Abstract
Terminal differentiation of male germ cells in Drosophila and mammals requires extensive cytoarchitectural remodeling, the elimination of many organelles, and a large reduction in cell volume. The associated process, termed spermatid individualization, is facilitated by the apoptotic machinery, including caspases, but does not result in cell death. From a screen for genes defective in caspase activation in this system, we isolated a novel F-box protein, which we termed Nutcracker, that is strictly required for caspase activation and sperm differentiation. Nutcracker interacts through its F-box domain with members of a Cullin-1-based ubiquitin ligase complex (SCF): Cullin-1 and SkpA. This ubiquitin ligase does not regulate the stability of the caspase inhibitors DIAP1 and DIAP2, but physically binds Bruce, a BIR-containing giant protein involved in apoptosis regulation. Furthermore, nutcracker mutants disrupt proteasome activity without affecting their distribution. These findings define a new SCF complex required for caspase activation during sperm differentiation and highlight the role of regulated proteolysis during this process.
Keywords: Caspase, SCF, Ubiquitin, IAP, Proteasome, Spermatogenesis, Drosophila
INTRODUCTION
Caspases are a family of cysteine proteases that are responsible for executing apoptosis, a form of programmed cell death that is essential for metazoan development and organismal homeostasis (Abraham and Shaham, 2004; Steller, 1995; Steller, 2008). Because of the inherent destructive function of these proteases, caspase activities are tightly regulated by both activators and inhibitors. Abnormal regulation of caspases is a hallmark of several diseases, including many types of cancer (Hanahan and Weinberg, 2000; Reed, 2003; Thompson, 1995; Vucic, 2008). The first level of caspase regulation is intrinsic to caspases themselves. Caspases are expressed as inactive zymogens that are cleaved to form the active enzyme, a step that is highly regulated, as there are multiple signaling pathways that govern their activation (Kornbluth and White, 2005). Furthermore, once caspases are activated by cleavage, their activity is held in check by a family of inhibitors, called inhibitor of apoptosis proteins (IAPs) (Deveraux and Reed, 1999; Salvesen and Duckett, 2002). These conserved proteins bind the active site of caspases through their baculovirus inhibitory repeat (BIR) domain, and only when they are removed can caspase substrates gain access to the protease (Hinds et al., 1999).
Post-translational modifications are another way by which caspases are regulated. The balance between pro- and anti-apoptotic factors is controlled by ubiquitin-dependent degradation, as well as by non-degradative ubiquitin modifications (Bader and Steller, 2009; Bergmann, 2010; Broemer and Meier, 2009; Vaux and Silke, 2005; Zhong and Belote, 2007). Some IAPs contain a RING domain that can act as a ubiquitin ligase, and the balance between their self- and caspase ubiquitylation is a major determinant of their activity (Ditzel et al., 2008; Lisi et al., 2000; Ryoo et al., 2002; Schile et al., 2008; Wilson et al., 2002; Yang et al., 2000).
An added level of complexity to caspase regulation is their non-apoptotic role in certain cells. Caspases are involved in diverse vital processes, including cellular signaling, differentiation and remodeling (Feinstein-Rotkopf and Arama, 2009; Kuranaga and Miura, 2007; Yi and Yuan, 2009). This type of activity requires very sensitive and nuanced control, as unrestrained caspase activity is destructive. For example, non-lethal caspase activity facilitates axonal branch removal during normal neuronal development in flies and mammals (Kuo et al., 2006; Nikolaev et al., 2009; Williams et al., 2006). However, it is thought to contribute to neurodegeneration when misregulated in Alzheimer's disease (Nikolaev et al., 2009).
In Drosophila, non-apoptotic caspase activation occurs at the final stage of sperm differentiation, also called individualization (Arama et al., 2003; Arama et al., 2007; Arama et al., 2006; Muro et al., 2006). This process is reminiscent of apoptosis in the sense that most of the cytoplasmic contents are exterminated to create highly motile sperm (Fuller, 1993; Tokuyasu et al., 1972) (Fig. 1A). Apoptotic regulators, such as Cytochrome c, Ark (the Drosophila homolog of Apaf-1), the initiator caspase Dronc (Nedd2-like caspase – FlyBase), and the IAP antagonist Hid (Wrinkled – FlyBase) have all been implicated in this process, suggesting that the basic molecular mechanisms of caspase activation are also conserved between this process and conventional apoptosis (Arama et al., 2003; Arama et al., 2006; Huh et al., 2004). A screen for genes that regulate caspase activation in this system uncovered a novel Cullin-3-based complex (Arama et al., 2007). Significantly, Cullin-3 was also recently implicated in the regulation of caspase-8 activation in mammals (Jin et al., 2009). Therefore, the isolation of additional genes from this screen is a promising avenue of research to identify novel players and/or pathways involved in the regulation of caspase activation.
Fig. 1.
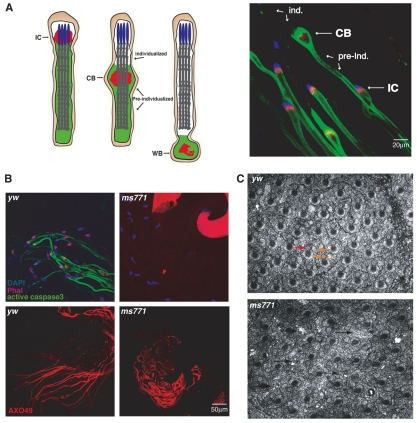
ms771 is a male-sterile mutant defective in caspase activity during sperm differentiation. (A) Diagram (left) and immunostaining (right) of spermatid individualization in Drosophila. At the final stage of differentiation, an actin-based individualization complex (IC) is formed around the elongated nuclei of 64 spermatids that are connected by cytoplasmic bridges. As the IC moves down the length of the spermatids, it expels the excess cytoplasm and unneeded organelles, leaving each spermatid engulfed in its own membrane. During this movement, the excess material accumulates around the IC to create the cystic bulge (CB). When the complex reaches the end of the tails, the CB turns into the waste bag (WB), which eventually degrades. In the right-hand panel, confocal images of several wild-type (yw) cysts illustrate the individualization (ind.) process. The cysts were stained with DAPI (nuclei, blue), phalloidin (IC, red) and for cleaved Caspase-3 (cytoplasm, green). (B) In contrast to cysts from yw testes, cysts from ms771 homozygotes do not stain for cleaved Caspase-3. The nuclei (DAPI stained) in the mutant elongate, but the IC does not form. Like wild-type cysts, ms771 cysts stain positively with AXO 49 antibody, a late differentiation marker, indicating that these deficiencies are not caused by a global differentiation defect. (C) Electron micrographs of cysts during individualization. Each spermatid within the cyst contains an axoneme (red arrow) and two mitochondrial derivatives (smaller and larger round structures, orange arrows). The ms771 mutant displays normal formation of these structures, but the space between the spermatids indicates that the overall cyst structure preceding individualization is defective (black arrows).
