Abstract
Neuroendocrine tumors (NETs) can arise from a variety of organs. They can vary widely in clinical behavior; consequently, optimizing their treatment plan can be problematic. NETs display diverse tumor biology; however, most secrete peptides such as chromogranin A into the circulation, consistent with their neuroendocrine origin. In this study, we sought to identify other potential markers for NETs by analyzing the secreted proteomes of three neuroendocrine cell lines. BON-1, NCI-H727, and SHP-77 cells were grown in serum-free media, and the secreted proteins were separated by SDS-PAGE and identified by LC-MS/MS. We identified 205 proteins of which 61 were secreted by two or more of the cell lines and 19 were secreted by all three lines. Mac-2-binding protein (Mac-2BP) was found to be secreted by all three cell lines, and this was confirmed by Western blotting. Immunohistochemical analysis found 29 of 33 NET cases from different primary sites to be positive for Mac-2BP. Serum Mac-2BP was significantly elevated in NET patients compared with healthy controls (p < 0.001). This study demonstrated that analysis of the secreted proteomes of neuroendocrine cell lines can identify potential biomarkers for NET. Initial assessment showed that serum Mac-2BP is significantly elevated in patients with NET and is expressed by the majority of NET tissues.
The incidence of neuroendocrine tumors (NETs)1 is 2–5 per 100,000, although recent epidemiological data suggest this is rising (1, 2) Their clinical behavior ranges from indolent to highly aggressive. Because of the indolent nature of many NETs, the prevalence of these tumors is relatively high, up to 35 per 100,000 population has been reported (3). The 5-year survival rate for patients with midgut metastatic disease is presently 40% (4).
NETs most commonly arise from the gastroenteropancreatic system; however, they can originate in other organs (2). NETs of the gut are thought to arise from cells of the diffuse endocrine system, which are characterized by the secretion of a variety of hormonal peptides and other bioactive molecules (1, 5). The majority of these tumors are non-functional in that they present without syndromic features secondary to hormone release; however, ∼40% of NETs are functional in that they secrete hormones into the circulation, leading to the development of clinical symptoms (6). Carcinoid syndrome is the most common clinical syndrome seen in patients with functional NETs and is thought to be due to release of serotonin and peptides such as kinins (6).
Chromogranin A (CgA) is the biochemical marker in the circulation currently used for monitoring and screening of NETs (7). However, this marker is neither 100% specific nor sensitive, especially for patients with low volume disease (8). Further markers are needed to aid the screening and management of NETs.
Proteins from cancer cells are secreted into the circulation. The serum levels of signature proteins may increase in particular cancers and correlate with cancer progression and proliferation. Consequently, secreted proteins are important as serum biomarkers for some cancers. Examples include carcinoembryonic antigen for colon cancer, CA-125 for ovarian cancer (9), and prostate-specific antigen for prostate cancer (10).
A number of studies have investigated the secreted proteomes of cultured cells in the search for marker proteins of different cancers (11, 12). This approach is based upon the assumption that the proteins secreted by the cell line will be representative of tumor cells in vivo. Studies have been performed using cell line models of prostate cancer, breast cancer (11), and head and neck cancer (13). Some putative markers were subsequently validated using immunological methods on limited numbers of clinical samples. However, some of these markers did not prove to be consistently linked with the disease when studied in additional clinical samples (11). This strategy appears to be justified for NET because these cells are known to secrete biologically active amines and proteins into the circulation.
The incidence of NETs is relatively low; therefore, establishing a screening program would not be feasible. However, the progression of the disease varies considerably, so reliable markers or a panel of validated markers would be useful for assessing the progression of the disease in patients who have already been diagnosed. This would enable optimization of management plans for patients.
The aim of this study was to identify putative protein markers, particularly those that could indicate the metastatic spread of NETs. We analyzed the secreted proteomes of three neuroendocrine cell lines and assessed a new putative NET marker using patient tissue and serum samples.
MATERIALS AND METHODS
Cell Lines
BON-1, a pancreatic neuroendocrine cell line, and two neuroendocrine lung cancer cell lines (NCI-H727 and SHP-77) were gifts from Prof. D. Hochhauser. BON-1 cells were maintained in Dulbecco's modified Eagle's medium/F-12 medium (1:1) supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin. NCI-H727 cells were maintained in RPMI 1640 medium supplemented with 10% FBS, penicillin, and streptomycin. SHP-727 cells were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated FBS. All cells were cultured in 75-cm2 flasks in a humidified incubator at 37 °C and 5% CO2.
Cell Culture
Approximately 106 cells per cell line were seeded into 75-cm2 tissue culture flasks. After ∼48 h when more than 80% confluent, the media were removed. Monolayers were washed in serum-free media and then incubated with 8 ml of serum-free media. After 24 h, the conditioned media (CM) were collected. Trypan blue exclusion indicated >99% viability of the attached cells. Medium with 10% FBS was added to the flasks and incubated for a further 48 h to ensure the attached cells were still viable. The CM were centrifuged at 2000 × g for 5 min to remove any cell debris and concentrated using a 10-kDa Centricon Mini-Prep (Millipore) to 200 μl. Concentrated CM samples were aliquoted and stored at −80 °C.
SDS-PAGE and Digestion
Samples of 30 μl of concentrated CM were fortified to 50 mm DTT, and 10 μl of 4× Laemmli buffer were added. Samples were separated by SDS-PAGE using 12% Tris-glycine gels. Samples were stained in colloidal brilliant blue dye (Sigma) for 24 h and then with fresh dye for a further 24 h. Gels were extensively destained in water and digitally imaged on a Bio-Rad GS800 scanner using Quantity One software (Bio-Rad). Gels were reduced in 50 mm DTT for 1 h and then alkylated in 30 mm iodoacetamide for 1 h. Each lane of proteins in the gel was cut into 50 bands. These were dried in vacuo prior to digestion with alkylated trypsin (Promega) overnight at 37 °C in 30 mm ammonium bicarbonate. The resulting mixture of peptides was extracted in 20% acetonitrile containing 0.1% trifluoroacetic acid.
Mass Spectrometry
Peptides were separated by reverse-phase HPLC: a 45-min linear gradient was developed from 3 to 50% acetonitrile in 0.1% formic acid on a C18 column (Dionex PepMap100, 100 mm × 75 μm) using an Ultimate 3000 system (Dionex). Data-determined acquisition was performed on a MicroMass Q-ToF Micro instrument (Waters) using ProteinLynx 4 software. Peak lists were generated by the MassLynx 4.0 PeptideAuto program using default parameters and used by Mascot 2.205 (Matrix Science, UK) to interrogate the human sequences (148,148 sequences) of the MSDB 20060831 non-redundant sequence database annotated August 31, 2006 (14). Search parameters allowed not more than a single missed cleavage site, all cysteine residues to be modified by carbamidomethylation, and variable oxidation of methionine residues. Precursor and fragment ion mass tolerance were set to 1.2 and 0.6 Da, respectively. Peptide identities were accepted provided that their Mowse scores were above 38 (p < 0.05). The assignments were manually verified by checking that there were several consecutive y or b ions. Proteins were accepted on the basis of the combined scores from more than one peptide ion, except for one protein identification that was accepted on the basis of data from a single peptide because it had eight of 10 consecutive y ions.
Analysis of Identified Proteins
Mascot accession numbers were uploaded as CSV files to Protein Center software (Proxeon) for the analysis of identified proteins. This software uses a knowledge base derived from the published literature to relate gene products to each other based on their interaction and function. The cellular localization of proteins in each sample was identified and examined in greater detail for proteins expressed in two or more cell lines.
Validation of Proteins
For validation of possible marker proteins, the following assessment was performed. Western blot analysis of the CM was performed to confirm that the correct protein had been identified, immunohistochemical analysis was performed on sections prepared from paraffin-embedded NET tissue, and serum analysis was performed on samples prepared from a cohort of NET patients and controls.
