Abstract
During embryonic development, Drosophila macrophages (haemocytes) undergo a series of stereotypical migrations to disperse throughout the embryo. One major migratory route is along the ventral nerve cord (VNC), where haemocytes are required for the correct development of this tissue. We show, for the first time, that a reciprocal relationship exists between haemocytes and the VNC and that defects in nerve cord development prevent haemocyte migration along this structure. Using live imaging, we demonstrate that the axonal guidance cue Slit and its receptor Robo are both required for haemocyte migration, but signalling is not autonomously required in haemocytes. We show that the failure of haemocyte migration along the VNC in slit mutants is not due to a lack of chemotactic signals within this structure, but rather to a failure in its detachment from the overlying epithelium, creating a physical barrier to haemocyte migration. This block of haemocyte migration in turn disrupts the formation of the dorsoventral channels within the VNC, further highlighting the importance of haemocyte migration for correct neural development. This study illustrates the important role played by the three-dimensional environment in directing cell migration in vivo and reveals an intriguing interplay between the developing nervous system and the blood cells within the fly, demonstrating that their development is both closely coupled and interdependent.
Keywords: Drosophila melanogaster, Haemocyte, Ventral nerve cord
INTRODUCTION
Embryonic haemocytes are the Drosophila equivalent of macrophages and play crucial roles during development. Following specification in the head mesoderm, haemocytes undertake multiple stereotyped migrations to disperse throughout the entire embryo by stage 15 of development (Wood and Jacinto, 2007). As they follow these migratory routes they secrete extracellular matrix and remove apoptotic corpses and cellular debris (Olofsson and Page, 2005; Sears et al., 2003; Tepass et al., 1994). When haemocytes are genetically ablated, or their migration inhibited, decreased matrix deposition is observed throughout the embryo and the normal morphogenesis of organs, such as the ventral nerve cord (VNC) and trachea, is perturbed (Defaye et al., 2009; Martinek et al., 2008; Olofsson and Page, 2005; Sears et al., 2003).
One major haemocyte migratory pathway is along the VNC, with haemocytes migrating down the midline on the dorsal and ventral surfaces of this structure immediately beneath the ventral epidermis. The PDGF/VEGF-related ligands Pvf2 and Pvf3 are expressed along the VNC and are believed to act as chemoattractants, directing haemocytes along this structure through activation of the PDGFR/VEGFR-related receptor Pvr (Cho et al., 2002; Wood et al., 2006). These same growth factors are also required for haemocyte survival (Bruckner et al., 2004) and regulation of cell size (Sims et al., 2009). Blocking Pvr signalling in haemocytes, either through the expression of dominant-negative versions of Pvr or the removal of both Pvf2 and Pvf3, prevents haemocytes from migrating along the VNC (Bruckner et al., 2004; Wood et al., 2006). Lesions in the gene single-minded (sim), which encodes a bHLH transcription factor required for development of the midline glia and neurons (Nambu et al., 1990; Nambu et al., 1991), which are themselves required for the generation of the initial axonal scaffold of the VNC (Thomas et al., 1988), also prevent haemocyte migration along the VNC (Cho et al., 2002; Paladi and Tepass, 2004). In sim mutants, Pvf signals are lost from the midline, which has been presumed to explain the resultant haemocyte migration defects (Cho et al., 2002; Paladi and Tepass, 2004). Failure of haemocytes to migrate along the length of the VNC or preventing their engulfment of apoptotic corpses leads to a failure of VNC condensation (Defaye et al., 2009; Olofsson and Page, 2005; Sears et al., 2003), a process that is required for the formation of the blood-brain barrier, resulting in lethality (Schwabe et al., 2005).
Following migration along their VNC substrate, haemocytes undertake a phase of lateral migration to the edges of the VNC coincident with, and dependent on, downregulation of Pvf expression at the midline (Wood et al., 2006). Aside from the involvement of Pvfs, nothing is known about what other signals might act in concert with these growth factors to orchestrate these elaborate migrations along and around the VNC. Numerous axonal chemoattractants and chemorepellents are expressed at the midline of the VNC, which is thought to be analogous to the floorplate of the developing vertebrate central nervous system (Jacobs, 2000). One such guidance cue is Slit, a component of the extracellular matrix secreted by the midline glia, which prevents inappropriate recrossing of the midline by commissural neurons through activation of Robo receptors (Battye et al., 1999; Kidd et al., 1999; Simpson et al., 2000). Slit is known to regulate the migration of vertebrate leukocytes (Wu et al., 2001). Hence, we hypothesised that neuronal guidance cues might also guide haemocyte migration, either directly through the activation of haemocyte receptors, or indirectly by ensuring that the normal spatial interactions take place between neural tissues and haemocytes.
Here, we show that Slit and Robo are both necessary for haemocyte migration along the VNC, but not autonomously in haemocytes. Instead, the structural defects in the VNC present in slit mutants prevent haemocyte migration, despite the continuing expression of Pvf chemoattractants along the VNC. We show that the VNC abnormalities in slit mutant embryos cause the formation of a physical barrier that prevents haemocytes from accessing their normal migratory routes. Blockages in haemocyte migration in turn affect the formation of the dorsoventral channels within the VNC, which might contribute to the failure of VNC condensation previously reported in mutants with disrupted haemocyte migration. These studies therefore reveal that haemocyte migration and VNC development are closely coupled and dependent upon one another.
MATERIALS AND METHODS
Fly lines and phenotype quantification
Haemocyte migration was followed by imaging GFP or GMA, the globular actin-binding domain of Moesin fused to GFP (Dutta et al., 2002), in w;srp-Gal4,UAS-GFP;crq-Gal4,UAS-GFP, w;;crq-Gal4,uas-GFP or w;srp-Gal4,UAS-GMA wild-type embryos. w;slitGA178/CTG;crq-Gal4,UAS-GFP, w;robo4,robo2x123/CTG;crq-Gal4,UAS-GFP and w;srp-Gal4,UAS-GMA;sim8/TTG flies were used to generate slit, robo1,2 and sim homozygotes with GFP/GMA-labelled haemocytes by selecting embryos that lacked CTG- or TTG-associated fluorescence. Phenotypes for these fly lines were quantified by scoring for the presence of GFP/GMA-positive haemocytes in each segment between the VNC and epidermis of immunostained stage 13/14 embryos.
Comm, Pvf2, Slit and dominant-negative Rac1 (N17Rac) were expressed specifically in haemocytes by crossing yw;;UAS-comm, w;UAS-Pvf2, yw;;UAS-slit or w;;UAS-N17Rac with w;srp-Gal4,UAS-GFP;crq-Gal4,UAS-GFP flies; the presence of GFP-labelled haemocytes along the entire length of the VNC was assessed using live embryos. The same UAS lines were crossed with w;sim-Gal4/CTG;sim-Gal4 or w;en-Gal4,UAS-GMA for expression along the ventral midline or in epithelial stripes, respectively. Embryos were immunostained for Fascin (Singed – FlyBase) to detect haemocytes along the VNC (Zanet et al., 2009). The percentage of haemocytes in contact with epithelial stripes was quantified from z-projections of confocal z-stacks of the ventral side of w;en-Gal4,UAS-GMA and w;en-Gal4,UAS-GMA/+;UAS-slit/+ embryos.
