Abstract
Background and purpose:
Female sexual arousal consists of a number of physiological responses resulting from increased genital blood. Vasoactive intestinal peptide (VIP), neuropeptide Y and to a lesser extent nitric oxide are neurotransmitters found in the vasculature of the genitalia. Neutral endopeptidase (NEP) modulates the activity of neuropeptides including VIP. The aim of this study was to investigate the control of genital blood flow by VIP and endogenous neuropeptides using a selective NEP inhibitor [UK-414,495, ((R)-2-({1-[(5-ethyl-1,3,4-thiadiazol-2-yl) carbamoyl]cyclopentyl}methyl) valeric acid)].
Experimental approach:
Vaginal and clitoral blood flow (VBF and CBF) were monitored using laser Doppler in terminally anaesthetized New Zealand rabbits. Increases in VBF and CBF were induced by either electrical stimulation of the pelvic nerve or by i.v. infusion of VIP.
Key results:
Stimulation of the pelvic nerve increased VBF and CBF, compared with basal flow. Increases were mimicked by infusion of exogenous VIP. UK-414,495 dose-dependently potentiated pelvic nerve-stimulated increases in VBF (EC50= 37 ± 9 nM; 3.6 × IC50 rabbit NEP). Nerve-stimulated increases in VBF and CBF were both enhanced after UK-414,495. UK-414,495 increased the amplitude and duration of VIP-induced increases in VBF. UK-414,495 had no effect on basal VBF or cardiovascular parameters.
Conclusions and implications:
Inhibition of NEP potentiates pelvic nerve-stimulated increases in genital blood flow. This suggests that the endogenous neurotransmitter mediating genital blood flow is a substrate for NEP (most likely VIP). NEP inhibitors may restore sexual arousal in women adversely affected by female sexual arousal disorder.
This article is commented on by Angulo, pp. 48–50 of this issue. To view this commentary visit http://dx.doi.org/10.1111/j.1476-5381.2010.00693.x
Keywords: female sexual arousal, vasocongestion, vaginal blood flow, vasoactive intestinal peptide, neutral endopeptidase, VIP, laser Doppler, rabbit
Introduction
Female sexual arousal consists of a number of physiological responses that are observed during sexual excitement. These changes such as vaginal, labial and clitoral engorgement or vasocongestion result from increases in genital blood flow. Vasocongestion leads to increased vaginal lubrication via plasma transudation, increased vaginal compliance (relaxation of vaginal smooth muscle), clitoral erection and increases in genital sensitivity (Levin, 1991; Berman et al., 1999b; 2000; 2001; Berman, 2005). Knowledge of these haemodynamic processes and their local regulation is poorly understood and requires further investigation.
Female sexual arousal disorder (FSAD) is defined as ‘a persistent or recurrent inability to attain or to maintain until completion of the sexual activity adequate lubrication-swelling response of sexual excitement’ (Leiblum, 1998). FSAD is a highly prevalent sexual disorder affecting up to 40% of women irrespective of age (Laumann et al., 1999). FSAD manifests itself as reduced vaginal, labial and clitoral engorgement, which leads to a lack of vaginal lubrication and a lack of pleasurable genital sensation, and ultimately personal distress. Vascular insufficiency and/or neuropathies are a potential underlying pathophysiology that could lead to reduced increases in genital blood flow during sexual arousal and chronic diseases, such as hypertension, atherosclerosis and diabetes or physical trauma that effect blood flow and/or sensation have all been implicated in genital arousal dysfunction. Hormonal imbalances, psychological disturbances and adverse effects of medications have also been implicated, but none have been conclusively proven to cause FSAD (Goldstein and Berman, 1998; Berman et al., 1999b). It may be possible to restore the sexual arousal response in women suffering from FSAD by enhancing genital blood flow directly using genitally acting vasoactive mechanisms or, indirectly, via centrally acting agents that enhance sexual desire and/or subjective arousal.
A number of vasoactive agents, including oral phosphodiesterase type 5 (PDE5) inhibitors (reviewed in Schoen and Bachmann, 2009), adrenoceptors antagonists (Rosen et al., 1999) and topically applied prostaglandins (Heiman et al., 2006), have been tested in exploratory clinical studies in women with FSAD and all have been associated with mixed results. Whether these are the most appropriate mechanisms based on the physiology of female genitalia and our understanding of the regulation of genital blood flow remains to be established.
Vasoactive intestinal peptide (VIP), neuropeptide Y (NPY) and to a lesser extent nitric oxide (NO) are the potential vasoactive neurotransmitter candidates found in nerves that innervate the vasculature of the vagina (Hoyle et al., 1996). VIP is a non-adrenergic non-cholinergic neurotransmitter that has been suggested to play a role in causing relaxation of the muscles that make up the vaginal wall and the clitoral corpus cavernosum (Ziessen et al., 2002). Plasma concentrations of VIP are elevated during sexual arousal, and exogenously administered VIP has been shown to increase vaginal blood flow (VBF) and lubrication in healthy volunteers but it is associated with adverse cardiovascular effects (Ottesen et al., 1982; 1983; 1987;). These data are certainly suggestive that VIP may play an important role in female genital arousal. Recently we have shown that, in addition to relaxing strips of vaginal wall smooth muscle, VIP, and a number of other neuropeptides, are potent vasodilators of rabbit vaginal artery (Aughton et al., 2008). The signal transduction pathway that mediates the effects of VIP in female genitalia is as yet uncharacterized but, like in other tissues, is thought to be mediated by elevated cAMP (Traish et al., 1999; Ückert et al., 2005a,b;).
Neutral endopeptidase or neprilysin (NEP; EC3.4.24.11) is a zinc-dependent neutral endopeptidase enzyme, which is involved in the breakdown of several bioactive oligopeptides, cleaving peptide bonds on the amino side of hydrophobic amino acid residues (Duggan et al., 1995; Gourlet et al., 1997; reviewed in Turner and Tanzawa, 1997; see Alexander et al., 2008). The peptides metabolized include natriuretic peptides (atrial natriuretic peptide, brain-type natriuretic peptide and C-type natriuretic peptide), bombesin, bradykinin, calcitonin gene-related peptide (CGRP), endothelins, enkephalins, neurotensin, substance P (sub P) and VIP. Some of these peptides have potent vasodilatory and neurohormone functions, diuretic and natriuretic activity or mediate behaviour effects. Many of these are thought to be involved in the sexual arousal response. We recently described a prototype development candidate UK-414,495 ((R)-2-({1-[(5-ethyl-1,3,4-thiadiazol-2-yl) carbamoyl]cyclopentyl}methyl) valeric acid), which is a novel, potent and selective inhibitor of NEP (R-13 in Pryde et al., 2006). In addition to being a potent inhibitor of human NEP, UK-414,495 is also a potent inhibitor of rabbit isolated native NEP (IC50= 10 nM; Pryde et al., 2006).