Western Blotting
Samples of 30 μl of concentrated CM were separated by SDS-PAGE, and proteins were transferred to PVDF membranes (Invitrogen iBlot) and soaked in blocking solution (5% (w/v) skimmed milk powder in TBS-T (20 mm Tris-HCl, pH 7.5, 0.5 m NaCl, 0.1% (v/v) Tween 20) for 2 h at room temperature. One membrane was incubated with 1% (w/v) skimmed milk powder in TBS-T, while a duplicate was incubated in the same buffer incorporating 1.5 μg/ml anti-Mac-2BP polyclonal mouse antibody (Bender Systems). Both membranes were incubated for 16 h at 4 °C. Membranes were washed with TBS-T three times for 10 min each and subsequently incubated in horseradish peroxidase-conjugated goat anti-mouse IgG antibody (diluted 1:5,000 in blocking solution) for 120 min at room temperature. After another three washes in TBS-T, immunoreactive bands were detected using ECL (GE Healthcare).
Immunohistochemistry
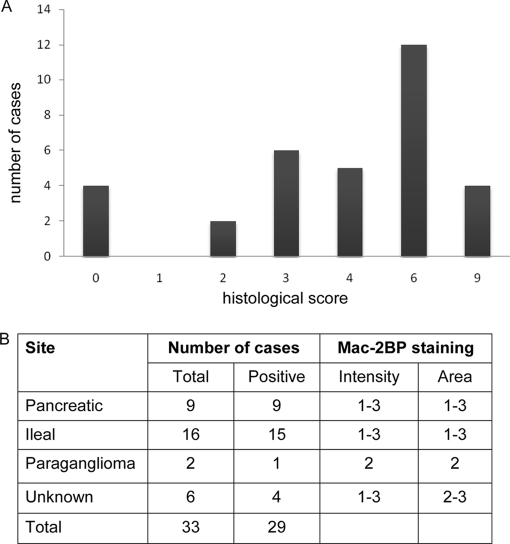
Immunohistochemical analysis of Mac-2BP was performed in NET tissue samples. The study population contained formalin-fixed paraffin-embedded tumor tissue from 33 patients with a histologically confirmed diagnosis of NET. Tumor tissue was available from 20 patients who had undergone an operation and tumor resection with a further 13 samples from tumor biopsies. The study population included all major NET subtypes including nine foregut, 16 midgut, two paragangliomas, and six NETs of unknown primary origin.
Sections of 3 μm of tumor tissue were dewaxed three times in xylene and rehydrated in ethanol. Slides were submersed in 10 mm citric acid (pH 6.0) for 2 min in a pressure cooker, cooled immediately in water, and transferred to fresh water at room temperature for 5 min. All slides were blocked in normal horse serum containing avidin and biotin for 40 min before incubation with anti-Mac-2BP rabbit polyclonal antibody (Bender Systems) at a dilution of 1:400 for 1 h at room temperature. Biotinylated secondary antibody was incubated with slides for 30 min. Bound antibody was visualized using a diaminobenzidine peroxidase substrate kit. Sections were counterstained with Mayer's hematoxylin for 3.5 min. As Mac-2BP expression is up-regulated in gastric carcinomas (15), these were selected as positive controls. Negative controls included substitution of the primary antibody with normal horse serum.
Tumor Classification
Tumors were classified according to their site of origin, level of differentiation, and initial mitotic index. Two examiners (R. S. and J. W.) independently interpreted each immunohistological result. Discordances were jointly reviewed to reach agreement or determine an average value for disputed sections. Slides were scored on the basis of intensity of staining as follows: 0 = negative, 1 = weakly positive, 2 = moderate, and 3 = strongly positive. The extent of tumor staining was also scored whereby 10 random high power fields were assessed to determine the average percentage of positive staining cells in which 1 = <25%, 2 = 25–75%, and 3 = >75%. The product of the density of staining and the percentage of tumor cells staining positive was used as the histological score, giving final values of 0, 1, 2, 3, 4, 6, and 9 (16).
Blood Analysis
For Mac-2BP, we enrolled 47 patients with NETs between July 2007 and March 2008. All patients had histological confirmation, including assessment of morphology and immunohistochemical analysis for neuron-specific enolase, CgA, synaptophysin, and PGP9.5. All cases were well or moderately differentiated tumors. The cohort of NET patients included three paragangliomas, 15 pancreatic, 25 small bowel, one large bowel, and 3 bronchial NETs. 24 healthy controls were also enrolled; control subjects had no history of cancer and were age- and sex-matched. The study was approved by the local Ethics Committee, and all participants gave written informed consent prior to obtaining samples.
Serum samples were obtained from each individual using a 12-gauge BD Vacutainer safety-Lok™ blood collection set system. Blood samples were collected in “gold top” tubes containing gel separator and clot activator and immediately placed on ice and allowed to stand for 30 min. Following this, the samples were centrifuged at 3000 rpm for 15 min. Sera were stored at −80 °C. Serological measurement of Mac-2BP was performed using an ELISA from Bender Systems. CgA analysis was performed on blood samples from patients using a radioimmunoassay kit (Roche Applied Science). As this assay has previously been validated and is currently used in clinical practice, no samples were run on normal healthy controls.
Statistical Analysis
Statistical software (GraphPad Prism Software, San Diego, CA) was used for the analysis. All values are given as median (plus range). As the values did not fit a standard distribution, non-parametric analysis was performed. The Mann-Whitney U test was used to compare patients and control groups, and the Kruskal-Wallis test was used to compare multiple groups. Spearman correlation of rank coefficient was used to analyze correlations between parameters. Spearman correlation was also used to analyze correlation between Mac-2BP immunohistochemistry and other parameters.
RESULTS
Identification of CM Proteins Using SDS-PAGE and Mass Spectrometry
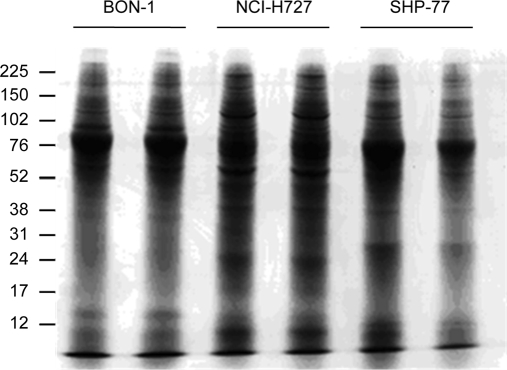
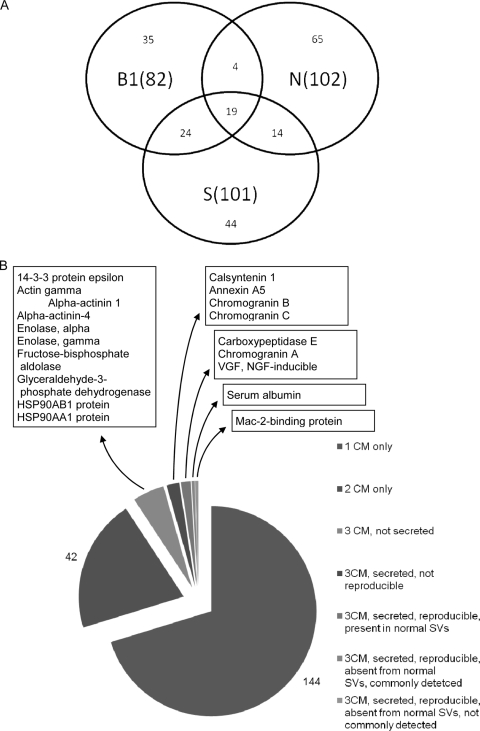
Using similar amounts of total protein from each CM (Fig. 1) and after contaminants including porcine trypsin and major human hair and skin keratins had been excluded, a total of 205 different proteins were identified in CM preparations from the three cell lines (supplemental Table 1). 82 proteins were identified in the BON-1 CM, 102 proteins were identified in the NCI-H727 CM, and 101 were identified in the SHP-77 CM. 42 proteins were identified in the CM from two cell lines only, whereas 19 proteins were identified in the CM from all three cell lines (Fig. 2).
Fig. 1.