Here, we describe a new ubiquitin ligase complex that regulates caspase activity during sperm individualization. From the genetic screen, we isolated an F-box protein, which we called Nutcracker, that shares some sequence similarity with the mammalian FBXO7 protein. Flies mutant for nutcracker are viable but male sterile, displaying elongated spermatids, severe individualization defects and an absence of activated caspases. We further show that Nutcracker physically interacts with SkpA and Cullin-1 (Lin-19-like – FlyBase), which are two main components of a Skp/Cullin/F-box (SCF) ubiquitin ligase complex, and that the F-box domain of Nutcracker is important for both complex binding and caspase activation. Nutcracker can also associate with Bruce, a giant BIR and UBC domain-containing IAP-like protein, indicating a direct link between this SCF complex and the apoptotic machinery. Finally, we show that loss of nutcracker function causes reduced proteasome activity without affecting their distribution or numbers. These findings are the first to demonstrate the role of an SCF ubiquitin ligase complex in caspase activation and proteasome regulation, and they suggest that the involvement of controlled proteolysis in caspase activation is broader than has been previously appreciated.
MATERIALS AND METHODS
Fly strains
yw flies were used as wild-type controls. The Zuker mutant Z3-4692 (ms771) was obtained from C. S. Zuker (UCSD); alpha6T;Alpha6T-GFP was obtained from John Belote (Syracuse University); the deficiency lines Df(3L)HR119 and Df(3L)GN34 and the PBac insertion PBac{WH}CG10855f07259 from the Bloomington Stock Center; and Df(3L)Exel6097 from Exelixis.
Genetic screen of the Zuker male-sterile collection lines
For technical details of the screen, see Arama et al., supplementary 4 (Arama et al., 2006).
Electron microscopy
Testes were sectioned and imaged as described (Arama et al., 2006).
Molecular biology
Drosophila Genomics Resource Center (DGRC) clone LD12948 was used to amplify the CG10855 (nutcracker) ORF. For rescuing the nutcrackerms771 mutant phenotypes, the CG10855 ORF was amplified by PCR (primers 1 and 2, see Table S1 in the supplementary material) and cloned into pHSP83(5′-3′UTRs) (Arama et al., 2006), which contained the 5′ and 3′ UTRs of Cytochrome c distal (Cyt-c-d). For antibody generation, the CG10855 ORF was amplified by PCR (primers 3 and 4) and cloned into pET41a(+) (Novagen), which contains an N-terminal GST tag. For antibody affinity purification, the CG10855 ORF was also cloned into pET101/D-TOPO (Invitrogen) (primers 5 and 6), which contains a C-terminal His tag. For testes expression of tagged proteins, a Protein A (PrA) tag was cloned into Casper-4 containing the don juan (DJ) promoter (primers 7 and 8), and full-length CG10855 was cloned in frame with it (primers 9 and 10). ΔFbox was created by PCR amplification of CG10855 without the last 174 nucleotides, and this product was also cloned in frame into Casper-4-DJ-PrA (primers 9 and 11). The construction of the Bruce ‘mini-gene’ was as detailed (Arama et al., 2007).
Antibody generation and tissue staining
Cleaved effector caspase antibody staining of young (0- to 2-day-old) adult testes was carried out as described (Arama et al., 2007), using a rabbit polyclonal anti-cleaved Caspase-3 (Asp175) antibody (Cell Signaling Technology) diluted 1:75. The only changes were that the subsequent TRITC-phalloidin (Sigma) incubation for staining of actin filaments was carried out during incubation with the secondary antibody, and that the slides were subsequently rinsed twice for 10 minutes each in PBS. Axonemal tubulin polyglycylation antibody staining was carried out using the mouse polyclonal antibody AXO 49 (a kind gift from Marie-Helene Bre, University of Paris-Sud, France) diluted 1:5000. Anti-CG10855 was created by injecting guinea-pig with full-length recombinant CG10855 (Cocalico Biologicals). CG10855 staining was carried out as described (Hime et al., 1996) using a 1:100 dilution of affinity-purified serum. The serum was purified by expressing a C-terminal His-tagged CG10855 in BL21 E. coli for 1.5 hours at 37°C. The bacterial lysate was run on SDS-PAGE, transferred onto an Immobilon-P membrane (Millipore) and blocked in 5% dried milk powder in PBST (0.1% Triton X-100 in PBS). Serum (500 μl) was pipetted onto the membrane and incubated for 3 hours, and, once removed, the purified antibody was eluted by 50 mM glycine (pH 2.5); the antibody was then neutralized with 0.1 M Tris (pH 9.0).
Genomic DNA Isolation and sequencing of the mutant alleles
Genomic DNA was isolated as described (Arama et al., 2007). This DNA (20 ng) was used to amplify CG10855 with primers corresponding to its 5′ and 3′ UTRs (primers 12-15, see Table S1 in the supplementary material). PCR reactions were carried out using DyNAzyme EXT DNA polymerase (NEB) according to the manufacturer's protocol. The products were purified using the High Pure PCR Product Purification Kit (Roche), concentrated by evaporation, and sequenced in a GENEWIZ sequencing facility.
RNA isolation and RT-PCR
Total RNA isolation and RT-PCR were conducted as described (Arama et al., 2007). Primers corresponding to the 5′ and 3′ UTRs (primers 12 and 13, see Table S1 in the supplementary material) were used to amplify CG10855 mRNA.
Western blot
To create testes lysate, testes were dissected in lysis buffer (20 mM Hepes pH 7.6, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM EDTA, 1 mM DTT), homogenized and spun at 14,000 rpm (20,800 g) for 15 minutes at 4°C. Protein concentration was determined by Bradford assay (BioRad), and 30-50 μg total protein, dissolved in SDS loading buffer, was separated by SDS-PAGE. After transfer, the Immobilon-P membrane was blocked with 5% dried milk powder in TBST (0.1% Tween 20 in PBS) for 1 hour, and incubated with primary antibody overnight at 4°C. The membrane was then washed three times in TBST, incubated in HRP-conjugated secondary antibody for 1 hour, and washed three more times with TBST before developing with ECL reagents (Amersham) and exposure to Kodak Biomax MR film. The following antibodies were used at a 1:1000 dilution: anti-Nutcracker (serum), anti-Cullin-1 (Zymed), anti-SkpA (a kind gift from T. Murphy, Carnegie Institution of Washington, Baltimore, MD, USA), anti-DIAP1 (a kind gift from H. D. Ryoo, NYU Medical School, NYC, NY, USA), anti-DIAP2 (a kind gift from P. Meier, Institute of Cancer Research, London, UK) and anti-Bruce (Arama et al., 2007). Anti-Alpha7 (Biomol) was used at 1:200.
