, caspase-8 cleaves and activates Bid. Subsequently, t-Bid translocates to the mitochondria where it can induce the oligomerization of Bax and/or Bak in the outer mitochondrial membrane leading to cytochrome c release. Afterwards, Apaf-1 binds cytochrome c and recruits caspase-9 to form a large complex, called apoptosome, which is responsible for the following avtivation of caspase-3. Anti-apoptotic Bcl-2 and Bcl-xL oppose the effecst of Bid and Bax.
Another target of ATLs appears to be the dual lipid and protein kinase phosphatidylinositol-3 kinase (PI3K, shown in Figure 4), which contributes to the recruitment and activation of various signalling components important for proliferation, differentiation, motility, survival and intracellular trafficking. Alterations in the PI3K pathway are mostly correlated with cancer. Perifosine has been characterized as a potent and efficient inhibitor of the PI3K-pathway (for a review, see Gills and Dennis, 2009). This alkylphospholipid is able to inhibit the phosphorylation and membrane activation of the PI3K downstream effector Akt/PKB in a large number of tumour cells (e.g. leukemia, prostate cancer, non-small cell lung cancer, breast cancer, human endometrial adenocarcinoma, human glioma and epithelial carcinoma cells) (Kondapaka et al., 2003; Ruiter et al., 2003; Dasmahapatra et al., 2004; Momota et al., 2005; Rahmani et al., 2005; Nyakern et al., 2006; Elrod et al., 2007; Engel et al., 2008; Na and Surh, 2008; Papa et al., 2008; Tazzari et al., 2008; Korkaya et al., 2009).
Figure 4.
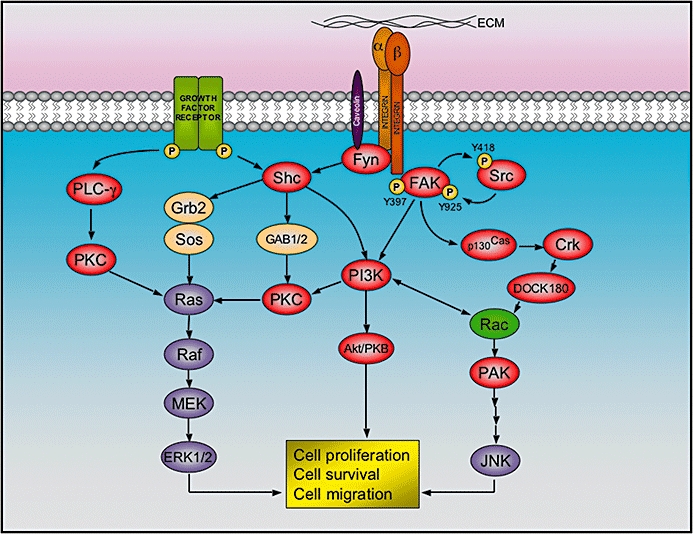
Integrin signalling at focal adhesions. After binding to the extracellular matrix (ECM), the cytoplasmic tail of integrin beta 1 recruits focal adhesion kinase (FAK), which is activated by auto-phosphorylation at the tyrosine 397, and thereby allow the binding and activation of Src by phosphorylation of tyrosine 418. The activated FAK/Src complex phosphorylates other kinases (i.e. p130Cas and PI3K), which in turn elicit a cascade of events that lead to cell proliferation, survival and migration. Alternatively, alpha subunits of certain integrins are able to recruit Fyn (a Src family member) via caveolin-1. This allows the activation of Shc, which combines with adaptor proteins Grb2 and SOS in order to activate the extracellular signal-regulated kinase (ERK)/MAP kinase pathway. As represented, the integrin pathway has reciprocal action with other signalling cascades triggered by several growth factor receptors.
Furthermore, ATLs affect MAPK pathways, which in concert regulate diverse cellular activities like gene expression, mitosis, metabolism, motility, survival, apoptosis and differentiation (for reviews, see Chen et al., 2001; Kyriakis and Avruch, 2001; Pearson et al., 2001, Figure 4). Treatment of different leukemic cell lines with edelfosine constitutively activates the MAP Kinase JNK, which results in increased mRNA levels of its substrate c-jun and induction of apoptosis (Gajate et al., 1998; Ruiter et al., 2003; Hideshima et al., 2006). This effect is enhanced when combined with radiation (Ruiter et al., 1999).
Additionally, edelfosine inhibits the Erk pathway, which is continuously activated in many cancers. Here, edelfosine is able to block the translocation of Raf-1 to the membrane, consequently inhibiting the interaction of Raf-1 with Ras and subsequently reducing Erk1/2 activity (Samadder and Arthur, 1999; Samadder et al., 2003; Na and Surh, 2008). In combination with other inhibitors and chemotherapeutic agents like temozolomide, ATLs inhibit Erk activity (Momota et al., 2005; Rahmani et al., 2005).
Protein kinase C (PKC) isozymes play a central role in cellular signalling and are involved in the regulation of cell proliferation, differentiation, apoptosis and angiogenesis. Therefore, dysfunction of PKC activity is associated with cancer of the prostate, breast, colon, pancreatic, liver and kidney (Griner and Kazanietz, 2007). Miltefosine and edelfosine inhibit phosphatidylserine-activated PKC in MDCK cells (Daniel et al., 1987; Geilen et al., 1991). Further studies have indicated that miltefosine and edelfosine do not interfere with PKC translocation, but decrease enzyme activity at the membrane and in the cytosol of cells (Berkovic et al., 1994). Finally, edelfosine is a stronger inhibitor than miltefosine.
Concept of glycosidated phospholipid analogues
In light of the severe side-effects of the existing anti-tumour lipids, efforts have been made to synthesize phospholipid analogues with high anti-proliferative capacity but less cytotoxicity.
In the late 1990s, we developed a novel concept by introducing carbohydrates or carbohydrate-related molecules to the chemical lead of APLs, which led to the synthesis of glycosidated phospholipids. Monosaccharides or monosaccharide-related molecules were introduced in the sn-2 position of the glycerol-backbone of lysophosphocholine or lyso-platelet-activating factor. The primary idea was to increase the hydrophilic properties of these compounds in order to confer higher water solubility without disrupting the hydrophobic character (given by the presence of the fatty acid) of the substance, thereby ensuring insertion of the substance into the plasma membrane. In subsequent studies, the use of monosaccharide-containing phospholipid analogues was also described by others. The group of Bittman replaced the phosphocholine head group by a carbohydrate moiety. This replacement resulted in improved efficacy in comparison to non-glycosidated, phosphocholine-containing compounds (Marino-albernas et al., 1996; Samadder et al., 1998).
Based on experiences with miltefosine (which is currently used for the topical treatment of skin metastases and cutaneous lymphomas (Unger et al., 1990; Dummer et al., 1992), the effects of glycosidated phospholipid analogues characterized by our group were primarily investigated in transformed cell lines derived from skin.
Structure of glycosidated phospholipids
Initially, d-glucose was introduced into phosphocholine (PC) and platelet-activating factor (PAF). The resulting compounds [Glc-PC and Glc-PAF, respectively (Figure 5)] displayed good anti-proliferative properties in HaCaT cells (Mickeleit et al., 1995; Mickeleit et al., 1998), a premalignant keratinocyte cell line (Boukamp et al., 1988), as well as in other tumour cells (von Haefen, unpublished data). Fluorescence-resonance-energy-transfer (FRET) analysis revealed that both Glc-PAF and Glc-PC intercalated in liposomes, and, moreover, were able to induce lesions in the plasma membrane of cells (Wiese et al., 2000). However, the effective concentrations of both glycosidated phospholipids were relatively close to their cytotoxic concentrations.