To test whether overexpression of Pvf2 could rescue slit haemocyte phenotypes w;slitGA178/CTG;slit-Gal4 flies were crossed with w;slitGA178,UAS-Pvf2/CyO (rescue) or w;slitGA178/CyO (control) flies. Fascin-immunostained CTG-negative embryos were then scored for haemocyte progression along the midline on the ventral surface of the VNC; embryos scored therefore comprise ~50% slitGA178±UAS-Pvf2/slitGA178;slit-Gal4/+ and ~50% slitGA178±UAS-Pvf2/CyO;slit-Gal4/+.
All lines were generated from flies available from the Bloomington Stock Centre with the exception of srp-Gal4 (a gift from K. Bruckner, UCSF, USA), UAS-comm and robo1,2 (gifts from G. Tear, KCL, UK).
Antibody staining and in situ hybridisation
Embryos were fixed and immunostained as previously described (Wood et al., 2006) using rabbit anti-GFP (Abcam, Cambridge, MA, USA; diluted 1:1000), mouse 1D4 anti-FasII (Fas2; DSHB, University of Iowa, USA; supernatant diluted 1:10), mouse C555.6D anti-Slit (DSHB; supernatant diluted 1:25) and mouse sn7c anti-Fascin (DSHB; concentrated antibody diluted 1:100). Prior to imaging, embryos were mounted in 1×PBS in 90% glycerol supplemented with 2.5% (w/v) DABCO (Sigma, St Louis, MO, USA).
Pvf2 and Pvf3 in situ hybridisation probes were as previously described (Wood et al., 2006) and were synthesised using the DIG RNA Labelling Kit (Roche Applied Science, Indianapolis, IN, USA). Whole-mount in situ hybridisations were carried out according to standard protocols and probes were detected using alkaline phosphatase-conjugated anti-DIG (Roche Applied Science). Pvf2 and Pvf3 in situs were mounted in Durcupan (Fluka/Sigma) and 50% glycerol, respectively.
Imaging and analysis
Live embryos were mounted as previously described (Wood and Jacinto, 2005; Evans et al., 2010). Time-lapse movies were produced on an LSM510 confocal microscope (Carl Zeiss, Oberkochen, Germany) at the University of Bath BioImaging Facility. z-stacks of five slices were taken every 3 minutes of haemocytes on the ventral side of the VNC and movies of maximum z-projections were assembled using ImageJ (NIH). Cell tracking and cell size quantification were also carried out using ImageJ.
Dextran injection assay
Embryos were dechorionated and mounted as per live imaging but were dried for 3 minutes using silica gel before being covered with Voltalef oil (no longer commercially available; halocarbon oil 700 from Sigma can be used as an alternative). Embryos were injected either anteriorly in the head or posteriorly between epidermis and VNC with 2.5 mg/ml 70 kDa rhodamine-dextran (Molecular Probes/Invitrogen, Carlsbad, CA, USA) using Femtotips II and a FemtoJet/InjectMan injection system (Eppendorf, Hamburg, Germany). z-stacks were collected of 1.5 μm sections of GFP-labelled haemocytes on the ventral midline and of rhodamine-dextran-labelled extracellular spaces. Cross-sectional areas of dorsoventral channels were measured using ImageJ.
RESULTS
Slit and Robo are required for haemocyte progression down the ventral midline
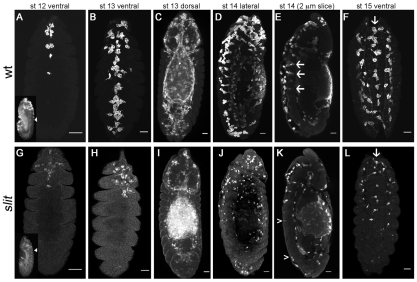
Haemocytes undergo a range of stereotyped migrations to disperse over the entirety of the Drosophila embryo during development (Wood and Jacinto, 2007). To follow these migrations we expressed UAS-GFP or UAS-GMA using the haemocyte-specific drivers srp-Gal4 (Bruckner et al., 2004) and/or crq-Gal4 (Wood et al., 2006). In wild-type embryos, haemocytes proceed posteriorly down the midline on both sides of the developing VNC in a narrow line from stage 12 onwards (Fig. 1A). By stage 13, the haemocytes from the head meet those that have been carried posteriorly during germ band retraction to form a single band of haemocytes along the ventral midline, immediately beneath the epidermis (Fig. 1B,D). A subset of these haemocytes can be found in the dorsoventral channels in the VNC (Fig. 1E). Haemocytes then migrate laterally from the midline to the edges of the VNC, giving rise to three parallel, longitudinal lines of haemocytes on the ventral side of the VNC by stage 15 (Fig. 1F).
Fig. 1.
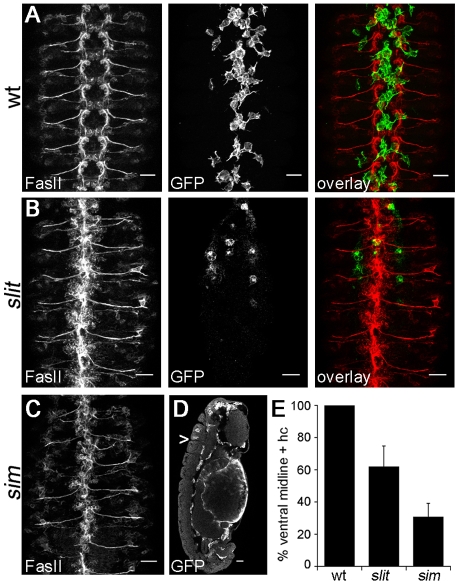
Haemocyte developmental migrations are perturbed in slit mutants. (A-L) Wild-type (w;srp-Gal4,UAS-GFP;crq-Gal4,UAS-GFP; wt) (A-F) and slit mutant (w;slitGA178;crq-Gal4,UAS-GFP) (G-L) Drosophila embryos were immunostained for GFP to show haemocyte dispersal. Ventral views of wild-type (A) and slit mutant (G) embryos reveal that haemocyte progression along the midline is only moderately delayed in the latter at stage 12. Lateral views of the same embryos (insets) show the extent of germ band retraction and the position of the posterior-most haemocyte along the ventral midline (arrowhead). By stage 13, haemocytes occupy the full length of the ventral midline in wild types (B), but make little progress posteriorly in slit mutant embryos (H). Dorsal views indicate that haemocytes reach other destinations equally well in each genotype (C,I). Lateral views at stage 14 reveal that whereas haemocytes continue to occupy the ventral midline in wild types (D), they are absent from large regions of the ventral surface of the VNC in slit mutants (J). Single confocal slices through the midline show haemocytes along both sides of the VNC and within dorsoventral channels in wild types (arrows in E), whereas only the dorsal side of the VNC is fully occupied in slit mutants (the region between arrowheads lacks haemocytes on the ventral side of the VNC) (K). By stage 15, three parallel lines of haemocytes are visible ventrally in both wild-type (F) and slit mutant (L) embryos (arrows indicate the midline), but haemocytes remain absent from much of the ventral side of the VNC in the latter. Anterior is up. Scale bars: 20 μm.