SDS-PAGE separation of CM preparations. Duplicate aliquots of CM from each cell line were fractionated by SDS-PAGE and stained with Coomassie Blue. Marker protein positions and molecular masses (kDa) are indicated.
Fig. 2.
Distribution of proteins secreted by three neuroendocrine cell lines. A, Venn diagram showing in parentheses the total number of different proteins identified in the CM for each cell line (B1, BON-1; N, NCI-H727; S, SHP-77) as well as the number of proteins identified exclusively in one, two, or all three cell lines. B, pie chart illustrating the progressive selection of candidate NET markers from the 205 CM proteins exclusively present in CM preparations from one (1 CM), two (2 CM), or three (3 CM) cell lines.
61 proteins were present in CM preparations from two or more cell lines (Table I), and 24 of these are known to be extracellular and not permanently bound to cells. The number of unique peptides identified from each of these proteins is also shown. All 205 proteins were identified from two or more unique peptide identifications (range, 2–20; mean, 6). For the 61 proteins in Table I, the ion score range was from 37 to 106 (mean, 67), and the Mascot score ranged from 40 to 421 (mean, 102).
Table I. Proteins secreted by two or more cell lines.
61 proteins were identified from two or more cell lines; proteins found reproducibly in three BON-1 CM analyses are shown in bold. Cellular location (Loc'n) was identified using Protein Center and extensive literature searches (C, cytoplasmic; E, extracellular and not constitutively membrane-bound; M, membrane-bound); in many cases more than one cellular location has been reported for proteins. Cell lines from which proteins were identified are indicated (B, BON-1; N, NCI-H727; S, SHP-77). Reference protein accession numbers are from the NCBI protein database (National Center for Biotechnology Information). Mass is the predicted mass of the precursor in daltons. Score is the Mowse score. Ion score is the highest Mowse score for individual peptides. MHC, major histocompatibility complex.
| Reference protein accession no. | Protein | Cell line | Loc'n | Mass | Score | Percent coverage | No. peptides | Ion score |
|---|---|---|---|---|---|---|---|---|
| NP_033562 | 14-3-3 protein ϵ | BNS | C | 29,043 | 171 | 34 | 12 | 67 |
| NP_006817 | 14-3-3 protein θ/τ | NS | C | 27,633 | 129 | 13 | 4 | 101 |
| NP_663723 | 14-3-3 protein ζ/δ | BS | C | 27,614 | 73 | 12 | 3 | 60 |
| NP_001605 | Actin γ | BNS | C | 41,662 | 151 | 5 | 6 | 106 |
| NP_006399 | AGR2 | BS | E | 19,848 | 46 | 14 | 4 | 37 |
| NP_000286 | α1-Antitrypsin | NS | E | 46,737 | 49 | 4 | 3 | 41 |
| NP_001624 | α1-Microglobulin | BS | E | 38,999 | 77 | 5 | 4 | 56 |
| NP_001123476 | α-Actinin 1 | BNS | C | 105,438 | 90 | 6 | 6 | 80 |
| NP_004915 | α-Actinin 4 | BNS | C | 104,723 | 90 | 6 | 9 | 80 |
| NP_000475 | Alzheimer disease amyloid β protein | BS | M, E | 86,944 | 141 | 11 | 12 | 75 |
| NP_001002858 | Annexin A2 | BS | C, E | 40,280 | 70 | 7 | 4 | 68 |
| NP_001145 | Annexin A5 | BNS | C, E | 35,806 | 55 | 8 | 5 | 56 |
| NP_004351 | Cadherin 1 | BS | M, E | 97,456 | 212 | 8 | 13 | 67 |
| NP_001009566 | Calsyntenin 1 | BNS | M, E | 109,662 | 135 | 6 | 9 | 89 |
| NP_006126 | CapZ α-1 | BN | C | 32,792 | 87 | 14 | 5 | 64 |
| NP_001864 | Carboxypeptidase E | BNS | E | 53,151 | 115 | 13 | 8 | 50 |
| NP_001279 | Chloride intracellular channel protein 1 | NS | M | 26,792 | 63 | 27 | 9 | 63 |
| NP_001266 | Chromogranin A | BNS | E | 50,688 | 95 | 3 | 4 | 64 |
| NP_001810 | Chromogranin B (secretogranin 1) | BNS | E | 78,276 | 72 | 5 | 5 | 72 |
| NP_003460 | Chromogranin C (secretogranin 2) | BNS | E | 70,810 | 97 | 7 | 5 | 81 |
| NP_005498 | Cofilin-1 | BS | C | 18,371 | 94 | 27 | 7 | 52 |
| NP_001814 | Creatine kinase B | BN | C | 42,513 | 120 | 8 | 2 | 90 |
| NP_000090 | Cystatin C | BS | E | 15,749 | 128 | 19 | 3 | 72 |
| NP_001952 | Elongation factor 2 (EF-2) | NS | C | 95,207 | 92 | 3 | 2 | 93 |
| NP_001419 | Enolase, α | BNS | C | 47,038 | 97 | 4 | 2 | 97 |
| NP_001967 | Enolase, β | NS | C | 46,801 | 167 | 10 | 6 | 100 |
| NP_001966 | Enolase, γ | BNS | C | 47,138 | 84 | 7 | 4 | 84 |
| NP_000025 | Fructose-bisphosphate aldolase | BNS | C | 39,289 | 49 | 9 | 2 | 49 |
| NP_000166 | Glucose-6-phosphate isomerase | NS | C, E | 63,016 | 103 | 7 | 6 | 66 |
| NP_036545 | Glutaminyl-peptide cyclotransferase | BS | C | 40,887 | 87 | 25 | 6 | 56 |
| NP_002037 | Glyceraldehyde-3-phosphate dehydrogenase | BNS | C | 35,922 | 49 | 6 | 4 | 49 |
| NP_004855 | Growth differentiation factor 15 | BS | E | 34,009 | 90 | 10 | 6 | 86 |
| NP_002131 | Heterogeneous nuclear ribonucleoprotein K transcript variant | 50,897 | 63 | 22 | 8 | 58 | ||
| NP_005336 | NS | C | 69,921 | 193 | 27 | 16 | 69 | |
| NP_002146 | Hsp 70 protein 1A/6/8 | 70,897 | ||||||
| NP_006588 | 70,767 | |||||||
| NP_005338 | Hsp 70 protein 5 | NS | C | 72,202 | 133 | 14 | 9 | 79 |
| NP_003290 | Hsp 90 β 1 (endoplasmin) | NS | C | 92,469 | 131 | 14 | 10 | 61 |
| NP_031381 | HSP90AB1 protein | BNS | C | 83,133 | 65 | 6 | 5 | 61 |
| NP_001017963 | HSP90AA1 protein | BNS | C | 98,030 | 421 | 25 | 20 | 99 |
| NP_002256 | Importin β, chain B | NS | C | 97,040 | 40 | 3 | 1 | 40 |
| NP_002207 | Inter-α-trypsin inhibitor heavy chain 2 | BS | E | 106,333 | 102 | 15 | 10 | 69 |
| NP_002264 | Keratin 8 | BS | C | 53,573 | 71 | 6 | 3 | 61 |
| NP_002291 | l-Lactate dehydrogenase, chain H | NS | C | 36,507 | 54 | 17 | 7 | 49 |
| NP_005557 | l-Lactate dehydrogenase, chain M | BS | C | 36,558 | 126 | 23 | 12 | 69 |
| NP_005558 | Mac-2-binding protein | BNS | E | 65,200 | 114 | 8 | 8 | 47 |
| NP_005908 | Malate dehydrogenase | BN | C | 36,295 | 155 | 29 | 11 | 64 |
| NP_002108 | MHC class I antigen; HLA-C | BS | M | 40,649 | 50 | 13 | 3 | 50 |
| NP_002406 | MIF | BS | E | 12,345 | 76 | 9 | 3 | 52 |
| NP_006175 | Nucleobindin 1 | NS | C | 53,748 | 77 | 2 | 2 | 77 |
| NP_002565 | Peroxiredoxin 1 | BS | C | 21,979 | 67 | 18 | 3 | 50 |
| NP_005800 | Peroxiredoxin-2 | BS | C | 21,761 | 45 | 5 | 2 | 45 |
| NP_005013 | Profilin | BS | C | 14,923 | 209 | 23 | 4 | 90 |
| NP_000468 | Serum albumin | BNS | E | 69,368 | 115 | 3 | 2 | 68 |
| NP_006746 | TALDO1 | NS | C | 37,409 | 56 | 8 | 6 | 46 |
| NP_003237 | Thrombospondin 1 | BS | E | 129,383 | 208 | 8 | 12 | 70 |
| NP_003245 | TIMP1 | BS | E | 23,189 | 50 | 5 | 2 | 50 |
| NP_000356 | Triose-phosphate isomerase | BS | C | 26,538 | 89 | 11 | 7 | 70 |
| NP_002760 | Trypsin (PRSS1) | BS | E | 26,558 | 43 | 12 | 4 | 43 |
| NP_003339 | Ubiquitin-protein ligase E2N | BS | C | 17,007 | 64 | 13 | 2 | 65 |
| NP_079493 | UL16-binding protein 2 | BS | M, E | 27,237 | 51 | 11 | 4 | 51 |
| NP_009057 | VCP | NS | C | 89,191 | 51 | 18 | 11 | 47 |
| NP_003369 | VGF, NGF-inducible | BNS | E | 67,275 | 152 | 22 | 18 | 97 |
Of the 61 proteins identified in CM preparations from two or more cell lines, chromogranins A, B, and C were detected in the CM from all three cell lines. CgA secretion is consistent with previous studies that had demonstrated that this protein is secreted by all three cell lines (11, 17–20). Chromogranin A was used as the positive controls for the analysis of each preparation and each cell line during this investigation.