Figure 5.
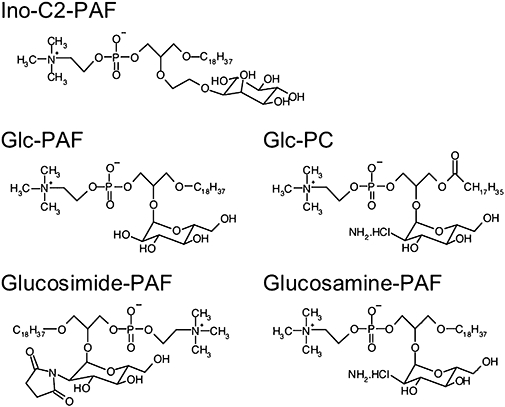
Structure of the glycosidated phospholipids Ino-C2- platelet-activating factor (PAF), Glc-PAF, Glc-PC, glucosimide-PAF and glucosamine-PAF.
Based on the structure of Glc-PAF, we synthesized a series of other glycosidated phospholipid analogues. These included derivatives with a shorter aliphatic side-chain, other carbohydrate-substitutes, or an ether link between the glycerol backbone and the carbohydrate-moiety. In addition to Glc-PC and C18-Glc-PAF, we synthesized C16-Glc-PAF, Glc-C2-PAF (not shown), Ino-C2-PAF (Fischer et al., 2006; Figure 5), glucosimide-PAF and glucosamine-PAF (Bartolmäs et al., 2005; Figure 5).
Biological activity of glycosidated phospholipids
It is generally thought that alkylphospholipids, which are only slightly different from platelet-activating factor (PAF), are able to bind to the G protein-coupled PAF receptor. As previously shown, however, edelfosine scarcely induces platelet aggregation and exerts its biological activity via ‘non-specific’ mechanisms (Kudo et al., 1987). Additionally, glycosidated phospholipids are not able to provoke signals that are normally induced following PAF receptor stimulation (Fischer, 2006).
To obtain information about the uptake of glycosidated phospholipids by HaCaT cells and the stability of these compounds within cells, we established an analytical method based on MALDI-TOF-MS. With this method, it was possible to clearly distinguish synthetic phospholipids from endogenous phospholipids by their characteristic molecular weights. The resulting data revealed that the analogues were taken up by HaCaT cells during the first hour of treatment. The compounds accumulated over time in cells and their stability was demonstrated by their detection after 48 h (Fischer et al., 2006).
In recent years, it has become increasingly clear that common ATLs influence membrane microdomains enriched in cholesterol, which represent essential platforms for the regulation of signal transduction events after uptake. In fact, it has been reported that modifications in the organization of lipid rafts by edelfosine play a decisive role in the induction of apoptosis in leukaemic cells (Gajate and Mollinedo, 2001; 2007; Gajate et al., 2004; 2009a,b; Mollinedo et al., 2004).
Nevertheless, detailed studies with Ino-C2-PAF and Glc-PAF do not show any influence on the structure or function of these membrane microdomains in Jurkat cells (von Haefen, unpublished data). As described later, this fact provides evidence of the differences between glycosidated and common anti-tumour phospholipids with regard to the induction of apoptosis in leukaemic cells.
Structure-activity relationship of glycosidated phospholipids with regard to cytotoxicity and proliferation
Due to their detergent-like character, glycosidated phospholipids like Glc-PAF and Glc-PC intercalate into cellular membranes. At higher concentrations, they exert a cytotoxic effect on cells by lesioning the plasma membrane and leading to rapid cell lysis (Wiese et al., 2000).
The anti-proliferative effects of these phospholipid analogues do not seem to be a simple consequence of their lytic properties because the proliferation of HaCaT cells was inhibited at non-toxic concentrations. In this regard, Ino-C2-PAF represents the most efficient glycosidated phospholipid analogue to be synthesized thus far. It inhibits the proliferation of HaCaT cells with an IC50 of 1.8 µM; its LC50 of 15 µM is clearly higher than the IC50. In contrast, the IC50 of Glc-PAF (4.8 µM) is very close to its LC50 (9 µM). The same results were obtained for miltefosine (IC50 of 3 µM and LC50 of 6 µM) (Danker et al., 2003).
The acyl-phospholipid Glc-PC (LC50 of 17 µM) is less toxic than glucosidated PAF-derivatives, but it is biologically less active (IC50 of 9 µM) (Mickeleit et al., 1995). Regarding the biological activity of ATLs, metabolic stability represents a very important and crucial attribute. In this context, alkylphospholipid analogues affect proliferation in a stronger manner than do acyl-phospholipids like Glc-PC. Ester bonds in the latter are presumably cleaved by endogenous esterases, where this confers metabolic instability and accounts for their restricted biological activity.
Due to steric hindrance, an ether linker had to be introduced between the inositol moiety and glycerol backbone of Ino-C2-PAF. To determine whether the linker or the inositol residue plays a more important role in improving growth inhibition (relative to Glc-PAF), an additional Glc-C2-PAF analogue was synthesized. Glc-C2-PAF inhibited the proliferation of HaCaT cells with an IC50 of 5 µM, which is comparable to the IC50 value of Glc-PAF (Fischer, 2006). This result indicates the importance of the cyclic alcohol inositol for its pharmacological effect. It is likely that the ether bond connecting inositol to glycerol is metabolically more stable than the acetal linkage between glucose and glycerol, which can be attacked by endogenous glucosidases.
Interestingly, the glucosamine-PAF, which possesses a LC50 of 30 µM, is the least toxic substance among the glycosidated APLs and only marginally inhibits cell proliferation (IC50 > 15 µM). In contrast, the related glucosimide-PAF is the most toxic substance (LC50 of 5 µM). Thus, its growth inhibitory capacity seems to be caused only by its cytotoxic effect (Bartolmäs et al., 2005).
In SCC-25 cells (a squamous carcinoma cell line from tongue), the anti-proliferative effects of the glycosidated phospholipid analogues are much stronger. In particular, Glc-PAF and Ino-C2-PAF act on these tumour cells in the sub-micromolar range (unpublished data).
Similar to other well-studied ATLs, Ino-C2-PAF is able to inhibit the proliferation of tumour cells in a selective manner. Studies with primary fibroblasts and peripheral blood cells have demonstrated that the cell growth of normal cells is only marginally influenced by this compound (Fischer, 2006; Fischer et al., 2006).
These results highlight the impact of the carbohydrate moiety and allow this new class of phospholipid analogues to serve as a new chemical lead.
Influence of glycosidated phospholipids on cell differentiation
The inhibition of the proliferation by glycosidated phospholipid analogues has been proposed to be due to the concerted interference of several processes and pathways. In vitro differentiation assays have demonstrated that the most active glycosidated phospholipid, Ino-C2-PAF, induced to some extent the differentiation of HaCaT cells (Fischer et al., 2006). At non-toxic concentrations of Ino-C2-PAF, the expression of the terminal differentiation marker involucrin increased. The expression of transglutaminase, another marker for terminal differentiation of keratinocytes, did not significantly change after treatment with Ino-C2-PAF; however, enzyme activity clearly increased (Fischer et al., 2006). Apparently, this is in clear contrast to the action of the APC miltefosine. Differentiation of HaCaT cells after treatment with miltefosine could not be detected. Nevertheless, this APC was able to induce apoptosis in HaCaT cells in a time and concentration-dependent manner (Wieder et al., 1998; Fischer, 2006). For Glc-PAF, similar results were observed at low but anti-proliferative concentrations (Mickeleit et al., 1998). In the presence of Ino-C2-PAF, an increased number of apoptotic HaCaT cells could be detected only after longer exposures and higher concentrations.