During development of the VNC a number of axonal guidance cues, such as Slit and Netrin, are expressed along the ventral midline (Jacobs, 2000). We hypothesised that some of these might be reiteratively used in haemocyte dispersal; indeed, Slit has previously been shown to inhibit the chemotaxis of vertebrate leukocytes (Wu et al., 2001). We therefore examined embryonic haemocyte migration in the loss-of-function mutant, slitGA178. In stark contrast to wild-type embryos, haemocytes in slit mutants were severely retarded in their migration down the ventral midline (Fig. 1G-L), such that by stage 13, when a continuous line of haemocytes is normally present along the midline, haemocytes in slit mutants have failed to progress along the VNC from both the anterior and posterior ends (Fig. 1H). This dramatic failure in haemocyte migration persisted through development (Fig. 1J-L), so that by stage 15 haemocytes remained absent from large portions of the ventral midline (Fig. 1L). The fact that haemocytes in these mutants migrated along wild-type routes elsewhere, reaching their appropriate destinations in the head, along the dorsal vessel and around the spiracles (compare Fig. 1C with 1I), suggests that their migration machinery remains intact along with their ability to follow other guidance cues. Interestingly, the migration defect in slit mutants appears specific to the ventral surface of the VNC because haemocytes were present from anterior to posterior on the dorsal side (Fig. 1J,K).
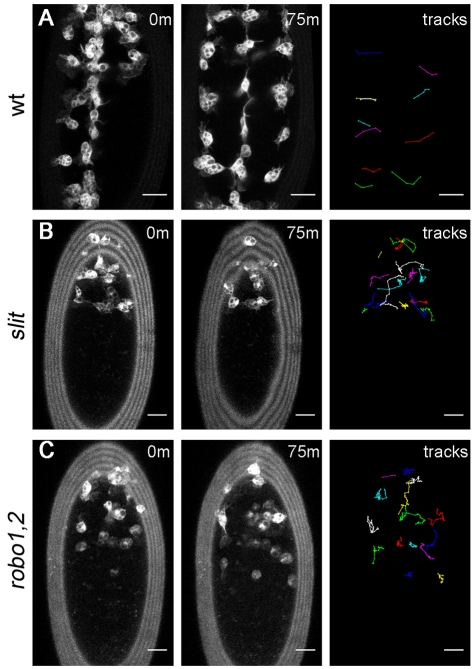
To understand more clearly how haemocyte dynamics are affected in slit mutants and to reveal the fate of stalled haemocytes, we produced live movies of haemocytes on the ventral side of the VNC from stage 13/14, when haemocytes would normally undergo lateral migration (Wood et al., 2006). Wild-type embryos exhibited a line of haemocytes down the midline, similar to immunostained embryos, which then migrated out from the ventral midline in a rapid and segmentally repeated pattern (Fig. 2A; see Movie 1 in the supplementary material). In slit mutants, haemocytes failed to make substantial progress down the midline, although overlays of haemocyte tracks showed that some were still able to migrate laterally, with an average speed of 1.8±0.6 μm/minute (Fig. 2B; see Movie 2 in the supplementary material), which is comparable to the average speed of 1.6±0.5 μm/minute for wild types.
Fig. 2.
Dynamic imaging reveals a failure in haemocyte progression along the ventral midline in slit and robo1,2 mutants. (A-C) GFP-expressing haemocytes in stage 13/14 wild-type (w;srp-Gal4,UAS-GFP;crq-Gal4,UAS-GFP) (A), slit (w;slitGA178;crq-Gal4,UAS-GFP) (B) and robo1,2 mutant (w;robo1,2;crq-Gal4,UAS-GFP) (C) Drosophila embryos were subjected to live imaging for 75 minutes as they migrated along the ventral midline. Positions of haemocytes at 0 and 75 minutes are shown. For clarity, only the laterally migrating wild-type tracks are shown, whereas all cell trajectories are shown for slit and robo1,2 mutants. Whereas haemocytes migrate laterally in each segment in wild-type embryos (A), haemocytes fail to cover the length of the ventral midline in slit (B) and robo1,2 (C) mutants and, consequently, lateral migration does not occur in every segment. Anterior is up. See Movies 1-3 in the supplementary material for full videos. Scale bars: 20 μm.
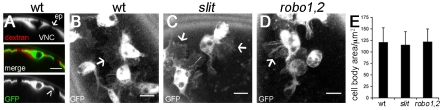
To test whether Robo, the receptor for Slit, is also required for haemocyte migration, movies were produced of robo4 and robo2x123 double mutants (termed robo1,2 mutants). The robo1,2 mutant embryos showed a similar phenotype to slit mutants, with haemocytes failing to progress down the midline (Fig. 2C; see Movie 3 in the supplementary material). These results therefore indicate that Slit is required for haemocyte migration along the midline, functioning via Robo, rather than via an alternative, unidentified Slit receptor. Despite failing to undergo normal developmental dispersal, haemocytes in slit and robo1,2 mutants exhibited a similar morphology and size to those in wild-type embryos, possessing spherical cell bodies that extend large lamellipodial projections (Fig. 3), again suggesting that their migratory machinery is unperturbed.
Fig. 3.
Haemocyte morphology is unaffected in slit and robo1,2 mutants. (A) Rhodamine-dextran (red in merged image) was injected into wild-type Drosophila embryos (w;srp-Gal4,UAS-GFP;crq-Gal4,UAS-GFP) with GFP-labelled haemocytes (green in merge) to label the extracellular spaces between the VNC and epidermis (ep). Orthogonal projections of haemocytes at the ventral midline reveal their thin lamellipodial protrusions (arrowhead). (B-D) z-projections show that haemocytes on the ventral side of the VNC in wild-type embryos (w;;crq-Gal4,UAS-GFP) (B) are morphologically indistinct from those containing the same Gal4 driver and UAS reporter in slit (C) and robo1,2 (D) mutants, extending large lamellipodial protrusions (arrows) from their phagosome-containing cell bodies. (E) No significant difference in haemocyte cell body area was found between wild types, slit and robo1,2 mutants (41, 54 and 35 haemocytes were measured, respectively). Error bars indicate s.d. Scale bars: 10 μm.
Slit-Robo signalling is not required within haemocytes
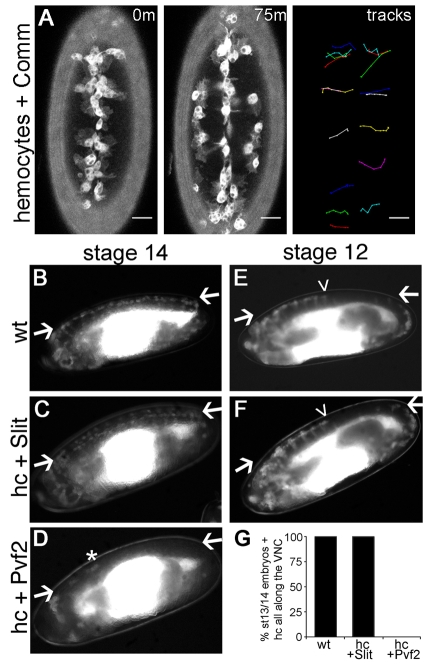
The Drosophila genome encodes three Robo receptors (robo1-3), each of which can be negatively regulated by Comm (Rajagopalan et al., 2000), a transmembrane protein that enables commissural neurons to overcome repulsion by Slit at the midline and therefore to cross this point (Tear et al., 1996). To determine whether Robo signalling is required autonomously in haemocytes we blocked Robo signalling by driving UAS-comm expression specifically in haemocytes. Surprisingly, we could find no defect in haemocyte migration upon expression of Comm, with haemocytes migrating down the midline and subsequently undergoing lateral migration at the expected stage of development (Fig. 4A; see Movie 4 in the supplementary material).