The reproducibility of each protein identified in a secreted proteome was assessed using the BON-1 cell line: repetition of the entire procedure demonstrated that 81% of the 82 BON-1 proteins in Table I could be reproducibly identified. However, when approximately 50% less protein was analyzed in a third series of experiments, only 46% of the initial set of proteins could be identified. In addition to establishing the importance of sample loading, variation in the presence of cytosolic, membrane, and extracellular matrix (ECM) proteins was also apparent in different CM preparations, indicating empirical variations in cell detachment and incorporation of ECM.
Cellular Location of CM Proteins
The cellular location of each protein identified using Protein Centre and NCBI databases revealed that many proteins have been reported to be present in more than one location. Verification and further refinement of protein localization was based on extensive mining of the research literature, although we cannot exclude the possibility that intracellular and cell surface proteins or fragments thereof are secreted by the cell lines used in this study. Of the 19 proteins found in CM preparations from all three cell lines, nine are known to be secreted (Table I). Furthermore, of these nine proteins, five were found in every run performed, including replicates. These five proteins (carboxypeptidase E, CgA, nerve growth factor (NGF)-inducible VGF, serum albumin, and Mac-2BP) were therefore the most suitable for consideration as markers (Fig. 2B).
Comparison between Proteins Secreted by NET Cell Lines and Normal Tissue
In this study, we opted to focus on proteins not detected in normal neuroendocrine secretory vesicles (SVs) as these may be more sensitive markers and may also be involved in tumor development or progression. We compared the soluble proteins from neuroendocrine SVs isolated from normal tissue (21) and the CM preparations to identify proteins that are not specific to transformed cells: 18 of the 205 proteins from NET cell line CMs were found in SVs. 10 of these proteins were present in the 61 proteins secreted by two or more NET lines, and three were present in the five secreted proteins found in every preparation (Fig. 2B). Finally, we excluded proteins found by Wang et al. (39) to be commonly identified in comparative proteomics studies of a variety of different tissues. Accordingly, albumin was not studied further, leaving Mac-2BP as the best candidate marker.
Assessment of Mac-2BP as Marker for NET
Western Blotting
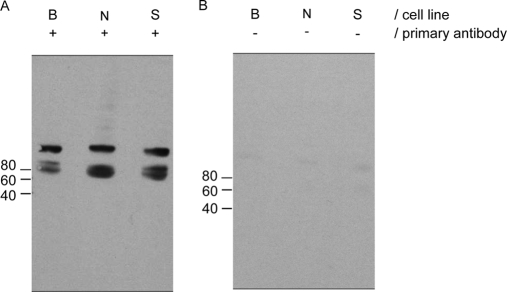
Mac-2BP has not previously been studied as a potential marker for NET and was selected for validation, initially by Western blotting. Blots were performed on the CM from all three cell lines to test for the presence of Mac-2BP using a parallel blot that was not probed with primary antibody as a negative control (Fig. 3). Mac-2BP was identified in the CM from all three cell lines, consistent with the identifications made by LC-MS/MS.
Fig. 3.
Western blotting of conditioned media to confirm Mac-2BP expression by all NET cell lines. Western blots of equal loadings (30 μl) of CM from each cell line (B, BON-1; N, NCI-H727; S, SHP-77) using anti-Mac-2BP antibodies (A) and no primary antibody (B). Marker protein positions and molecular masses (kDa) are indicated.
Immunohistochemistry
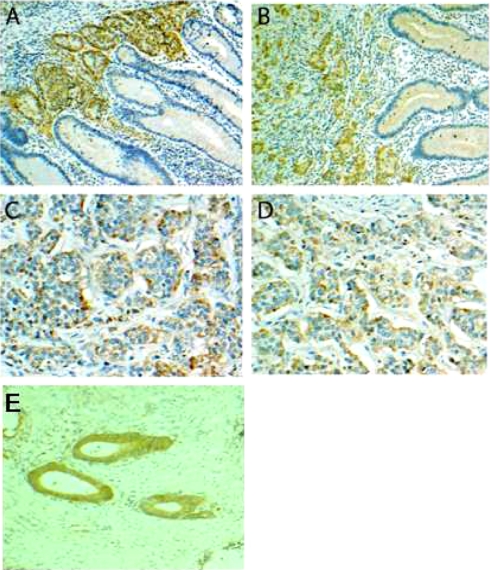
To test whether Mac-2BP was elevated in different types of tumors and surrounding stromal tissue, immunohistochemistry was performed on biopsy samples from 33 patients with previously diagnosed NET. 29 of these samples had positive staining for Mac-2BP (Fig. 4A) localized in each case to tumor areas. Staining was predominantly cytoplasmic with no nuclear staining apparent (Fig. 5) and was seen in all types of neuroendocrine tumor examined (pancreatic, ileal, and paraganglioma and tumors of unknown primary origin) (Fig. 4B). The strength of staining showed no apparent correlation with tumor type. The four cases with no detectable staining for Mac-2BP were all well differentiated neuroendocrine carcinomas. We found no significant correlation between tumor grade and staining for Mac-2BP.
Fig. 4.
Immunohistochemical analysis of Mac-2BP expression in NET tissue. A, tumor samples from a panel of different NET types were scored according to the intensity of immunohistochemical staining for Mac-2BP (histological score). B, details of the intensity and scoring ranges are shown for each NET type, showing the number of cases in total; the number of cases with positive uptake (i.e. score >2); the intensity scored on a scale of 1–3 where 1 = weak, 2 = moderate, and 3 = intense; and the area scored on a range of 1–3 where 1 = <25%, 2 = 25–75%, and 3 = >75% positively stained.
Fig. 5.
Immunohistochemical staining of NET and normal tissue for Mac-2BP. Fixed tissue sections were stained with anti-Mac-2BP antibody (brown), and nuclei were counterstained with hematoxylin (blue). A, ileal NET tissue shows predominantly cytoplasmic staining of the clusters of NET cells. The surrounding mucosa appears normal with no visible staining. Magnification, ×200. B, midgut NET clearly demonstrating staining of clusters of NET cells and negative staining of the surrounding tissue. Magnification, ×200. C, pancreatic NET showing cytoplasmic staining in the tumor cells. Magnification, ×400. D, ileal NET clearly containing NET and normal cells. Normal tissue shows no evidence of epithelial staining, whereas adjacent NET cells are clearly stained. Magnification, ×400. E, gastric cancer control showing moderate staining of gastric cancer cells with minimal background staining. Magnification, ×200.