Induction of apoptosis by glycosidated phospholipids
While Ino-C2-PAF induces mainly differentiation in transformed keratinocytes and apoptosis only to a lesser extent, Glc-PAF and Ino-C2-PAF can induce apoptotic cell death in the leukaemia cell lines Jurkat and BJAB. Again, Ino-C2-PAF was more effective than Glc-PAF. Additionally, miltefosine and Glc-PC were ineffective in these defined cell lines. Both Ino-C2-PAF and Glc-PAF trigger a CD95/Fas ligand- and receptor-independent atypical death-inducing-signalling-complex (DISC, for overview, see Figure 3) that relies on the intrinsic apoptotic pathway via the endoplasmic reticulum, as well as the mitochondria (von Haefen, unpublished data). This is in clear contrast to studies with edelfosine and perifosine, which also induce apoptosis in lymphoid leukaemic cells, but mediate their apoptotic action in the target cell via a Fas-dependent mechanism (Gajate and Mollinedo, 2007; Gajate et al., 2009a,b;). This seems to be achieved by the modulation of cholesterol-rich microdomains, which results in the redistribution and clustering of Fas/CD95, Fas-associated Death Domain-containing proteins, and procaspase-8 into lipid rafts that form the DISC complex (Gajate and Mollinedo, 2001; 2007; Gajate et al., 2004; 2009b; Mollinedo et al., 2004). These discrepancies in matters of apoptosis induction point to different modes of action for glycosidated phospholipid analogues and common ATLs.
Glycosidated phospholipids influence integrin-dependent cell-matrix adhesion and migration
In addition to cell growth, migration of malignant cells or metastasis is considered as one of the hallmarks of tumour progression. While all known ATLs inhibit cell proliferation, their influence on cell motility differs depending on both the particular analogue and the cell system used.
Whereas Ino-C2-PAF and Glc-PAF reduce the motility of HaCaT and SCC-25 cells at relatively low concentrations, the related compounds Glc-PC, glucosamine-PAF, and glucosimide-PAF increase the migration of a keratinocyte cell line (Bartolmäs et al., 2005). Other ether lipids (e.g. miltefosine and edelfosine) have been previously reported to influence cellular motility as well. Both miltefosine and edelfosine reduce the capacity of malignant murine MO4 cells to invade precultured heart fragments (Schallier et al., 1991). At high concentrations, edelfosine reduces both invasion and haptotactic migration of transitional cell carcinoma (TCC) cells (Slaton et al., 1994). Recently, however, edelfosine was shown to inhibit cell–cell adhesion, and subsequently to induce the invasiveness of breast cancer cells (Van slambrouck et al., 2008). The particular effects of edelfosine and the specific differences between edelfosine and Ino-C2-PAF may be explained by a cell-type specificity of the compounds. Variations may also arise from the different methodical approaches used in the different studies. The structure and stability of the PAF-derived compounds may also contribute to their differential impact on migration and migration-relevant signalling pathways.
Integrin-mediated attachment to the extracellular matrix (ECM) is a prerequisite for the controlled movement of cells (Vicente-Manzanares et al., 2005). In reference to the skin, modifications of adhesive interactions with other cells and extracellular matrix components are required for the development and differentiation of keratinocytes (Kaufmann et al., 1990). In both malignant and benign hyperproliferative disorders of the epidermis, integrin-dependent adhesion is often impaired (De Luca et al., 1994; Schön et al., 1996).
While analogues affect migration in a different manner, all glycosidated analogues increase cell attachment to the integrin β1 ligands collagen IV, laminin-111 and fibronectin (Bartolmäs et al., 2005). This increase in adhesion is not achieved by up-regulation of the β1 integrin subunit. In fact, activation of β1 integrins by glycosidated phospholipids analogues is achieved by increased β1 integrin clustering, which results in the formation of enlarged focal adhesions. Subsequent to ligand binding, integrins induce various signal transduction pathways (Hynes, 2002). Since integrins lack intrinsic enzymatic activity, their signalling function is dependent on non-receptor tyrosine kinases like the focal adhesion kinase (FAK) and Src (Figure 4). FAK is activated upon integrin engagement and physically interacts with the β1 cytoplasmic tail, as well as various signalling molecules at the site of focal adhesions (Schaller et al., 1995; Danker et al., 1998; Chen et al., 2002). Once activated, FAK forms a dual kinase complex with Src that is subsequently able to phosphorylate various adaptor proteins like p130Cas and paxillin (Mitra et al., 2005).
Although integrins are activated after treatment with glycosidated phospholipids (especially Ino-C2-PAF), subsequent integrin signalling cascades (e.g. the FAK/Src pathways) are attenuated by Ino-C2-PAF-treatment (Figure 6). In skin cells, the non-receptor tyrosine kinase FAK has been shown to control cytoskeletal dynamics and focal adhesion disassembly. Accordingly, FAK-null keratinocytes display perturbed motility and fail to emigrate out of skin explants (Schober et al., 2007). Furthermore, FAK autophosphorylation has been shown to be required for the efficient recruitment of Src to focal adhesions (Schaller et al., 1994). Thus, the reduction of cellular motility caused by Ino-C2-PAF may be due to the inhibition of FAK autophosphorylation on tyrosine residue 397 and the consequent reduction in Src activation, which in turn negatively affects the disassembly of focal complexes and results in enlarged focal adhesions.
Figure 6.
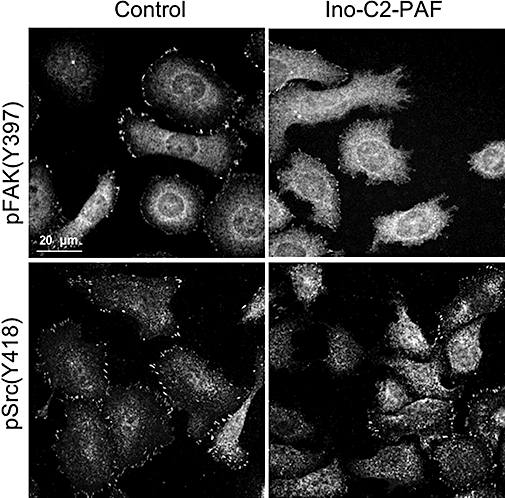
Treatment of HaCaT cells with Ino-C2-platelet-activating factor (PAF) attenuates phosphorylation of the cytoplasmic tyrosine kinases FAK and Src. HaCaT cells maintained under sub-confluent conditions and treated with either 5 µM Ino-C2-PAF or under control conditions were labelled for pSrc (Y418) and pFAK (Y397).
Mechanisms of action of glycosidated phospholipids
Modification of signalling pathways
The exact mechanism of action of glycosidated phospholipid analogues remains to be elucidated. Nevertheless, their influence on the biological processes outlined might result from intercalation into the phospholipid membrane of the target cell, which allows them to influence signal transduction pathways and particular biological processes. Thus, the mechanism by which glycosidated phospholipids act on cells follows a certain pattern. After incorporation into the plasma membrane, receptor systems are uncoupled from their specific pathways. Different modes have been observed. Treatment of leukaemia cell lines with Ino-C2-PAF or Glc-PAF leads to the formation of an atypical death-inducing signalling complex (DISC) and the subsequent induction of apoptosis (for overview see Figure 3). However, apoptosis induced by both glycosidated phospholipids proceeds in the absence of CD95/Fas death-ligand expression and is independent of blockade of a putative death-ligand/receptor interaction. In keratinocytes, integrins are clustered and activated upon treatment with Ino-C2-PAF. Nonetheless, subsequent integrin-dependent signalling events (e.g. phosphorylation of signalling molecules like FAK, Src and p130Cas) are inhibited.