Immunoprecipitations
For testes immunoprecipitation (IP), testes were dissected in IP buffer (50 mM Hepes pH 7.5, 150 mM NaCl, 15 mM MgCl2, 10% glycerol, 1% Triton X-100, 1 mM EDTA, 1 mM DTT), lysed (as for western blot), and equal amounts of protein were incubated for 2 hours at 4°C with Dynabeads (Invitrogen) that had been conjugated to rabbit IgG according to the manufacturer's protocol. The beads were washed five times in lysis buffer (except that detergent was reduced to 0.1%), and eluted in SDS loading buffer by boiling.
Proteasome activity assay
Testes were lysed as for western blot. Protein concentration was measured and equal amounts of lysates were added at a 1:1 volume ratio to the Proteasome-Glo Proteasome Activity Detection Kit (Promega). The assay was carried out according to the manufacturer's protocol. All reactions were conducted in a 96-well plate and read on a SpectraMax M2 micro-plate reader (Molecular Devices).
RESULTS
A male-sterile mutant defective in caspase activation during sperm individualization
In order to identify novel genes involved in caspase activation during sperm differentiation, we conducted a screen for genes that control caspase activation in this system, as described (Arama et al., 2007). In brief, ~850 fly lines from the Zuker stock collection that were previously shown to have individualization defects (Wakimoto et al., 2004) were screened for a lack of caspase activation in spermatids using anti-cleaved Caspase-3 antibody (Arama et al., 2003). This antibody detects the activity of Drosophila effector caspases such as Dcp-1 and Drice (Ice – FlyBase), but is also a marker for Caspase-9-like Dronc (nc – FlyBase) activity (Fan and Bergmann, 2009). Several complementation groups/genes were identified that displayed complete loss of caspase activation. To test whether the block in caspase activation is direct and not a consequence of premature developmental arrest of these mutant spermatids, we stained testes from these lines with the AXO 49 antibody, a late developmental marker that detects polyglycylation of axonemal tubulin, a modification that accumulates at the onset of individualization (Arama et al., 2007; Bre et al., 1996; Bre et al., 1998; Rogowski et al., 2009). One of these cleaved caspase-negative and polyglycylation-positive mutants, which is represented in this screen by a single allele termed ms771, was homozygote viable with no gross defects apart from male sterility (Fig. 1B). Consistent with the idea that this mutant is specific for caspase activation, ultrastructural analysis portrayed morphologically intact mitochondria and axoneme, although some vacuolar structures were detected, indicating individualization defects (Fig. 1C).
The ms771 mutant maps to an uncharacterized F-box protein, nutcracker
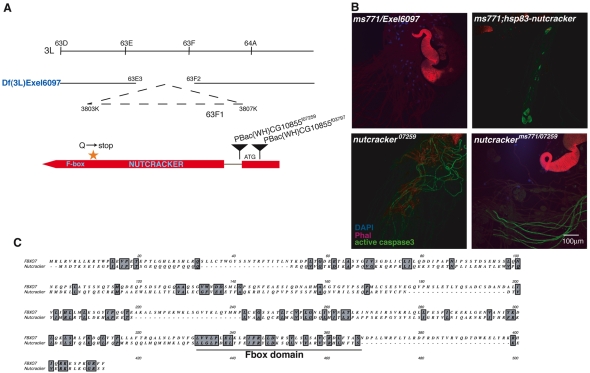
To genetically map ms771, the deficiency ‘kit’ of large genomic deletion lines was screened for deletions that fail to complement its sterility. Two overlapping lines, Df(3L)HR119 and Df(3L)GN34, corresponding to regions 63C2;63F7 and 63E8-9;64A8-9, respectively, failed to complement the sterility phenotype of ms771, and the transheterozygotes stained negative for active Caspase-3. A smaller deficiency in the overlapping region, Df(3L)Exel6097 (Fig. 2A), also failed to complement sterility and caspase staining (Fig. 2B). This deficiency deletes 15 genes, and the coding regions of several of them were PCR amplified from ms771 mutants and sequenced. A premature stop codon mutation was identified in a novel gene (CG10855) that encodes a putative F-box protein. This uncharacterized F-box protein displays limited amino acid conservation with the mammalian F-box protein FBXO7, and in both proteins the F-box domain is uncommonly close to the C-terminus (Fig. 2C). We termed CG10855 nutcracker, for ‘novel ubiquitin targeting complex required for activating caspases’.
Fig. 2.
ms771 maps to nutcracker (CG10855), which encodes an uncharacterized F-box protein expressed in testes. (A) nutcracker genomic region. nutcracker is located at position 63F1, within the region removed by the deficiency Df(3L)Exel6097. Two piggyback insertions (inverted triangles) are annotated for nutcracker: PBac{WH}CG10855f03797 is inserted upstream of the ATG start site, and PBac{WH}CG1085507259 (nutcracker07259) is inserted in the intronic region. An orange star indicates the location of the premature stop codon mutation in nutcrackerms771 that results in the truncation of the F-box domain. (B) The ms771 mutation maps to nutcracker. The deficiency Df(3L)Exel6097 fails to complement ms771 sterility and staining, and both these defects are rescued by reintroducing nutcracker ORF. nutcracker07259 also has individualization defects and is hypomorphic for Caspase-3 staining. The staining is slightly reduced, but not eliminated, in the transheterozygote nutcrackerms771/07259. (C) The F-box protein Nutcracker shares sequence homology with the F-box-only protein FBXO7. Alignment of the Drosophila Nutcracker protein against human FBXO7 (accession number CAG30377). The proteins exhibit highest similarity in their F-box domains. Overall, they share 17 identical amino acids (5% of Nutcracker, 2% of FBXO7), and 36 similar amino acids (11% of Nutcracker, 7% of FBXO7). Not shown are the last ~100 amino acids of FBXO7, as it is a longer protein.
We next looked for possible transposon-derived mutations in nutcracker and identified a piggyBac insertion in the intron of nutcracker (nutcracker07259), which was obtained from the Bloomington Stock Center (Fig. 2A). Complementation analysis with the nutcrackerms771 mutant resulted in a failure of nutcracker07259 to complement the male sterility of the former, indicating that nutcracker07259 is indeed an allele of nutcracker. Staining of testes from the nutcracker07259 mutants revealed individualization defects, although they still exhibited some level of active caspases, suggesting that it is a weak allele of nutcracker (Fig. 2B). Finally, transgenic expression of an intact nutcracker gene using the testis-specific promoter of the Hsp83 gene restored proper caspase activation, spermatid individualization and fertility in both nutcracker mutants, indicating that all the male sterility-associated phenotypes are due to mutations in nutcracker (Fig. 2B).