Serum Mac-2BP Levels in Patients with NET
Mac-2BP levels were assessed in serum samples from 47 patients and 24 healthy control subjects (Fig. 6A). The group of patients with NET taken as a whole (3.31 μg/ml; range, 0.82–10.66 μg/ml) had significantly higher serum Mac-2BP levels than the control group (2.30 μg/ml; range, 1.16–3.56 μg/ml; p < 0.001). When compared according to primary site of NET, serum Mac-2BP was significantly elevated in NETs that originated from the midgut (3.34 μg/ml; range, 0.82–10.66 μg/ml) compared with controls (p < 0.001) as well as pancreatic NET (2.67 μg/ml; range 1.37–10.50, μg/ml) when compared with controls (p < 0.05). There was no significant difference in Mac-2BP levels between patients with pancreatic or midgut primary tumors. There was a positive correlation between CgA and Mac-2BP in patients with midgut NETs using a Spearman rank correlation (r = 0.36, p = 0.013) but no such correlation in pancreatic NETs.
Fig. 6.
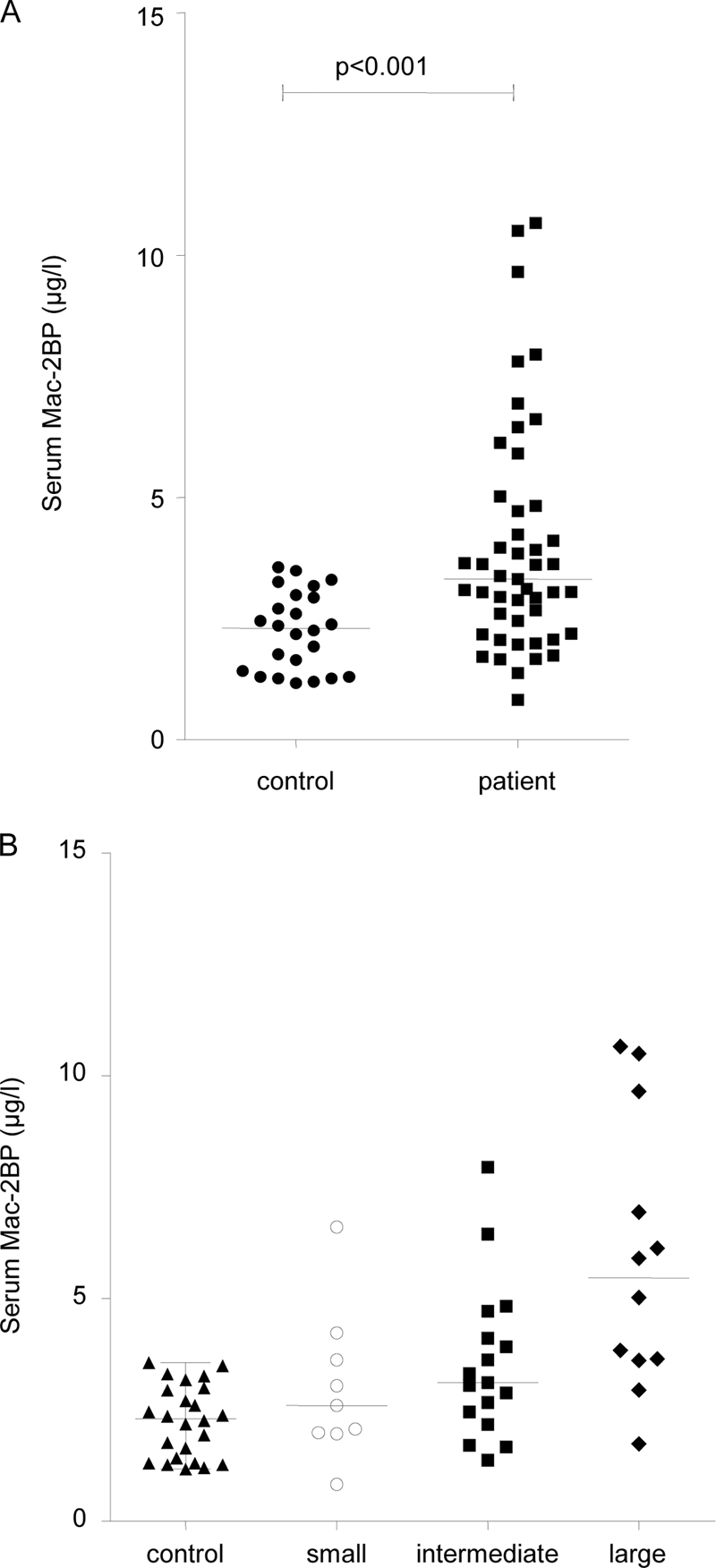
Serum Mac-2BP protein levels in patients and controls. Serum levels of Mac-2BP were assayed using an ELISA test. A, a Mann-Whitney U test demonstrates that serum Mac-2BP levels are significantly higher in patients than controls (p < 0.001). B, a Kruskal-Wallis one-way analysis of variance test shows a significant difference in Mac-2BP levels in NET patients with no metastases and small, intermediate, and large volume liver metastases (p = 0.024). Bars indicate median value for the group.
From radiological assessment of cross-sectional imaging performed within 2 months of blood sampling, visual assessments (by R. S.) were made of the volume of liver metastases present. Three categories were created: low volume liver metastases (involving <25% of liver), intermediate (involving 25–50% of liver), and large volume liver metastases (>50% of liver). When comparing volume of liver metastases and Mac-2BP levels using Kruskal-Wallis one-way analysis of variance test (Fig. 6B), there was a significant difference between the groups (p = 0.024). This indicates that higher Mac-2BP levels occur in serum with greater tumor burden.
Assessment of Sensitivity and Specificity of Serum Mac-2BP as Marker for NET
To investigate whether levels of Mac-2BP in the circulation could be useful as a marker of NET, serum samples were taken from patients and control subjects. The levels of Mac-2BP were measured in the serum by ELISA. Receiver operator characteristic (ROC) curves for serum Mac-2BP were constructed to determine the cutoff values (for set sensitivity and associated specificity of the assay for Mac-2BP) for all NETs. ROC curves are a graphical method of assessing the characteristic of a diagnostic test by plotting sensitivity (true positive rate) against 1 − specificity (false positive rate). These parameters are used as measures of the proportion of positives and negatives, respectively, that are correctly identified. Accuracy of the test is measured by the area under the ROC curve: an area of 1 represents a perfect test, whereas an area of 0.5 represents a random association; generally, >0.75 is regarded as a good marker. If the area under the curve (AUC) was 0.75, then on average a patient will have a more abnormal Mac-2BP level than 75% of controls. If the test was perfect, then every patient would have a more abnormal test result than every control, and hence the AUC would equal 1. The AUC for all NETs was 0.77, which shows that Mac-2BP is a good marker of NET. Serum Mac-2BP ≥2.41 μg/ml was a highly sensitive marker (≥75%) for NETs; the associated specificity relative to the control group was 59.3% (Table II). For the detection of midgut NET, the area under the curve was slightly higher at 0.79 (Fig. 7B), and at ≥75% sensitivity, the specificity was 70.8% (cutoff value >2.91 μg/ml).
Table II. Assessment of serum Mac-2BP as marker for all gastroenteropancreatic (GEP) NETs and midgut NETs.