Small GTPases are molecular switches that regulate the actin cytoskeleton but are also able to influence cell polarity, microtubule dynamics, membrane transport pathways and transcription factor activity (Etienne-Manneville and Hall, 2002). These GTPases are regulated by integrin engagement and are affected by Ino-C2-PAF treatment. In haptotactic migration assays, we showed that the inhibition of migration induced by Ino-C2-PAF could be rescued by transfection of a constitutively active variant of Rac1 or partially rescued by inhibition of the RhoA signalling pathway (Figure 7). These findings indicate that this phospholipid analogue interferes with the precisely regulated balance between different small GTPases.
Figure 7.
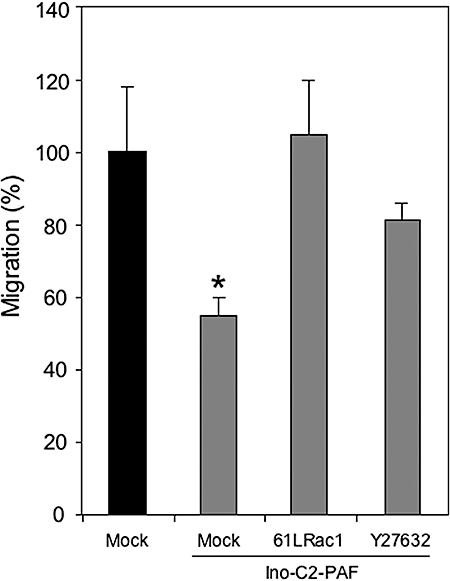
Inhibition of migration induced by Ino-C2-platelet-activating factor (PAF) can be rescued by transfection with a constitutively active variant of Rac1 or partially rescued by treatment with the pharmacological inhibitor Y27632 (RhoA signalling pathway inhibitor). Significant differences in migration compared with mock-transfected control cells are marked with an asterisk.
Influence on gene expression
Signalling pathways can be regulated at different levels through transcriptional and translational control, as well as post-translational modifications. Most studies with ATLs deal with mechanisms of post-translational modulation, such as the phosphorylation of key enzymes of the PI3K/Akt and MAPK pathways, cleavage of pro-enzymes like caspases in apoptosis, and conformational modifications that permit the activation and/or translocation of specific proteins to definite sub-cellular regions.
Until now, only a few publications have presented data obtained from expression studies after treatment with ATLs. Furthermore, these data result from a targeted search that considers only a group of genes (e.g. genes correlated with perifosine cytotoxicity) (Zhang et al., 2008).
In the context of cancer, the use of DNA microarray analysis has been established as a valuable tool for the identification and understanding of genes associated with cellular processes like proliferation, metastasis, apoptosis and multi-drug resistance.
In biological and pharmacological experiments, it is usually possible to analyse the expression of only a single or a small group of genes. With the advent of the first DNA microarrays, this problem has been overcome; the simultaneous analysis of global gene expression now provides additional information about regulatory mechanisms, biochemical pathways and other cellular functions. Moreover, this technique has become increasingly significant with regard to drug development, since gene expression microarrays permit the collection of a broad pharmacological and toxicological profile on a genome-wide basis (for a review, see Clarke et al., 2004).
DNA microarrays that contain sequences representing the currently known human genome were incubated with cDNA derived from mRNA of HaCaT cells treated with Ino-C2-PAF, Glc-PAF and edelfosine. Of these, Ino-C2-PAF had the strongest influence on gene expression. Approximately 600 genes were differentially expressed after a 24-h incubation with Ino-C2-PAF, whereas edelfosine regulated roughly 250 genes. Glc-PAF had the weakest influence on transcription, and its targets were mostly comprised of those genes regulated by Ino-C2-PAF (manuscript in preparation).
Interestingly, gene ontology analysis revealed that Ino-C2-PAF is able to down-regulate a broad spectrum of genes associated with the regulation of innate and acquired immune responses, as well as with inflammation. Additionally, edelfosine possesses an anti-inflammatory function. In fact, edelfosine induces the expression of the anti-inflammatory protein interleukin-10. It also inhibits neutrophil-endothelium interactions through the shedding of l-selectin from lipid rafts in two murine models (Mollinedo et al., 2009).
These data portray glycosidated phospholipids not only as anti-proliferative substances, but also as negative regulators of the immune system. Therefore, these compounds could also be tested as therapeutic tools to treat inflammatory hyperproliferative diseases like psoriasis.
Summary and outlook
The search for specific anti-cancer drugs that do not interfere with DNA synthesis or influence the cytoskeleton but affect other targets (e.g. the plasma membranes) offers a promising future line of research. In addition to the anti-tumour phospholipid analogues already established, a novel class of synthetic phospholipids has been described. In this class (known as the glycosidated phospholipid analogues), monosaccharides or monosaccharide-related molecules are introduced into the chemical lead of lyso-platelet-activating factor. All members of this novel subfamily inhibit cell proliferation, and some of them inhibit cell migration.
The efficacy of Ino-C2-PAF clearly separates it from the other glycosidated phospholipids. Data from DNA microarray experiments show that Ino-C2-PAF has a stronger impact on gene expression than edelfosine, the prototypical anti-tumour ether lipid.
Due to their entirely distinct actions, these membrane-active compounds may also be suitable for counteracting drug resistance when used in combination with other known cytostatic drugs. The real potential of glycosidated phospholipids remains to be evaluated in animal experiments.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (FOR 463), Berliner Krebsgesellschaft, and Sonnenfeld-Stiftung.
Glossary
Abbreviations:
- MALDI-TOF-MS
matrix assisted laser desorption ionization-time of flight-mass spectrometry
Conflicts of interest
We certify that there are no conflicts of interest.