Fig. 4.
Slit does not directly regulate haemocyte migration. (A) GFP- and Comm-expressing haemocytes in stage 13/14 Drosophila embryos were subjected to live imaging for 75 minutes as they migrated on the ventral midline. The positions of haemocytes at 0 and 75 minutes and their associated tracks as they leave the midline reveal that Comm expression has no effect on haemocyte progression down the midline or on lateral migration. For the full video, see Movie 4 in the supplementary material. Anterior is up. Scale bars: 20 μm. (B-F)To verify that Slit does not directly regulate haemocyte migration, srp-Gal4 and crq-Gal4 were used to drive haemocyte-specific expression of UAS-GFP alone (wt), UAS-GFP and UAS-slit (hc + Slit), or UAS-GFP and UAS-Pvf2 (hc + Pvf2). Haemocytes cover the VNC at stage 14 when wt (B) or expressing Slit (C), whereas Pvf2 expression causes retention in the head (D). Few escapees were observed (asterisk), with D representing the least severe retention observed. Compared with wild types (E), Slit expression did not accelerate exit from the head at stage 12 (F). Lateral views with anterior to the left and ventral up. Arrows indicate VNC; arrowheads indicate the progression of haemocytes along the VNC. (G) Percentage of embryos with haemocytes all along the VNC at stage 13/14 (35, 60 and 78 embryos were scored for wt, hc + Slit and hc + Pvf2, respectively).
To confirm that Slit plays no direct role in haemocyte guidance, UAS-slit expression was driven in haemocytes using srp-Gal4 and crq-Gal4. Haemocyte-specific expression of Slit failed to divert haemocytes from their normal patterns of dispersal (Fig. 4B,C,E-G), neither preventing nor accelerating exit from the head. By contrast, expression of Pvf2 prevented their exit and subsequent migration along the VNC (Fig. 4D,G). Consistently, the expression of Slit in epithelial stripes or along the ventral midline using en-Gal4 or sim-Gal4, respectively, also failed to disrupt migration, nor were haemocytes attracted to Slit-expressing epithelial stripes (see Fig. S1 in the supplementary material). These data demonstrate that haemocytes do not respond either attractively or repulsively to Slit during embryonic dispersal and that Slit-Robo signalling is not required autonomously in haemocytes. Therefore, the haemocyte phenotype observed in slit and robo1,2 mutants must be due to indirect effects upon these cells.
Disruption of the VNC perturbs haemocyte migration
It is well known that Slit and Robo are required for neuronal guidance at the midline, and disruption to Slit signalling leads to a collapsed axonal scaffold (Dickson and Gilestro, 2006). This greatly disorganised axonal structure within the VNC provided a severely perturbed substrate for haemocyte migration in slit mutant embryos (compare Fig. 5A with 5B). Similar, but more severe VNC defects were present in sim mutants (Fig. 5C) (Nambu et al., 1990; Nambu et al., 1991); in the absence of functional Sim expression, the ectodermal cells that produce the midline glia, VUM neurons and RP1 neurons fail to differentiate correctly (Nambu et al., 1990). The greater severity of VNC phenotypes observed in sim mutants correlated with a further restriction in haemocyte progression along the ventral midline (Fig. 5D,E). At stage 14, haemocytes were present along the ventral midline between epidermis and VNC in every segment in all wild-type embryos, whereas on average only 62±13% and 31±9% of the ventral midline contained haemocytes in slit and sim mutants, respectively (Fig. 5E; see Fig. S2A in the supplementary material). Imaging haemocyte migration dynamically in sim mutants revealed that haemocytes are almost completely unable to progress down the midline and can only reach mid-trunk segments by migrating along the lateral edges of the VNC (see Fig. S2B and Movie 5 in the supplementary material), which is potentially where laterally positioned chemoattractants are present. As in wild-type and slit mutant embryos, haemocytes remained able to migrate along the dorsal side of the VNC in sim mutants (Fig. 5D), suggesting that the structure and/or cues present here are less disrupted than on the ventral side.
Fig. 5.
Haemocyte migration and VNC development are perturbed in both slit and sim mutants. (A-D) Stage 13/14 wild-type (A), slit (B) and sim mutant (C,D) Drosophila embryos with GFP-expressing haemocytes were co-immunostained for the neuronal marker FasII (red in overlay) and GFP (green in overlay) to show VNC morphology and haemocyte position, respectively. Only haemocytes on the ventral side of the VNC are shown for clarity. z-projections of FasII staining (ventral view) reveal that the regular repeating structure of the wild-type VNC (A) is collapsed in slit (B) and sim (C) mutants. Although haemocytes are able to migrate along the dorsal surface of the VNC in sim mutants, single 1.5 μm confocal slices of laterally orientated embryos (D) reveal that very few segments contain haemocytes on the ventral side of the VNC at the midline (the arrowhead indicates the posterior-most haemocyte). (E) Quantification of haemocyte distribution along the VNC at stage 13/14. The number of segments containing haemocytes on the ventral side of the VNC is expressed as a percentage of the total number of segments for each embryo. A significant difference (P<0.05, Student's t-test) was found between each genotype when 44 wild-type, 63 slit and 40 sim embryos were scored; error bars indicate s.d. Anterior is up. Scale bars: 20 μm.
Haemocyte chemoattractants are not absent in slit mutants
Pvf2 and Pvf3 are crucial for the migration of haemocytes along the developing VNC (Cho et al., 2002; Bruckner et al., 2004) and are thought to function redundantly with one another (Wood et al., 2006). It is known that the expression of Pvf2 is lost in sim mutants (Cho et al., 2002), which has been presumed to explain the absence of haemocytes on the midline in this mutant. Given the similar defects in haemocyte migration observed in sim and slit mutants and the fact that Sim is required for Slit expression (Nambu et al., 1990), a loss of Pvf expression in slit mutants could explain the reduction in haemocyte progression along the VNC. In order to determine whether this is the case, we investigated Pvf expression in slit mutant embryos.
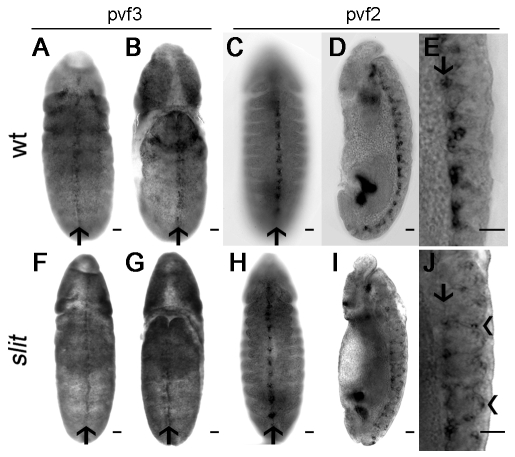
In wild-type embryos, Pvf3 is expressed along the ventral midline during stage 12 (Wood et al., 2006) and in situ hybridisations revealed that Pvf3 expression is unaffected in slit mutants (Fig. 6A,B,F,G). Pvf2 is expressed later than Pvf3, with transcripts peaking at late stage 12/early stage 13 in wild-type embryos. Consistent with a dependence on Sim for expression (Cho et al., 2002; Wood et al., 2006), Pvf2 transcripts were present along the dorsal midline of the developing VNC (Fig. 6C-E). In slit mutants, when viewed ventrally, Pvf2 expression appeared normal (Fig. 6H). However, consistent with the ventral displacement of midline glia in these mutants (Sonnenfeld and Jacobs, 1994), a subset of Pvf2-expressing cells was found at the ventral surface of the VNC (Fig. 6I,J).