Because VIP has been shown to have physiological effects on the female genitalia, and NEP has been shown to degrade VIP, plus as in hamster cheek pouch inhibition of NEP leads to increased VIP-induced vasodilatation (Suzuki et al., 1996), our hypothesis was that inhibition of NEP will potentiate the endogenous vasorelaxant effect of bioactive peptides released during arousal and ultimately this may have the clinical effect of enhancing genital engorgement and restoring sexual arousal in women with FSAD. The aim of this study was to use an anaesthetized rabbit model of genital arousal to investigate the identity of endogenous vasoactive agents/mechanisms that mediate vaginal and clitoral blood flow, focusing in on the peptide substrates of NEP. To do this we used VIP and UK-414,495 as pharmacological probes. Additionally this study was designed to be a predictor for investigating the pharmacokinetic/pharmacodynamic properties of UK-414,495 on rabbit VBF and aiding dose setting for this development compound.
Methods
Anaesthetic protocol
All experiments were conducted in accordance with the UK Animals (Scientific Procedures) Act (1986) and Home Office guidelines.
Female New Zealand rabbits (∼2.5 kg) were pre-medicated with a combination of medetomidine (Domitor®, Pfizer, Ltd., Sandwich, UK) 0.5 mL·kg−1 i.m. and ketamine (Vetalar®, Pfizer) 0.25 mL·kg−1 i.m. while oxygen intake was maintained via a face mask. The rabbits were tracheotomized using a Portex™ uncuffed endotracheal tube (ID 3 mm), connected to a ventilator and maintained at a ventilation rate of 30–40 breaths·min−1, with an approximate tidal volume of 18–20 mL, and a maximum airway pressure of 10 cm H2O. Anaesthesia was then switched to isoflurane and ventilation continued with O2 at 2 L·min−1. The right marginal ear vein was cannulated using a 23 G or 24 G catheter, and Lactated Ringer solution perfused at 0.5 mL·min−1. The rabbit was maintained at 3% isoflurane during invasive surgery, dropping to 2% for maintenance anaesthesia.
Cannulation of vessels
The left groin area of the rabbit was shaved and a vertical incision was made approximately 5 cm in length along the thigh. The femoral vein was exposed, isolated and then cannulated with a PVC catheter (17 G) for the infusion of drugs and compounds. Cannulation was repeated for the femoral artery; the catheter was inserted to a depth of 10 cm to ensure that the catheter reached the abdominal aorta. This arterial catheter was linked to a Gould system to record blood pressure. Samples for blood gas analysis were also taken via the arterial catheter. Systolic and diastolic pressures were measured, and the mean arterial pressure calculated using the formula (diastolic × 2 + systolic) ÷ 3. Heart rate was measured via the pulse oxymeter and Po-ne-mah data acquisition software system (Ponemah Physiology Platform, Gould Instrument Systems Inc., Valley View, OH, USA).
Stimulation of pelvic nerve
A ventral midline incision was made into the abdominal cavity. The incision was about 5 cm in length just above the pubis. The fat and muscle was bluntly dissected away to reveal the hypogastric nerve, which runs down the body cavity. It was essential to keep close to the side curve of the pubis wall in order to avoid damaging the femoral vein and artery, which lie above the pubis. The sciatic and pelvic nerves lie deeper and were located after further dissection on the dorsal side of the rabbit. Once the sciatic nerve had been identified, the pelvic nerve was easily located. The term ‘pelvic nerve’ is loosely applied; anatomy books on the subject fail to identify the nerves in sufficient detail. The pelvic nerve is known to innervate the female genitalia and it is documented that stimulation of the pelvic nerve causes an increase in genital blood flow. We are confident that the nerve we were stimulating in this study was the pelvic nerve, because: (i) upon stimulation we observe an increase in vaginal and clitoral blood flow; and (ii) we had traced the passage of the nerve from the female genitalia, through the pelvic ganglion back to its spinal origin in the S2/S4 region. The pelvic nerve was freed away from surrounding tissue and a Harvard bipolar stimulating electrode was placed around the nerve. The pelvic nerve was slightly lifted to give some tension, then the electrode was secured in position. Approximately 1 mL of light paraffin oil was placed around the pelvic nerve and electrode. This acts as a protective lubricant to the pelvic nerve and prevents blood contamination of the electrode. The electrode was connected to a Grass S88 stimulator. The pelvic nerve was stimulated using the following parameters: 5 V, pulse width 0.5 ms, duration of stimulus 10 s and a frequency range of 2–16 Hz. Reproducible responses were obtained when the nerve was stimulated every 15 min. A frequency response curve was determined at the start of each experiment in order to determine the optimum frequency to use as a submaximal response, normally 4 Hz. The compound(s) to be tested were infused, via the femoral vein, using a Harvard 22 infusion pump allowing a continuous stimulation cycle every 15 min.
Positioning of laser Doppler probes
A ventral midline incision was made, at the caudal end of the pubis, to expose the pubic area. Connective tissue was removed to expose the tunica of the clitoris, ensuring that the wall was free from small blood vessels. The external vaginal wall was also exposed by removing any connective tissue. One laser Doppler flow probe was inserted 3 cm into the vagina, so that half the probe shaft was still visible. A second probe was positioned so that it lay just above the external clitoral wall. The position of these probes was then adjusted until a signal was obtained. Both probes were clamped in position. Vaginal and clitoral blood flow was recorded either as numbers directly from the Flowmeter (ALF 21D) using Po-ne-mah data acquisition software, or indirectly from a Gould chart recorder trace. Calibration was set at the beginning of the experiment (0–125 mL·min−1 per 100 g tissue).
Infusion of VIP
The doses of VIP infused were 6, 20 and 60 µg·kg−1 i.v. and were infused in a volume of 1.0 mL of saline. VIP was infused using a Harvard 22 pump, infusing at 500 µL·min−1 via a three-way tap into the femoral vein. After VIP infusion, the catheter was flushed with heparin-treated saline (Hepsaline) so that no VIP was left in the catheter. For experiments using VIP infusions, there was a need for an initial sensitizing dose–response curve (60 µg·kg−1), in order that reproducible responses could be obtained. An initial infusion of Hepsaline (50 U·mL−1) was infused to act as a negative control.