References
- Andreesen R, Modolell M, Munder PG. Selective sensitivity of chronic myelogenous leukemia cell populations to alkyl-lysophospholipids. Blood. 1979;54:519–523. [PubMed] [Google Scholar]
- Andreesen R, Modolell M, Weltzien HU, Eibl H, Common HH, Lohr GW, et al. Selective destruction of human leukemic cells by alkyl-lysophospholipids. Cancer Res. 1978;38:3894–3899. [PubMed] [Google Scholar]
- Ausili A, Torrecillas A, Aranda FJ, Mollinedo F, Gajate C, Corbalan-Garcia S, et al. Edelfosine is incorporated into rafts and alters their organization. J Phys Chem B. 2008;112:11643–11654. doi: 10.1021/jp802165n. [DOI] [PubMed] [Google Scholar]
- Baburina I, Jackowski S. Apoptosis triggered by 1-o-octadecyl-2-o-methyl-rac-glycero-3-phosphocholine is prevented by increased expression of ctp:Phosphocholine cytidylyltransferase. J Biol Chem. 1998;273:2169–2173. doi: 10.1074/jbc.273.4.2169. [DOI] [PubMed] [Google Scholar]
- Bartolmäs T, Heyn T, Mickeleit M, Fischer A, Reutter W, Danker K. Glucosamine-glycerophospholipids that activate cell-matrix adhesion and migration. J Med Chem. 2005;48:6750–6755. doi: 10.1021/jm050558n. [DOI] [PubMed] [Google Scholar]
- Berkovic D, Berkovic K, Fleer EA, Eibl H, Unger C. Inhibition of calcium-dependent protein kinase C by hexadecylphosphocholine and 1-o-octadecyl-2-o-methyl-rac-glycero-3-phosphocholine do not correlate with inhibition of proliferation of HL60 and K562 cell lines. Eur J Cancer. 1994;30A:509–515. doi: 10.1016/0959-8049(94)90428-6. [DOI] [PubMed] [Google Scholar]
- Boggs KP, Rock CO, Jackowski S. Lysophosphatidylcholine and 1-o-octadecyl-2-o-methyl-rac-glycero-3-phosphocholine inhibit the CDP-choline pathway of phosphatidylcholine synthesis at the CTP:Phosphocholine cytidylyltransferase step. J Biol Chem. 1995a;270:7757–7764. doi: 10.1074/jbc.270.13.7757. [DOI] [PubMed] [Google Scholar]
- Boggs KP, Rock CO, Jackowski S. Lysophosphatidylcholine attenuates the cytotoxic effects of the antineoplastic phospholipid 1-o-octadecyl-2-o-methyl-rac-glycero-3-phosphocholine. J Biol Chem. 1995b;270:11612–11618. doi: 10.1074/jbc.270.19.11612. [DOI] [PubMed] [Google Scholar]
- Boggs K, Rock CO, Jackowski S. The antiproliferative effect of hexadecylphosphocholine toward HL60 cells is prevented by exogenous lysophosphatidylcholine. Biochim Biophys Acta. 1998;1389:1–12. doi: 10.1016/s0005-2760(97)00145-8. [DOI] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratton DL, Dreyer E, Kailey JM, Fadok VA, Clay KL, Henson PM. The mechanism of internalization of platelet-activating factor in activated human neutrophils. Enhanced transbilayer movement across the plasma membrane. J Immunol. 1992;148:514–523. [PubMed] [Google Scholar]
- Cabaner C, Gajate C, Macho A, Munoz E, Modolell M, Mollinedo F. Induction of apoptosis in human mitogen-activated peripheral blood t-lymphocytes by the ether phospholipid ET-18-OCH3: Involvement of the Fas receptor/ligand system. Br J Pharmacol. 1999;127:813–825. doi: 10.1038/sj.bjp.0702606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, et al. MAP kinases. Chem Rev. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- Chen BH, Tzen JT, Bresnick AR, Chen HC. Roles of Rho-associated kinase and myosin light chain kinase in morphological and migratory defects of focal adhesion kinase-null cells. J Biol Chem. 2002;277:33857–33863. doi: 10.1074/jbc.M204429200. [DOI] [PubMed] [Google Scholar]
- Chignard M, Le Couedic JP, Tence M, Vargaftig BB, Benveniste J. The role of platelet-activating factor in platelet aggregation. Nature. 1979;279:799–800. doi: 10.1038/279799a0. [DOI] [PubMed] [Google Scholar]
- Clarke PA, Poele R, Workman P. Gene expression microarray technologies in the development of new therapeutic agents. Eur J Cancer. 2004;40:2560–2591. doi: 10.1016/j.ejca.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Clive S, Gardiner J, Leonard RC. Miltefosine as a topical treatment for cutaneous metastases in breast carcinoma. Cancer Chemother Pharmacol. 1999;44(Suppl):S29–S30. doi: 10.1007/s002800051114. [DOI] [PubMed] [Google Scholar]
- Daniel LW, Etkin LA, Morrison BT, Parker J, Morris-Natschke S, Surles JR, et al. Ether lipids inhibit the effects of phorbol diester tumor promoters. Lipids. 1987;22:851–855. doi: 10.1007/BF02535543. [DOI] [PubMed] [Google Scholar]
- Daniel LW, Waite M, Wykle RL. A novel mechanism of diglyceride formation. 12-o-tetradecanoylphorbol-13-acetate stimulates the cyclic breakdown and resynthesis of phosphatidylcholine. J Biol Chem. 1986;261:9128–9132. [PubMed] [Google Scholar]
- Danker K, Fischer A, Reutter W. Modified and modifying sugars as a new tool for the development of therapeutic agents – glycosidated phospholipids as a new type of antiproliferative agents. In: Wong C-H, editor. Carbohydrate-Based Drug Discovery. Weinheim: Wiley-VCH; 2003. pp. 875–882. [Google Scholar]
- Danker K, Gabriel B, Heidrich C, Reutter W. Focal adhesion kinase pp125FAK and the beta 1 integrin subunit are constitutively complexed in HaCaT cells. Exp Cell Res. 1998;239:326–331. doi: 10.1006/excr.1997.3916. [DOI] [PubMed] [Google Scholar]
- Dasmahapatra GP, Didolkar P, Alley MC, Ghosh S, Sausville EA, Roy KK. In vitro combination treatment with perifosine and UCN-01 demonstrates synergism against prostate (PC-3) and lung (A549) epithelial adenocarcinoma cell lines. Clin Cancer Res. 2004;10:5242–5252. doi: 10.1158/1078-0432.CCR-03-0534. [DOI] [PubMed] [Google Scholar]
- De Luca M, Pellegrini G, Zambruno G, Marchisio PC. Role of integrins in cell adhesion and polarity in normal keratinocytes and human skin pathologies. J Dermatol. 1994;21:821–828. doi: 10.1111/j.1346-8138.1994.tb03296.x. [DOI] [PubMed] [Google Scholar]
- Diomede L, Colotta F, Piovani B, Re F, Modest EJ, Salmona M. Induction of apoptosis in human leukemic cells by the ether lipid 1-octadecyl-2-methyl-rac-glycero-3-phosphocholine. A possible basis for its selective action. Int J Cancer. 1993;53:124–130. doi: 10.1002/ijc.2910530123. [DOI] [PubMed] [Google Scholar]
- Dummer R, Roger J, Vogt T, Becker J, Hefner H, Sindermann H, et al. Topical application of hexadecylphosphocholine in patients with cutaneous lymphomas. Prog Exp Tumor Res. 1992;34:160–169. doi: 10.1159/000420841. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, Constantinescu CS. Platelet activating factor/platelet activating factor receptor pathway as a potential therapeutic target in autoimmune diseases. Inflamm Allergy Drug Targets. 2009;8:182–190. doi: 10.2174/187152809788681010. [DOI] [PubMed] [Google Scholar]
- Elrod HA, Lin YD, Yue P, Wang X, Lonial S, Khuri FR, et al. The alkylphospholipid perifosine induces apoptosis of human lung cancer cells requiring inhibition of akt and activation of the extrinsic apoptotic pathway. Mol Cancer Ther. 2007;6:2029–2038. doi: 10.1158/1535-7163.MCT-07-0004. [DOI] [PubMed] [Google Scholar]