Fig. 6.
Expression of Pvf ligands is not lost in slit mutants. (A,B,F,G) In situ hybridisations on stage 12 wild-type (A,B) and slit mutant (F,G) Drosophila embryos show that Pvf3 is expressed along the midline on both the ventral (A,F) and dorsal (B,G) sides in both genotypes (arrows indicate the midline). (C,H) In stage 13/14 wild-type (C) and slit mutant (H) embryos, in situ hybridisation indicates that Pvf2 is expressed at the ventral midline (arrows). (D,I) Lateral views at stage 13/14 reveal Pvf2 expression to be restricted to the dorsal side of the VNC in wild-type embryos (D), but displaced from the dorsal aspect of the VNC in slit mutants (I). (E,J) High-magnification images of the ventral surface of embryos from D,I show tight clustering of the Pvf2 signal on the dorsal aspect of the VNC (arrow) in wild types (E), whereas some Pvf2-expressing cells are displaced ventrally (arrowheads) in slit mutants (J). Anterior is up. Scale bars: 20 μm.
Haemocytes were not reduced in size in slit mutants (Fig. 3), as might be expected should their exposure to Pvfs be decreased given the proposed positive role of Pvr signalling in controlling their size (Sims et al., 2009), suggesting at best a minimal reduction in Pvf levels. To further verify that defects in haemocyte migration were not due to a subtle decrease in Pvf signals, we overexpressed UAS-Pvf2 along the ventral midline, using slit-Gal4 in a slit mutant background. There was no significant difference in haemocyte migration along the midline on the ventral surface of the VNC compared with control slit mutant embryos (Table 1). Performing the same experiment using sim-Gal4 also failed to rescue migration along the VNC (data not shown). Since increasing Pvf2 levels failed to rescue haemocyte migration, it seems that any potential reduction in Pvf levels does not contribute to this slit phenotype; instead, haemocytes are acutely sensitive to the VNC defects present in these mutants.
Table 1.
Expression of Pvf2 fails to rescue haemocyte migration defects in slit mutants
VNC defects create a physical barrier to haemocyte migration
We had anticipated that the haemocyte phenotype in slit mutants would be due to a loss of Pvf chemoattractant expression or encasement of Pvf-expressing midline cells within a collapsed axonal scaffold, rendering Pvfs inaccessible to haemocytes. However, Pvf3 expression appears normal and Pvf2 expression is present at the ventral surface of the VNC, a position presumably more exposed to haemocytes than in the wild-type situation (Fig. 6E,J). It has previously been shown that in the absence of haemocyte migration the VNC fails to develop correctly, with the clearest consequence being a failure in VNC condensation (Olofsson and Page, 2005; Martinek et al., 2008; Defaye et al., 2009). Consequently, to visualise the extracellular spaces surrounding the VNC and indirectly probe its structure, we adapted a protocol of Schwabe et al. (Schwabe et al., 2005), injecting live embryos anteriorly or posteriorly with 70 kDa rhodamine-dextran (Fig. 7A); injection at either site yielded identical results (see Fig. S3 in the supplementary material). Since Drosophila has an open circulatory system that lacks blood vessels, this extracellular space is used as a substitute conduit for blood cells. Haemocytes migrate within this extracellular interface between tissues, and injecting dextran therefore enables imaging of the locations within the embryo that are accessible to haemocytes.
Fig. 7.
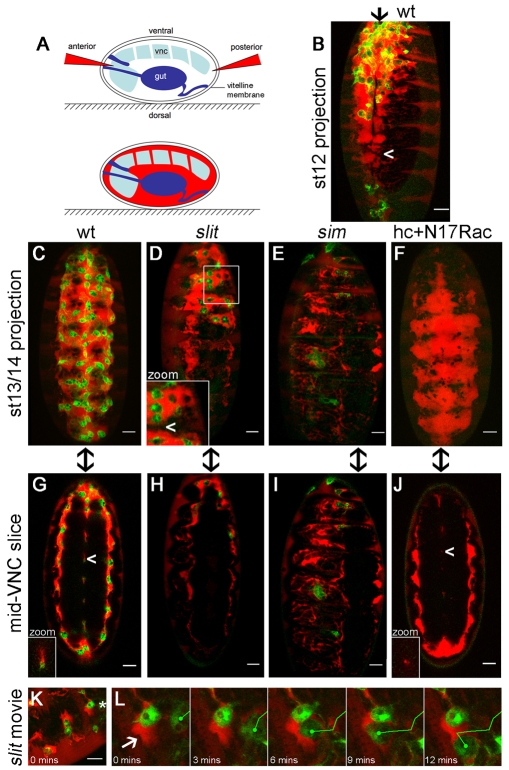
VNC defects create physical barriers to haemocyte migration. (A) Rhodamine-dextran (red) was injected into live Drosophila embryos with GFP-labelled haemocytes (green in B-L) to reveal extracellular space. Dextran was excluded from tissues such as the VNC and gut but spread between the epidermis and VNC and through dorsoventral channels in the VNC. (B,C) Dextran pooled along the ventral midline in stage 12 (B) and 13/14 (C) wild-type embryos, but was more restricted at mid-trunk segments at stage 12 (arrowhead, B). (D)By contrast, VNC-epidermal contacts created physical barriers (arrowhead in inset) to posterior movement of dextran and haemocytes in stage 13/14 slit mutants. (E) The restriction in dextran-accessible space and haemocyte progression was more severe in sim mutants. (F) Haemocytes are not responsible for VNC-epidermal separation, as dextran localised along the entire VNC at stage 13/14 when haemocyte migration was blocked by haemocyte-specific expression of N17Rac. (G-J) Single 1.5 μm confocal sections through the middle of the VNC highlight dorsoventral channels in stage 13/14 wild-type embryos (G), which are absent in slit (H) and sim mutants (I) and greatly reduced in embryos containing N17Rac-expressing haemocytes (J). Arrowheads indicate channels, which are shown at the same scale in insets (G,J). Anterior is up and arrows indicate the ventral midline in B-J. (K) Single image from Movie 7 in the supplementary material, showing the confinement of haemocytes by dextran-inaccessible barriers in the anterior of a stage 13/14 slit mutant embryo. (L) A haemocyte (asterisk in K) is deflected by a physical barrier (arrow) during the movie. Scale bars: 20 μm.