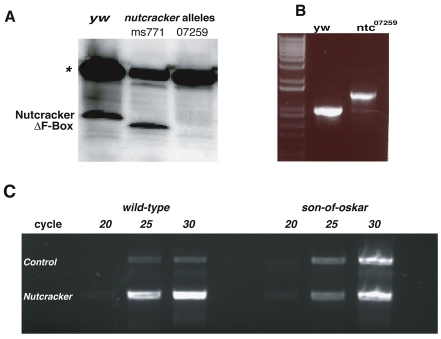
We also generated an antibody to full-length Nutcracker. A band of ~35 kDa, corresponding to the predicted size of Nutcracker, was detected in testes lysates by western blotting (Fig. 3A). By contrast, a smaller band was detected in lysates from nutcrackerms771 testes, consistent with the finding that this allele contains a premature stop codon that deletes the entire F-box domain (Fig. 3A). The fact that this stable, but truncated, protein still displays the mutant phenotypes suggests that the F-box domain is important for the physiological role of Nutcracker in sperm differentiation.
Fig. 3.
ms771 harbors a truncated Nutcracker protein. (A) Western blot of testes lysates using the Nutcracker antibody detects a band of the predicted size (~35 kDa). Analysis of testes lysates taken from the mutant flies indicate that whereas nutcrackerms771 (ms771) harbors a truncated form of Nutcracker protein, the nutcracker07259 (07259) mutant is a protein null. The asterisk indicates a non-specific band that serves as a loading control. (B) RT-PCR analysis of nutcracker mRNA transcripts collected from wild-type (yw) or nutcracker07259 (ntc07259) adult testes. Compared with the transcript of predicted size found in wild-type testis, the nutcracker07259 transcript contains a ~600 nt insertion. Sequencing analysis of the corresponding band revealed that this insertion is located in the middle of the transcript, resulting in a frame shift in the ORF. (C) nutcracker is expressed in the germ line. Semi-quantitative RT-PCR of nutcracker mRNA using total mRNA taken from either wild-type or son-of-oskar males. More transcripts were detected in the wild type, indicating that it is preferentially expressed in testes.
Analysis of nutcracker07259 mRNA by RT-PCR revealed a 600 bp insertion that causes a frame shift, which was likely to be a result of failed splicing due to the transposon insertion (Fig. 2A, Fig. 3B). This mutation results in complete elimination of Nutcracker protein as detected by western analysis (Fig. 3A). However, because this allele behaves as a genetic hypomorph, it is likely that a small amount of wild-type protein is still produced, but at levels below western blot detection.
To further characterize nutcracker expression, we first examined its mRNA distribution. We extracted mRNA from either wild-type or son-of-oskar [males born to oskar mutant females (Lehmann and Nusslein-Volhard, 1986; Arama et al., 2007)] males, which lack germ cells and functioning testes, and compared nutcracker levels by semi-quantitative RT-PCR. This analysis revealed that more mRNA is found in the wild-type animal (cycle 25, Fig. 3C), indicating that nutcracker mRNA is preferentially expressed in testes.
Nutcracker is part of an SCF ubiquitin ligase complex
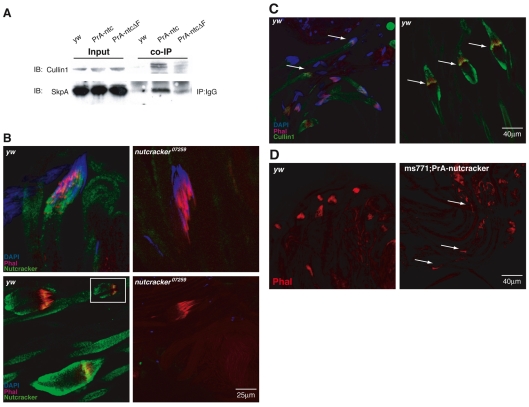
F-box-containing proteins can function as substrate-binding adaptors in SCF ubiquitin ligase complexes, which mediate the ubiquitylation of substrate proteins, thus targeting them for degradation by the 26S proteasome (Cardozo and Pagano, 2004; Kipreos and Pagano, 2000). These complexes comprise an Skp protein that links the F-box protein to the Cullin-1 scaffolding protein. To examine whether Nutcracker is in complex with other SCF members in the testis, we tagged Nutcracker with Protein A (PrA) and placed them downstream of the don juan (DJ) promoter and 5′ UTR and upstream of the Cyt-c-d 3′ UTR (DJ-PrA-ntc), which are specifically and highly expressed in spermatids (Arama et al., 2006; Santel et al., 1998; Santel et al., 1997). Transgenic fly lines were generated and checked for expression by western blotting (not shown). These transgenes restored caspase staining, but failed to rescue the sterility phenotype of the nutcracker mutants, possibly owing to misexpression of this construct or domain obstruction by the PrA tag. Co-immunoprecipitation (co-IP) experiments using lysates of testes from these transgenic flies showed that PrA-ntc specifically binds both endogenous SkpA and Cullin-1, suggesting that Nutcracker functions in an SCF complex (Fig. 4A). To investigate the role of the F-box domain in mediating this interaction, we also constructed a truncated form of PrA-tagged Nutcracker by deleting the F-box domain (PrA-ntcΔF), similar to the stop codon mutation in nutcrackerms771. Unlike full-length Nutcracker, PrA-ntcΔF was unable to enrich Cullin-1 or SkpA in co-IP experiments (Fig. 4A). These results confirm that Nutcracker forms an SCF complex that is mediated through its F-box domain, and raises the possibility that the phenotypes observed in nutcrackerms771 are caused by the inability to form this complex.
Fig. 4.
Nutcracker and Cullin-1 form an SCF complex and colocalize with actin at the individualization complex. (A) The F-box protein Nutcracker co-immunoprecipitates (co-IPs) with members of the SCF complex. Protein A (PrA)-tagged Nutcracker (PrA-ntc) was expressed in testes and its endogenous interacting partners were identified by western blot. Co-IP followed by western blot analysis using either Cullin-1 or SkpA antibodies demonstrates that Nutcracker binds both Cullin-1 and SkpA, which are both part of the SCF ubiquitin ligase (the middle lane of the co-IP). The interaction is hindered by the removal of the F-box domain (PrA-ntcΔF), suggesting that Nutcracker is part of this complex (right-hand lane of the co-IP). The input panel indicates that equal amounts of either SkpA or Cullin-1 were present in the lysate at the beginning of the experiment. (B) Nutcracker antibody staining (green). As the IC forms around the elongated nuclei, Nutcracker protein accumulates around it (upper left). After the IC begin to move, Nutcracker localizes around the bulge, intertwined within the complex, as can be seen in the two planes shown (lower left). The staining completely disappears in nutcracker07259, which occasionally displays formed (yet defective) ICs (right-hand panels). The ICs are stained with phalloidin (red), and the nuclei are stained with DAPI (blue). (C) Cullin-1 staining (green) of yw testes showing similar staining pattern to Nutcracker (arrows point to the staining around the nuclei in the left panel, and to the staining around the IC in the right panel). (D) Nutcracker misexpression affects IC integrity. DJ-PrA-ntc was expressed in the nutcrackerms771 background. The DJ promoter drives high levels of expression at later stages of differentiation, so Nutcracker is overexpressed and mistimed. This misexpression does not rescue sterility, but does restore some cleaved Caspase-3 staining (not shown). The IC, which does not form in nutcrackerms771, does form in this background (phalloidin, red), but its formation is altered and the actin cones are scattered (white arrows).
