Abstract
Background and purpose:
The regulatory guidelines (ICHS7B) for the identification of only drug-induced long QT and pro-arrhythmias have certain limitations.
Experimental approach:
Conduction time (CT) was measured in isolated Purkinje fibres, left ventricular perfused wedges and perfused hearts from rabbits, and sodium current was measured in Chinese hamster ovary cells, transfected with Nav1.5 channels.
Key results:
A total of 355 compounds were screened for their effects on CT: 32% of these compounds slowed conduction, 65% had no effect and 3% accelerated conduction. Lidocaine and flecainide, which slow conduction, were tested in more detail as reference compounds. In isolated Purkinje fibres, flecainide largely slowed conduction and markedly increased triangulation, while lidocaine slightly slowed conduction and did not produce significant triangulation. Also in isolated left ventricular wedge preparations, flecainide largely slowed conduction in a rate-dependent manner, and elicited ventricular tachycardia (VT). Lidocaine slightly slowed conduction, reduced Tp–Te and did not induce VT. Similarly in isolated hearts, flecainide markedly slowed conduction, increased Tp–Te and elicited VT or ventricular fibrillation (VF). The slowing of conduction and induction of VT/VF with flecainide was much more evident in a condition of ischaemia/reperfusion. Lidocaine abolished ischaemia/reperfusion-induced VT/VF. Flecainide blocked sodium current (INa) preferentially in the activated state (i.e. open channel) with slow binding and dissociation rates in a use-dependent manner, and lidocaine weakly blocked INa.
Conclusion and implications:
Slowing conduction by blocking INa could be potentially pro-arrhythmic. It is possible to differentiate between compounds with ‘good’ (lidocaine-like) and ‘bad’ (flecainide-like) INa blocking activities in these models.
Keywords: conduction time, INa, drug, ventricular tachycardia (VT), ventricular fibrillation (VF)
Introduction
Drug-induced QT prolongation and the appearance of torsades de pointes (TdPs) are recognized as potential adverse effects associated with the use of a broad range of cardiovascular and non-cardiovascular drugs (Haverkamp et al., 2000; Cubeddu, 2003; Shah, 2004). Current established regulatory guidelines (CPMP/986/96, 1997 and Food and Drug Administration ICH S7B, 2005) recommend the use of preclinical studies to detect QT prolongation as a surrogate biomarker of drug-induced TdP.
Inhibition of the rapidly activating delayed rectifier potassium current (IKr) of cardiac cells, which is the key current responsible for phase 3 repolarization of the cardiac action potential and conveyed by the human ether-ā-go-go-related gene-encoded voltage-dependent potassium channel (hERG channels), is the most common mechanism of these drugs to induce QT prolongation (ion channel nomenclature follows Alexander et al., 2009). Drug-induced long QT and the risk for TdPs are relatively well known, thanks to extensive studies in recent years and our increased understanding of the mechanisms for the long QT syndrome (Roden, 2008). Conversely, relatively little is known about the pro-arrhythmic potential of sodium current (INa) blocking agents, although the Cardiac Arrhythmia Suppression Trial (The CAST Investigators, 1989) showed that flecainide and encainide (class Ic anti-arrhythmic) were associated with an increased incidence of sudden cardiac death in post-infarction patients (Sellers and DiMarco, 1984; Echt et al., 1991). This finding raised questions concerning the pro-arrhythmic potential of non-cardiovascular compounds with INa blocking activities, like encainide and flecainide, and again points to a gap in the regulatory guidelines for cardiovascular safety testing. Indeed, in a recent study (Lu et al., 2008), we showed that these guidelines have limitations in that they do not cover the pro-arrhythmic potential of drug-induced QT shortening. In this report, we elaborate on the importance of detecting drugs with ‘bad’INa blocking activities like flecainide (drug-induced slowing of conduction or widening of the QRS complex with strong use dependence) which can induce potentially fatal ventricular tachycardia or fibrillation (VT or VF) in the absence of QT prolongation. The exact mechanism of the pro-arrhythmic effect of flecainide remains elusive. On the other hand, the INa blocking agent lidocaine (class Ib) has been extensively used in the clinic for many years, especially as an anaesthetic, and exhibits an excellent safety profile even in patients with myocardial infarction (Zehender et al., 1990; Wyman et al., 2004). Therefore, non-cardiovascular compounds with a ‘lidocaine-like’ effect on INa may be considered to be safe.
There is a need to demonstrate the potential of preclinical models to discriminate between compounds, which block INa with ‘flecainide-like’ actions and have a high pro-arrhythmic potential, and those, which block INa with ‘lidocaine-like’ actions without being pro-arrhythmic. In the present study, we have investigated the effects of flecainide and lidocaine, as two reference compounds, on human cardiac INa, and on isolated rabbit Purkinje fibres, arterially perfused left ventricular wedge preparations or Langendorff-perfused rabbit hearts to evaluate whether these preclinical models could differentiate between ‘good’ and ‘bad’INa blockers.
Methods
All animal care and experimental procedures were in accordance with ‘The Provision of the European Convention’ on the protection of vertebrate animals which are used for experimental and other scientific purposes, and with ‘the Appendices A and B’, made at Strasbourg on March 18, 1986 (Belgian Act of October 18, 1991).
Rabbit isolated Purkinje fibres
Electrophysiological experiments were performed on isolated Purkinje fibres from female rabbits, using conventional microelectrode techniques. The approach was similar to that used in recent studies from our laboratories (Lu et al., 2001; 2008;). Briefly, Purkinje fibres, isolated from rabbits weighing 2.5–3 kg, were isolated within 5 min after killing the animal and placed in a tissue bath. The cardiac tissues were perfused with gassed (95% O2 and 5% CO2) Tyrode's solution of the following composition (in mM): NaCl, 136.9; KCl, 4; CaCl2, 1.8; MgCl2, 1.04; NaHCO3, 11.9; NaH2PO4, 0.42; and glucose, 5.5. The preparations were fixed at the bottom of a 2.5 mL perfusion organ bath by two small needles, and continuously superfused with oxygenated Tyrode's solution at a rate of 2.7 mL·min−1 together with solutions of the reference compounds or solvent at a rate of 300 µL·min−1. The temperature in the bath was kept at 36.5 ± 1.0°C.
The Purkinje fibres were stimulated at a basal rate of 1 Hz through bipolar Teflon-coated silver wire electrodes that were connected to a pulse generator and an isolation transformer. Stimuli consisted of regular pulses of 0.5–1 ms duration, delivered at an intensity of twice the diastolic threshold. Intracellular potentials were recorded with glass microelectrodes filled with 2.7 M KCl, with tip resistances between 5 and 65 MΩ. The microelectrode was connected to the headstage of the microelectrode amplifier (Hugo Sachs Elektronik: HSE; type 695; March-Hugstetten, Germany). Action potential signals were acquired by action potential software (Notocord-Hem 3.5, Paris, France) at a sampling rate of 20 kHz, filtered at 100 Hz. The signals were stored on a personal computer for subsequent analysis. The optimal stimulation site of the preparation was determined as the site where a normal and stable action potential could be induced. The maximum rate of rise of the action potential was obtained by electronic differentiation (Vmax in V/s), which was subsequently used as the parameter for the conduction time (CT) in this setting. The amplitude of action potential (AAP in mV) and action potential duration (APD) at 40, 50 or at 90% repolarization (APD40, APD50 or APD90 in ms), and triangulation (APD90–APD40) were determined from the recordings.
Control values (baseline values before contact with solvent or compound) were determined after a stabilization period, when the measured variables had reached a steady state. The electrophysiological parameters were again measured during the experimental period.
After baseline values had been recorded at 1 Hz, solvent or one of the drug solutions (such as flecainide or lidocaine; at four concentrations (0.01–10 µM) or (0.1–100 µM); n= 5–7 for each compound), was continuously superfused for 15 min for each concentration at a stimulation rate of 1 Hz.
Isolated, arterially perfused left ventricular wedge from rabbits
The methods used for isolation, perfusion and recording of transmembrane activity from the arterially perfused ventricular wedge preparation, as well as the viability and electrical stability of the preparation, have been described previously (Yan and Antzelevitch, 1999; Yan et al., 2001). Briefly, female rabbits weighing 2.5–3 kg were anaesthetized with ketamine (40–50 mg per kg IV) and anticoagulated with heparin. The chest was opened via a left thoracotomy, and the heart was excised and placed in a cardioplegic solution consisting of cold (4°C) normal Tyrode's solution. Transmural wedges with dimensions of approximately 1.5 cm wide and 2–3 cm long were dissected from the left ventricle. The wedge tissue was cannulated via the left anterior descending artery or the circumflex artery, and perfused with the cardioplegic solution. The preparation was then placed in a small tissue bath and arterially perfused with warm Tyrode's solution containing 4 mM K+ buffered with 95% O2 and 5% CO2 (T: 35.7 ± 0.1°C; perfusion pressure: 40–50 mm Hg). The ventricular wedge was allowed to equilibrate in the tissue bath until electrically stable, usually 1 h. The preparation was paced at basic cycle lengths (BCLs) of 1000 and 2000 ms. A brief period (30–60 s) of faster pacing at a BCL of 500 ms or less was introduced between BCLs of 1000 and 2000 ms using bipolar silver electrodes insulated except at the tips and applied to the endocardial surface. The purpose of the faster pacing frequency was to examine use-dependent conduction delay and resultant VT/VF. During experiments in which QRS duration increased more than 10 ms at a BCL of 2000 ms, acceleration of the pacing rate was performed until marked conduction delay and VT/VF were observed. However, the faster pacing rate was limited to 60 s and at BCLs from 500 to 400 ms in order to avoid myocardial ischaemia.
Transmembrane action potentials in wedge preparations were recorded simultaneously from epicardial and endocardial sites by use of two separate intracellular floating microelectrodes. A transmural electrocardiogram (ECG) was recorded concurrently in all experiments. APD was measured at 90% repolarization (APD90). Transmural dispersion of repolarization (TDR) was defined as the interval from the peak to the end of the T wave (QTend–QTpeak). The QT interval was defined as the time from the onset of the QRS to the point at which the final downslope of the T wave crosses the isoelectric line. A TdP risk score relative to the effects on QT interval, TDR/QT ratio, early afterdepolarizations (EADs), R-on-T and TdP were also calculated with the criteria similar to that in previous studies (Wang et al., 2008). QRS duration was taken as the parameter for the CT.