When stage 12 wild-type embryos were injected and analysed by confocal microscopy, we found that dextran pooled in a cavity between the epidermis and VNC (Fig. 3A, Fig. 7B). Therefore, it is possible that a physical barrier that excludes the diffusion of dextran to lateral positions might also prevent lateral migration until later in development. By stage 13/14, when haemocytes occupied the entire length of the ventral midline immediately beneath the epidermis (Fig. 7C), the dextran was also able to reach the lateral edges of the VNC and pass within the funnel-shaped dorsoventral channels (Fig. 7G; see Fig. S4A in the supplementary material). Analysing dextran-injected stage 13/14 slit mutant embryos, we discovered that the flow of dextran between epidermis and VNC at the midline was restricted to areas containing haemocytes (Fig. 7D) and that dorsoventral channels were absent, consistent with a collapsed VNC (Fig. 7H). z-projections showed that, unlike in wild-type embryos, areas on the midline where the dextran pools were not continuous in slit mutants, with dextran presumably accessing these regions from the lateral edges of the VNC (Fig. 7D). A physical barrier to the diffusion of dextran was even more obvious in stage 13/14 sim mutants: here, haemocytes completely failed to migrate onto the ventral surface of the VNC at the midline, correlating with a failure of dextran to pool between epidermis and VNC (Fig. 7E). Again, dorsoventral channels were absent, although dextran-accessible spaces were present at the lateral edges of the VNC (Fig. 7I), which explains why progress could be made posteriorly down this route (Fig. 5D; see Movie 5 in the supplementary material). The physical barrier to haemocyte migration was particularly obvious when haemocytes in slit mutants were subjected to live imaging following dextran injection: haemocytes were confined to rhodamine-dextran-labelled spaces between the VNC and epidermis, failing to move into areas from which dextran was excluded (Fig. 7K,L; see Movies 6 and 7 in the supplementary material). Haemocyte migration could be deflected by collisions with the boundaries between dextran-accessible and dextran-inaccessible space (Fig. 7L) and haemocytes repeatedly ‘bounced off’ these barriers before a non-constrained route was found (see Movies 6 and 7 in the supplementary material).
To determine whether haemocytes are required to create the void between epidermis and VNC into which they migrate, we expressed a dominant-negative version of the small GTPase Rac1 (N17Rac) specifically in haemocytes, which is known to block haemocyte migration from the head (Paladi and Tepass, 2004; Stramer et al., 2005). Unlike in sim mutants, dextran was able to pool between the epidermis and VNC in embryos containing N17Rac-expressing haemocytes (Fig. 7F; see Fig. S4 and Movie 8 in the supplementary material). Thus, haemocytes are not required for the separation of these two tissues during development. Interestingly, the dorsoventral channels within the VNC were of reduced diameter in the absence of haemocytes (Fig. 7J; see Fig. S4 and Movie 8 in the supplementary material), with an average luminal area per segment of 83±22 μm2 in wild types and 15±9 μm2 in N17Rac embryos (P<0.0005, Student's t-test), emphasising the importance of haemocytes to VNC morphogenesis.
Similarly, the separation of epidermis from the VNC was clear at stage 13/14 in fixed wild types and in embryos that contain N17Rac-expressing haemocytes when viewed by conventional brightfield microscopy, whereas separation was partial in slit mutants and had almost completely failed in sim mutants (see Fig. S5 in the supplementary material). Since haemocytes are not required for the separation of the VNC from the epidermis and are restricted to areas that have separated in slit mutants (Fig. 7D), the failure of these two structures to separate adequately as a consequence of defective VNC morphogenesis causes a physical barrier to haemocyte migration along the ventral midline (Fig. 8).
Fig. 8.
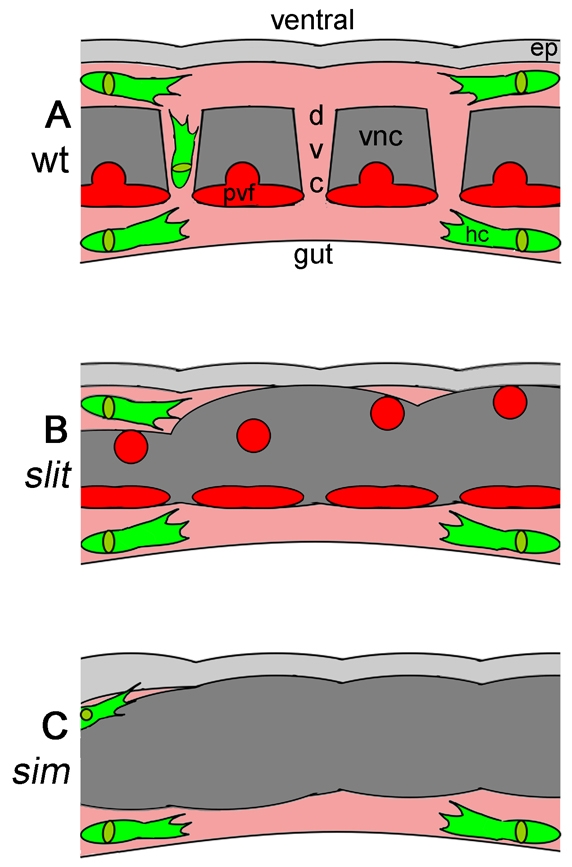
VNC-epidermal separation and haemocyte migration are developmentally coupled. (A-C) Schematic of the VNC and surrounding embryonic environment at stage 13 in wild-type (A), slit (B) and sim (C) Drosophila embryos. The embryos are viewed laterally at the midline; anterior is to the left. For normal development, the epidermis (light grey) and VNC (dark grey) must separate to enable haemocytes (green) to migrate along the VNC through dextran-accessible space (pink) by following the attractive Pvf ligands secreted by Sim-expressing midline cells (red). This allows haemocytes to reach mid-trunk segments and remodel the developing VNC (A). In slit mutants, despite the presence of Pvfs, a defective VNC results in its failure to separate from the epidermis, blocking haemocyte migration (B). Pvf expression is lost and VNC-epidermal separation defects are enhanced in sim mutants, completely preventing haemocyte progress along the ventral midline (C). ep, epidermis; dvc, dorsoventral channel; vnc, ventral nerve cord; hc, haemocyte.
The importance of haemocyte migration to VNC development is well known (Sears et al., 2003; Olofsson and Page, 2005; Defaye et al., 2009). Here, we show that a reciprocal relationship exists between haemocytes and the VNC, as defects in VNC development also prevent haemocyte migration along this structure (Fig. 8). Additionally, these studies underscore the importance of coordinating developmental events to ensure the correct morphogenesis of developing tissues.
DISCUSSION
Here we show that haemocyte migration is dependent upon the correct development of the VNC. Haemocytes fail to migrate along the VNC midline in slit and robo1,2 mutants, which have related VNC defects due to axonal pathfinding defects and glial mispositioning. However, Robo signalling is not required in haemocytes, nor are haemocytes attracted or repelled by Slit. Instead, VNC defects in these mutants result in a physical barrier to haemocyte progression down the ventral midline. Previously, it was assumed that haemocytes fail to migrate down the midline in sim mutants because of a loss of Pvf ligand expression (Cho et al., 2002; Paladi and Tepass, 2004); however, slit mutants exhibit a similar, although less severe, phenotype to sim mutants, despite maintaining Pvf2 and Pvf3 expression. Therefore, it seems that the failure of the VNC and epidermis to separate and consequently provide a suitable migratory substrate is also a crucial factor in the regulation of haemocyte migration. The failure in separation might be due to both axonal pathfinding defects and glial mispositioning, resulting in a failure of midline cells to relinquish contact with the epidermis; apoptosis of midline cells has previously been shown to contribute to separation (Page and Olofsson, 2008). Lastly, we show that in the absence of haemocyte migration along the VNC, the dorsoventral channels in this structure fail to form correctly, underlining the importance of haemocyte migration for correct morphogenesis of the VNC.