Infusion of UK-414,495
To achieve steady-state plasma concentrations of UK-414,495 a loading dose was administered followed by infusion of a steady-state dose. The inhibitor and saline vehicle controls were infused at the same rate. NEP inhibitors were left for 30 min prior to a VIP dose–response curve, or for 15 min prior to pelvic nerve stimulation. UK-414,495 was infused to achieve steady-state plasma concentrations at 1, 3, 10, 30 and 100 × IC50 values based on the rabbit isolated enzyme IC50 (10.2 nM). The loading dose was administered i.v. as a 1 mL infusion volume at a rate of 0.5 mL·min−1. The cannula was immediately flushed with 0.3 mL saline to purge the cannula of any remaining loading dose. This was followed by the maintenance dose, which was infused at a constant rate of 20 µL·min−1. Blood samples were collected and free plasma drug levels for each infusion of UK-414,495 were quantified using mass spectroscopy (LC-MS-MS; Pryde et al., 2006).
UK-414,495 has demonstrated good transdermal penetration across intact human stratum corneum which, given the low molecular weight (339) and its log D (0.5), would be expected (D. McCleverty, Pfizer Global R&D, unpubl. obs.). The vaginal wall is not expected to present such a barrier and therefore we would expect to see a greater flux in vivo across the vaginal wall. Following its administration, UK-414,495 in contact with the vaginal wall is predicted to be well absorbed. The formulation used contained UK-414,495 at 90% saturation in 50% propylene glycol/50% water made viscous with carboxymethyl cellulose giving a final concentration of approximately 2.5 mg·mL−1. The administration of 0.2 mL provides a maximal dose of 0.5 mg. UK-414,495 was applied topically to the internal vaginal wall via an ‘in house’ designed applicator. Basically, pieces of tubing (ID 3 mm, OD 4 mm) 10 cm in length were cut and attached to a 1 mL syringes. The syringes were each filled with a control gel (containing no UK-414,495) or the formulation described above. The tubing was inserted 2 cm into the vagina, and 0.2 mL of the gel was gently injected to avoid disturbing the laser Doppler probe. The addition of gel caused no major distension to the vagina and there was no excessive leakage of the gels from within the vagina during non-stimulated or stimulated periods.
Data analysis
The Po-ne-mah data acquisition software system (Ponemah Physiology Platform) was obtained from Gould Instrument Systems Inc. (8333 Rockside Road, Valley View, OH, USA). Laser Doppler, ALF 21D was supplied by Linton Instruments (Diss, Norfolk, UK). All data are reported as mean ± SEM. Significant changes were identified using Student's t-tests (independent or paired as appropriate) versus baseline.
Drugs
The drug and molecular target nomenclature used in this paper are in agreement with BJP's Guide to Receptors and Channels (Alexander et al., 2008). VIP was purchased from Bachem (H-3775) and UK-414,495 (R-13 in Pryde et al., 2006) was synthesized at Pfizer Global R&D. For infusion studies, both VIP and UK-414,495 were dissolved in saline, and saline was used as the vehicle control. For topical delivery studies, the formulation used for UK-414,495 was 50% propylene glycol/50% water made viscous with carboxymethyl cellulose.
Medetomidine (Domitor®) was obtained from Pfizer Animal Health-VM 3649/4001; ketamine (Vetalar®) from Pharmacia and Upjohn-VM 14851/4009 and isoflurane (Isoflo®vet) from Schering-Plough, Animal Health-346/49/A.
Results
UK-414,495 significantly enhanced the female sexual arousal response
Stimulation of the pelvic nerve induced frequency-dependent increases in vaginal and clitoral blood flow. These values, both absolute and relative to baseline, were of similar amplitude to those previously observed in human studies and animal models of arousal (Park et al., 1997; Min et al., 2000;Berman et al., 2001; 2003; Angulo et al., 2003; 2006; Kim et al., 2004). Submaximal stimulation of the pelvic nerve resulted in reproducible increases in genital blood flow that were maintained for up to 5 h (data not shown).
Pelvic nerve-stimulated (PNS) increases in VBF were significantly potentiated by escalating doses of UK-414,495. Submaximal increases in VBF were significantly enhanced c. 33% compared with baseline control increases following i.v. infusion of UK-414,495 (37.3 ± 2.7 vs. 28.2 ± 1.2 mL·min−1 per 100 g tissue; P < 0.01), at free plasma concentrations of approximately 3× rabbit NEP IC50 (Figure 1). At this dose, significant enhancement was observed 45 min after infusion commenced. A maximal potentiation of VBF was observed at approximately 10 × IC50, this equated to approximately a 53% enhancement of control increases (45.4 ± 2.5 vs. 28.2 ± 1.2 mL·min−1 per 100 g tissue; n= 3–10). Increasing the dose, up to 100 × IC50, had no additional effect on VBF. Control vehicle infusions had no effect on nerve-stimulated increases in VBF (27 vs. 31 mL·min−1 per 100 g tissue; n= 2).
Figure 1.
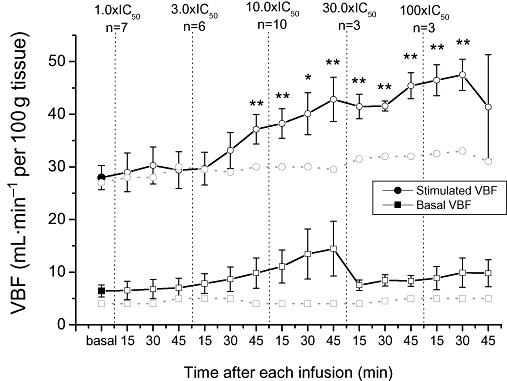
((R)-2-({1-[(5-ethyl-1,3,4-thiadiazol-2-yl) carbamoyl]cyclopentyl}methyl) valeric acid) (UK-414,495), a selective inhibitor of neutral endopeptidase (NEP), dose-dependently enhanced pelvic nerve-stimulated (PNS) increases in vaginal blood flow (VBF) in the anaesthetized rabbit model of sexual arousal. Repetitive PNS, at submaximal stimulation frequencies, at 15 min intervals induced reproducible increases in VBF (solid circle). Infusion of increasing concentrations of UK-414,495 (at 1, 3, 10, 30 and 100 × IC50 for rabbit native NEP) significantly enhanced the peak increase in VBF (open circles) compared with the mean baseline control flow increases. UK-414,495 had no effect on basal (unstimulated) VBF (open squares) compared with baseline control flow (solid square). Vehicle infusion had no effect on nerve-stimulated and unstimulated VBF (shaded circles and squares). All changes were monitored using laser Doppler technologies and data are expressed as mean ± SEM (n= 3–10), *P < 0.05, **P < 0.01 Student's t-test (paired vs. baseline control). Note that the small non-significant increases in basal VBF observed at 10 × IC50 arose from one animal out of 10 where the baseline increased. At 45 min after the highest dose of UK-414,495, the nerve-mediated response was lost in a number of animals.