Rabbit Langendorff-perfused hearts
The method for determining various cardiac electrophysiological properties was similar to and has been already described in detail (Lu et al., 2008). Briefly, Langendorff experiments were performed on the hearts isolated from female albino rabbits (about 2.5 kg). The heart was perfused at a constant pressure of 80 cm H2O with a bicarbonate buffer (in mM: NaCl, 118; KCl, 3.5; NaHCO3, 22; MgCl2, 1.1; NaH2PO4, 0.4; CaCl2, 1.8; glucose, 5; pyruvate, 2; and creatine, 0.038, gassed with 95% O2 and 5% CO2, pH adjusted to 7.4, at 37°C). The bundle of His was cut, and a stimulating electrode was sutured on each side of the distal His bundle. Recording electrodes were placed in the left ventricular endocardium, and left and right epicardium respectively.
Stimulation current was adjusted, and automaticity, escape cycle lengths, CTs and APDs for cycle lengths at 2000, 1500, 1000, 750, 500, 300 and 250 ms were determined. Trains of 5 s at cycle lengths of 250, 500, 750 and 1000 ms were recorded. APD90 or APD60, triangulation (APD90–APD30), short-term beat-to-beat instability of the APD90, intraventricular CT and coronary flow were measured during experimental periods. A bath ECG from Lead II, reflecting the propagation of action potential waves Q, R, S, J and T, was recorded. ECG parameters such as QRS duration, QT interval, JT interval, Tp–Te [= QTpeak–QTend], rTp–Te (Tp–Te/QT interval * 100) (Antzelevitch et al., 1999; Yan et al., 2001) were also measured. QRS duration or the intraventricular CT was taken as the parameter for the CT. Intraventricular time was measured at the interval between the electrical stimulation and the onset of the depolarization of the action potential.
The solvent or drug solutions (four concentrations of the test compound from 0.001 to 100 µM; n= 6–8 for each compound) were perfused for 30 min per concentration.
In ischaemia/reperfusion experiments, hearts were subjected to four periods of 15 min of global ischaemia followed by 15 min of reperfusion. Compound or solvent was given during the reperfusion period.
Monophasic action potentials from the inside of the left ventricle, the right and left epicardium and ECG were recorded and digitized at 1 kHz (12 bits). For the conduction data, sampling was done at 10 kHz (each channel). Data were analysed beat by beat during the experiment, and the results were compressed and saved to disc.
Human cardiac INa
Human cardiac INa were studied at single-cell level using the single electrode, whole-cell configuration of the patch clamp technique (Hamill et al., 1981). A Chinese hamster ovary cell line (CHO) with a stable transfection of the SCN5A gene, encoding the human cardiac Na channel Nav1.5, was used (Gellens et al., 1992). The cells were cultured in Iscove's modified Dulbecco's medium (IMDM, Gibco, Invitrogen, Ltd., Paisley, UK), which was supplemented with (amounts indicated hereafter are added to 500 mL IMDM): 50 mL fetal bovine serum (Bio-Whittaker, Walkersville, MD, USA), 5 mL non-essential amino acids 100× (Gibco) and 10 mL hypoxanthine/thymidine supplement 50× (Gibco). Immediately before use, 400 µg·mL−1 geneticin (Gibco) was added to the medium. The cells were incubated at 37°C in 5% CO2 atmosphere (in air). Medium was refreshed three times a week. When 60–70% confluence was reached, cells were subcultured (twice a week), and the cells were seeded 1 day before use in Petri dishes.
The extracellular bath solution contained (in mM): 100, CsCl; 50, NaCl; 4, KCl; 1, MgCl2; 1.8, CaCl2; 10, HEPES; and 5, glucose (pH 7.4 with CsOH). The pipette solution contained (in mM): 100, CsF; 30, CsCl; 12, NaF; 5, HEPES; and 5, EGTA (pH 7.2 with CsOH).
The Petri dish was constantly perfused with the solvent control solution at room temperature (21–23°C). Solvent control solution and test solutions were applied to the cell under study using a Y-tube system, allowing a rapid change of solutions in the vicinity of the cell under study. The membrane current of the cells was measured at distinct membrane potentials with the patch clamp technique by means of an EPC-9 patch clamp amplifier (HEKA, Lambrecht/Pfalz, Germany). Data were acquired and analysed using the programs Patchmaster (version 2.05; HEKA), DataAccess (Bruxton, Seattle, WA, USA), Igor (version 5.01; WaveMetrics, Lake Oswego, OR, USA) and SigmaPlot 2001 (SPSS Inc., Chicago, IL, USA), running on a PC. The current signals were low-pass filtered and subsequently digitized. After disruption of the membrane, the cell capacitance and the series resistance were compensated using the circuit of the EPC-9 patch clamp amplifier. In all experiments, a 50–70% series resistance compensation was achieved. After establishing whole-cell configuration, a 5 min equilibration period was allowed for internal perfusion of the cell with the pipette solution. The P/4 method (Bezanilla and Armstrong, 1977) was used to correct for leak current. A range of different experimental conditions were used with these cells, as shown below.
To determine the concentration-dependent effect at the holding potentials of −140 and −40 mV, the following pulse protocol was used: a 5 ms test pulse to –20 mV from the holding potential of –140 mV, followed by a conditioning pulse of 10 s to –40 mV to inactivate the INa, a 10 ms pulse to −140 mV and the second 3 ms test pulse to −20 mV. The sodium peak current was determined at test pulses of −20 mV, at −140 mV and after the conditioning pulse to –40 mV. The cycle rate was 15 s. Test pulses were given for 5 min, to quantify the INa under control conditions. While continuing the pulse protocol, perfusion was switched from solvent control solution to drug-containing solution. The effect of the drug was evaluated after 5 min of drug application. The concentration–response relationship was calculated using the Hill equation:
For the steady-state inactivation curve, currents were elicited by 20 ms test pulses to –20 mV, following 500 ms conditioning pulses varying from −140 to –30 mV with 10 mV increments. The holding potential was −140 mV, and the pulse rate was 0.1 Hz. The peak current amplitudes at −20 mV were normalized by their corresponding controls at the pre-pulse voltage of −140 mV. Normalized inactivation sodium currents were fitted by the Boltzmann equation: 1/[1 + exp(V1/2−V)/k].
Recovery from inactivation was measured from a holding potential of −140 mV with a 500 ms depolarizing pre-pulse to –20 mV, followed by a variable recovery period from 0.7 ms to 48 s, which in turn was followed by a 20 ms test pulse to –20 mV. The cycle rate was 30 or 60 s, respectively.
Development of inactivated-state block was measured from a holding potential of –140 mV with a depolarizing pre-pulse to –20 mV of variable length (5 ms to 10.24 s), followed by a 10 ms long recovery to the holding potential, which in turn was followed by the 20 ms test pulse to −20 mV. The cycle rate was 30 s. For the recovery of inactivation and the development of inactivated-state block, the peak current amplitudes of the test pulses were normalized to the current amplitude of the pre-pulses. The time constants were described by exponential equations.
Use-dependent effect: after a resting period of 2 min at a holding potential of –100 mV, trains of depolarizing pulses to –20 mV following a 10 ms pre-pulse to –140 mV were delivered to assess the use-dependent effect (after the 30th stimulus). The stimulation frequency was 1 Hz. A use-dependent block is the increase in drug-induced block of a voltage-activated current upon application of a train of voltage pulses following a rest period. The current amplitudes were normalized to the maximal peak current of the first pulse in the presence of the compound or solvent. The onset of use-dependent block at 1 Hz was described by a single exponential equation.
Recovery from use-dependent block: A use-dependent block of cardiac INa in the presence of flecainide or solvent was elicited with a train of pulses (100 repeats at 3 Hz to a pulse of −20 mV from a holding potential at –100 mV), and then for a varying length of time the cell was hyperpolarized to −140 mV, and finally, the test pulse of −20 mV was elicited. The peak current of this test pulse was measured and normalized, with the peak amplitude of a test pulse elicited with 0.033 Hz. The data were fit with a double or triple exponential function.
Effects of drugs on hERG current
The experimental approach we used is similar to that in other studies (Dubin et al., 2005; Lu et al., 2008). The cell preparation used was a human embryonic kidney cell line (HEK293) with a stable transfection of hERG (purchased from Prof Craig January's group, University of Wisconsin, Madison, WI, USA). The cells were cultured in medium (minimum essential medium, Gibco) supplemented with (amounts added to 500 mL medium): 5 mL l-glutamine–penicillin–streptomycin (Sigma, St Louis, MO, USA); 50 mL fetal bovine serum (Bio-Whittaker); 5 mL non-essential amino acids 100× (Gibco); 5 mL sodium pyruvate 100 mM (Gibco); and 4 mL geneticin 50 mg·mL−1 (Gibco) at 37°C in 5% CO2 atmosphere. Before use, the cells were seeded in small Petri dishes pre-coated with poly-l-lysine (Biocoat, Becton Dickinson, Franklin Lakes, NJ, USA). Experiments were carried out 1–2 days after plating.
The conventional patch clamp recording experiments were conducted at room temperature. The bath solution contained (in mM) 150, NaCl; 4, KCl; 1, MgCl2; 1.8, CaCl2; 10, HEPES; 5, glucose (pH 7.4 with NaOH), and the pipette solution contained (in mM) 120, KCl; 10, HEPES; 5, EGTA; 4, ATP-Mg2; 2, MgCl2; 0.5, CaCl2 (pH 7.2 with KOH). Solutions were applied to the cell under study using a Y-tube system, allowing a rapid delivery and mixing of solutions in the vicinity of the cell under study. The membrane current of the cells was measured at distinct membrane potentials with the patch clamp technique by means of an EPC-9 patch clamp amplifier (HEKA). Data were acquired and analysed using the programs Pulse and PatchmasterPro (HEKA), Data Access (Bruxton), Igor (WaveMetrics) and Excel (Microsoft). After disruption of the membrane, the cell capacitance and the series resistance were compensated (90%) using the circuit of the EPC-9 patch clamp amplifier. The holding potential was –80 mV. The hERG-mediated current (K+-selective outward current) was determined as the maximal tail current at −40 mV after a 2 s depolarization to +60 mV. Pulse cycling rate was 15 s. Before each test pulse, a short pulse (0.5 s) to −60 mV was given to determine leak current. The protocol consisted of a 5 min equilibration period (no pulses), 5 min in control solution and then 5 min for each concentration of the drug. Up to three concentrations were tested per cell (0.001–100 µM). Rundown of the hERG current was taken into account by extrapolating the gradual decrease in current measured during the 5 min in control solution to the remaining duration of the experiment. The effect of the drug was measured after 5 min of drug application by dividing the measured current by the extrapolated current. In addition to the conventional patch clamp, for some compounds, the hERG test was performed by an automated patch clamp system (PatchXpress 7000A, Molecular Devices, Silicon Valley, CA, USA). This experimental approach was similar to that described earlier (Dubin et al., 2005). We used astemizole as a routine positive control.