Interdependence of VNC development and haemocyte migration
The migration of haemocytes along the VNC is a key process in VNC morphogenesis (Sears et al., 2003; Olofsson and Page, 2005; Defaye et al., 2009). Conversely, perturbing VNC development blocks haemocyte migration along the midline and prevents haemocytes from reaching mid-trunk neuromeres and other potential sites of function; hence, haemocyte migration and VNC development are interdependent processes. This interdependence is particularly fascinating given that the key growth factors in haemocyte migration (Pvf2 and Pvf3) also regulate glial cell migrations within the VNC (Learte et al., 2008). Reiterative use of growth factors, such as the Pvfs, reduces the number of genes required to regulate the multitude of processes crucial for development. Furthermore, using the same genes to regulate more than one process enables temporal and spatial coupling of such processes. In this example, sim coordinates haemocyte migration and epidermis-VNC separation through the expression of genes such as slit and Pvf2.
Most VNC defects caused by failures in haemocyte migration are visible at later stages of development, but here we detect an early (stage 13/14) structural difference in the VNC, with a reduction in the diameter of the dorsoventral channels when haemocytes are prevented from leaving the head. It remains to be seen whether this phenotype is purely due to the lack of haemocytes sitting in these channels or whether haemocyte-derived matrix is required for their normal formation. The precise function of these channels is unknown, but they are lined by a specific subset of glia (Ito et al., 1995) and so presumably fulfil an important role. One potential purpose might be to facilitate haemocyte migration, enabling cues produced dorsally to diffuse through to the ventral surface.
Implications for the regulation of haemocyte migration
Several papers have focused on the role of the Pvfs in haemocyte migration along the VNC midline (Cho et al., 2002; Wood et al., 2006). Here, we demonstrate that expression of Pvfs alone is not sufficient for this process (or even required for migration along the dorsal side of the VNC) and that the integrity of the VNC is also fundamental. We cannot exclude the loss of other chemoattractants in slit and robo1,2 mutants, but the failure of the VNC to separate from the epidermis seems a more likely explanation: first, unlike sim mutants, slit mutants maintain expression of many midline genes (Sonnenfeld and Jacobs, 1994); second, the sim and slit haemocyte phenotypes are very similar, suggesting that those genes that are lost play minor roles, if any, in the regulation of this process.
Our current understanding of haemocyte migration along the VNC is as follows: haemocytes require activation of Pvr by Pvfs to direct migration into the germ band and along the VNC (Cho et al., 2002; Wood et al., 2006) and for their survival (Bruckner et al., 2004). Concurrently, the VNC must separate from the epidermis to provide space for them to move down the midline (Fig. 8A), where they can secrete matrix and remove dying glial and neuronal cells. This process is aberrant in slit mutants and haemocytes can only reach mid-trunk neuromeres by moving to the lateral edges of the VNC to bypass regions where the epidermis and VNC have failed to separate. Cell-cell repulsion between haemocytes exiting the head and germ band might also help drive haemocytes onto the attractive ‘track’ of Pvfs and matrix (derived from glia and preceding haemocytes), enabling them to spread along the developing VNC, and might also contribute to lateral migration. Other partially redundant cues might also aid haemocyte dispersal.
The regulation of cell migration by the presence or absence of physical barriers has been shown in other systems. For example, dendritic cells migrate into lymphatic vessels through ‘preformed portals’ in the basement membrane (Pflicke and Sixt, 2009). Conversely, the three-dimensional environment can physically constrain cells, inhibiting their dispersal, such that its remodelling by distinct leading cells (e.g. fibroblasts) is required before invasive migration can begin (Gaggioli et al., 2007; Wolf et al., 2007). Therefore, it seems likely that the availability or constriction of such routes is an important means to regulate cell migration.
Evolutionary perspectives
The reciprocal nature of VNC development and haemocyte migration shows us the importance of coordinating developmental events. Haemocytes are programmed to track along the VNC by following Pvf signals and to migrate into the gap between the separating VNC and epidermis at the exact time when they are needed to clear apoptotic cells (Rogulja-Ortmann et al., 2007). Vertebrate endothelial cells and hematopoietic lineages are thought to be derived from common hemangioblast precursors (Choi et al., 1998); like haemocytes, vertebrate endothelial cells respond to VEGF ligands and, additionally, to a variety of neuronal guidance cues, enabling the vasculature to closely follow the pattern of nerves (Carmeliet and Tessier-Lavigne, 2005; Mukouyama et al., 2002). Although Drosophila possess an open circulatory system, as opposed to the closed vasculature of vertebrates, it is intriguing that haemocytes use proteins that are related to those employed in vertebrate haematopoiesis (Serpent and Lozenge are members of the GATA and RUNX transcription factor families, respectively) and to those employed in the generation of a vascular network (Pvr and the Pvfs are related to VEGFRs and VEGFs, respectively) (Evans et al., 2003), particularly as haemocyte routes, similar to those of vertebrate endothelial cells, closely follow the routes of neuronal tracts. Therefore, because the control of haemocyte migration appears to have evolved in an analogous way to endothelial guidance in vertebrates, haemocytes might represent a useful model with which to study the evolution of neurovascularisation and could represent an early step in the evolution of a vascular network.