UK-414,495 had no effect on basal genital blood flow in the absence of pelvic nerve stimulation. The small non-significant increases shown in Figure 1 in basal VBF observed at 10 × IC50 arose from one animal out of 10 where the baseline increased. Control vehicle infusions had no effect on unstimulated VBF (4 vs. 5 mL·min−1 per 100 g tissue; n= 2). This reinforces our view that this agent will enhance the arousal response by potentiating endogenously released peptides, and will not induce arousal in the absence of sexual stimulation. Additionally, UK-414,495 had no significant effects on either heart rate or mean arterial blood pressure (data not shown).
Analysis of plasma samples allowed investigation of the pharmacodynamic relationship between the extent of NEP inhibition and corresponding effect on VBF. UK-414,495 produced a sigmoidal dose-dependent potentiation of PNS-induced increases in VBF following i.v. infusion in the anaesthetized rabbit. VBF became significantly elevated (P < 0.01), above control flow changes, at a free plasma concentrations of 43 ± 9 nM (Figure 2). Maximal potentiation was observed at approximately 103 ± 12 nM. On fitting a sigmoidal curve through the data, the dose required to enhance VBF to 50% of the maximum achievable increase in flow could be calculated (termed EC50). The EC50 for UK-414,495-mediated potentiation in VBF was 37.0 ± 8.9 nM and this value is equivalent to 3.6 × IC50 for UK-414,495 against native rabbit NEP.
Figure 2.
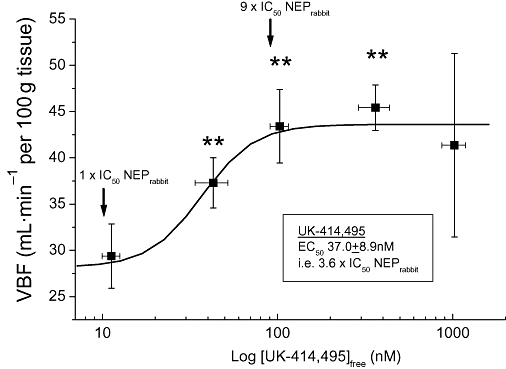
Dose–response relationship for UK-414,495 [((R)-2-({1-[(5-ethyl-1,3,4-thiadiazol-2-yl) carbamoyl]cyclopentyl}methyl) valeric acid)] in the anaesthetized rabbit. The amplitude of pelvic nerve-stimulated (PNS)-induced increase in vaginal blood flow (VBF) and the free plasma concentration of UK-414,495 were measured 45 min after infusion. The dose required to enhance VBF to 50% of the maximum achievable increase in flow was determined from this relationship. Arrows illustrate relationship between free concentrations of UK-414,495 and IC50 potency against rabbit native neutral endopeptidase (NEP). All changes were monitored using laser Doppler technologies and data are expressed as mean ± SEM (n= 3–10), **P < 0.01 Student's t-test (paired vs. baseline control).
In a separate study both vaginal and clitoral blood flow were simultaneously monitored following an i.v. bolus injection of the NEP inhibitor. UK-414,495 (1 mg·kg−1 i.v. bolus) significantly potentiated PNS increases in both vaginal and clitoral blood flow (128 ± 49% and 208 ± 77%, respectively; Figure 3) compared with baseline control increases. The magnitude of the increases observed could not be compared with the increases seen in the above infusion studies due to lower baseline conditions.
Figure 3.
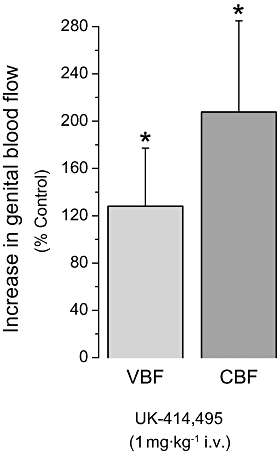
((R)-2-({1-[(5-ethyl-1,3,4-thiadiazol-2-yl) carbamoyl]cyclopentyl}methyl) valeric acid) (UK-414,495) enhanced both vaginal and clitoral blood flow in the anaesthetized rabbit model of sexual arousal. Submaximal increases in genital blood flow were induced by pelvic nerve stimulation (PNS). UK-414,495 enhanced vaginal blood flow (VBF, c. 128%) and clitoral blood flow (CBF, 208%) compared with control increases. All changes were monitored using laser Doppler technologies and data are expressed as mean ± SEM (n= 3–4), *P < 0.05 Student's t-test versus baseline control.
UK-414,495 enhanced vasoactive intestinal polypeptide (VIP)-induced increases in VBF
The identity of the neurotransmitter candidate(s) released during sexual arousal and/or pelvic nerve stimulation is currently unidentified. Ottesen et al. have shown that infusion of VIP results in increases in VBF and lubrication in healthy volunteers (Ottesen et al., 1982; 1983; 1987;). To investigate whether UK-414,495 could be enhancing neuronally released VIP, we monitored the amplitude and duration of VIP-induced increases in VBF in the presence and absence of UK-414,495.
Exogenous VIP (6–60 µg·kg−1 i.v. bolus) induced dose-dependent increases in VBF (see Figure 4A). VBF was significantly increased, by 14.6 ± 3.5 mL·min−1 per 100 g tissue, after i.v. administration of VIP (60 µg·kg−1). The VIP-induced increase was roughly equivalent to the submaximal increased observed during pelvic nerve stimulation. Lower doses induced smaller increases for example 20 µg·kg−1, elevated blood flow by 3.3 ± 0.7 mL·min−1 per 100 g tissue. UK-414,495 (i.v. infusion) significantly enhanced the amplitude of VIP (20 and 60 µg·kg−1)-induced increases in VBF by 67% and 60% respectively (Figure 4A).
Figure 4.