Data analysis
All values are expressed as mean and standard error of the mean (SEM). Statistically significant differences between solvent and compound were calculated based on their changes from baseline with the Wilcoxon–Mann–Whitney test. For the evaluation of differences in the incidence of VT or VF, Fisher's exact test was used. Two-tailed probabilities of less than 0.05 were considered to indicate statistically significant differences.
Materials
A total of 355 novel compounds from Johnson & Johnson's (J & J) worldwide research projects were used. Reference compounds such as flecainide and lidocaine were obtained from Sigma. The compounds were dissolved in either pyrogen-free water (acidified with tartaric acid to obtain a pH of approximately 4.0), buffer solution or in dimethyl sulfoxide (DMSO, final bath concentration of 0.1 or 0.3%). Similar solutions without compound were used as solvent controls. Other compounds were supplied as shown; ketamine (Pfizer, Exton, PA, USA) heparin (LEO Pharma, Wilrijk, Belgium) and astemizole (J & J, Beerse, Belgium).
Results
Criteria for the assessment of effects of compounds on conduction and hERG
A slowing of conduction translates into a reduction in Vmax of the action potential in isolated rabbit Purkinje fibres, an increase in intraventricular CT in isolated rabbit hearts or a widening of the QRS complex in isolated rabbit wedge preparations or in isolated rabbit hearts. A slowing of conduction (an indirect measure of INa inhibition) was defined as a reduction of Vmax by ≥15% in isolated rabbit Purkinje fibres, an increase in the intraventricular CT by ≥15% in isolated Langendorff-perfused rabbit hearts or an increased QRS duration by ≥10% in isolated rabbit arterially perfused left ventricular wedge or isolated rabbit hearts, by the compound at any concentration (net effects, after correction for solvent effects; n= 5–8 per compound). The compound was considered as having no effect on conduction if it induced a solvent-corrected change from baseline between −15 and +15% in Vmax or intraventricular CT, or between −10 and +10% in QRS duration. An acceleration of conduction was defined as an increase in Vmax by ≥15% in isolated rabbit Purkinje fibres, a decrease in intraventricular CT by ≥15% in isolated rabbit hearts or a shortening of QRS duration by ≥10% in isolated wedge preparations or isolated hearts, at any tested concentration.
An effect was defined as ‘hERG inhibition’ if a compound inhibited hERG current by ≥20% at a concentration ≤3 µM, as ‘hERG stimulation’ (or activation of hERG) if a compound activated hERG current ≥10% at a concentration ≤3 µM, or was considered as ‘no effect’ if a compound was associated with <20% inhibition or <10% activation of hERG current at a concentration ≤3 µM (n= 3–5 per compound and per concentration) or if effects appeared only at concentrations >3 µM.
A total of 355 compounds were tested in both the hERG assay and one of three in vitro assays (isolated rabbit Purkinje fibres, isolated arterially perfused rabbit left ventricular wedge or isolated Langendorff-perfused rabbit hearts).
Effects on conduction (Vmax, intraventricular CT or QRS duration)
Of these 355 compounds, 32.1% (n= 114) were found to slow conduction, while 65.4% (n= 232) were found to have no effect and 2.5% (n= 9) were found to accelerate conduction (see upper part of Figure 1). These nine compounds only slightly accelerated conduction (by ≤25%), while flecainide slowed conduction by more than 100%.
Figure 1.
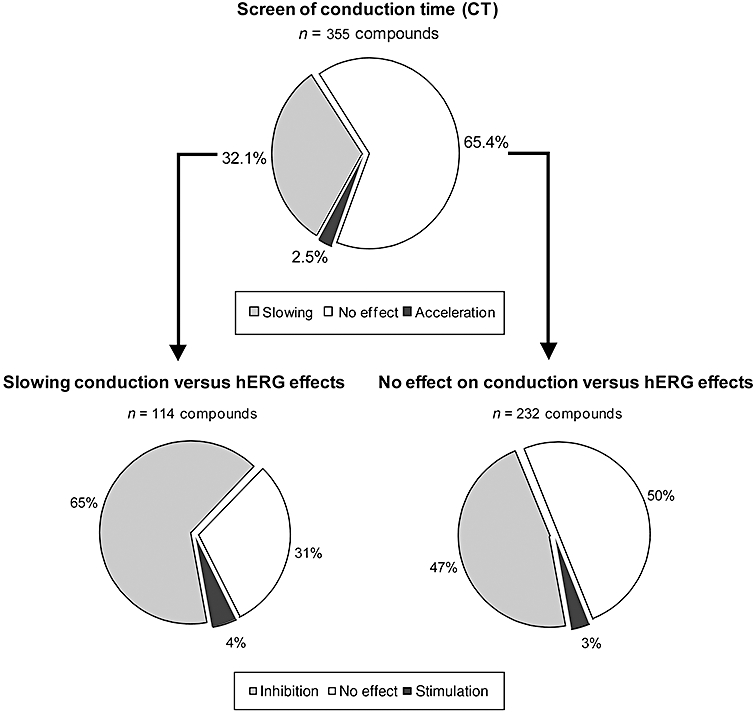
Upper part: effect of 355 compounds on the CT. Slowing of conduction was defined as a reduction of Vmax or an increase in intraventricular CT by ≥15% (net effect), or an increase in the QRS duration by ≥10% (net effect) by a compound in rabbit isolated Purkinje fibres, in isolated Langendorff-perfused hearts or in isolated left ventricular, arterially perfused, wedge preparations at any tested concentration (n= 5–7). Acceleration of conduction was defined as an increase in Vmax or a reduction of CT by ≥15% (net effect) or a shortening of QRS duration by ≥10% (net effect) in the Purkinje fibre, in Langendorff-perfused hearts or in the wedge preparations, at any tested concentration. The compound was considered as having no effect if it induced a change from baseline in Vmax or in the intraventricular CT between −15 and +15%, or a change from baseline in QRS duration between −10 and +10%. Lower part: effect of 114 compounds, which slowed conduction (left), and 232 compounds, having no effect on conduction, on the hERG current. Inhibition: ≥20% inhibition of hERG current at a concentration ≤3 µM; no effect: <20% inhibition or <10% activation of hERG current at a concentration ≤3 µM or effects appeared only at high concentrations (>3 µM); hERG stimulation (activation): ≥10% activation of hERG at a concentration ≤3 µM (n= 3–5 per concentration).
Effects on hERG
Of these 355 compounds, 52% (n= 186) were found to block hERG current, 44% (n= 156) were without effect and 4% (n= 13) were found to activate/stimulate hERG current. Of the 114 compounds that slowed conduction in one of the in vitro assays, 65% (n= 74) inhibited hERG, 31% (n= 35) had no effect on hERG, while 4% (n= 5) were found to activate hERG current (lower part of Figure 1).
Of the 232 compounds without effect on CT, 47% (n= 108) inhibited hERG, 50% (n= 116) had no effect on hERG, while 3% (n= 8) were found to activate hERG current (see lower part of Figure 1). Of the nine compounds found to accelerate conduction, four inhibited hERG and five had no effect on hERG.
Effects of lidocaine and flecainide on hERG
Lidocaine had no effect on hERG current (net effect of 14.9% inhibition of hERG at 100 µM), whereas flecainide blocked the hERG current with an IC50 of 1.7 µM. As a positive control, astemizole blocked the hERG current by 86% at 10 nM (n= 5).
Effects of flecainide and lidocaine on rabbit isolated Purkinje fibres
Compared to solvent (n= 14), lidocaine at increasing concentrations from 0.1 to 100 µM (n= 7) did not significantly change triangulation, resting membrane potential and did not elicit EADs. Lidocaine at 100 µM (Figure 2) tended to reduce Vmax, but significantly shortened APD40, APD50 and APD90 (all P < 0.05).
Figure 2.
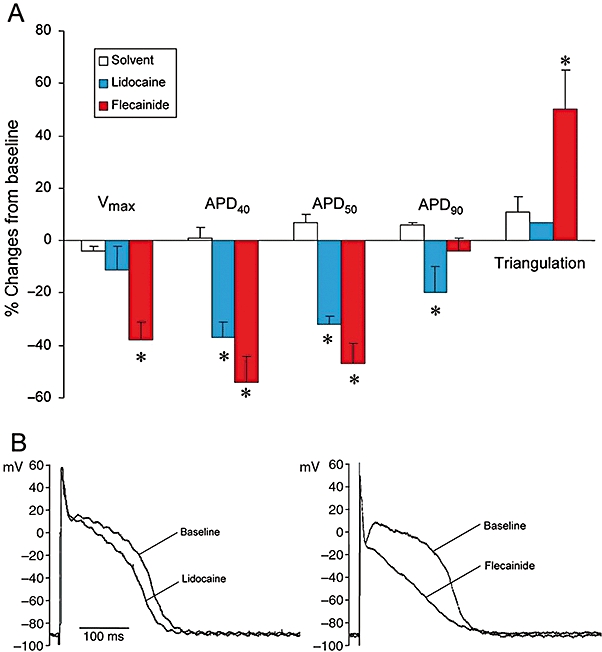
Effects of lidocaine (100 µM) and flecainide (10 µM) in rabbit isolated Purkinje fibres. Upper part: effects on the duration of the action potential at 40, 50 and 90% repolarization (APD40, APD50 and APD90), triangulation and Vmax.*P < 0.05, significantly different from effects of solvent. Data are mean ± SEM. Lower part: effect of lidocaine (100 µM) and flecainide (10 µM) on the morphology of the action potential in a Purkinje fibre at a stimulation rate of 1 Hz. The figure shows that lidocaine has much weaker effects on the action potential than flecainide.
At 10 µM, flecainide (n= 8) clearly shortened APD40 and APD50, markedly reduced Vmax and also significantly increased triangulation of the action potential (all P < 0.05). However, flecainide did not significantly change APD90 (Figure 2A). Figure 2B shows an example of the effects of lidocaine (100 µM) and flecainide (10 µM) on the morphology of the action potential in an isolated rabbit Purkinje fibre.
The major differences between lidocaine and flecainide on the Purkinje fibre can be summarized as follows: (i) flecainide largely increased triangulation, while lidocaine did not; and (ii) flecainide largely decreased Vmax, while lidocaine did not.
Effects of flecainide and lidocaine in Langendorff-perfused rabbit hearts without ischaemia and reperfusion
Compared to solvent (n= 6), lidocaine (n= 6) at increasing concentrations from 0.1 to 100 µM did not significantly change the duration of the JT interval, duration of the action potential at 90% repolarization (APD90), triangulation of the action potential, transmural dispersion (Tp–Te of the T wave), instability of the action potential and coronary flow (data not shown). Lidocaine (100 µM) slightly increased QRS duration (P < 0.05), while intraventricular CT was not significantly affected (upper part of Figure 3). Lidocaine did not elicit any incidence of VT, VF and in-excitability.
Figure 3.