Supplementary Material
Acknowledgements
We thank Cheryll Tickle for critical reading of the manuscript; Stephanie Bunt, Delia Cosgrove, Pippa Tucker, Adrian Rogers and Sergio Simoes for technical assistance and support; and Guy Tear, Katja Bruckner, the Bloomington Stock Centre and DSHB for flies and reagents. This work was funded by the Wellcome Trust. Deposited in PMC for release after 6 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.046797/-/DC1
References
- Battye R., Stevens A., Jacobs J. R. (1999). Axon repulsion from the midline of the Drosophila CNS requires slit function. Development 126, 2475-2481 [DOI] [PubMed] [Google Scholar]
- Bruckner K., Kockel L., Duchek P., Luque C. M., Rorth P., Perrimon N. (2004). The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev. Cell 7, 73-84 [DOI] [PubMed] [Google Scholar]
- Carmeliet P., Tessier-Lavigne M. (2005). Common mechanisms of nerve and blood vessel wiring. Nature 436, 193-200 [DOI] [PubMed] [Google Scholar]
- Cho N. K., Keyes L., Johnson E., Heller J., Ryner L., Karim F., Krasnow M. A. (2002). Developmental control of blood cell migration by the Drosophila VEGF pathway. Cell 108, 865-876 [DOI] [PubMed] [Google Scholar]
- Choi K., Kennedy M., Kazarov A., Papadimitriou J. C., Keller G. (1998). A common precursor for hematopoietic and endothelial cells. Development 125, 725-732 [DOI] [PubMed] [Google Scholar]
- Defaye A., Evans I., Crozatier M., Wood W., Lemaitre B., Leulier F. (2009). Genetic ablation of Drosophila phagocytes reveals their contribution to both development and resistance to bacterial infection. J. Innate Immun. 1, 322-334 [DOI] [PubMed] [Google Scholar]
- Dickson B. J., Gilestro G. F. (2006). Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu. Rev. Cell Dev. Biol. 22, 651-675 [DOI] [PubMed] [Google Scholar]
- Dutta D., Bloor J. W., Ruiz-Gomez M., VijayRaghavan K., Kiehart D. P. (2002). Real-time imaging of morphogenetic movements in Drosophila using Gal4-UAS-driven expression of GFP fused to the actin-binding domain of moesin. Genesis 34, 146-151 [DOI] [PubMed] [Google Scholar]
- Evans C. J., Hartenstein V., Banerjee U. (2003). Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev. Cell 5, 673-690 [DOI] [PubMed] [Google Scholar]
- Evans I. R., Zanet J., Wood W., Stramer B. M. (2010). Live imaging of Drosophila melanogaster embryonic hemocyte migrations. J. Vis. Exp. 12, 1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggioli C., Hooper S., Hidalgo-Carcedo C., Grosse R., Marshall J. F., Harrington K., Sahai E. (2007). Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 9, 1392-1400 [DOI] [PubMed] [Google Scholar]
- Ito K., Urban J., Technau G. M. (1995). Distribution, classification, and development of Drosophila glial cells in the late embryonic and early larval ventral nerve cord. Roux's Arch. Dev. Biol. 204, 284-307 [DOI] [PubMed] [Google Scholar]
- Jacobs J. R. (2000). The midline glia of Drosophila: a molecular genetic model for the developmental functions of glia. Prog. Neurobiol. 62, 475-508 [DOI] [PubMed] [Google Scholar]
- Kidd T., Bland K. S., Goodman C. S. (1999). Slit is the midline repellent for the robo receptor in Drosophila. Cell 96, 785-794 [DOI] [PubMed] [Google Scholar]
- Learte A. R., Forero M. G., Hidalgo A. (2008). Gliatrophic and gliatropic roles of PVF/PVR signaling during axon guidance. Glia 56, 164-176 [DOI] [PubMed] [Google Scholar]
- Martinek N., Shahab J., Saathoff M., Ringuette M. (2008). Haemocyte-derived SPARC is required for collagen-IV-dependent stability of basal laminae in Drosophila embryos. J. Cell Sci. 121, 1671-1680 [DOI] [PubMed] [Google Scholar]
- Mukouyama Y. S., Shin D., Britsch S., Taniguchi M., Anderson D. J. (2002). Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 109, 693-705 [DOI] [PubMed] [Google Scholar]
- Nambu J. R., Franks R. G., Hu S., Crews S. T. (1990). The single-minded gene of Drosophila is required for the expression of genes important for the development of CNS midline cells. Cell 63, 63-75 [DOI] [PubMed] [Google Scholar]
- Nambu J. R., Lewis J. O., Wharton K. A., Jr, Crews S. T. (1991). The Drosophila single-minded gene encodes a helix-loop-helix protein that acts as a master regulator of CNS midline development. Cell 67, 1157-1167 [DOI] [PubMed] [Google Scholar]
- Olofsson B., Page D. T. (2005). Condensation of the central nervous system in embryonic Drosophila is inhibited by blocking hemocyte migration or neural activity. Dev. Biol. 279, 233-243 [DOI] [PubMed] [Google Scholar]
- Page D. T., Olofsson B. (2008). Multiple roles for apoptosis facilitating condensation of the Drosophila ventral nerve cord. Genesis 46, 61-68 [DOI] [PubMed] [Google Scholar]
- Paladi M., Tepass U. (2004). Function of Rho GTPases in embryonic blood cell migration in Drosophila. J. Cell Sci. 117, 6313-6326 [DOI] [PubMed] [Google Scholar]
- Pflicke H., Sixt M. (2009). Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J. Exp. Med. 206, 2925-2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S., Vivancos V., Nicolas E., Dickson B. J. (2000). Selecting a longitudinal pathway: Robo receptors specify the lateral position of axons in the Drosophila CNS. Cell 103, 1033-1045 [DOI] [PubMed] [Google Scholar]
- Rogulja-Ortmann A., Luer K., Seibert J., Rickert C., Technau G. M. (2007). Programmed cell death in the embryonic central nervous system of Drosophila melanogaster. Development 134, 105-116 [DOI] [PubMed] [Google Scholar]
- Schwabe T., Bainton R. J., Fetter R. D., Heberlein U., Gaul U. (2005). GPCR signaling is required for blood-brain barrier formation in Drosophila. Cell 123, 133-144 [DOI] [PubMed] [Google Scholar]
- Sears H. C., Kennedy C. J., Garrity P. A. (2003). Macrophage-mediated corpse engulfment is required for normal Drosophila CNS morphogenesis. Development 130, 3557-3565 [DOI] [PubMed] [Google Scholar]
- Simpson J. H., Bland K. S., Fetter R. D., Goodman C. S. (2000). Short-range and long-range guidance by Slit and its Robo receptors: a combinatorial code of Robo receptors controls lateral position. Cell 103, 1019-1032 [DOI] [PubMed] [Google Scholar]
- Sims D., Duchek P., Baum B. (2009). PDGF/VEGF signaling controls cell size in Drosophila. Genome Biol. 10, R20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenfeld M. J., Jacobs J. R. (1994). Mesectodermal cell fate analysis in Drosophila midline mutants. Mech. Dev. 46, 3-13 [DOI] [PubMed] [Google Scholar]
- Stramer B., Wood W., Galko M. J., Redd M. J., Jacinto A., Parkhurst S. M., Martin P. (2005). Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J. Cell Biol. 168, 567-573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tear G., Harris R., Sutaria S., Kilomanski K., Goodman C. S., Seeger M. A. (1996). commissureless controls growth cone guidance across the CNS midline in Drosophila and encodes a novel membrane protein. Neuron 16, 501-514 [DOI] [PubMed] [Google Scholar]
- Tepass U., Fessler L. I., Aziz A., Hartenstein V. (1994). Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development 120, 1829-1837 [DOI] [PubMed] [Google Scholar]
- Thomas J. B., Crews S. T., Goodman C. S. (1988). Molecular genetics of the single-minded locus: a gene involved in the development of the Drosophila nervous system. Cell 52, 133-141 [DOI] [PubMed] [Google Scholar]
- Wolf K., Wu Y. I., Liu Y., Geiger J., Tam E., Overall C., Stack M. S., Friedl P. (2007). Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat. Cell Biol. 9, 893-904 [DOI] [PubMed] [Google Scholar]
- Wood W., Jacinto A. (2005). Imaging cell movement during dorsal closure in Drosophila embryos. Methods Mol. Biol. 294, 203-210 [DOI] [PubMed] [Google Scholar]
- Wood W., Jacinto A. (2007). Drosophila melanogaster embryonic haemocytes: masters of multitasking. Nat. Rev. Mol. Cell Biol. 8, 542-551 [DOI] [PubMed] [Google Scholar]
- Wood W., Faria C., Jacinto A. (2006). Distinct mechanisms regulate hemocyte chemotaxis during development and wound healing in Drosophila melanogaster. J. Cell Biol. 173, 405-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Y., Feng L., Park H. T., Havlioglu N., Wen L., Tang H., Bacon K. B., Jiang Z., Zhang X., Rao Y. (2001). The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature 410, 948-952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanet J., Stramer B., Millard T., Martin P., Payre F., Plaza S. (2009). Fascin is required for blood cell migration during Drosophila embryogenesis. Development 136, 2557-2565 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.