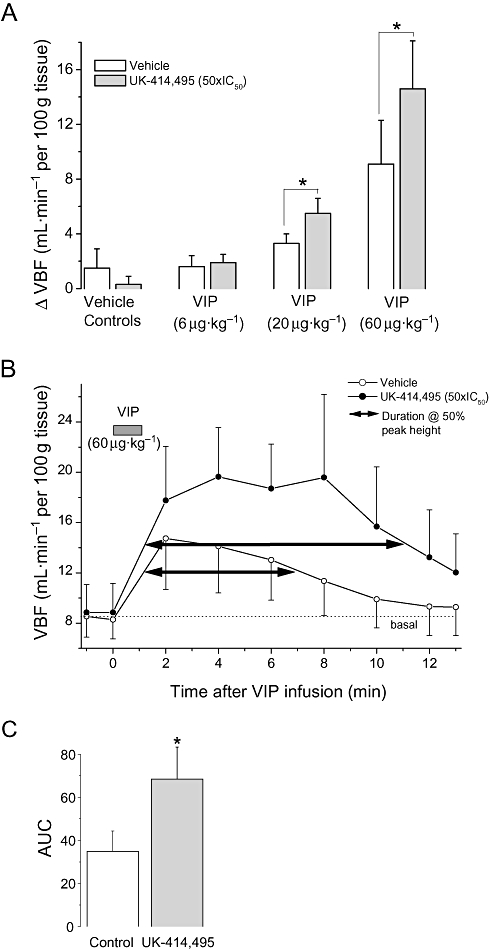
((R)-2-({1-[(5-ethyl-1,3,4-thiadiazol-2-yl) carbamoyl]cyclopentyl}methyl) valeric acid) (UK-414,495) enhanced vasoactive intestinal peptide (VIP)-induced increases in vaginal blood flow (VBF) in the anaesthetized rabbit model of sexual arousal. Intravenous infusion of VIP (6, 20 and 60 µg·kg−1) produced a significant enhancement in VBF when compared with basal flow (ΔVBF). Pretreatment with UK-414,495 (50 × IC50) significantly enhanced VIP-induced increases in VBF (A and B). In addition to enhancing the amplitude of blood flow changes, UK-414,495 also significantly increased the area under the curve (AUC, C) and the duration of VIP-mediated increases in VBF (the duration is measured at 50% of peak response). UK-414,495 had no effects on basal VBF. All changes were monitored using laser Doppler technologies, and data are expressed as mean ± SEM (n= 3–4), *P < 0.05 Student's unpaired t-test, compared with increases observed during vehicle infusions.
UK-414,495 significantly enhanced VIP (60 µg·kg−1; i.v.)-induced increases in VBF, compared with increases during vehicle infusions, in the absence of PNS (Figure 4). Pretreatment with UK-414,495 produced a 67% increase in the peak amplitude of the blood flow response, a 96% increase in area under the curve (calculated from Figure 4B) and increased the duration of the VIP-induced blood flow response from 5.9 to 9.8 min (i.e. 66% increase at 50% of peak response). The free plasma concentration of UK-414,495 was later calculated to be 557 ± 50 nM (approximately 50 × IC50 rabbit native NEP).
Effects of topically applied UK-414,495 on VBF
UK-414,495, when applied intravaginally (0.5 mg in 0.2 mL vehicle), prior to pelvic nerve stimulation, enhanced nerve-stimulated increases in VBF within 15 min of application (c. 73% 1 h after administration; Figure 5) compared with gel vehicle control. The degree of enhancement observed is comparable to the increases seen after i.v. infusion of the compound – yet occurred when the free plasma concentration of UK-414,495 was 8.0 ± 1.6 nM (a concentration that would not be expected to cause a potentiation of VBF; see Figure 2).
Figure 5.
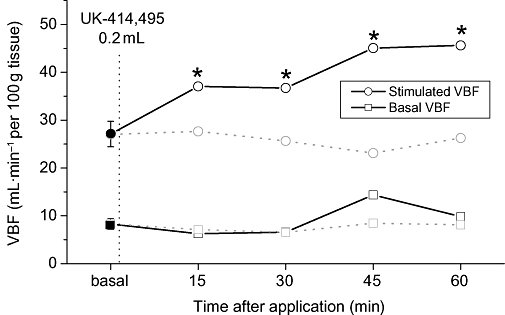
Topical/intravaginal application of UK-414,495 [((R)-2-({1-[(5-ethyl-1,3,4-thiadiazol-2-yl) carbamoyl]cyclopentyl}methyl) valeric acid)] enhanced pelvic nerve-stimulated (PNS) increases in vaginal blood flow (VBF) in the anaesthetized rabbit model of sexual arousal. Intravaginal application of a set concentration of UK-414,495 (0.2 mg·mL−1) enhanced the peak increase in VBF (open circles) compared with the baseline control flow increases (solid circle). Vehicle had no effect on stimulated or basal (unstimulated) VBF (shaded squares and circles, respectively). All changes were monitored using laser Doppler technologies, and data are expressed as mean ± SEM (n= 4), *P < 0.05 Student's paired t-test, compared with baseline control responses.
After the initial insertion of the tubing or application of the 0.2 mL of the vehicle gel, the non-stimulated/basal VBF did not appear to change, neither were any changes observed on stimulated VBF (data not shown).
Discussion and conclusions
This study has shown that stimulation of the pelvic nerve induces increases in vaginal and clitoral blood flow and these increases in VBF can be mimicked by infusion of exogenous VIP. Pretreatment with UK-414,495, a selective inhibitor of NEP, dose-dependently potentiated these PNS increases in VBF, by approximately 33% at unbound drug concentrations equivalent to 4 × IC50 for native rabbit NEP and maximal enhancement (∼53%) at 10 × IC50. UK-414,495 had no effect on systemic cardiovascular parameters. Also, UK-414,495 had no effect on basal (non-stimulated) genital blood flow. However, PNS increases in vaginal and clitoral blood flow were both significantly enhanced after NEP inhibition. Also, pretreatment with UK-414,495 significantly enhanced the amplitude and duration of VIP-induced increases in vaginal flow. In addition, intravaginal topical application of UK-414,495 enhanced PNS increases in VBF (∼73%) at unbound plasma concentrations that would not be expected to cause a potentiation of VBF, strongly suggesting a local vaginal effect.
The vaginal artery, the vaginal branch of the uterine artery, the internal pudendal artery and the middle branches of the middle rectal artery are all involved in supplying blood to the genitalia. During female sexual arousal genital blood flow increases via an enhanced arterial blood supply (Levin, 1991; Berman et al., 1999a; 2000; 2003; Berman, 2005). The pelvic nerve, which originates from S2/S4 spinal regions, innervates the female genitalia and has branches terminating in the lower vagina, clitoris and related blood vessels. By electrically stimulating the pelvic nerve we can mimic the increased blood flow observed during sexual arousal. The increased arterial blood flow is not mirrored by increased venous drainage thus allowing the capillary networks to become engorged with blood. Vaginal lubrication, via increased plasma transudation is one of the first pelvic responses observed during sexual stimulation.