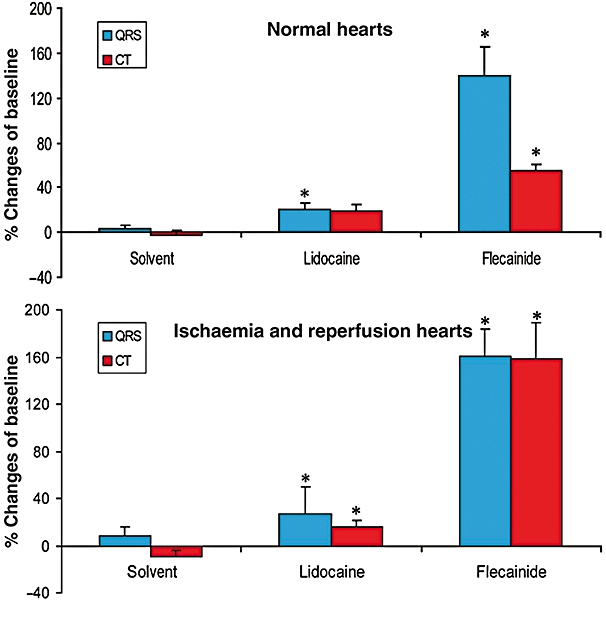
Effects of lidocaine (100 µM) and flecainide (10 µM) on QRS duration and intraventricular conduction time (CT) in Langendorff-perfused hearts without (upper part of the figure) and with ischaemia and reperfusion (lower part of the figure).*P < 0.05, significantly different from effects of solvent. The figure shows that lidocaine has much weaker effects on QRS duration and CT than flecainide. Data are mean ± SEM.
On the other hand, flecainide (n= 6) at 10 µM markedly increased QRS duration and CT (P < 0.05; upper part of Figure 3). Furthermore, flecainide prolonged the QT interval (P < 0.05). The prolongation of the QT interval by the compound was secondary to the marked increase in QRS duration of the compound because it did not significantly prolong the JT interval or APD90. Flecainide, however, did not significantly change the triangulation and instability of the action potential, APD90, Tp–Te of the T wave and coronary flow (data not shown).
At 10 µM, flecainide elicited VT, VF and in-excitability in three, one and six out of the six hearts, respectively (vs. in 0 out of the 12 hearts with solvent, and 0 out of the six hearts with lidocaine).
In summary, flecainide at 10 µM markedly increased QRS duration and CT, and elicited 100% incidence of in-excitability and 50% incidence of VT in isolated Langendorff-perfused rabbit hearts without ischaemia and reperfusion. On the other hand, lidocaine at 100 µM slightly increased QRS duration, did not relevantly change CT and did not elicit in-excitability or VT/VF.
Effects of flecainide and lidocaine in Langendorff-perfused rabbit hearts with ischaemia and reperfusion
All hearts were subjected to four cycles of treatments, each including a 15 min period of global ischaemia followed by a 15 min period of reperfusion. Increasing concentrations of compound or solvent were given during the reperfusion period.
In the solvent-perfused group (n= 10), ischaemia caused a shortening of the QT interval, JT interval and APD90; a large ST segment elevation; and elicited VT and VF in 30% (max during the third period of ischaemia) and 10% (max during the first and fourth periods of ischaemia), respectively (Figure 4). On the other hand, reperfusion (reflow) resulted in a normalization of all ECG and APD parameters, elicited VT in 30% of the hearts (max during the third period of reperfusion), but no VF.
Figure 4.
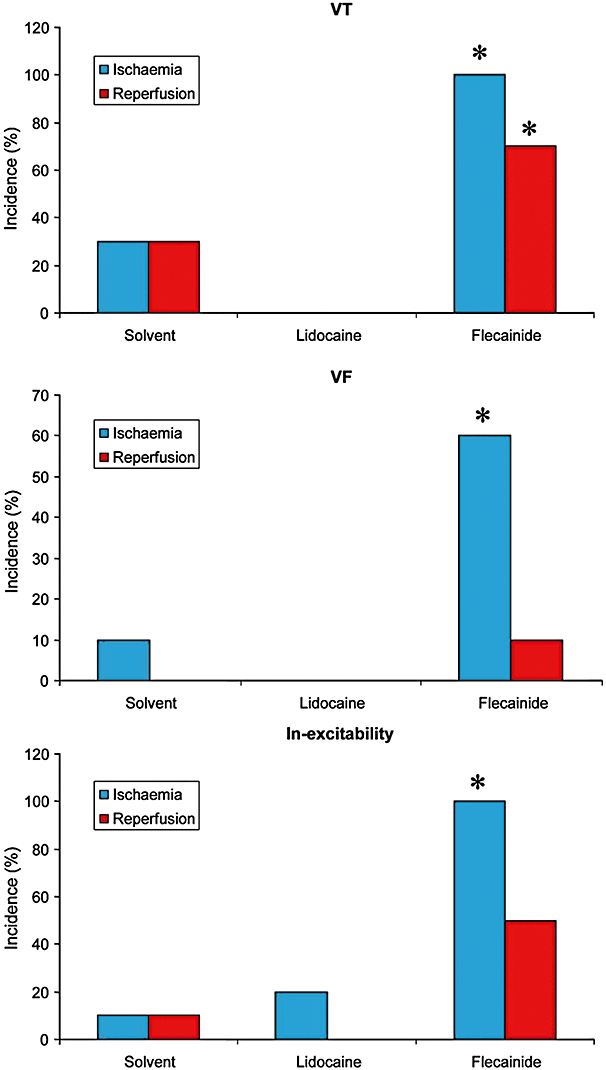
Effects of lidocaine (100 µM) and flecainide (10 µM) on the incidence of VT, VF and in-excitability in Langendorff-perfused hearts in a condition of ischaemia and reperfusion.*P < 0.05, significantly different from effects of solvent. Flecainide increased the incidence of ischaemia- and reperfusion-induced arrhythmias, while lidocaine abolished ischaemia- and reperfusion-induced VT and VF.
Relative to solvent (n= 10), lidocaine (n= 10) at 10 µM did not significantly change ECG and MAP parameters. At 100 µM, lidocaine significantly increased CT (P < 0.05) and tended to increase QRS duration (P= 0.055; lower part of Figure 3). Lidocaine prevented ischaemia- and reperfusion-induced VT/VF (Figure 4).
Flecainide at 10 µM (n= 10) significantly increased QRS duration and CT (both P < 0.05; lower part of Figure 3). Flecainide at 10 µM also tended to increase short-term instability of the action potential (9.8 ms from baseline vs. 2.3 ms with solvent; P > 0.05), and significantly increased Tp–Te by 110% (vs. 14% of baseline with solvent and 30% of baseline with lidocaine; P < 0.05). Furthermore, flecainide (10 µM) markedly increased the incidence of both ischaemia-induced VT during the third period of ischaemia (P < 0.05 vs. solvent) and reperfusion-induced VT during the fourth period of reperfusion (P < 0.05 vs. solvent). Moreover, at 10 µM, flecainide increased the incidence of VF to 60% during the third period of ischaemia (vs. 0% with solvent; P < 0.05) and to 10% during the third and fourth periods of reperfusion (vs. 0% with solvent). Furthermore, at 10 µM, flecainide also markedly increased the incidence of in-excitability to 100% during the fourth period of ischaemia versus 10% with solvent or 20% with lidocaine (P < 0.05), and during the fourth period of reperfusion as well (Figure 4).
In summary, flecainide markedly increased QRS duration, CT, Tp–Te and instability of the action potential, and increased the incidence of ischaemia-induced VT, VF and in-excitability, and the incidence of reperfusion-induced VT and in-excitability (Figure 4). Lidocaine slightly increased QRS duration and CT, did elicit in-excitability, but did not significantly change instability, and abolished ischaemia- and reperfusion-induced VT and VF (Figure 4). Figure 5 shows an example of the effects of lidocaine (100 µM) and flecainide (10 µM) on ECG, and the monophasic action potentials during ischaemia and reperfusion in Langendorff-perfused hearts. During ischaemia, the QT interval and duration of the action potential shortened, and the ST segment of the ECG was elevated or depressed. Lidocaine prevented ischaemia- and reperfusion-induced arrhythmias, while flecainide elicited VF at 11 min after the onset of ischaemia, and again after 2 min of reperfusion (not shown).
Figure 5.
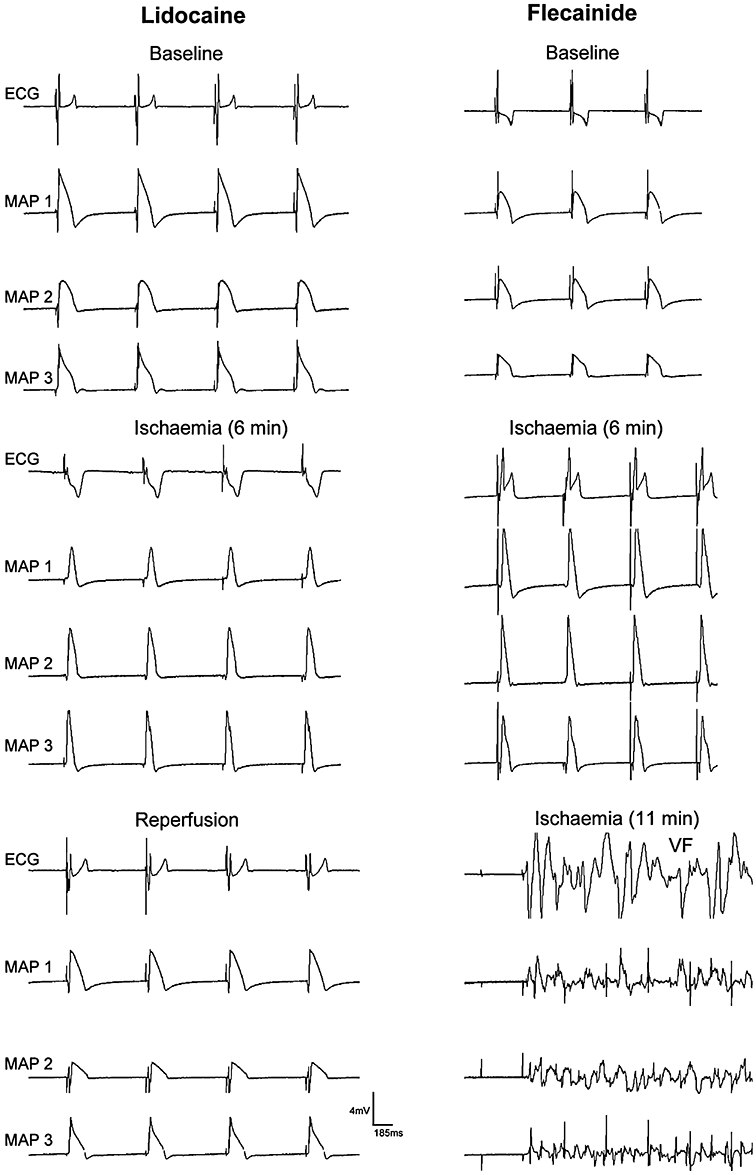
Effects of lidocaine (100 µM) and flecainide (10 µM) on the ECG and epicardial monophasic action potential in an isolated Langendorff-perfused heart during ischaemia and reperfusion. MAP1, the left ventricular epicardial monophasic action potential (MAP); MAP2, right ventricular MAP; MAP3, left intraventricular MAP recording.