Nutcracker and Cullin-1 colocalize with actin cones at the individualization complex
At the onset of individualization, actin organizes into cone structures that form around the elongated nuclei to create the individualization complex (IC) (Fig. 1A) (Noguchi and Miller, 2003). As the IC moves, it expels and ‘pushes out’ most of the cytoplasm and unneeded organelles, resulting in the formation of an inflated structure called the cystic bulge (CB) (Fuller, 1993). We visualized Nutcracker localization in the context of the morphological events during individualization. For immunostaining, we affinity purified Nutcracker serum and used it to stain testes (Fig. 4B). Nutcracker partially colocalized with the forming complex, although they did not completely overlap. As the complex moved, Nutcracker staining was detected within the CB, but the most prominent staining was observed around the outer layer of the IC itself, at the base of the cones. Various focal planes of the CB showed that the majority of the protein accumulates around the outer borders of the IC, and only slightly intertwines between the cones. This indicates that the protein might be anchored in the vicinity of the sites of cone attachment to the outer membrane. We also stained testes from nutcracker07279 flies, which failed to display a protein band by western blot. Similarly, we were unable to detect protein by immunostaining (Fig. 4B), demonstrating the specificity of the antibody in detecting Nutcracker.
We investigated whether this localization of Nutcracker corresponded to the localization of the SCF complex. The pattern of Cullin-1 staining at ICs in yw testes was similar to that of Nutcracker (Fig. 4C), placing these proteins in the same vicinity on the IC. Together with the biochemical data, this strongly suggests their physical interaction in vivo and their combined role in individualization.
nutcracker mutants display defects in individualization complex formation
nutcrackerms771 mutant testes are defective in IC formation, as no actin cone formation is observed around the elongated nuclei (Fig. 1B). When Nutcracker is misexpressed in this background, as in the DJ-PrA-ntc transgene, these phenotypes are partially rescued: flies displayed cleaved Caspase-3 staining and some formed ICs at the tip of the cyst (Fig. 4D). However, these ICs appeared irregular, were mostly scattered and, in some cases, individual actin cones could be observed scattered along the length of the cyst. This might explain why sterility is not rescued, and indicates that the timing and level of Nutcracker expression are important for IC integrity. Furthermore, in the testes of the hypomorphic allele nutcracker07259, which stain positive for cleaved Caspase-3, some irregular ICs were observed (Fig. 4B), further suggesting that actin filament formation and organization and caspase activation are sensitive to the levels of Nutcracker.
Nutcracker physically interacts with Bruce, a giant IAP-like protein
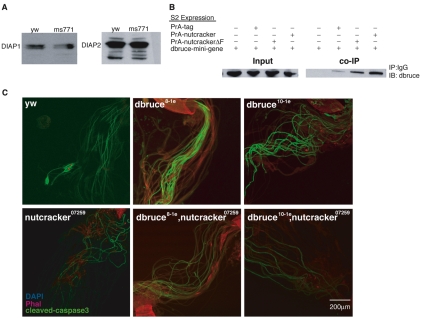
In Drosophila, two IAPs have been shown to directly inhibit active caspases: DIAP1 and DIAP2 (Thread and Inhibitor of apoptosis 2 – FlyBase) (Hay et al., 1995); however, only DIAP1 is strictly needed for controlling caspases in vivo (Goyal et al., 2000; Lisi et al., 2000). IAPs are regulated by ubiquitin-mediated degradation, which removes them from caspases, thus allowing proteolysis (Ryoo et al., 2002; Wilson et al., 2002). We examined whether a Nutcracker-containing SCF ubiquitin ligase mediates the degradation of these proteins. We checked steady-state levels of DIAP1 protein in nutcrackerms771 mutants by western blot and found no differences with the wild type, suggesting that Nutcracker does not regulate DIAP1 stability (Fig. 5A). Furthermore, co-IP experiments using testes lysates, and those conducted by co-expressing tagged DIAP1 and Nutcracker proteins in S2 cells, failed to detect an interaction between these proteins (data not shown). We also checked DIAP2, which is able to inhibit caspases in vitro (Ribeiro et al., 2007) and has a role in Drosophila immunity (Kleino et al., 2005). The levels of this inhibitor were also unchanged in nutcrackerms771 mutants (Fig. 5A). These results suggest that Nutcracker regulates caspase activation independently of the known direct inhibitors of caspases.
Fig. 5.
Nutcracker physically interacts with Bruce, a giant IAP-like protein. (A) Western blots of total protein lysates from yw or nutcrackerms771 testes using either DIAP1 or DIAP2 antibodies indicate that Nutcracker does not affect the levels of these direct caspase inhibitors. (B) The F-box protein Nutcracker physically binds Bruce. PrA, PrA-ntc or PrA-ntcΔF were expressed in S2 cells together with the Bruce ‘mini-gene’ construct. Western blot analysis of this co-IP experiment revealed that Bruce binds both full-length and truncated Nutcracker, suggesting that Bruce is an interacting protein but that its interaction with Nutcracker is independent of the SCF complex. (C) Genetic interaction between Bruce and nutcracker. Caspase staining (green) of the hypomorphic allele nutcracker07259 is unchanged in double mutants with Bruce8-1e or Bruce10-1e.
Next, we investigated another IAP, Bruce, which has a known role in spermatogenesis (Arama et al., 2003) and has been shown to inhibit apoptosis induced by Reaper (Vernooy et al., 2002). Bruce is a large (~500 kDa) protein that contains both a BIR domain and the UBC domain found in E2 conjugating enzymes. Mutations in Bruce are male sterile and cause nuclear degeneration, presumably by excess caspase activation (Arama et al., 2003). To check whether this protein is a potential substrate of Nutcracker, we conducted co-IP experiments by co-expressing PrA-ntc and a shorter version of Bruce (consisting of about half of the protein, including the BIR and UBC domains, as the entire gene is yet to be cloned) in S2 cells. In this experiment, the Bruce ‘mini-gene’ product physically interacted with both the full-length and truncated forms of Nutcracker (Fig. 5B).