- Engel JB, Honig A, Schonhals T, Weidler C, Hausler S, Krockenberger M, et al. Perifosine inhibits growth of human experimental endometrial cancers by blockade of Akt phosphorylation. Eur J Obstet Gynecol Reprod Biol. 2008;141:64–69. doi: 10.1016/j.ejogrb.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho gtpases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Exton JH. Effects of extracellular ATP on phosphatidylcholine phospholipase signaling systems. Ann N Y Acad Sci. 1990;603:246–254. doi: 10.1111/j.1749-6632.1990.tb37676.x. [DOI] [PubMed] [Google Scholar]
- Fischer A. 2006. Aufklärung der Wirkung eines neuartigen, modifizierten Phospholipidanalogons: inositol-C2-PAF. PhD Thesis, FU Berlin, Germany. [Google Scholar]
- Fischer A, Muller D, Zimmermann-Kordmann M, Kleuser B, Mickeleit M, Laabs S, et al. The ether lipid Inositol-C2-PAF is a potent inhibitor of cell proliferation in HaCaT cells. Chembiochem. 2006;7:441–449. doi: 10.1002/cbic.200500336. [DOI] [PubMed] [Google Scholar]
- Fleer EA, Berkovic D, Unger C, Eibl H. Cellular uptake and metabolic fate of hexadecylphosphocholine. Prog Exp Tumor Res. 1992;34:33–46. doi: 10.1159/000420830. [DOI] [PubMed] [Google Scholar]
- Fleer EA, Kim DJ, Nagel GA, Eibl H, Unger C. Cytotoxic activity of lysophosphatidylcholine analogues on human lymphoma Raji cells. Onkologie. 1990;13:295–300. doi: 10.1159/000216779. [DOI] [PubMed] [Google Scholar]
- Föller M, Huber SM, Lang F. Erythrocyte programmed cell death. IUBMB Life. 2008;60:661–668. doi: 10.1002/iub.106. [DOI] [PubMed] [Google Scholar]
- Gajate C, Del Canto-Janez E, Acuna AU, Amat-Guerri F, Geijo E, Santos-Beneit AM, et al. Intracellular triggering of Fas aggregation and recruitment of apoptotic molecules into Fas-enriched rafts in selective tumor cell apoptosis. J Exp Med. 2004;200:353–365. doi: 10.1084/jem.20040213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajate C, Gonzalez-Camacho F, Mollinedo F. Involvement of raft aggregates enriched in Fas/CD95 death-inducing signaling complex in the antileukemic action of edelfosine in Jurkat cells. Plos One. 2009a;4:e5044. doi: 10.1371/journal.pone.0005044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajate C, Gonzalez-Camacho F, Mollinedo F. Lipid raft connection between extrinsic and intrinsic apoptotic pathways. Biochem Biophys Res Commun. 2009b;380:780–784. doi: 10.1016/j.bbrc.2009.01.147. [DOI] [PubMed] [Google Scholar]
- Gajate C, Mollinedo F. The antitumor ether lipid ET-18-OCH3 induces apoptosis through translocation and capping of Fas/CD95 into membrane rafts in human leukemic cells. Blood. 2001;98:3860–3863. doi: 10.1182/blood.v98.13.3860. [DOI] [PubMed] [Google Scholar]
- Gajate C, Mollinedo F. Biological activities, mechanisms of action and biomedical prospect of the antitumor ether phospholipid ET-18-OCH3 (edelfosine), a proapoptotic agent in tumor cells. Curr Drug Metab. 2002;3:491–525. doi: 10.2174/1389200023337225. [DOI] [PubMed] [Google Scholar]
- Gajate C, Mollinedo F. Edelfosine and perifosine induce selective apoptosis in multiple myeloma by recruitment of death receptors and downstream signaling molecules into lipid rafts. Blood. 2007;109:711–719. doi: 10.1182/blood-2006-04-016824. [DOI] [PubMed] [Google Scholar]
- Gajate C, Santos-Beneit AM, Macho A, Lazaro M, Hernandez-De Rojas A, Modolell M, et al. Involvement of mitochondria and caspase-3 in ET-18-OCH3-induced apoptosis of human leukemic cells. Int J Cancer. 2000;86:208–218. doi: 10.1002/(sici)1097-0215(20000415)86:2<208::aid-ijc10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Gajate C, Santos-Beneit A, Modolell M, Mollinedo F. Involvement of c-jun NH2-terminal kinase activation and c-jun in the induction of apoptosis by the ether phospholipid 1-o-octadecyl-2-o-methyl-rac-glycero-3-phosphocholine. Mol Pharmacol. 1998;53:602–612. doi: 10.1124/mol.53.4.602. [DOI] [PubMed] [Google Scholar]
- Geilen CC, Haase R, Buchner K, Wieder T, Hucho F, Reutter W. The phospholipid analogue, hexadecylphosphocholine, inhibits protein kinase C in vitro and antagonises phorbol ester-stimulated cell proliferation. Eur J Cancer. 1991;27:1650–1653. doi: 10.1016/0277-5379(91)90438-j. [DOI] [PubMed] [Google Scholar]
- Geilen CC, Wieder T, Reutter W. Hexadecylphosphocholine inhibits translocation of CTP:Choline-phosphate cytidylyltransferase in Madin-Darby Canine Kidney cells. J Biol Chem. 1992;267:6719–6724. [PubMed] [Google Scholar]
- Gills JJ, Dennis PA. Perifosine: update on a novel Akt inhibitor. Curr Oncol Rep. 2009;11:102–110. doi: 10.1007/s11912-009-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- Haase R, Wieder T, Geilen CC, Reutter W. The phospholipid analogue hexadecylphosphocholine inhibits phosphatidylcholine biosynthesis in Madin-Darby Canine Kidney cells. FEBS Lett. 1991;288:129–132. doi: 10.1016/0014-5793(91)81018-4. [DOI] [PubMed] [Google Scholar]
- Heczková B, Slotte JP. Effect of anti-tumor ether lipids on ordered domains in model membranes. FEBS Lett. 2006;580:2471–2476. doi: 10.1016/j.febslet.2006.03.079. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053–4062. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlihan WJ, Lohmeyer M, Workman P, Cheon SH. Phospholipid antitumor agents. Med Res Rev. 1995;15:157–223. doi: 10.1002/med.2610150302. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Jimenez-Lopez JM, Carrasco MP, Segovia JL, Marco C. Hexadecylphosphocholine inhibits phosphatidylcholine biosynthesis and the proliferation of HEPG2 cells. Eur J Biochem. 2002;269:4649–4655. doi: 10.1046/j.1432-1033.2002.03169.x. [DOI] [PubMed] [Google Scholar]
- Kaufmann R, Weber L, Klein CE. [Integrins-new receptor molecules: their significance for the differentiation, regeneration and immune response of the skin] Hautarzt. 1990;41:256–261. [PubMed] [Google Scholar]
- Kelley EE, Modest EJ, Burns CP. Unidirectional membrane uptake of the ether lipid antineoplastic agent edelfosine by L1210 cells. Biochem Pharmacol. 1993;45:2435–2439. doi: 10.1016/0006-2952(93)90224-k. [DOI] [PubMed] [Google Scholar]
- Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2:1093–1103. [PubMed] [Google Scholar]
- Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. Plos Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo I, Nojima S, Chang HW, Yanoshita R, Hayashi H, Kondo E, et al. Antitumor activity of synthetic alkylphospholipids with or without PAF activity. Lipids. 1987;22:862–867. doi: 10.1007/BF02535545. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Lang PA, Kempe DS, Tanneur V, Eisele K, Klarl BA, Myssina S, et al. Stimulation of erythrocyte ceramide formation by platelet-activating factor. J Cell Sci. 2005;118:1233–1243. doi: 10.1242/jcs.01730. [DOI] [PubMed] [Google Scholar]