The neurotransmitters that are released during sexual arousal, or pelvic nerve stimulation, are currently unidentified. Nerves containing CGRP, NPY, NO synthase (NOS), sub P and VIP, innervating the vasculature and microvasculature of the vagina and clitoris, have all been identified immunohistochemically (NPY > VIP >> NOS > CGRP > SP; Hoyle et al., 1996; Burnett et al., 1997; Hauser-kronberger et al., 1999). Ottesen et al. (1982; 1983; 1987;) provided support for VIP as a neurotransmitter candidate by demonstrating that plasma concentrations of VIP are doubled during sexual arousal and, importantly, that administration of exogenous VIP to healthy women, either intravenously or directly into the vaginal wall, increased VBF and lubrication, although this was associated with hypotensive effects. Studies investigating the mechanism(s) by which VIP exerts its effects in the female genitalia are limited and there is no evidence that VIP is released from the neurones during sexual arousal.
Based on our data obtained in this study, we propose that during sexual arousal parasympathetic neurones release neuropeptides. One or more of these peptides, and specifically one that is metabolized by NEP, plays an important role in female genital arousal and participates in the local control of blood flow. The most likely peptide released is VIP, although we cannot rule out the possibility that other NEP substrates, such as endothelins or naturetic peptides (see below), are involved. In the vagina and clitoris neuronally released VIP then activates the adenylate cyclase/cAMP pathway, based on VIP receptor coupling studies, which ultimately results in genital engorgement. The biological activity of VIP is terminated by NEP, and, hence, NEP inhibition prolongs the biological activity of the released peptide. The presence of NEP mRNA and protein in human and rabbit vagina has been confirmed by Northern and Western analyses (data not shown). However, there is evidence that other NEP metabolized peptides are involved in this response; for example, elevated natriuretic peptides have been implicated in persistent genital sexual arousal in women following withdrawal from antidepressant drug therapy (Bell et al., 2007). Interestingly some of these neurotransmitter candidates are elevated in disease conditions that are often concomitant with FSAD and as such may be associated with the pathophysiology of this condition. Endothelins may increase vaginal contractility, impeding vasocongestion, as ET increases tension of isolated vaginal smooth muscle strips (Ückert et al., 2005a) and plasma levels are elevated in hypertension/cardiovascular disease (Kirkby et al., 2008;Versari et al., 2009). However, there are no reports of elevated, or reduced, neuropeptide levels in women with FSAD. Agents that enhance the nitrergic/cGMP pathway, such as PDE5 inhibitors, have been shown to potentiate nerve-stimulated increases vaginal and clitoral blood flow in rabbits (Min et al., 2000; Angulo et al., 2006), rats (Kim et al., 2004) and dogs (Angulo et al., 2003). This suggests that NO is also likely to be involved in the regulation of genital blood flow; however, these increases in genital blood flow do not appear to translate to broad clinical efficacy in women with FSAD.
This study does have a number of limitations that need to be taken into account. First, the rabbit model we used has not been validated and the translation of results obtained in the rabbit to humans is currently unknown, especially when one considers that the link between blood flow and subjective arousal remains controversial. This will only be resolved when a therapeutic agent that improves sexual function in women also displays efficacy in the rabbit model. Second, the rabbit does not undergo an oestrus cycle and so does not ovulate until mounted, resulting in a dioestrus state with low circulating oestrogen levels (and the reason why rabbits were chosen in order to control for variability in circulating sex hormone levels). Third, we assessed genital blood flow (a biomarker of sexual arousal) following electrical stimulation of the pelvic nerve, and in humans there may be additional afferent/efferent neuronal pathways and paracrine factors involved in this response that are absent from the model used in this study.
Female sexual arousal disorder manifests itself as reduced genital engorgement, which leads to a lack of vaginal lubrication, a lack of pleasurable genital sensation, and ultimately personal distress. Vascular insufficiency and/or neuropathies are a potential underlying pathophysiology that could lead to reduced increases in genital blood flow. Despite the link between genital blood flow and subjective sexual arousal still remaining unproven, there is growing evidence from a number of exploratory clinical studies in women with FSAD that increases in genital blood flow are associated with increases in subjective arousal. Intravaginal delivery of the vasodilator alprostadil has been shown to induce genital vasocongestion and patients reported greater assessments of physical and emotional arousal, and sexual satisfaction (Heiman et al., 2006). Yet oral phentolamine, despite having no clear effect on VBF, had a mild, positive effect on subjective measures of arousal including lubrication and pleasurable sensations (Rosen et al., 1999). The most studied clinical agent in FSAD is sildenafil, a PDE5 inhibitor, with at least 10 clinical studies published (reviewed in Schoen and Bachmann, 2009). Sildenafil has been shown to improve sexual arousal in specific subgroups of FSAD and normal sexual desire appears to be critical for its efficacy (Berman et al., 2003), which makes sense, given that the drive to be sexual needs to be present for the genital haemodynamic event to occur. Only a few studies have looked at both genital blood flow and subjective reporting, and these have shown that only in specific cases of FSAD, increasing genital blood flow translates to improved subjective arousal whereas the broad FSAD population showed no response to sildenafil (Sipski et al., 2000; Basson and Brotto, 2003; Caruso et al., 2006). Based on our growing knowledge of female genital physiology, the neurotransmitter candidates present in the genitalia and the effects of exogenous administration of VIP in women, it is questionable whether the mechanisms that have been tested in women with FSAD are the most appropriate. It is possible that they may have contributed to the increasing confusion and sub-division of this distressing condition.
In conclusion, this study clearly demonstrates that inhibition of NEP potentiates both vaginal and clitoral blood flow in an anaesthetized rabbit model of female sexual arousal. Moreover, these data indicate that the endogenous neurotransmitter mediating these vascular effects is a substrate for NEP. Importantly, inhibition of NEP had no effect on basal genital blood flow, and this reinforces our view that NEP inhibitors will not induce sexual arousal in the absence of sexual stimulation. Moreover, inhibition of NEP may offer an opportunity to enhance the endogenous of VIP and avoid the hypotensive effects associated with exogenous administration of, for example, VIP. The data demonstrate the importance of peptidergic neurotransmission in the female genital arousal response. Clinical usage of NEP inhibitors may help us explain the link between female genital blood flow and subjective arousal. Further preclinical and clinical studies are warranted and they may provide a novel mechanism to help women who are adversely affected by FSAD.