In order to define the genetic interaction between nutcracker and Bruce, we generated double mutants by recombining Bruce mutants onto the nutcracker07259 chromosome. We rationalized that if Bruce is the substrate of Nutcracker, then the decreased amount of caspase staining in this hypomorphic nutcracker mutant might be elevated in double mutants. We used two Bruce mutants previously isolated in our laboratory (J. Agapite, K. McCall and H.S., unpublished) (Arama et al., 2003). One mutant, 8-1e, was characterized as harboring a deletion of the BIR domain of Bruce, whereas the other, 10-1e, contains a deletion of almost the entire protein, including the UBC domain (but still contains the N-terminal BIR domain). When we stained the double mutants Bruce8-1e, nutcracker07259 or Bruce10-1e, nutcracker07259 we saw no change in caspase staining (Fig. 5C) as compared with the nutcracker single mutant. Furthermore, morphological analysis of either double mutant did not reveal more severe individualization defects than with single mutants. These data argue against a simple linear pathway in which Nutcracker activates caspases by inhibiting Bruce.
Proteasome activity is reduced in nutcracker mutants
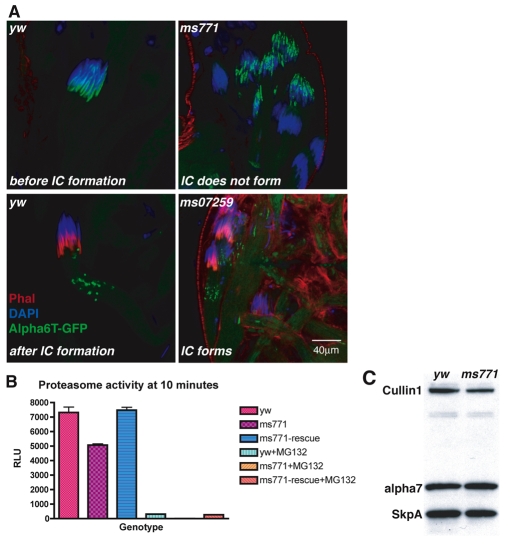
Proteasome activity is important for spermatid individualization (Zhong and Belote, 2007). In particular, IC movement and caspase activation are abnormal in a mutant for a testis-specific proteasome subunit, alpha6T (Prosα6T – FlyBase). Given that some aspects of the nutcracker mutant phenotypes resemble the loss of alpha6T function, we explored whether nutcracker has an effect on proteasomes.
We utilized a GFP-tagged Alpha6T protein (Zhong and Belote, 2007) to ask whether proteasome distribution and numbers are altered in a nutcracker mutant background. Alpha6T-GFP is a faithful reporter of proteasome localization, as it can rescue alpha6T mutant phenotypes and is fully incorporated into proteasomes (Zhong and Belote, 2007). Alpha6T colocalized with the elongated nuclei just before IC formation (Fig. 6A, top right), but moved ahead of the actin cones after the IC forms (Fig. 6A, bottom right). In nutcrackerms771, in which no ICs form, Alpha6T-GFP persisted in the nuclei (Fig. 6A, top left). By contrast, in the hypomorphic nutcracker07259, in which occasional ICs form, Alpha6T-GFP was detected at its normal location ahead of the actin cones (Fig. 6A, bottom left). These data indicate that the movement and overall distribution of proteasomes do not depend on nutcracker function, but on IC formation.
Fig. 6.
Nutcracker affects proteasome activity but not distribution or numbers. (A) Proteasome distribution during individualization. GFP-tagged Alpha6T is a marker for proteasome localization (Zhong and Belote, 2007). Alpha6T is detected in elongated nuclei before the IC forms (top left), and after IC formation moves to the base of it (bottom left). Testes of nutcrackerms771 display no Alpha6T outside the nuclei (top right). By contrast, in the nutcracker07259 hypomorphic allele, in which occasional ICs are formed, Alpha6T is detected at its normal location ahead of the IC (bottom right). (B) Testes from nutcrackerms771 display decreased proteasome activity. Total protein was extracted from the indicated genotype and proteasome activity detected by measuring fluorogenic peptide hydrolysis after 10 minutes. Measurements are plotted as relative luminescence units (RLU). Each genotype sample was split and a proteasome inhibitor (MG132) was added to half the sample to confirm the specificity of the read-out. Wild type, yw; nutcrackerms771, ms771; nutcrackerms771;Hsp83-nutcracker, ms771-rescue. (C) The levels of the proteasome subunit Alpha7 are unchanged in the nutcrackerms771 mutant. Total protein was extracted from either wild-type (yw) or nutcrackerms771 (ms771) testes and the amounts of the indicated proteins were determined by western blot analysis. Amounts of other SCF members (Cullin-1 and SkpA) were also assayed and used as loading controls.
We next asked whether nutcracker influences proteasome activity directly. We assayed proteasome activity in wild-type, nutcrackerms771 or nutcrackerms771 flies that express a nutcracker rescue construct. Testes from these genotypes were lysed and differences in proteasome activity were detected by measuring the hydrolysis of a fluorogenic peptide. Compared with wild-type testes, proteasome activity was reduced in nutcrackerms771 mutants (Fig. 6B). This activity was restored by reintroducing nutcracker into the mutant background, indicating that the decreased activity is indeed caused by reduced nutcracker function. Furthermore, in order to investigate whether this reduced activity is caused by a reduction in proteasome numbers in the mutant, we looked at the levels of three different proteasome subunits. Assaying individual subunit proteins is a good indicator of proteasome number because the majority of proteasome subunits in the cell are incorporated into the proteasome complex (Glickman and Raveh, 2005). Compared with the wild type, the levels of Alpha7 (Prosα7 – FlyBase), Rpn3 and Rpt4 were unchanged in nutcrackerms771 mutant testes (Alpha7 is shown in Fig. 6C; data not shown). These data demonstrate that equal numbers of proteasomes are formed in the mutant, suggesting that nutcracker acts directly on proteasome activity. Taken together, these results indicate that nutcracker controls caspase activation and spermatid individualization by regulating proteasome activity.
DISCUSSION
Nutcracker is a novel F-box protein that controls caspase activation
In this work, we have studied the regulation of non-apoptotic caspase activation during Drosophila spermatogenesis in order to understand the role of controlled proteolysis during differentiation and cellular remodeling. We identified a new component of this regulatory pathway – a novel F-box protein that we termed Nutcracker. Nutcracker is strictly required for caspase activation during spermatogenesis. The fact that Nutcracker can form a complex with bone fide components of an SCF ubiquitin ligase in the testis and that a mutant with a deleted F-box domain abrogates both the formation of this complex and caspase activation in spermatids, strongly suggest that the role of Nutcracker in spermatids is intimately associated with this SCF complex.