- Marino-Albernas JR, Bittman R, Peters A, Mayhew E. Synthesis and growth inhibitory properties of glycosides of 1-o-hexadecyl-2-o-methyl-sn-glycerol, analogs of the antitumor ether lipid ET-18-OCH3 (edelfosine) J Med Chem. 1996;39:3241–3247. doi: 10.1021/jm960164j. [DOI] [PubMed] [Google Scholar]
- Mickeleit M, Wieder T, Arnold M, Geilen CC, Mulzer J, Reutter W. A glucose-containing ether lipid (Glc-PAF) as an antiproliferative analogue of the platelet-activating factor. Angew Chem Int Ed. 1998;37:351–353. doi: 10.1002/(SICI)1521-3773(19980216)37:3<351::AID-ANIE351>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Mickeleit M, Wieder T, Buchner K, Geilen CC, Mulzer J, Reutter W. Glc-PC, a new type of glucosidic phospholipid. Angew Chem Int Ed Engl. 1995;34:2667–2669. [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Modolell M, Andreesen R, Pahlke W, Brugger U, Munder PG. Disturbance of phospholipid metabolism during the selective destruction of tumor cells induced by alkyl-lysophospholipids. Cancer Res. 1979;39:4681–4686. [PubMed] [Google Scholar]
- Mollinedo F, Fernandez-Luna JL, Gajate C, Martin-Martin B, Benito A, Martinez-Dalmau R, et al. Selective induction of apoptosis in cancer cells by the ether lipid ET-18-OCH3 (edelfosine): molecular structure requirements, cellular uptake, and protection by bcl-2 and bcl-x(L) Cancer Res. 1997;57:1320–1328. [PubMed] [Google Scholar]
- Mollinedo F, Gajate C, Martin-Santamaria S, Gago F. ET-18-OCH3 (edelfosine): a selective antitumour lipid targeting apoptosis through intracellular activation of Fas/CD95 death receptor. Curr Med Chem. 2004;11:3163–3184. doi: 10.2174/0929867043363703. [DOI] [PubMed] [Google Scholar]
- Mollinedo F, Gajate C, Morales AI, Canto-Janez E, Justies N, Collia F, et al. Novel anti-inflammatory action of edelfosine lacking toxicity with protective effect in experimental colitis. J Pharmacol Exp Ther. 2009;329:439–449. doi: 10.1124/jpet.108.148254. [DOI] [PubMed] [Google Scholar]
- Mollinedo F, Martinez-Dalmau R, Modolell M. Early and selective induction of apoptosis in human leukemic cells by the alkyl-lysophospholipid ET-18-OCH3. Biochem Biophys Res Commun. 1993;192:603–609. doi: 10.1006/bbrc.1993.1458. [DOI] [PubMed] [Google Scholar]
- Momota H, Nerio E, Holland EC. Perifosine inhibits multiple signaling pathways in glial progenitors and cooperates with temozolomide to arrest cell proliferation in gliomas in vivo. Cancer Res. 2005;65:7429–7435. doi: 10.1158/0008-5472.CAN-05-1042. [DOI] [PubMed] [Google Scholar]
- Mulder E, van Deenen LL. Metabolism of red-cell lipids. 3. Pathways for phospholipid renewal. Biochim Biophys Acta. 1965;106:348–356. doi: 10.1016/0005-2760(65)90043-3. [DOI] [PubMed] [Google Scholar]
- Munder PG. Antitumor activity of alkyllysophospholipids. Human Cancer Immunol. 1982;3:17–29. [Google Scholar]
- Munder PG, Ferber E, Modolell M, Fischer H. The influence of various adjuvants on the metabolism of phospholipids in macrophages. Int Arch Allergy Appl Immunol. 1969;36:117–128. doi: 10.1159/000230731. [DOI] [PubMed] [Google Scholar]
- Munder PG, Modolell M. Adjuvant induced formation of lysophosphatides and their role in the immune response. Int Arch Allergy Appl Immunol. 1973;45:133–135. doi: 10.1159/000231016. [DOI] [PubMed] [Google Scholar]
- Munder PG, Modolell M, Andreesen R, Weltzien HU, Westphal O. Lysophosphatidylcholine (lysolecithin) and its synthetic analogues. Immune modulating and other biologic effects. Immunopathology. 1979;2:187–203. [Google Scholar]
- Munder PG, Westphal O. Antitumoral and other biomedical activities of synthetic ether lysophospholipids. Chem Immunol. 1990;49:206–235. [PubMed] [Google Scholar]
- Na HK, Surh YJ. The antitumor ether lipid edelfosine (ET-18-OCH3) induces apoptosis in H-Ras transformed human breast epithelial cells: by blocking Erk1/2 and p38 mitogen-activated protein kinases as potential targets. Asia Pac J Clin Nutr. 2008;17(Suppl. 1):204–207. [PubMed] [Google Scholar]
- Noseda A, White JG, Godwin PL, Jerome WG, Modest EJ. Membrane damage in leukemic cells induced by ether and ester lipids: an electron microscopic study. Exp Mol Pathol. 1989;50:69–83. doi: 10.1016/0014-4800(89)90057-9. [DOI] [PubMed] [Google Scholar]
- Nyakern M, Cappellini A, Mantovani I, Martelli AM. Synergistic induction of apoptosis in human leukemia T cells by the Akt inhibitor perifosine and etoposide through activation of intrinsic and Fas-mediated extrinsic cell death pathways. Mol Cancer Ther. 2006;5:1559–1570. doi: 10.1158/1535-7163.MCT-06-0076. [DOI] [PubMed] [Google Scholar]
- Papa V, Tazzari PL, Chiarini F, Cappellini A, Ricci F, Billi AM, et al. Proapoptotic activity and chemosensitizing effect of the novel Akt inhibitor perifosine in acute myelogenous leukemia cells. Leukemia. 2008;22:147–160. doi: 10.1038/sj.leu.2404980. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Prescott SM, Zimmerman GA, McIntyre TM. Platelet-activating factor. J Biol Chem. 1990;265:17381–17384. [PubMed] [Google Scholar]
- Rahmani M, Reese E, Dai Y, Bauer C, Payne SG, Dent P, et al. Coadministration of histone deacetylase inhibitors and perifosine synergistically induces apoptosis in human leukemia cells through Akt and Erk1/2 inactivation and the generation of ceramide and reactive oxygen species. Cancer Res. 2005;65:2422–2432. doi: 10.1158/0008-5472.CAN-04-2440. [DOI] [PubMed] [Google Scholar]
- Ruiter GA, Zerp SF, Bartelink H, van Blitterswijk WJ, Verheij M. Alkyl-lysophospholipids activate the SAPK/JNK pathway and enhance radiation-induced apoptosis. Cancer Res. 1999;59:2457–2463. [PubMed] [Google Scholar]
- Ruiter GA, Zerp SF, Bartelink H, van Blitterswijk WJ, Verheij M. Anti-cancer alkyl-lysophospholipids inhibit the phosphatidylinositol 3-kinase-Akt/PKB survival pathway. Anticancer Drugs. 2003;14:167–173. doi: 10.1097/00001813-200302000-00011. [DOI] [PubMed] [Google Scholar]
- Samadder P, Arthur G. Decreased sensitivity to 1-o-octadecyl-2-o-methyl-glycerophosphocholine in MCF-7 cells adapted for serum-free growth correlates with constitutive association of Raf-1 with cellular membranes. Cancer Res. 1999;59:4808–4815. [PubMed] [Google Scholar]
- Samadder P, Byun HS, Bittman R, Arthur G. Glycosylated antitumor ether lipids are more effective against oncogene-transformed fibroblasts than alkyllysophospholipids. Anticancer Res. 1998;18:465–470. [PubMed] [Google Scholar]
- Samadder P, Richards C, Bittman R, Bhullar RP, Arthur G. The antitumor ether lipid 1-q-octadecyl-2-o-methyl-rac-glycerophosphocholine (ET-18-OCH3) inhibits the association between Ras and Raf-1. Anticancer Res. 2003;23:2291–2295. [PubMed] [Google Scholar]
- Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60Src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Otey CA, Hildebrand JD, Parsons JT. Focal adhesion kinase and paxillin bind to peptides mimicking beta integrin cytoplasmic domains. J Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallier DC, Bruyneel EA, Storme GA, Hilgard P, Mareel MM. Antiinvasive activity of hexadecylphosphocholine in vitro. Anticancer Res. 1991;11:1285–1292. [PubMed] [Google Scholar]
- Schober M, Raghavan S, Nikolova M, Polak L, Pasolli HA, Beggs HE, et al. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J Cell Biol. 2007;176:667–680. doi: 10.1083/jcb.200608010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön M, Schön MP, Geilen CC, Hoffmann M, Hakyi N, Orfanos CE, et al. Cell-matrix interactions of normal and transformed human keratinocytes in vitro are modulated by the synthetic phospholipid analogue hexadecylphosphocholine. Br J Dermatol. 1996;135:696–703. [PubMed] [Google Scholar]
- Slaton JW, Hampton JA, Selman SH. Exposure to alkyllysophospholipids inhibits in vitro invasion of transitional cell carcinoma. J Urol. 1994;152:1594–1598. doi: 10.1016/s0022-5347(17)32485-0. [DOI] [PubMed] [Google Scholar]
- Snyder F. Platelet-activating factor: The biosynthetic and catabolic enzymes. Biochem J. 1995;305(Pt 3):689–705. doi: 10.1042/bj3050689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnowski GS, Mountain IM, Stock CC, Munder PG, Weltzien HU, Westphal O. Effect of lysolecithin and analogs on mouse ascites tumors. Cancer Res. 1978;38:339–344. [PubMed] [Google Scholar]
- Tazzari PL, Tabellini G, Ricci F, Papa V, Bortul R, Chiarini F, et al. Synergistic proapoptotic activity of recombinant trail plus the akt inhibitor perifosine in acute myelogenous leukemia cells. Cancer Res. 2008;68:9394–9403. doi: 10.1158/0008-5472.CAN-08-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tertoolen LG, Kempenaar J, Boonstra J, de Laat SW, Ponec M. Lateral mobility of plasma membrane lipids in normal and transformed keratinocytes. Biochem Biophys Res Commun. 1988;152:491–496. doi: 10.1016/s0006-291x(88)80064-0. [DOI] [PubMed] [Google Scholar]
- Unger C, Eibl H. Hexadecylphosphocholine (D18506) in the topical treatment of skin metastases: a phase-I trial. Onkologie. 1988;11:295–296. doi: 10.1159/000216563. [DOI] [PubMed] [Google Scholar]
- Unger C, Peukert M, Sindermann H, Hilgard P, Nagel G, Eibl H. Hexadecylphosphocholine in the topical treatment of skin metastases in breast cancer patients. Cancer Treat Rev. 1990;17:243–246. doi: 10.1016/0305-7372(90)90054-j. [DOI] [PubMed] [Google Scholar]
- Unger C, Sindermann H, Peukert M, Hilgard P, Engel J, Eibl H. Hexadecylphosphocholine in the topical treatment of skin metastases in breast cancer patients. Prog Exp Tumor Res. 1992;34:153–159. doi: 10.1159/000420840. [DOI] [PubMed] [Google Scholar]
- Van Blitterswijk WJ, van der Bend RL, Kramer IM, Verhoeven AJ, Hilkmann H, de Widt J. A metabolite of an antineoplastic ether phospholipid may inhibit transmembrane signalling via protein kinase C. Lipids. 1987;22:842–846. doi: 10.1007/BF02535541. [DOI] [PubMed] [Google Scholar]
- Van Blitterswijk WJ, Verheij M. Anticancer alkylphospholipids: mechanisms of action, cellular sensitivity and resistance, and clinical prospects. Curr Pharm Des. 2008;14:2061–2074. doi: 10.2174/138161208785294636. [DOI] [PubMed] [Google Scholar]
- Van der Luit AH, Budde M, Ruurs P, Verheij M, van Blitterswijk WJ. Alkyl-lysophospholipid accumulates in lipid rafts and induces apoptosis via raft-dependent endocytosis and inhibition of phosphatidylcholine synthesis. J Biol Chem. 2002;277:39541–39547. doi: 10.1074/jbc.M203176200. [DOI] [PubMed] [Google Scholar]
- Van der Luit AH, Budde M, Zerp S, Caan W, Klarenbeek JB, Verheij M, et al. Resistance to alkyl-lysophospholipid-induced apoptosis due to downregulated sphingomyelin synthase 1 expression with consequent sphingomyelin- and cholesterol-deficiency in lipid rafts. Biochem J. 2007;401:541–549. doi: 10.1042/BJ20061178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Veer E, Van der Weide D, Heijmans HS, Hoekstra D. Translocation of fluorescent ether phospholipid, but not its diacyl counterpart, after insertion in plasma membranes of control and plasmalogen-deficient fibroblasts. Biochim Biophys Acta. 1993;1146:294–300. doi: 10.1016/0005-2736(93)90368-a. [DOI] [PubMed] [Google Scholar]
- Van Slambrouck S, Hilkens J, Steelant WF. Ether lipid 1-o-octadecyl-2-o-methyl-3-glycero-phosphocholine inhibits cell-cell adhesion through translocation and clustering of E-cadherin and episialin in membrane microdomains. Oncol Rep. 2008;19:123–128. [PubMed] [Google Scholar]
- Vicente-Manzanares M, Webb DJ, Horwitz AR. Cell migration at a glance. J Cell Sci. 2005;118:4917–4919. doi: 10.1242/jcs.02662. [DOI] [PubMed] [Google Scholar]
- Vink SR, van Blitterswijk WJ, Schellens JH, Verheij M. Rationale and clinical application of alkylphospholipid analogues in combination with radiotherapy. Cancer Treat Rev. 2007;33:191–202. doi: 10.1016/j.ctrv.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Vrablic AS, Albright CD, Craciunescu CN, Salganik RI, Zeisel SH. Altered mitochondrial function and overgeneration of reactive oxygen species precede the induction of apoptosis by 1-o-octadecyl-2-methyl-rac-glycero-3-phosphocholine in p53-defective hepatocytes. FASEB J. 2001;15:1739–1744. doi: 10.1096/fj.00-0300com. [DOI] [PubMed] [Google Scholar]
- Wieder T, Orfanos CE, Geilen CC. Induction of ceramide-mediated apoptosis by the anticancer phospholipid analog, hexadecylphosphocholine. J Biol Chem. 1998;273:11025–11031. doi: 10.1074/jbc.273.18.11025. [DOI] [PubMed] [Google Scholar]
- Wiese A, Wieder T, Mickeleit M, Reinohl S, Geilen CC, Seydel U, et al. Structure-dependent effects of glucose-containing analogs of platelet activating factor (PAF) on membrane integrity. Biol Chem. 2000;381:135–144. doi: 10.1515/BC.2000.019. [DOI] [PubMed] [Google Scholar]
- Wirtz KW. Phospholipid transfer proteins. Annu Rev Biochem. 1991;60:73–99. doi: 10.1146/annurev.bi.60.070191.000445. [DOI] [PubMed] [Google Scholar]
- Wolf P, Nghiem DX, Walterscheid JP, Byrne S, Matsumura Y, Matsumura Y, et al. Platelet-activating factor is crucial in psoralen and ultraviolet A-induced immune suppression. Inflammation. 2006;169:795–805. doi: 10.2353/ajpath.2006.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ST, Goodin S. Overcoming drug resistance in patients with metastatic breast cancer. Pharmacotherapy. 2009;29:954–965. doi: 10.1592/phco.29.8.954. [DOI] [PubMed] [Google Scholar]
- Zhang W, Liu W, Poradosu E, Ratain MJ. Genome-wide identification of genetic determinants for the cytotoxicity of perifosine. Hum Genomics. 2008;3:53–70. doi: 10.1186/1479-7364-3-1-53. [DOI] [PMC free article] [PubMed] [Google Scholar]