Effects of flecainide and lidocaine in rabbit arterially perfused left ventricular wedge
Flecainide (n= 7) caused a significant increase in the QRS duration at concentrations of 3 and 10 µM (Figure 6A and C). The effect of flecainide on the QRS duration at 10 µM was markedly use dependent (Figure 6), increasing the QRS duration significantly at all three BCL conditions (2000, 1000 and 500 ms) (P < 0.05).
Figure 6.
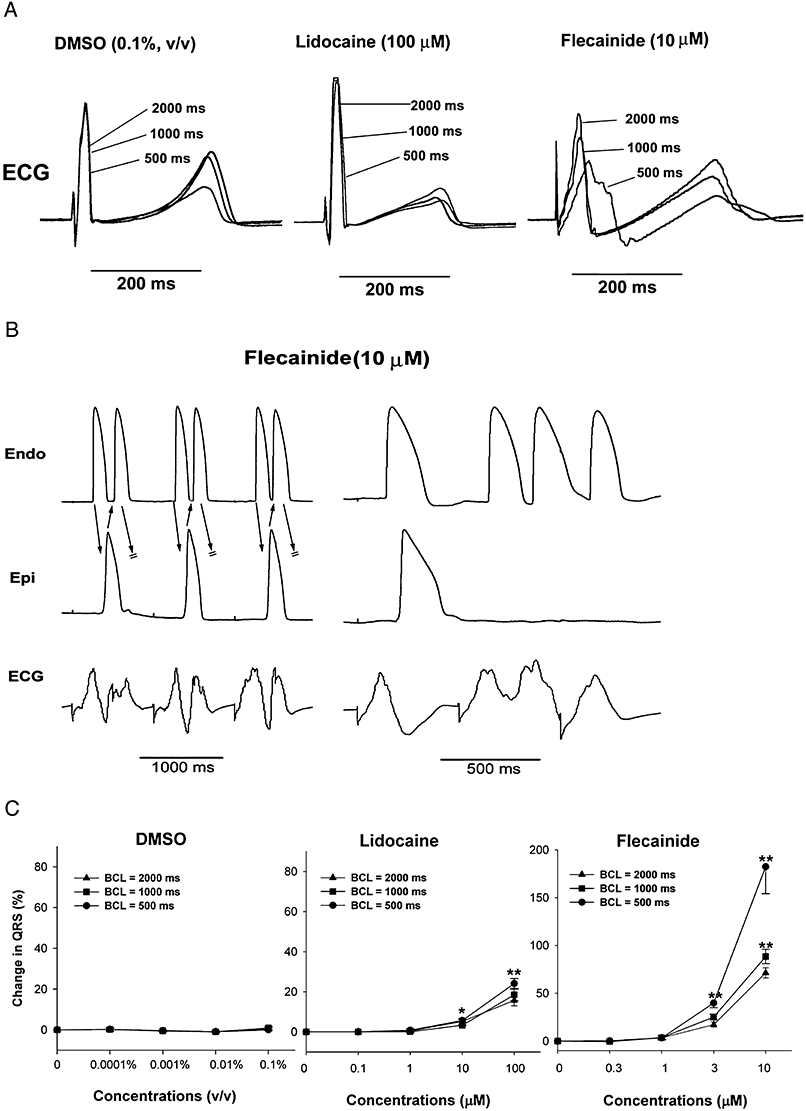
Effects of lidocaine (100 µM) and flecainide (10 µM) in the isolated rabbit left ventricular arterially perfused wedge preparation. (A) The effects of solvent (DMSO), lidocaine (100 µM) and flecainide (10 µM) on ECG at stimulation cycle lengths of 2000, 1000 and 500 ms. Note that lidocaine weakly increased QRS duration in a non-rate-dependent manner, while flecainide strongly increased QRS duration in a rate-dependent manner. (B) Flecainide-induced ectopic beats and VT. Note that the slowing of conduction between the endocardial (Endo) and epicardial (Epi) layers caused re-entrant beats, that is, extra-systoles (left panel) and three beats of VT (right panel). Stimulation at basic cycle length = 500 ms. (C) The effects of solvent (DMSO), lidocaine and flecainide on QRS duration. Note that lidocaine slightly, but significantly, increased QRS duration (slowing of conduction), and flecainide markedly increased QRS duration in a concentration-dependent and a rate-dependent manner (n= 7 per group). **P < 0.01, significantly different from effects of solvent.
Flecainide at 3 and 10 µM caused a slight, but significant QT prolongation by 15 and 13% from 306 ± 10.2 ms to 351 ± 10.1 ms, and 345 ± 10.6 (vs. 0.4 ± 0.9% and 0 ± 1.0% with solvent; P < 0.05) at a BCL of 2000 ms.
Flecainide increased the Tp–Te interval at 0.3 and 3 µM (+5 ± 1.0% and 19 ± 2.5% of baseline vs. –2 ± 1.0% and –2 ± 1.0% of baseline with solvent; P < 0.05) at a BCL of 2000 ms. At the higher dose (10 µM) tested, the increase in the Tp–Te interval was attenuated (−10 ± 5.5% from baseline vs. –3 ± 0.9% of baseline with solvent; P > 0.05).
In the concentration range tested (0.3–10 µM), no EAD-dependent R-on-T and TdP were observed in any of the seven preparations treated with flecainide. A slight, but significant, increase in TdP score was observed only at 3 µM (2 ± 0.3 vs. – 0.3 ± 0.2 with solvent; P < 0.05). Interestingly, flecainide at a concentration of 10 µM caused VT and VF in four out of seven preparations at faster pacing rates at which marked conduction delay (QRS widening) was present (P < 0.05 when compared with the solvent group, Figure 6B). The average cycle length at which VT/VF occurred was 469 ± 6.3 ms.
Lidocaine, on the other hand, slightly, but significantly, shortened the QT interval at 10 µM (to 259 ± 34 ms from baseline of 280 ± 36 ms) and 100 µM (to 251 ± 33 ms), and slightly increased the QRS duration at 100 µM by 11% (Figure 6A and C). These effects were accompanied by a slight, but significant, relative decrease in the TdP score to –1.6 ± 0.2 and −1.7 ± 0.2 from baseline of 0 ± 0 at 10 and 100 µM respectively. Lidocaine also slightly, but significantly, reduced Tp–Te by 11–31%, and Tp–Te by 6–23% from baseline starting at a concentration of 1 µM.
Inhibition of the sodium current by lidocaine was only weakly use-dependent (Figure 6A and C), and no arrhythmias were observed in the tested concentration range.
The major differences between lidocaine and flecainide on the rabbit wedge preparation were: flecainide largely widened the QRS complex in a rate dependence manner, increased Tp–Te and rTp–Te and elicited a high incidence of VT, while lidocaine caused a slight increase in QRS duration at a concentration 10 times higher than that of flecainide, and reduced Tp–Te and rTp–Te without arrhythmia generation.
Effects of flecainide and lidocaine on human cardiac INa
In order to disclose differences between class Ic (‘flecainide-like’) and class Ib (‘lidocaine-like’) INa blockers, we first determined the concentration-dependent effects of flecainide and lidocaine on the human cardiac sodium current in CHO cells, expressing the Nav1.5 channels at two different holding potentials. Flecainide reduced the INa in a concentration-dependent manner with an IC50 of 10.7 µM at a holding potential of –140 mV (n= 4–7), and to a similar extent with an IC50 of 6.3 µM at a depolarized holding potential of −40 mV (n= 4–7). Lidocaine reduced the INa in a concentration-dependent manner with an IC50 of 680 µM at a holding potential of –140 mV (n= 5–7), and with a higher affinity with an IC50 of 44 µM at a depolarized holding potential of –40 mV (n= 5–6). These results already suggest that lidocaine has a higher affinity for the inactivated state of the Nav1.5 channel than for its resting or activated state. In contrast, the effect of flecainide on the INa did not increase with inactivation of the Na channels.
Preferential binding of drugs to the inactivated state of the Nav1.5 channel results in a hyperpolarizing shift in the steady-state inactivation curve. To determine whether a shift in the steady-state inactivation curve can or cannot be detected with lidocaine or flecainide, we used a two-pulse protocol. Sodium currents were elicited with 20 ms test pulses to –20 mV following 500 ms conditioning pre-pulses varying from −140 to –30 mV with 10 mV increments. The holding potential was –140 mV. The resulting inward peak currents were normalized to their respective control values at the pre-pulse voltage of −140 mV. The normalized currents were fitted with the Boltzmann function. Lidocaine (300 µM) shifted the half maximal voltage (V1/2) of inactivation to a hyperpolarizing direction by −24.5 mV (n= 5) (vs. −11.3 mV with solvent; n= 5). As expected, flecainide (10 µM) caused no significant shift in the steady-state inactivation curve (shift in V1/2 at 10 µM: −10.8 mV; n= 6 vs. −10.8 mV with solvent; n= 6).
The effects of lidocaine (100 µM) and flecainide (10 µM) on the recovery from inactivation are described in Figure 7 (upper part). A two-pulse protocol was used to determine the recovery from inactivation. A 500 ms pre-pulse was applied from a holding potential of –140 to –20 mV. After various recovery intervals (0.7 ms to 48 s) to the holding potentials of –140 mV, a test pulse was applied to –20 mV for 20 ms (see inset of Figure 7, upper part). The data were fit with a double or triple exponential function (Figure 7, upper part). Under control conditions, around 77.6 ± 4.9% of the cardiac sodium current rapidly recovered with a fast time constant (2.1 ± 0.3 ms), and the remaining current recovered slowly with a time constant of 49.4 ± 10.6 ms (n= 6). Lidocaine prolonged the recovery from inactivation. Lidocaine (100 µM) significantly increased both the fast and the slow time constants (τrec f= 59.4 ± 6.9 ms vs. 1.6 ± 0.2 ms with control; τrec s= 326.2 ± 29.9 ms vs. 33.5 ± 8.8 ms with control; n= 6). The percentage of current recovering with the fast time constant was reduced, and the percentage of current recovering with the slow time constant was increased (Af 33.4 ± 5.8% vs. 84.9 ± 3.7% with control; As 58.5 ± 7.0% vs. 19.9 ± 1.5% with control). The recovery from inactivation in the presence of flecainide was best fitted with a triple exponential function. In contrast, flecainide at 10 µM had no significant effects on both fast and slow time constant of recovery of inactivation of human cardiac INa (τrec f= 1.9 ± 0.1 ms vs. 1.6 ± 0.1 ms with control; τrec s= 33.2 ± 4.6 ms vs. 36.6 ± 4.5 ms with control; n= 6). A third much slower time constant appeared where 11.0 ± 2.3% of the current recovered with a time constant of 35.6 ± 5.9 s. The percentage of current recovering with the fast time constant was slightly, but significantly, reduced, whereas the percentage of current recovering with the second slow time constant was not different to control (Af 71.1 ± 4.3% vs. 77.8 ± 4.5% with control; As 17.8 ± 0.5% vs. 13.9 ± 2.2% with control).