The detection rate (sensitivity) is the total true positive results divided by the true positive and false negative results. The false positive rate (FPR) is 100% − specificity. CI is the confidence interval. Likelihood ratio (LR) is sensitivity divided by 1 − specificity. At levels of >3.58 μg/ml, Mac-2BP is 100% specific for NETs.
| Detection rate (sensitivity) | CI | Mac-2BP in all GEP NETs |
Mac-2BP in midgut NETs |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cutoff | FPR | CI | LR | Cutoff | FPR | CI | LR | ||
| % | % | μg/ml | % | % | μg/ml | % | % | ||
| 47 | 32.1–61.9 | >3.58 | 0 | 85.6–100 | >3.59 | 0 | 85.6–100 | ||
| 75 | 59.7–86.0 | >2.41 | 41.7 | 22.2–63.4 | 1.80 | >2.91 | 29.2 | 12.2–44.6 | 2.56 |
| 80 | 66.7–90.9 | >2.06 | 58.3 | 37.4–78.2 | 1.37 | >2.30 | 50.0 | 29.2–61.8 | 1.60 |
| 85 | 71.7–93.8 | >1.97 | 58.3 | 36.4–77.2 | 1.46 | >2.02 | 58.3 | 36.4–77.9 | 1.46 |
| 90 | 76.9–96.5 | >1.72 | 66.6 | 45.3–84.3 | 1.35 | >1.94 | 58.2 | 37.0–77.7 | 1.55 |
| 95 | 85.5–99.5 | >1.65 | 70.2 | 45.3–84.3 | 1.35 | >1.70 | 66.6 | 44.7–84.3 | 1.43 |
Fig. 7.
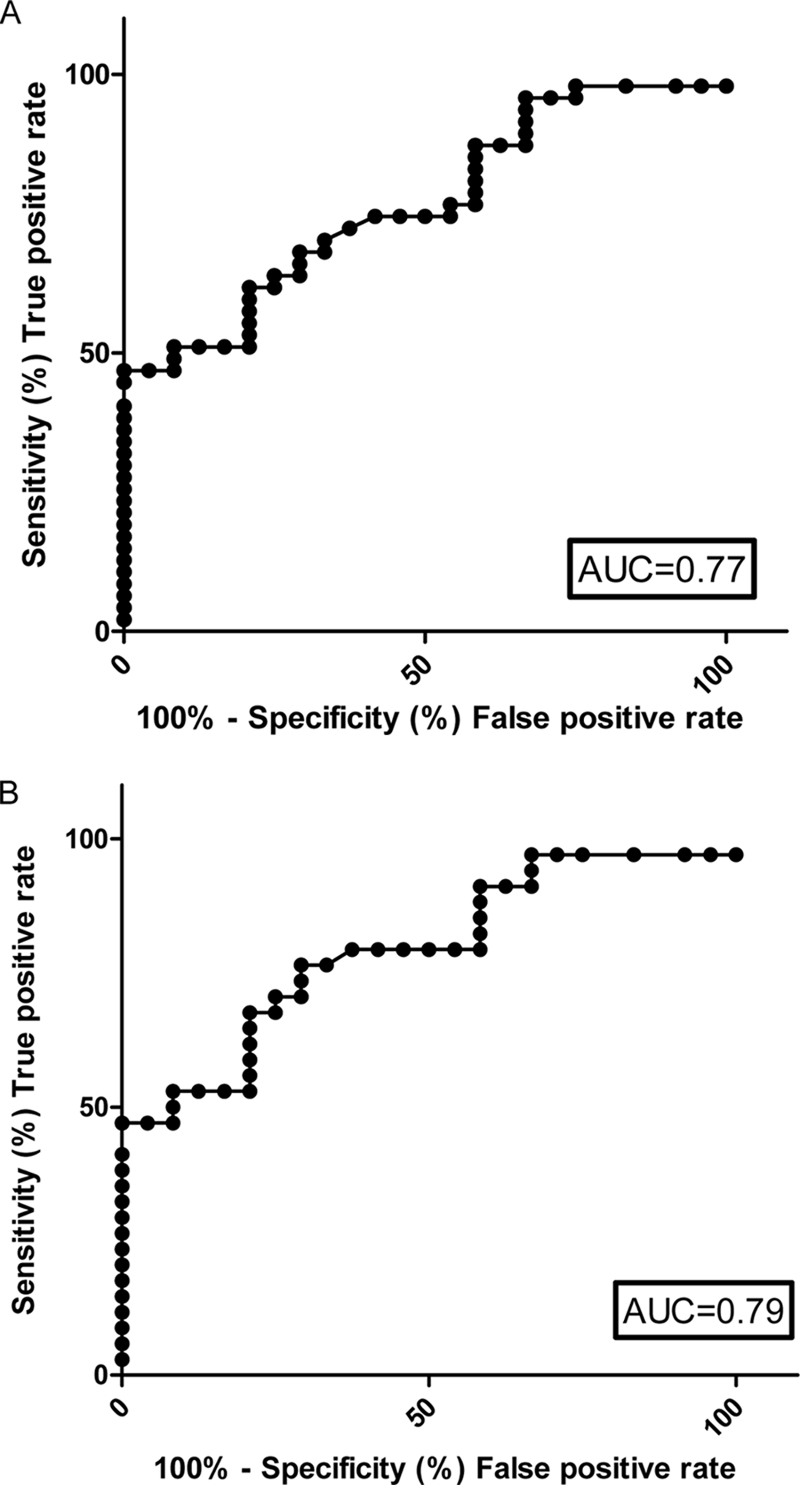
Assessment of serum Mac-2BP as NET marker. ROC curves and the corresponding AUC are shown for serum Mac-2BP levels in all types of NET (A) and serum Mac-2BP from patients with midgut primary NETs (B).
Because this marker is not proposed for population screening but for monitoring the disease status of NET patients, a high specificity in identifying patients with disease is more useful than a high sensitivity. Mac-2BP achieves 100% specificity for identifying disease in all of the NET patients when the cutoff value was increased to >3.58 μg/ml, corresponding to a detection rate of 47% (Table II).
Combination of Mac-2BP and Chromogranin A as Biochemical Markers of NET
The CgA assay (at a cutoff >60 pg/ml) correctly identified 72.3% (34 of 47) of the NET cases in our patient population. Serum Mac2-BP at a cutoff of >3.58 μg/ml had 100% specificity for NET (Table II). Using this threshold, an additional five cases (10.6%) were identified as positive for NET. The combination of these two biochemical markers correctly identified 82.9% of the NET cases in this cohort of patients.
DISCUSSION
In this study, we analyzed samples of serum-free media that had been conditioned by three neuroendocrine cell lines: NCI-H727, BON-1, and SHP-77. Protein identifications were only accepted when at least two peptides from the protein produced a significant Mowse score, apart from one case where a convincing series of y ions was identified. This stringency may have reduced the number of proteins identified, but it increases the confidence of identifications. We identified nine extracellular proteins including Mac-2BP that are secreted by all three cell lines. Mac-2BP was expressed in 88% of immunohistochemical samples, and serum levels of Mac-2BP were significantly higher in patients than in controls.
BON-1 is a pancreatic neuroendocrine cell line (19), and NCI-H727 and SHP-77 are bronchial neuroendocrine tumor cell lines. In the present study, the established NET serum markers, chromogranins A, B, and C, were identified in the CM of all three lines, consistent with the neuroendocrine phenotype of these cells. These proteins have not been reported in the secretomes of several non-NET cell lines (11, 13, 22–24) and were found in normal neuroendocrine secretory vesicles (21). However, these proteins possess limitations as NET biomarkers (see below).
Several other proteins identified here in the CM of NET cell lines have also been identified in other cancer cell lines and have postulated roles in cancer, including heat shock protein 70, heat shock protein 90, laminin α-5 chain, thioredoxin reductase, annexin A2, glutathione S-transferase, Mac-2BP, and NGF-inducible VGF (13, 25–27). Consequently, they are potential markers of different types of tumors. Furthermore, a number of these proteins may be involved in the pathophysiological development or progression of tumors, including NETs. Among the nine proteins of specific interest to this study because they were secreted by all three cell lines, we chose to focus on Mac-2BP for validation because it was reproducibly detected in replicate CM preparations and was not found in normal neuroendocrine secretory vesicles or as a commonly occurring component of tissue proteomes.
Other proteins identified in this study may still prove of value as markers: a study by Rindi et al. (27) has previously established that NGF-inducible VGF is expressed in the majority of NETs, and it is likely that combinations of markers will prove to be more specific than individual markers. Future work will address the potential value of other proteins in this set, individually and in combination.