Glossary
Abbreviations:
- CBF
clitoral blood flow
- CGRP
calcitonin gene-related peptide
- FSAD
female sexual arousal disorder
- NEP
neutral endopeptidase (neprilysin, EC3.4.24.11)
- NO
nitric oxide
- NPY
neuropeptide Y
- PDE
phosphodiesterase
- PDE5
phosphodiesterase type 5
- PNS
pelvic nerve-stimulated
- sub P
substance P
- VBF
vaginal blood flow
- VIP
vasoactive intestinal peptide
- UK-414,495
((R)-2-({1-[(5-ethyl-1,3,4-thiadiazol-2-yl) carbamoyl]cyclopentyl}methyl) valeric acid)
Statement of conflicts of interest
At the time of this study all the authors were full time employees of Pfizer Global R&D. AMN is currently an employee of Solace Pharmaceuticals. The other authors are currently employees of Pfizer Global R&D.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo J, Cuevas P, Cuevas B, Bischoff E, Sáenz de Tejada I. Vardenafil enhances clitoral and vaginal blood flow responses to pelvic nerve stimulation in female dogs. Int J Impot Res. 2003;15(2):137–141. doi: 10.1038/sj.ijir.3900985. [DOI] [PubMed] [Google Scholar]
- Angulo J, Cuevas P, Cuevas B, Bischoff E, de Tejada IS. Antidepressant-induced inhibition of genital vascular responses is reversed by vardenafil in female rabbits. J Sex Med. 2006;3(6):988–995. doi: 10.1111/j.1743-6109.2006.00326.x. [DOI] [PubMed] [Google Scholar]
- Aughton KL, Hamilton-Smith K, Gupta J, Morton JS, Wayman CP, Jackson VM. Pharmacological profiling of neuropeptides on rabbit vaginal wall and vaginal artery smooth muscle in vitro. Br J Pharmacol. 2008;155(2):236–243. doi: 10.1038/bjp.2008.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson R, Brotto LA. Sexual psychophysiology and effects of sildenafil citrate in oestrogenised women with acquired genital arousal disorder and impaired orgasm: a randomised controlled trial. BJOG. 2003;110(11):1014–1024. [PubMed] [Google Scholar]
- Bell C, Richardson D, Goldmeier D, Crowley T, Kocsis A, Hill S. Persistent sexual arousal in a woman with associated cardiac defects and raised atrial natriuretic peptide. Int J STD AIDS. 2007;18(2):130–131. doi: 10.1258/095646207779949592. [DOI] [PubMed] [Google Scholar]
- Berman JR. Physiology of female sexual function and dysfunction. Int J Impot Res. 2005;17(Suppl. 1):S44–S51. doi: 10.1038/sj.ijir.3901428. [DOI] [PubMed] [Google Scholar]
- Berman JR, Berman LA, Werbin TJ, Flaherty EE, Leahy NM, Goldstein I. Clinical evaluation of female sexual function: effects of age and estrogen status on subjective and physiologic sexual responses. Int J Impot Res. 1999a;11(Suppl. 1):S31–S38. doi: 10.1038/sj.ijir.3900468. [DOI] [PubMed] [Google Scholar]
- Berman JR, Berman L, Goldstein I. Female sexual dysfunction: Incidence, pathophysiology, evaluations and treatment options. Urology. 1999b;54:385–391. [PubMed] [Google Scholar]
- Berman JR, Adhikari SP, Goldstein I. Anatomy and physiology of female sexual function and dysfunction: classification, evaluation and treatment options. Eur Urol. 2000;38(1):20–29. doi: 10.1159/000020247. [DOI] [PubMed] [Google Scholar]
- Berman JR, Berman LA, Lin H, Flaherty E, Lahey N, Goldstein I, et al. Effect of sildenafil on subjective and physiologic parameters of the female sexual response in women with sexual arousal disorder. J Sex Marital Ther. 2001;27(5):411–420. doi: 10.1080/713846815. [DOI] [PubMed] [Google Scholar]
- Berman JR, Berman LA, Toler SM, Gill J, Haughie S, Sildenafil Study Group Safety and efficacy of sildenafil citrate for the treatment of female sexual arousal disorder: a double-blind, placebo controlled study. J Urol. 2003;170(6 Pt 1):2333–2338. doi: 10.1097/01.ju.0000090966.74607.34. [DOI] [PubMed] [Google Scholar]
- Burnett AL, Calvin DC, Silver RI, Peppas DS, Docimo SG. Immunohistochemical description of nitric oxide synthase isoforms in human clitoris. J Urol. 1997;158:75–78. doi: 10.1097/00005392-199707000-00020. [DOI] [PubMed] [Google Scholar]
- Caruso S, Rugolo S, Agnello C, Intelisano G, Di Mari L, Cianci A. Sildenafil improves sexual functioning in premenopausal women with type 1 diabetes who are affected by sexual arousal disorder: a double-blind, crossover, placebo-controlled pilot study. Fertil Steril. 2006;85(5):1496–1501. doi: 10.1016/j.fertnstert.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Duggan KA, Jones DM, Ye VZ, Davis RE, Macdonald GJ. Effects of endopeptidase 24.11 inhibition on plasma and tissue concentrations of vasoactive intestinal peptide. Clin Sci. 1995;89:267–271. doi: 10.1042/cs0890267. [DOI] [PubMed] [Google Scholar]
- Goldstein I, Berman JR. Vasculogenic female sexual dysfunction: vaginal engorgement and clitoral erectile insufficiency syndromes. Int J Impot Res. 1998;10:S84–S90. [PubMed] [Google Scholar]
- Gourlet P, Vandermeers A, Robberecht P, Deschodt-Lanckman M. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating peptide (PACAP-27, but not PACAP-38) degradation by the neutral endopeptidase EC 3.4.24.11. Biochem Pharmacol. 1997;54(4):509–515. doi: 10.1016/s0006-2952(97)00207-4. [DOI] [PubMed] [Google Scholar]
- Hauser-Kronberger C, Cheung A, Hacker GW, Graf AH, Dietze O, Frick J. Peptidergic innervation of the human clitoris. Peptides. 1999;20:539–543. doi: 10.1016/s0196-9781(99)00005-4. [DOI] [PubMed] [Google Scholar]
- Heiman JR, Gittelman M, Costabile R, Guay A, Friedman A, Heard-Davison A, et al. Topical alprostadil (PGE1) for the treatment of female sexual arousal disorder: in-clinic evaluation of safety and efficacy. J Psychosom Obstet Gynaecol. 2006;27(1):31–41. doi: 10.1080/01674820500237973. [DOI] [PubMed] [Google Scholar]