Figure 7.
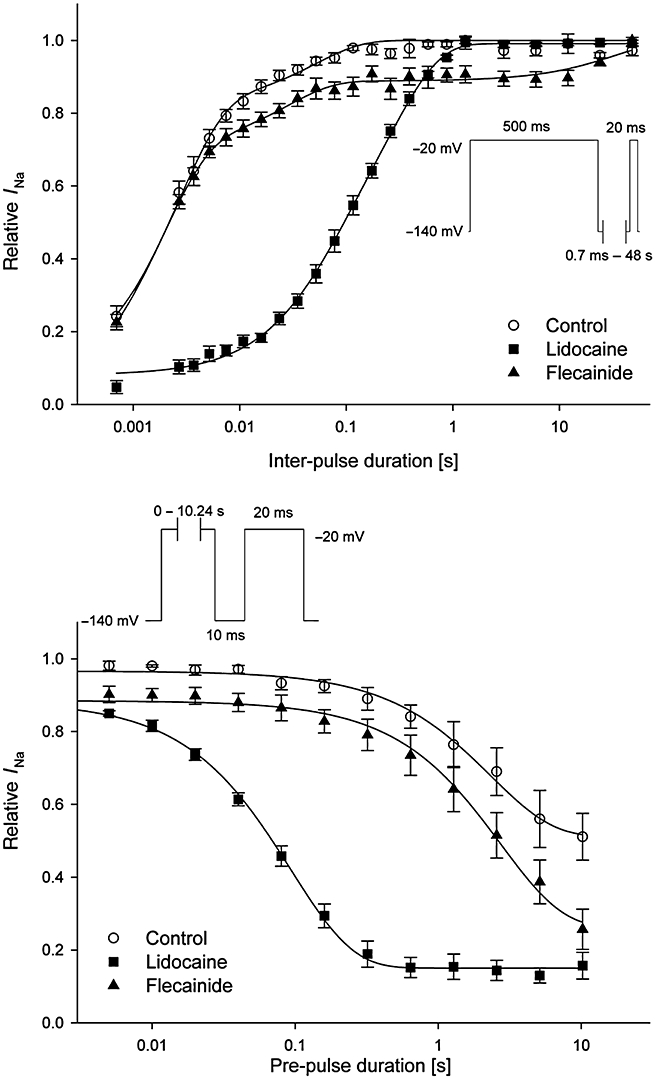
Effects of lidocaine and flecainide on the recovery and development of inactivated-state block of the human cardiac Nav1.5 channels in CHO cells transfected with the corresponding cDNA. Upper part: recovery from inactivation: the data were fitted to a double exponential function for solvent (n= 6) and lidocaine (100 µM; n= 6), and by a triple exponential function for flecainide (10 µM; n= 6). In contrast to flecainide, lidocaine prolonged the recovery from inactivation. Lower part: development of inactivated-state block: the data were fitted to a single exponential function for solvent (n= 6), lidocaine (100 µM; n= 6) and flecainide (10 µM; n= 6). Lidocaine, in contrast to flecainide, developed an inactivated-state block. Data are mean ± SEM.
The development of the inactivated state block by lidocaine and flecainide was determined by a two-pulse protocol varying the duration of the pre-pulse to –20 mV (5 ms to 10.24 s; Figure 7, lower part). The peak current amplitude of the test pulse was normalized to the peak current amplitude of the corresponding pre-pulse (Figure 7, lower part of the figure). Under control conditions, the longer the pre-pulse, the longer the cardiac Nav1.5 channels were in the inactivated state, and the peak current amplitude of the test pulse was reduced compared to the peak current amplitude of the pre-pulse. In the presence of lidocaine, the development of inactivated-state block was best fitted by a single exponential function. The block of the INa by lidocaine (100 µM) developed with a fast time constant of 96.3 ± 5.4 ms (vs. 3.3 ± 0.3 s with solvent control; n= 6). In contrast, flecainide at 10 µM had no effect on the amount of inactivated-state block by prolongation of the single depolarizing pulses up to 10 s (3.1 ± 0.4 s vs. 3.1 ± 0.5 s with solvent control; n= 6).
In order to determine the binding rates for flecainide, we investigated the drug-induced block (use-dependent block) of flecainide (10 µM). Following a rest period, cardiac INa was elicited with 20 ms test pulses to –20 mV following a 10 ms pre-pulse to –140 mV from a holding potential of –100 mV at a stimulation frequency of 1 Hz. In Figure 8 (upper part of the figure), the onset of use-dependent block is shown. Peak current amplitudes were normalized to the maximal current of the first pulse. To assess the use-dependent effects, the ratios after 30 stimuli obtained at 1 Hz were compared with time-matched solvent controls. Under control conditions, repetitive stimulation of the cardiac Nav1.5 channels increased the current by 7.2 ± 4.3% when measured after 30 stimuli (Figure 8, upper part; n= 5). Flecainide at a concentration of 10 µM had a use-dependent effect at 1 Hz stimulation frequency, and the current was reduced by 42.9 ± 2.0% (n= 5). The onset of use-dependent block in the presence of flecainide could be fitted with a single exponential function with time constant of 10.8 ± 2.4 stimuli (Figure 8, upper part).
Figure 8.
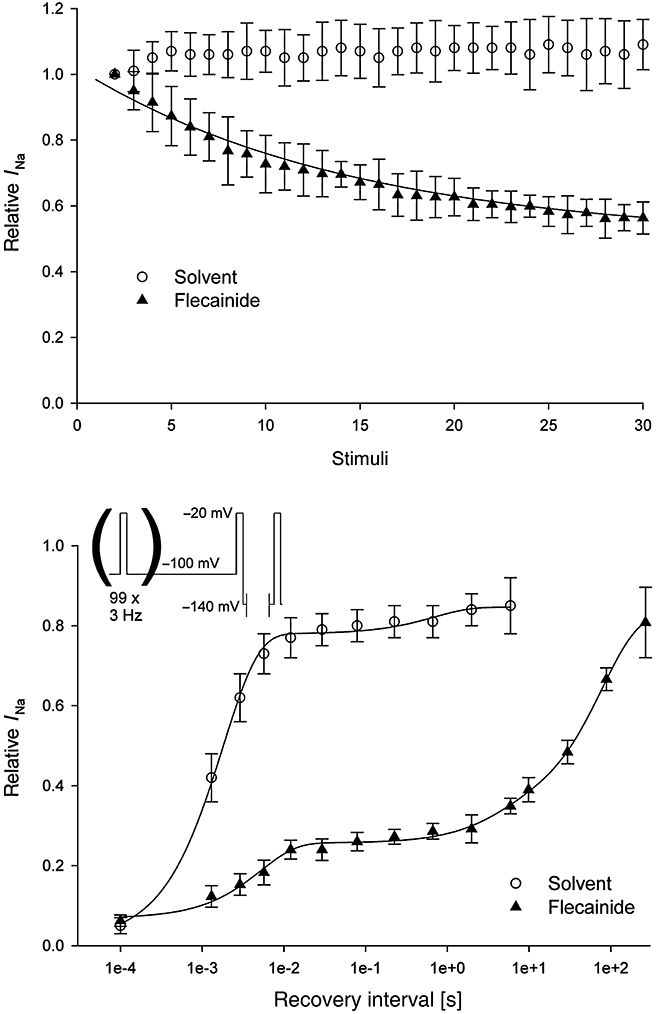
Use-dependent effect and recovery of block by flecainide on the human cardiac Nav1.5 channels and the resulting INa. Upper part: use-dependent effect of flecainide (10 µM; n= 5). The data were fitted to a single exponential function. Lower part: recovery from use-dependent block. The data were fitted to a double exponential function for solvent (n= 5), and by a triple exponential function for flecainide (10 µM; n= 5). Data are mean ± SEM.
To characterize the effect of flecainide on the recovery from use-dependent block, we elicited a block of the sodium currents by flecainide with a train of pulses (100 repeats at 3 Hz to a test pulse of –20 mV from a holding potential of –100 mV). For a varying length of time, the cell was hyperpolarized to –140 mV, and the second test pulse to –20 mV was elicited (Figure 8, lower part). The peak current of this test pulse was measured and normalized with the peak amplitude of the test pulse elicited with 0.033 Hz and plotted versus inter-pulse duration (Figure 8, lower part). Under control conditions, the cardiac INa rapidly recovered from use-dependent block with fast time constant of 2.0 ± 0.3 ms with 72.9 ± 5.9% of the current already recovered, and with a slow time constant of 536.8 ± 185.7 ms, with 11.1 ± 2.4% of the current recovered (n= 5). The recovery from use-dependent block in the presence of flecainide (10 µM) was best fitted with a triple exponential function. Only 16.9 ± 4.3% of the current recovered with a fast time constant of 4.8 ± 1.1 ms, and 8.1 ± 2.1% with a slower time constant of 860.9 ± 613.8 ms. The majority of the currents (53.5 ± 4.5%) recovered with a long, third time constant of 76.9 ± 12.5 s (n= 5).
In summary, flecainide blocks cardiac Nav1.5 channels preferentially in the activated state with slow binding and unbinding rates, whereas lidocaine blocks these channels preferentially in the inactivated state with fast binding and unbinding rates (Figures 7 and 8).