Mac-2BP is elevated in patients with a variety of different cancers, including breast, nasopharyngeal, and lung cancers (28–30). It is associated with poor survival and metastatic spread of liver and lung cancers (31, 32). This study indicates that Mac-2BP is also elevated in patients with NETs. (i) Mac-2BP levels were significantly elevated in both pancreatic and midgut NETs. (ii) Elevation of serum Mac-2BP was related to volume of liver metastases. (iii) CgA is currently the most commonly used NET marker, but it does not offer accurate prognostic information, and its accuracy is dependent on the assay used. Furthermore, CgA is labile, and blood samples need to be collected on ice and stored frozen prior to analysis. Consequently, additional studies are warranted to assess a role for Mac-2BP as a biochemical NET marker.
Using a threshold of >3.58 μg/ml, Mac-2BP was a 100% specific marker in identifying NETs in this study. The low likelihood ratio of Mac-2BP and its high false positive rate mean that it currently would not be useful as a sole screening marker. We found that serum levels of Mac 2-BP >3.58 μg/ml can identify additional cases of NET that would not have been identified using the standard assay for CgA. Mac-2BP could be used as part of a panel of markers for diagnosing NETs, although a large scale study would be needed to validate this approach. The other role for serum levels of Mac-2BP could be in identifying patients with progressive disease or increases in tumor load because Mac-2BP showed a significant trend with increasing tumor load.
Mac-2 BP, also known as 90K, is an oligomeric glycoprotein composed of subunits of ∼90 kDa (33) and binds galectin-1, -3, and -7. Several lines of evidence support a role for galectins in tumor invasion and metastasis. These proteins have been reported to mediate apoptosis, cell proliferation, and angiogenesis (26, 34). The mechanisms underlying the role of Mac-2BP and galectins in cancer may be related to the ability of these proteins to interact with and modulate cell-cell and cell-matrix adhesion and apoptosis (35, 36). Inohara and Raz (37) found that Mac-2BP can mediate homotypic cell adhesion and the formation of multicellular aggregates by cross-linking galectin-1 and -3 residues on adjacent tumor cells. This process appears to be critical for cancer cell survival in the bloodstream and may be a vital step in metastatic diffusion. Moreover, Mac-2BP and galectins are also located in the ECM where they may play a role in cell attachment by binding to β1 integrins, collagens, and fibronectin (36, 38).
Further studies will be required to assess the suitability and reliability of Mac-2BP as a marker of NET. It will be necessary to determine whether there is diurnal variation of Mac-2BP by sampling serum from patient and controls subjects several times a day. A study to determine changes in serum Mac-2BP following different treatments such as chemotherapy, radiotargeted therapy, and surgical resection would be of benefit to identify whether Mac-2BP is related to level of disease activity. Finally, larger studies could assess whether Mac-2BP would be useful as a prognostic marker in patients with NETs.
In summary, the identification of the secreted proteome of NET cell lines has established a panel of possible biomarkers for NET. Extensive studies will be required to rigorously assess the potential of Mac-2BP as a marker for the clinical management of NET and to elucidate the pathophysiological role of Mac-2BP. This study provides evidence for the utility of this combination of CM preparation, LC-MS/MS, and immunological methods to identify putative serum markers from cultured cells and their initial assessment using clinical samples. This combination is a powerful strategy for finding and evaluating potential serum markers for NETs and other types of cancer.
Supplementary Material
Acknowledgments
We thank Dr. T. Luong for help with preparation of histopathology figures and Amy Kirkwood for help with statistical analysis.
* This work was supported in part by the Royal Free Hampstead National Health Service (NHS) Trust (to the Royal Free Centre for Biomedical Science).
 This article contains supplemental Table 1.
This article contains supplemental Table 1.
1 The abbreviations used are:
- NET
- neuroendocrine tumor
- CgA
- chromogranin A
- Mac-2BP
- Mac-2-binding protein
- FBS
- fetal bovine serum
- CM
- conditioned media
- 2-D
- two-dimensional
- ROC
- receiver operator characteristic
- ECM
- extracellular matrix
- NGF
- nerve growth factor
- SV
- secretory vesicle
- AUC
- area under the curve.
REFERENCES
- 1.Caplin M. E., Buscombe J. R., Hilson A. J., Jones A. L., Watkinson A. F., Burroughs A. K. (1998) Carcinoid tumour. Lancet 352, 799–805 [DOI] [PubMed] [Google Scholar]
- 2.Modlin I. M., Oberg K., Chung D. C., Jensen R. T., de Herder W. W., Thakker R. V., Caplin M., Delle Fave G., Kaltsas G. A., Krenning E. P., Moss S. F., Nilsson O., Rindi G., Salazar R., Ruszniewski P., Sundin A. (2008) Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 9, 61–72 [DOI] [PubMed] [Google Scholar]
- 3.Yao J. C., Hassan M., Phan A., Dagohoy C., Leary C., Mares J. E., Abdalla E. K., Fleming J. B., Vauthey J. N., Rashid A., Evans D. B. (2008) One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol 26, 3063–3072 [DOI] [PubMed] [Google Scholar]
- 4.Gustafsson B. I., Kidd M., Modlin I. M. (2008) Neuroendocrine tumors of the diffuse neuroendocrine system. Curr. Opin. Oncol 20, 1–12 [DOI] [PubMed] [Google Scholar]
- 5.de Herder W. W. (2007) Biochemistry of neuroendocrine tumours. Best Pract. Res. Clin. Endocrinol. Metab 21, 33–41 [DOI] [PubMed] [Google Scholar]
- 6.Kaltsas G., Grossman A. B. (2007) Clinical features of gastroenteropancreatic tumours, in Handbook of Neuroendocrine Tumours (Caplin M. E., Kvols L. eds) 1st Ed., pp. 53–82, BioScientifica, Bristol, UK [Google Scholar]
- 7.Rindi G., Bordi C. (2005) Endocrine tumours of the gastrointestinal tract: aetiology, molecular pathogenesis and genetics. Best Pract. Res. Clin. Gastroenterol 19, 519–534 [DOI] [PubMed] [Google Scholar]
- 8.Eriksson B., Oberg K., Stridsberg M. (2000) Tumor markers in neuroendocrine tumors. Digestion 62, Suppl. 1, 33–38 [DOI] [PubMed] [Google Scholar]
- 9.Bast R. C., Jr., Feeney M., Lazarus H., Nadler L. M., Colvin R. B., Knapp R. C. (1981) Reactivity of a monoclonal antibody with human ovarian carcinoma. J. Clin. Investig 68, 1331–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balk S. P., Ko Y. J., Bubley G. J. (2003) Biology of prostate-specific antigen. J. Clin. Oncol 21, 383–391 [DOI] [PubMed] [Google Scholar]
- 11.Kulasingam V., Diamandis E. P. (2007) Proteomics analysis of conditioned media from three breast cancer cell lines: a mine for biomarkers and therapeutic targets. Mol. Cell. Proteomics 6, 1997–2011 [DOI] [PubMed] [Google Scholar]
- 12.Sardana G., Marshall J., Diamandis E. P. (2007) Discovery of candidate tumor markers for prostate cancer via proteomic analysis of cell culture-conditioned medium. Clin. Chem 53, 429–437 [DOI] [PubMed] [Google Scholar]