- Hoyle CH, Stones RW, Robson T, Whitley K, Burnstock G. Innervation of vasculature and microvasculature of the human vagina by NOS and neuropeptide containing nerves. J Anat. 1996;188:633–644. [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Jeong SJ, Munarriz R, Kim NN, Goldstein I, Traish AM. An in vivo rat model to investigate female vaginal arousal response. J Urol. 2004;171(3):1357–1361. doi: 10.1097/01.ju.0000109868.19569.d7. [DOI] [PubMed] [Google Scholar]
- Kirkby NS, Hadoke PW, Bagnall AJ, Webb DJ. The endothelin system as a therapeutic target in cardiovascular disease: great expectations or bleak house? Br J Pharmacol. 2008;153(6):1105–1119. doi: 10.1038/sj.bjp.0707516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281(6):537–544. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- Leiblum SR. Definition and classification of female sexual disorders. Int J Impot Res. 1998;10:S104–S106. doi: 10.1201/b14618-47. [DOI] [PubMed] [Google Scholar]
- Levin RJ. VIP, vagina, clitoral and periurethral glans – an update on human female genital arousal. Exp Clin Endocrinol. 1991;98(2):61–69. doi: 10.1055/s-0029-1211102. [DOI] [PubMed] [Google Scholar]
- Min K, Kim NN, McAuley I, Stankowicz M, Goldstein I, Traish AM. Sildenafil augments pelvic nerve-mediated female genital sexual arousal in the anesthetized rabbit. Int J Impot Res. 2000;12(Suppl. 3):S32–S39. doi: 10.1038/sj.ijir.3900610. [DOI] [PubMed] [Google Scholar]
- Ottesen B, Ulrichsen H, Fahrenkrug J, Larsen JJ, Wagner G, Schierup L, et al. Vasoactive intestinal polypeptide and the female genital tract: relationship to reproductive phase and delivery. Am J Obstet Gynecol. 1982;143(4):414–420. doi: 10.1016/0002-9378(82)90083-7. [DOI] [PubMed] [Google Scholar]
- Ottesen B, Gerstenberg T, Ulrichsen H, Manthorpe T, Fahrenkrug J, Wagner G. Vasoactive intestinal polypeptide (VIP) increases vaginal blood flow and inhibits smooth muscle activity in women. Eur J Clin Invest. 1983;13:321–324. doi: 10.1111/j.1365-2362.1983.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Ottesen B, Pedersen B, Nielsen J, Dalgaard D, Wagner G, Fahrenkrug J. Vasoactive intestinal polypeptide (VIP) provokes vaginal lubrication in normal women. Peptides. 1987;8:797–800. doi: 10.1016/0196-9781(87)90061-1. [DOI] [PubMed] [Google Scholar]
- Park K, Goldstein I, Andry C, Siroky MB, Krane RJ, Azadzoi KM. Vasculogenic female sexual dysfunction: the hemodynamic basis for vaginal engorgement insufficiency and clitoral erectile insufficiency. Int J Impot Res. 1997;9:27–37. doi: 10.1038/sj.ijir.3900258. [DOI] [PubMed] [Google Scholar]
- Pryde DC, Maw GN, Planken S, Platts MY, Sanderson V, Corless M, et al. Novel selective inhibitors of neutral endopeptidase for the treatment of female sexual arousal disorder. Synthesis and activity of functionalized glutaramides. J Med Chem. 2006;49(14):4409–4424. doi: 10.1021/jm060133g. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Phillips NA, Gendrano NC, Ferguson DM. Oral phentolamine and female sexual arousal disorder: a pilot study. J Sex Marital Ther. 1999;25(2):137–144. doi: 10.1080/00926239908403987. [DOI] [PubMed] [Google Scholar]
- Schoen C, Bachmann G. Sildenafil citrate for female sexual arousal disorder: a future possibility? Nat Rev Urol. 2009;6(4):216–222. doi: 10.1038/nrurol.2009.25. [DOI] [PubMed] [Google Scholar]
- Sipski ML, Rosen RC, Alexander CJ, Hamer RM. Sildenafil effects on sexual and cardiovascular responses in women with spinal cord injury. Urology. 2000;55(6):812–815. doi: 10.1016/s0090-4295(00)00493-3. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Gao XP, Olopade CO, Rubinstein I. Neutral endopeptidase modulates VIP-induced vasodilation in hamster cheek pouch vessels in situ. Am J Physiol. 1996;271(2 Pt 2):R393–R397. doi: 10.1152/ajpregu.1996.271.2.R393. [DOI] [PubMed] [Google Scholar]
- Traish A, Moreland RB, Huang YH, Kim NN, Berman J, Goldstein I. Development of human and rabbit vaginal smooth muscle cell cultures: effects of vasoactive agents on intracellular levels of cyclic nucleotides. Mol Cell Biol Res Commun. 1999;2:131–137. doi: 10.1006/mcbr.1999.0164. [DOI] [PubMed] [Google Scholar]
- Turner AJ, Tanzawa K. Mammalian membrane metallopeptidases: NEP, ECE, KELL, and PEX. FASEB J. 1997;11:355–364. doi: 10.1096/fasebj.11.5.9141502. [DOI] [PubMed] [Google Scholar]
- Ückert S, Ehlers V, Nüser V, Oelke M, Kauffels W, Scheller F, et al. In vitro functional responses of isolated human vaginal tissue to selective phosphodiesterase inhibitors. World J Urol. 2005a;23(6):398–404. doi: 10.1007/s00345-005-0014-6. [DOI] [PubMed] [Google Scholar]
- Ückert S, Oelke M, Waldkirch E, Stief CG, Albrecht K, Tröger HD, et al. Cyclic adenosine monophosphate and cyclic guanosine monophosphate-phosphodiesterase isoenzymes in human vagina: relation to nitric oxide synthase isoforms and vasoactive intestinal polypeptide-containing nerves. Urology. 2005b;65(3):604–610. doi: 10.1016/j.urology.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br J Pharmacol. 2009;157(4):527–536. doi: 10.1111/j.1476-5381.2009.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziessen T, Moncada S, Cellek S. Characterization of the non-nitrergic NANC relaxation responses in the rabbit vaginal wall. Br J Pharmacol. 2002;135(2):546–554. doi: 10.1038/sj.bjp.0704481. [DOI] [PMC free article] [PubMed] [Google Scholar]