Discussion and conclusions
The hERG (IKr)-testing paradigms recommended in the present regulatory guidelines (Food and Drug Administration ICH S7B, 2005) for predicting drug-induced QT prolongation may not be sufficient to eliminate other drug-induced cardiac arrhythmias. Our recent study (Lu et al., 2008) shows that the ICH guidelines fail to address the question of precipitation of other potentially more life-threatening, drug-induced cardiac arrhythmias such as VT/VF (non-TdPs) associated with drug-induced shortening of the QT interval. In the present study, our data show that the ICH guidelines also partially fail to address the possibility of potential drug-induced VT/VF (i.e. non-TdP) associated with drug-induced widening of QRS duration or slowing of conduction. Indeed, our current screening data of 355 compounds indicate that many compounds (32% of 355 compounds: n= 114) slowed conduction (expressed as widening the QRS complex of the ECG in the wedge preparation and isolated Langendorff hearts, reduction in Vmax in isolated Purkinje fibres or increase in intraventricular CT in isolated hearts), and some of these compounds may have ‘flecainide-like’ effects and may be pro-arrhythmic (VT/VF: non-TdPs). Slowing of conduction has been shown to be associated with sudden cardiac arrhythmic death in both man (The CAST Investigators, 1989) and experimental models (Antzelevitch et al., 1999; Akar et al., 2004), and many drugs have the ability to slow conduction in the myocardium, and elicit arrhythmias (Antzelevitch, 2006).
The CAS Trial has shown that sodium current blockers such as flecainide (class Ic) were associated with an increased incidence of sudden cardiac death in post-infarct patients (The CAST Investigators, 1989), while other sodium current blockers like lidocaine (class Ib) have been used widely and safely as both an anaesthetic agent and for treatment of ischaemia-induced ventricular arrhythmias. Therefore, there may be a key difference between these two subtypes of compounds, from a cardiovascular safety perspective, and thus it is very important to discriminate between ‘lidocaine-like’ and ‘flecainide-like’ properties of non-cardiovascular drugs. A new medical entity with ‘lidocaine-like’ properties could be considered to be safe for further development. On the other hand, a compound with ‘flecainide-like’ actions could be considered to be dangerous in certain patient populations, and its development programme should be stopped. Therefore, there is a need for pre-clinical safety models that are able to differentiate between ‘lidocaine-like’ and ‘flecainide-like’ effects. Our present study outlines a strategy to discriminate drugs with ‘lidocaine-like’ and ‘flecainide-like’ effects using different in vitro experimental models.
Flecainide (a prototypic type Ic anti-arrhythmic drug) and lidocaine (class Ib) have markedly different effects on INa. The class Ic anti-arrhythmic drug flecainide has been generally considered to be an activated, open-state inhibitor of the cardiac Nav1.5 channel (e.g. Anno and Hondeghem, 1990; Carmeliet and Mubagwa, 1998). In contrast, the class Ib anti-arrhythmic drug lidocaine is considered to have a high affinity for the inactivated state of the cardiac Nav1.5 channel (Bean et al., 1983; Carmeliet and Mubagwa, 1998). Lidocaine is widely and safely used for the prevention and treatment of ventricular tachyarrhythmias (extra beats, VT, VF) occurring in the pre-hospital and early hospital phases of acute myocardial infarction (Zehender et al., 1990; Wyman et al., 2004). Flecainide, on the other hand, has a pro-arrhythmic potential, previously identified in both the clinic (The CAST Investigators, 1989) and in experimental models of ischaemia (Brugada et al., 1991; Ranger and Nattel, 1995; Amitzur et al., 2003; Clements-Jewery et al., 2006). The depressant action of lidocaine on the impulse conduction in normal myocardium is claimed to be much weaker than that of flecainide, yet lidocaine's anti-arrhythmic effect has been demonstrated both in experimental models (Aidonidis et al., 1994; Bellemin-Baurreau et al., 1994) and in the clinic (Zehender et al., 1990; Wyman et al., 2004). Other drugs with class Ib (‘lidocaine-like’) actions such as the anti-epileptic phenytoin (Ragsdale et al., 1996) have also been shown to be safe in clinical use (Lathers and Schraeder, 2002; Walczak, 2003).
The whole-cell voltage clamp technique was used to investigate differences in the effect of flecainide and lidocaine on cardiac INa in CHO cells transfected with Nav1.5 channels. Lidocaine showed higher affinity to bind to the inactivated state of the channels than to the activated state (IC50 680 µM vs. 44 µM). In addition, lidocaine shifted the steady-state inactivation curve significantly to the hyperpolarized direction. Lidocaine significantly prolonged the recovery from inactivation and showed a fast development of block. All these observations suggest that lidocaine binds to the inactivated state with fast binding and dissociation rates as reported earlier (Bean et al., 1983; Bellemin-Baurreau et al., 1994; Carmeliet and Mubagwa, 1998). In contrast, flecainide showed no difference in affinity for the activated and inactivated state (IC50 10.7 µM vs. 6.3 µM). In addition, there was no shift in the steady-state inactivation curve, there was no effect on recovery from inactivation and no development of inactivated-state block during longer depolarizing pre-pulses in the presence of flecainide. However, unlike lidocaine, flecainide showed a strong use-dependent effect. All these observations confirm results reported earlier (e.g. Ranger et al., 1989; Anno and Hondeghem, 1990; Carmeliet and Mubagwa, 1998). Flecainide preferentially blocks the Nav1.5 channels in the activated state with slow binding and slow dissociation rates (Figure 8).
In the isolated Purkinje fibres, lidocaine had much weaker effects on INa, shown by a lack of effect in Vmax. Furthermore, lidocaine had no effect on triangulation of the action potential, while flecainide very clearly increased triangulation in isolated Purkinje fibres (Figure 2). In isolated rabbit hearts without ischaemia/reperfusion, lidocaine also had much weaker effects on conduction than flecainide, demonstrated by a smaller effect on QRS duration and a lack of effect on CT with lidocaine (Figure 3). Furthermore, flecainide elicited VT in three out of the six hearts without ischaemia/reperfusion, while lidocaine did not induce VT in the same model. Similarly, in the left ventricular arterially perfused wedge, flecainide markedly increased QRS duration in a use-dependent manner, and elicited a high incidence of VT/VF at a fast stimulation rate of 500 ms (2 Hz). The incidence of VT/VF was associated with increases in dispersion of repolarization in the ventricle (Tp–Te and rTp–Te) in this model. On the other hand, lidocaine weakly widened QRS duration, slightly reduced the dispersion (Tp–Te and rTp–Te) without any incidence of VT/VF in the wedge model.
Interestingly, in the Langendorff-perfused hearts, in a condition of ischaemia, flecainide massively inhibited INa shown by a marked slowing of conduction compared to normal hearts, and had a high pro-arrhythmic incidence of VT/VF. On the other hand, lidocaine slightly increased QRS duration, and even protected against ischaemia- and reperfusion-induced VT/VF in an isolated rabbit heart model of global ischaemia.
An increase in rate of sudden death in patients taking flecainide and encainide has been reported in The CAST Investigators (1989), and is likely to be the consequence of malignant ventricular arrhythmias. Several studies show that Ic anti-arrhythmic drugs like flecainide indeed enhance ischaemia-induced VT and VF in isolated rat, feline and rabbit perfused hearts (Amitzur et al., 2003; Clements-Jewery et al., 2006), in dogs in vivo (Patterson et al., 1988; Aidonidis et al., 1994; Ranger and Nattel, 1995) and in clinical trials (Greenberg et al., 1995). This is confirmed by our finding that flecainide resulted in a more marked depression of conduction and more pro-arrhythmic biomarker changes in ischaemic/reperfused myocardia compared to normal myocardia (Figure 3). The reduction in conduction velocity was demonstrated by a widening in QRS duration, an increased CT and by induction of in-excitability in isolated hearts. Our results confirm early studies in other experimental models, which show that flecainide indeed enhances ischaemia-induced VT/VF (Kou et al., 1987; Sakai et al., 1989; Brugada et al., 1991).
The exact mechanism of flecainide-induced arrhythmias remains unclear both in clinical (Levine et al., 1989; Ranger et al., 1989) and experimental studies (Kou et al., 1987; Sakai et al., 1989; Brugada et al., 1991; Clements-Jewery et al., 2006). In the present study, flecainide resulted in a more marked slowing of conduction in ischaemic compared to normal myocardium (Figure 3). This was also shown in other studies with experimental models (Kou et al., 1987; Sakai et al., 1989; Brugada et al., 1991; Gutstein et al., 2001; Amitzur et al., 2003). Flecainide, by markedly slowing ventricular conduction without comparably prolonging the repolarization period (APD or refractory period), may favour the development of large re-entrant circuits particularly during acute myocardial ischaemia, in which conduction is already slow. Other potential mechanisms involved in the pro-arrhythmic effect of flecainide in the present study are likely to be due to the large dispersion of ventricular refractoriness caused by the inhomogeneous abbreviation of the action potential within the ventricle and within different layers of the ventricular wall: transmural dispersion of repolarization (Antzelevitch et al., 1999; Yan and Antzelevitch, 1999). Our present study confirms that flecainide significantly increased transmural dispersion (Tp–Te) which in turn favours functional re-entry, especially in a condition of ischaemia/reperfusion.
The ICH S7B guidelines were introduced following the withdrawal of drugs from the market for causing TdP. Because this phenomenon is associated with hERG-current (IKr) blockade and QT prolongation, the guidelines focus on the detection of QT-prolonging hERG-blocking drugs. However, the guidelines do not help to eliminate the possibility for ‘flecainide-like’ drug-induced pro-arrhythmia. One of the major reasons for the withdrawal of drugs from the market is cardiac side effects during clinical use. Therefore, assessment of the pro-arrhythmic liability early in development is important in the ‘go/no go’ decision-making process for selecting a potential new drug. There have been no appropriate models validated to detect ‘flecainide-like’ drug-induced arrhythmias in the past, and our results provide a range of different models (CHO cells transfected with the human Nav1.5 channels, isolated Purkinje fibres, isolated left ventricular wedges and isolated perfused hearts), which could distinguish between ‘good’ (‘lidocaine-like’) and ‘bad’ (‘flecainide-like’) INa blocking activities of non-cardiovascular drugs.
In conclusion, while the current ICHS7B and E14 regulatory guidelines (Darpo et al., 2006) may be useful for predicting drug-induced QT prolongation, revision of these guidelines may be warranted in the future to address, in more detail, the potential liability for drug-induced widening of QRS duration (slowing conduction) and associated fatal arrhythmias. Such a revision may help to eliminate the potential for ‘flecainide-like’ fatal arrhythmias (VF) with non-cardiovascular drugs.
Acknowledgments
The authors wish to thank Dr R.Towart (JNJ) and Dr B. Loenders (JNJ) for their scientific comments, Mr D. Borgers (K15, Belgium) for the appropriate figures and Heart Rhythm Solutions for performing experiments in the isolated wedge model.