- 13.Mlynarek A. M., Balys R. L., Su J., Hier M. P., Black M. J., Alaoui-Jamali M. A. (2007) A cell proteomic approach for the detection of secretable biomarkers of invasiveness in oral squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg 133, 910–918 [DOI] [PubMed] [Google Scholar]
- 14.Perkins D. N., Pappin D. J., Creasy D. M., Cottrell J. S. (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 [DOI] [PubMed] [Google Scholar]
- 15.Park Y. P., Choi S. C., Kim J. H., Song E. Y., Kim J. W., Yoon D. Y., Yeom Y. I., Lim J. S., Kim J. W., Paik S. G., Lee H. G. (2007) Up-regulation of Mac-2 binding protein by hTERT in gastric cancer. Int. J. Cancer 120, 813–820 [DOI] [PubMed] [Google Scholar]
- 16.Shah T., Hochhauser D., Frow R., Quaglia A., Dhillon A. P., Caplin M. E. (2006) Epidermal growth factor receptor expression and activation in neuroendocrine tumours. J. Neuroendocrinol 18, 355–360 [DOI] [PubMed] [Google Scholar]
- 17.Gazdar A. F., Helman L. J., Israel M. A., Russell E. K., Linnoila R. I., Mulshine J. L., Schuller H. M., Park J. G. (1988) Expression of neuroendocrine cell markers L-dopa decarboxylase, chromogranin A, and dense core granules in human tumors of endocrine and nonendocrine origin. Cancer Res 48, 4078–4082 [PubMed] [Google Scholar]
- 18.Ono K., Suzuki T., Miki Y., Taniyama Y., Nakamura Y., Noda Y., Watanabe M., Sasano H. (2007) Somatostatin receptor subtypes in human non-functioning neuroendocrine tumors and effects of somatostatin analogue SOM230 on cell proliferation in cell line NCI-H727. Anticancer Res 27, 2231–2239 [PubMed] [Google Scholar]
- 19.Parekh D., Ishizuka J., Townsend C. M., Jr., Haber B., Beauchamp R. D., Karp G., Kim S. W., Rajaraman S., Greeley G., Jr., Thompson J. C. (1994) Characterization of a human pancreatic carcinoid in vitro: morphology, amine and peptide storage, and secretion. Pancreas 9, 83–90 [DOI] [PubMed] [Google Scholar]
- 20.von Wichert G., Edenfeld T., von Blume J., Krisp H., Krndija D., Schmid H., Oswald F., Lother U., Walther P., Adler G., Seufferlein T. (2008) Protein kinase D2 regulates chromogranin A secretion in human BON neuroendocrine tumour cells. Cell. Signal 20, 925–934 [DOI] [PubMed] [Google Scholar]
- 21.Wegrzyn J., Lee J., Neveu J. M., Lane W. S., Hook V. (2007) Proteomics of neuroendocrine secretory vesicles reveal distinct functional systems for biosynthesis and exocytosis of peptide hormones and neurotransmitters. J. Proteome Res 6, 1652–1665 [DOI] [PubMed] [Google Scholar]
- 22.Huang L. J., Chen S. X., Huang Y., Luo W. J., Jiang H. H., Hu Q. H., Zhang P. F., Yi H. (2006) Proteomics-based identification of secreted protein dihydrodiol dehydrogenase as a novel serum markers of non-small cell lung cancer. Lung Cancer 54, 87–94 [DOI] [PubMed] [Google Scholar]
- 23.Lin C. Y., Tsui K. H., Yu C. C., Yeh C. W., Chang P. L., Yung B. Y. (2006) Searching cell-secreted proteomes for potential urinary bladder tumor markers. Proteomics 6, 4381–4389 [DOI] [PubMed] [Google Scholar]
- 24.Martin D. B., Gifford D. R., Wright M. E., Keller A., Yi E., Goodlett D. R., Aebersold R., Nelson P. S. (2004) Quantitative proteomic analysis of proteins released by neoplastic prostate epithelium. Cancer Res 64, 347–355 [DOI] [PubMed] [Google Scholar]
- 25.Wu C. C., Chien K. Y., Tsang N. M., Chang K. P., Hao S. P., Tsao C. H., Chang Y. S., Yu J. S. (2005) Cancer cell-secreted proteomes as a basis for searching potential tumor markers: nasopharyngeal carcinoma as a model. Proteomics 5, 3173–3182 [DOI] [PubMed] [Google Scholar]
- 26.Grassadonia A., Tinari N., Iurisci I., Piccolo E., Cumashi A., Innominato P., D'Egidio M., Natoli C., Piantelli M., Iacobelli S. (2004) 90K (Mac-2 BP) and galectins in tumor progression and metastasis. Glycoconj. J 19, 551–556 [DOI] [PubMed] [Google Scholar]
- 27.Rindi G., Licini L., Necchi V., Bottarelli L., Campanini N., Azzoni C., Favret M., Giordano G., D'Amato F., Brancia C., Solcia E., Ferri G. L. (2007) Peptide products of the neurotrophin-inducible gene vgf are produced in human neuroendocrine cells from early development and increase in hyperplasia and neoplasia. J. Clin. Endocrinol. Metab 92, 2811–2815 [DOI] [PubMed] [Google Scholar]
- 28.Iacobelli S., Sismondi P., Giai M., D'Egidio M., Tinari N., Amatetti C., Di Stefano P., Natoli C. (1994) Prognostic value of a novel circulating serum 90K antigen in breast cancer. Br. J. Cancer 69, 172–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchetti A., Tinari N., Buttitta F., Chella A., Angeletti C. A., Sacco R., Mucilli F., Ullrich A., Iacobelli S. (2002) Expression of 90K (Mac-2 BP) correlates with distant metastasis and predicts survival in stage I non-small cell lung cancer patients. Cancer Res 62, 2535–2539 [PubMed] [Google Scholar]
- 30.Zeimet A. G., Natoli C., Herold M., Fuchs D., Windbichler G., Daxenbichler G., Iacobelli S., Dapunt O., Marth C. (1996) Circulating immunostimulatory protein 90K and soluble interleukin-2-receptor in human ovarian cancer. Int. J. Cancer 68, 34–38 [DOI] [PubMed] [Google Scholar]
- 31.Ozaki Y., Kontani K., Hanaoka J., Chano T., Teramoto K., Tezuka N., Sawai S., Fujino S., Yoshiki T., Okabe H., Ohkubo I. (2002) Expression and immunogenicity of a tumor-associated antigen, 90K/Mac-2 binding protein, in lung carcinoma. Cancer 95, 1954–1962 [DOI] [PubMed] [Google Scholar]
- 32.Valentini A. M., Iacovazzi P. A., Correale M., Pirrelli M., Armentano R., Iacobelli S., Tinari N., Iurisci I., Caruso M. L. (2005) Immunohistochemical and serological 90K/Mac-2BP detection in hepatocellular carcinoma patients: different behaviour of two monoclonal antibodies. Med. Chem 1, 185–189 [DOI] [PubMed] [Google Scholar]
- 33.Iacobelli S., Bucci I., D'Egidio M., Giuliani C., Natoli C., Tinari N., Rubistein M., Schlessinger J. (1993) Purification and characterization of a 90 kDa protein released from human tumors and tumor cell lines. FEBS Lett 319, 59–65 [DOI] [PubMed] [Google Scholar]
- 34.Matarrese P., Fusco O., Tinari N., Natoli C., Liu F. T., Semeraro M. L., Malorni W., Iacobelli S. (2000) Galectin-3 overexpression protects from apoptosis by improving cell adhesion properties. Int. J. Cancer 85, 545–554 [PubMed] [Google Scholar]
- 35.Tinari N., Kuwabara I., Huflejt M. E., Shen P. F., Iacobelli S., Liu F. T. (2001) Glycoprotein 90K/MAC-2BP interacts with galectin-1 and mediates galectin-1-induced cell aggregation. Int. J. Cancer 91, 167–172 [DOI] [PubMed] [Google Scholar]
- 36.Inohara H., Akahani S., Raz A. (1998) Galectin-3 stimulates cell proliferation. Exp. Cell Res 245, 294–302 [DOI] [PubMed] [Google Scholar]
- 37.Inohara H., Raz A. (1995) Functional evidence that cell surface galectin-3 mediates homotypic cell adhesion. Cancer Res 55, 3267–3271 [PubMed] [Google Scholar]
- 38.Sasaki T., Brakebusch C., Engel J., Timpl R. (1998) Mac-2 binding protein is a cell-adhesive protein of the extracellular matrix which self-assembles into ring-like structures and binds beta1 integrins, collagens and fibronectin. EMBO J 17, 1606–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H., Kachman M. T., Schwartz D. R., Cho K. R., Lubam D. M.Comprehensive proteome analysis of ovarian cancers using liquid phase separation, mass mapping and tandem mass spectrometry: a strategy for identification of candidate cancer biomarkers. Proteomics 2004: 4:2476–95 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.