Most F-box proteins also possess another protein-interaction domain, usually comprising WD40 or LRR motifs, that is responsible for binding the ubiquitylation substrate (Cardozo and Pagano, 2004; Kipreos and Pagano, 2000). Nutcracker belongs to the class of F-box proteins that do not contain a known protein-protein interaction domain, and differs topologically from most F-box proteins in that its F-box domain is at the very C-terminus (Kirk et al., 2008). Sequence alignments with several F-box-only proteins revealed that Nutcracker shares some limited amino acid similarity with the mammalian FBXO7 protein, which also contains the F-box domain at the C-terminus (Fig. 2C). Although the sequence conservation is limited primarily to the F-box domain, it is possible that these two proteins share functional properties, as do other proteins that are conserved only within limited regions. For example, the C. elegans p53 protein displays less than 20% overall primary sequence similarity to the human protein, mostly in the active sites, but has been demonstrated to function in related cellular processes (Derry et al., 2001; Schumacher et al., 2001). Since FBXO7 has been shown to regulate the stability of cIAP1 (BIRC2) (Chang et al., 2006), it is possible that these two E3 ligases have a conserved function in caspase regulation.
The ubiquitin-proteasome system is implicated in regulating caspase activity. Several studies have shown that the ubiquitylation and degradation of DIAP1 is a means of displacing it from caspases when apoptosis is favored (Bader and Steller, 2009). Also, ubiquitylation of caspases themselves contributes to their regulation by preventing a critical mass of full-length caspases from auto-activation in a normal setting (Ditzel et al., 2008; Schile et al., 2008). The wide variety of other ubiquitin-modifying proteins that regulate apoptosis and caspase activity, including Bruce (Vernooy et al., 2002), Morgue (Hays et al., 2002; Schreader et al., 2003; Wing et al., 2002) and Uba1 (Lee et al., 2008), imply the existence of an elaborate regulatory network that is controlled by ubiquitylation.
In our screen we isolated another ubiquitin ligase, a Cullin-3-based complex, which indicates that caspase activation in this system is tightly controlled by ubiquitin modifications. These two complexes could regulate the stability of the same substrate, as is the case for regulation of Cubitus interruptus (Ci) stability in Hedgehog signaling by both Cullin-1-based and Cullin-3-based complexes (Jiang, 2006; Ou et al., 2002), or they might target multiple important substrates. Alternatively, the E3 ligases isolated in our screen might play non-degradative roles in controlling caspase activity. For example, mono-ubiquitylation affects the targeted localization of proteins (Haglund and Dikic, 2005) and these ubiquitin ligases might control the proper localization of caspase regulators. Another possibility is that these E3 ligases mediate the non-classical Lys63 ubiquitin chain addition that is important for protein-protein interaction. Thus, instead of degradation, these proteins might actually control interactions between caspase regulators.
Nutcracker binds the IAP Bruce
Although DIAP1 is the only Drosophila BIR-containing protein that has been shown to directly inhibit caspases in vivo, Bruce has been implicated in modifying apoptosis in several death paradigms, and mutations in its mammalian homolog cause defects associated with excess cell death (Bartke et al., 2004; Hao et al., 2004; Lotz et al., 2004). Here, we show that Bruce can physically bind Nutcracker, and that this interaction is independent of the F-box domain. Therefore, Bruce might be a substrate of Nutcracker. However, we were unable to determine the steady-state levels of Bruce in nutcracker mutants, so it is as yet unclear whether it is indeed a substrate or a complex partner. The fact that Bruce also binds to another E3 ligase isolated in our screen (Arama et al., 2007) suggests that this protein is a common regulator of caspase activation during individualization.
Nutcracker affects proteasome activity
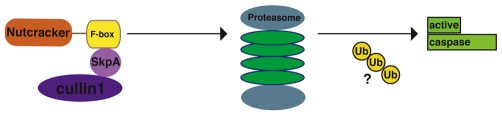
We show that nutcracker mutants cause a reduction in proteasome activity. This decreased activity does not seem to be due to proteasome mislocalization or a reduction in their numbers, suggesting that Nutcracker controls proteasome activity directly. It is possible that Nutcracker modifies proteasome regulators, which could include, for example, proteins of the regulatory particle of the proteasome. An attractive model is that Nutcracker functions through proteasomes to activate caspases (Fig. 7). As mentioned above, caspase activity is tightly controlled by the ubiquitin proteasome system (Bader and Steller, 2009; Broemer and Meier, 2009). Therefore, it is possible that local activation of proteasomes controls localized caspase activation.
Fig. 7.
A model for caspase activation by the Drosophila Nutcracker SCF complex. Our model suggests that Nutcracker forms a complex with members of an SCF ubiquitin ligase complex. This complex can then regulate caspase activation, possibly by modifying proteins that activate the proteasome; these could include, for example, components of the proteasome regulatory particle or other associated proteins. Caspase activation could then be facilitated by ubiquitin-mediated regulation.
Many questions remain regarding the non-lethal role of caspases in cellular remodeling. For instance, is it a specialized activation that is governed by dedicated proteins, and to what extent are known apoptotic regulators involved in this process? Another intriguing question is how cells tolerate a certain level of caspase activation and avoid destruction by these potentially deadly proteases. Answers to these questions will not only uncover novel caspase regulators, but might also help us to understand how diseased cells, such as cancer cells, manage to escape cell death.