Glossary
Abbreviations:
- AAP
amplitude of the action potential
- APD
duration of the action potential
- BCLs
basic cycle lengths
- CAST
Cardiac Arrhythmia Suppression Trial
- CHO
Chinese hamster ovary cell line
- CPMP
current established regulatory guidelines
- CT
conduction time
- DMSO
dimethyl sulphoxide
- EAD
early afterdepolarization
- ECG
electrocardiogram
- hERG
human ether-à-go-go-related gene
- ICH
International Conference on Harmonisation
- IKr
the rapidly activating delayed rectifier potassium current
- INa
sodium current
- MAP
monophasic action potential
- SCN5A
gene encoding α-subunit of the human cardiac sodium channel Nav1.5
- TdPs
torsades de pointes
- TDR
maximal transmural dispersion of repolarization
- Tp–Te, QTpeak–QTend
measure of transmural dispersion
- VF
ventricular fibrillation
- Vmax
the maximum rate of rise of the action potential
- VT
ventricular tachycardia
Conflict of interest
None.
References
- Aidonidis I, Brachmann J, Rizos I, Toutouzas P, Kubler W. Lidocaine converts inducible ventricular fibrillation into sustained ventricular tachycardia in conscious dogs with recent myocardial infarction. Cardiology. 1994;85:378–387. doi: 10.1159/000176739. [DOI] [PubMed] [Google Scholar]
- Akar FG, Spragg DD, Tunin RS, Kass DA, Tomaselli GF. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ Res. 2004;95:717–725. doi: 10.1161/01.RES.0000144125.61927.1c. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitzur G, Shenkar N, Mueller M, Kraft P, Noviko I, Eldar M, et al. Refractoriness and conduction interaction during modulation of non-ischemic ventricular fibrillation by flecainide. Cardiovasc Drugs Ther. 2003;17:237–247. doi: 10.1023/a:1026124224369. [DOI] [PubMed] [Google Scholar]
- Anno T, Hondeghem LM. Interactions of flecainide with guinea-pig cardiac sodium channels. Importance of activation unblocking to the voltage dependence of recovery. Circ Res. 1990;66:789–803. doi: 10.1161/01.res.66.3.789. [DOI] [PubMed] [Google Scholar]
- Antzelevitch C. Brugada syndrome. Pacing Clin Electrophysiol. 2006;29:1130–1159. doi: 10.1111/j.1540-8159.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch C, Yan GX, Shimizu W. Transmural dispersion of repolarization and arrhythmogenicity: the Brugada syndrome versus the long QT syndrome. J Electrocardiol. 1999;32(Suppl.):158–165. doi: 10.1016/s0022-0736(99)90074-2. [DOI] [PubMed] [Google Scholar]
- Bean BP, Cohen CJ, Tsien RW. Lidocaine block of cardiac sodium channels. J Gen Physiol. 1983;81:613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellemin-Baurreau J, Poizot A, Hicks PE, Armstrong JM. An in vitro method for the evaluation of antiarrhythmic and antiischemic agents by using programmed electrical stimulation of rabbit heart. J Pharmacol Toxicol Methods. 1994;31:31–40. doi: 10.1016/1056-8719(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Bezanilla F, Armstrong CM. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977;70:549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugada J, Boersma L, Kirchhof C, Allessie M. Proarrhythmic effects of flecainide: experimental evidence for increased susceptibility to reentrant arrhythmias. Circulation. 1991;84:1808–1818. doi: 10.1161/01.cir.84.4.1808. [DOI] [PubMed] [Google Scholar]
- Carmeliet E, Mubagwa K. Antiarrhythmic drugs and cardiac ion channels: mechanisms of action. Prog Biophys Mol Biol. 1998;70:1–72. doi: 10.1016/s0079-6107(98)00002-9. [DOI] [PubMed] [Google Scholar]
- Clements-Jewery H, Kanaganaygam GS, Kabra R, Curtis MJ. Actions of flecainide on susceptibility to phase-2 ventricular arrhythmias during infarct evolution in rat isolated perfused hearts. Br J Pharmacol. 2006;147:468–475. doi: 10.1038/sj.bjp.0706633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubeddu LX. QT prolongation and fatal arrhythmias: a review of clinical implications and effects of drugs. Am J Ther. 2003;10:452–457. doi: 10.1097/00045391-200311000-00013. [DOI] [PubMed] [Google Scholar]
- Darpo B, Nebout T, Sager PT. Clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs: the International Conference on Harmonization of Technical Requirement for Registration of Pharmaceuticals for Human Use E14 guidance. J Clin Pharmacol. 2006;46:498–507. doi: 10.1177/0091270006286436. [DOI] [PubMed] [Google Scholar]
- Dubin AE, Nasser N, Rohrbacher J, Hermans AN, Marrannes R, Grantham C, et al. Identifying modulators of hERG channel activity using the PatchXpress planar patch clamp. J Biomol Screen. 2005;10:168–181. doi: 10.1177/1087057104272295. [DOI] [PubMed] [Google Scholar]
- Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmias Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. HRS: International Conference on Harmonisation; guidance on S7B nonclinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals; availability. Fed Regist. 2005;70:61133–61134. [PubMed] [Google Scholar]
- Gellens ME, George Al, Chen L, Chahine M, Horn R, Barchi RL, et al. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proc Natl Acad Sci USA. 1992;89:554–558. doi: 10.1073/pnas.89.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg HM, Dwyer EMJ, Hochman JS, Steinberg JS, Echt DS, Peters RW. Interaction of ischemia and encainide/flecainide treatment: a proposed mechanism for the increased mortality in CAST I. Br Heart J. 1995;74:631–635. doi: 10.1136/hrt.74.6.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, et al. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333–339. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Haverkamp W, Breithardt G, Camm AJ, Janse MJ, Rosen MR, Antzelevitch C, et al. The potential for QT prolongation and pro-arrhythmia by non-anti-arrhythmic drugs: clinical and regulatory implications: report on a Policy Conference on the European Society of Cardiology. Cardiovasc Res. 2000;47:219–233. doi: 10.1016/s0008-6363(00)00119-x. [DOI] [PubMed] [Google Scholar]
- Kou WH, Nelson SD, Lynch JJ, Montgomery DG, DiCarlo L, Lucchesi BR. Effect of flecainide acetate on prevention of electrical induction of ventricular tachycardia and occurrence of ischemic ventricular fibrillation during the early postmyocardial infarction period: evaluation in a conscious canine model of sudden death. J Am Coll Cardiol. 1987;9:359–365. doi: 10.1016/s0735-1097(87)80389-3. [DOI] [PubMed] [Google Scholar]
- Lathers CM, Schraeder PL. Clinical pharmacology: drugs as a benefit and/or risk in sudden unexpected death in epilepsy? J Clin Pharmacol. 2002;42:123–136. doi: 10.1177/00912700222011157. [DOI] [PubMed] [Google Scholar]
- Levine JH, Morganroth J, Kadish AH. Mechanisms and risk factors for proarrhythmias with type Ia compared with Ic antiarrhythmic drug therapy. Circulation. 1989;80:1063–1069. doi: 10.1161/01.cir.80.4.1063. [DOI] [PubMed] [Google Scholar]
- Lu HR, Mariën R, Saels A, De Clerck F. Species plays an important role in drug-induced prolongation of action potential duration and early afterdepolarizations in isolated Purkinje fibers. J Cardiovasc Electrophysiol. 2001;12:93–102. doi: 10.1046/j.1540-8167.2001.00093.x. [DOI] [PubMed] [Google Scholar]
- Lu HR, Vlaminckx E, Hermans AN, Rohrbacher J, Van Ammel K, Towart R, et al. Predicting drug-induced changes in QT interval and arrhythmias: QT-shortening drugs point to gaps in the ICHS7B guidelines. Br J Pharmacol. 2008;154:1427–1438. doi: 10.1038/bjp.2008.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson E, Gibson JK, Lucchesi BR. Antiarrhythmic and arrhythmogenic actions of methyl lidocaine during the recovery phase after canine myocardial infarction. Pharmacology. 1988;36:73–83. doi: 10.1159/000138362. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci USA. 1996;93:9270–9275. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranger S, Nattel S. Determinants and mechanisms of flecainide-induced promotion of ventricular tachycardia in anesthetized dogs. Circulation. 1995;92:1300–1311. doi: 10.1161/01.cir.92.5.1300. [DOI] [PubMed] [Google Scholar]
- Ranger S, Talajic M, Lemery R, Roy D, Nattel S. Amplification flecainide-induced ventricular conduction slowing by exercise. A potentially significant clinical consequence of use-dependent sodium channel blockade. Circulation. 1989;79:1000–1006. doi: 10.1161/01.cir.79.5.1000. [DOI] [PubMed] [Google Scholar]
- Roden DM. Long-QT syndrome. N Engl J Med. 2008;358:169–176. doi: 10.1056/NEJMcp0706513. [DOI] [PubMed] [Google Scholar]
- Sakai T, Ogawa S, Hosokawa M, Miyazaki T, Sakurai K, Nakamura Y. Electrophysiological effects of flecainide in a canine 7 day old myocardial infarction model. Cardiovas Res. 1989;23:177–183. doi: 10.1093/cvr/23.3.177. [DOI] [PubMed] [Google Scholar]
- Sellers TD, DiMarco JP. Sinusoidal ventricular tachycardia associated with flecainide acetate. Chest. 1984;85:647–649. doi: 10.1378/chest.85.5.647. [DOI] [PubMed] [Google Scholar]
- Shah RR. Pharmacogenetic aspects of drug-induced torsade de pointes. Drug Saf. 2004;27:145–172. doi: 10.2165/00002018-200427030-00001. [DOI] [PubMed] [Google Scholar]
- The CAST Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406–412. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- Walczak T. Do antiepileptic drugs play a role in sudden unexpected death in epilepsy? Drug Saf. 2003;26:673–683. doi: 10.2165/00002018-200326100-00001. [DOI] [PubMed] [Google Scholar]
- Wang D, Patel C, Cui C, Yan G-X. Preclinical assessment of drug-induced proarrhythmias: role of the arterially perfused rabbit left ventricular wedge preparation. Pharmacol Ther. 2008;119:141–151. doi: 10.1016/j.pharmthera.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Wyman MG, Wyman RM, Cannom DS, Criley JM. Prevention of primary fibrillation in acute myocardial infarction with prophylactic lidocaine. Am J Cardiol. 2004;94:545–551. doi: 10.1016/j.amjcard.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation. 1999;100:1660–1666. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- Yan GX, Wu Y, Liu T, Wang J, Marinchak RA, Kowey PR. Phase 2 early afterdepolarization as a trigger of polymorphic ventricular tachycardia in acquired long-QT syndrome: direct evidence from intracellular recordings in the intact left ventricular wall. Circulation. 2001;103:2851–2856. doi: 10.1161/01.cir.103.23.2851. [DOI] [PubMed] [Google Scholar]
- Zehender M, Kasper W, Just H. Lidocaine in the early phase of acute myocardial infarction: the controversy over prophylactic or selective use. Clin Cardiol. 1990;13:534–539. doi: 10.1002/clc.4960130805. [DOI] [PubMed] [Google Scholar]