Supplementary Material
Acknowledgements
We thank C. Zuker for the male-sterile mutant collection and M. Cahill for collecting and shipping the stocks; B. Wakimoto for the annotated list of mutants; M. H. Bre, T. Murphy, P. Meier and H. D. Ryoo for antibodies; J. Belote for the alpha6T;Alpha6T-GFP line; S. Benjamin for help with the immunofluorescence images; and S. Benjamin, M. Suzanne, M. Pratt, T. Gorenc and Y. Kaplan for critically reading the manuscript. H.S. is an investigator of the Howard Hughes Medical Institute. This work was supported by NIH grant RO1GM60124 to H.S. Deposited in PMC for release after 6 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.050088/-/DC1
References
- Abraham M. C., Shaham S. (2004). Death without caspases, caspases without death. Trends Cell Biol. 14, 184-193 [DOI] [PubMed] [Google Scholar]
- Arama E., Agapite J., Steller H. (2003). Caspase activity and a specific cytochrome C are required for sperm differentiation in Drosophila. Dev. Cell 4, 687-697 [DOI] [PubMed] [Google Scholar]
- Arama E., Bader M., Srivastava M., Bergmann A., Steller H. (2006). The two Drosophila cytochrome C proteins can function in both respiration and caspase activation. EMBO J. 25, 232-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arama E., Bader M., Rieckhof G. E., Steller H. (2007). A ubiquitin ligase complex regulates caspase activation during sperm differentiation in Drosophila. PLoS Biol. 5, e251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader M., Steller H. (2009). Regulation of cell death by the ubiquitin-proteasome system. Curr. Opin. Cell Biol. 21, 878-884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke T., Pohl C., Pyrowolakis G., Jentsch S. (2004). Dual role of BRUCE as an antiapoptotic IAP and a chimeric E2/E3 ubiquitin ligase. Mol. Cell 14, 801-811 [DOI] [PubMed] [Google Scholar]
- Bergmann A. (2010). The role of ubiquitylation for the control of cell death in Drosophila. Cell Death Differ. 17, 61-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bre M. H., Redeker V., Quibell M., Darmanaden-Delorme J., Bressac C., Cosson J., Huitorel P., Schmitter J. M., Rossler J., Johnson T., et al. (1996). Axonemal tubulin polyglycylation probed with two monoclonal antibodies: widespread evolutionary distribution, appearance during spermatozoan maturation and possible function in motility. J. Cell Sci. 109, 727-738 [DOI] [PubMed] [Google Scholar]
- Bre M. H., Redeker V., Vinh J., Rossier J., Levilliers N. (1998). Tubulin polyglycylation: differential posttranslational modification of dynamic cytoplasmic and stable axonemal microtubules in paramecium. Mol. Biol. Cell 9, 2655-2665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broemer M., Meier P. (2009). Ubiquitin-mediated regulation of apoptosis. Trends Cell Biol. 19, 130-140 [DOI] [PubMed] [Google Scholar]
- Cardozo T., Pagano M. (2004). The SCF ubiquitin ligase: insights into a molecular machine. Nat. Rev. Mol. Cell Biol. 5, 739-751 [DOI] [PubMed] [Google Scholar]
- Chang Y. F., Cheng C. M., Chang L. K., Jong Y. J., Yoo C. Y. (2006). The F-box protein Fbxo7 interacts with human inhibitor of apoptosis protein cIAP1 and promotes cIAP1 ubiquitination. Biochem. Biophys. Res. Commun. 342, 1022-1026 [DOI] [PubMed] [Google Scholar]
- Derry W. B., Putzke A. P., Rothman J. H. (2001). Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science 294, 591-595 [DOI] [PubMed] [Google Scholar]
- Deveraux Q. L., Reed J. C. (1999). IAP family proteins-suppressors of apoptosis. Genes Dev. 13, 239-252 [DOI] [PubMed] [Google Scholar]
- Ditzel M., Broemer M., Tenev T., Bolduc C., Lee T. V., Rigbolt K. T., Elliott R., Zvelebil M., Blagoev B., Bergmann A., et al. (2008). Inactivation of effector caspases through nondegradative polyubiquitylation. Mol. Cell 32, 540-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Bergmann A. (2009). The cleaved-Caspase-3 antibody is a marker of Caspase-9-like DRONC activity in Drosophila. Cell Death Differ. 17, 534-539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein-Rotkopf Y., Arama E. (2009). Can't live without them, can live with them: roles of caspases during vital cellular processes. Apoptosis 14, 980-995 [DOI] [PubMed] [Google Scholar]
- Fuller M. T. (1993). The Development of Drosophila melanogaster: Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Glickman M. H., Raveh D. (2005). Proteasome plasticity. FEBS Lett. 579, 3214-3223 [DOI] [PubMed] [Google Scholar]
- Goyal L., McCall K., Agapite J., Hartwieg E., Steller H. (2000). Induction of apoptosis by Drosophila reaper, hid and grim through inhibition of IAP function. EMBO J. 19, 589-597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund K., Dikic I. (2005). Ubiquitylation and cell signaling. EMBO J. 24, 3353-3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. (2000). The hallmarks of cancer. Cell 100, 57-70 [DOI] [PubMed] [Google Scholar]
- Hao Y., Sekine K., Kawabata A., Nakamura H., Ishioka T., Ohata H., Katayama R., Hashimoto C., Zhang X., Noda T., et al. (2004). Apollon ubiquitinates SMAC and caspase-9, and has an essential cytoprotection function. Nat. Cell Biol. 6, 849-860 [DOI] [PubMed] [Google Scholar]
- Hay B. A., Wassarman D. A., Rubin G. M. (1995). Drosophila homologs of baculovirus inhibitor of apoptosis proteins function to block cell death. Cell 83, 1253-1262 [DOI] [PubMed] [Google Scholar]
- Hays R., Wickline L., Cagan R. (2002). Morgue mediates apoptosis in the Drosophila melanogaster retina by promoting degradation of DIAP1. Nat. Cell Biol. 4, 425-431 [DOI] [PubMed] [Google Scholar]
- Hime G. R., Brill J. A., Fuller M. T. (1996). Assembly of ring canals in the male germ line from structural components of the contractile ring. J. Cell Sci. 109, 2779-2788 [DOI] [PubMed] [Google Scholar]
- Hinds M. G., Norton R. S., Vaux D. L., Day C. L. (1999). Solution structure of a baculoviral inhibitor of apoptosis (IAP) repeat. Nat. Struct. Biol. 6, 648-651 [DOI] [PubMed] [Google Scholar]
- Huh J. R., Vernooy S. Y., Yu H., Yan N., Shi Y., Guo M., Hay B. A. (2004). Multiple apoptotic caspase cascades are required in nonapoptotic roles for Drosophila spermatid individualization. PLoS Biol. 2, E15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J. (2006). Regulation of Hh/Gli signaling by dual ubiquitin pathways. Cell Cycle 5, 2457-2463 [DOI] [PubMed] [Google Scholar]
- Jin Z., Li Y., Pitti R., Lawrence D., Pham V. C., Lill J. R., Ashkenazi A. (2009). Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell 137, 721-735 [DOI] [PubMed] [Google Scholar]
- Kipreos E. T., Pagano M. (2000). The F-box protein family. Genome Biol. 1, REVIEWS3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk R., Laman H., Knowles P. P., Murray-Rust J., Lomonosov M., Meziane xEl K., McDonald N. Q. (2008). Structure of a conserved dimerization domain within the F-box protein Fbxo7 and the PI31 proteasome inhibitor. J. Biol. Chem. 283, 22325-22335 [DOI] [PubMed] [Google Scholar]
- Kleino A., Valanne S., Ulvila J., Kallio J., Myllymäki H., Enwald H., Stöven S., Poidevin M., Ueda R., Hultmark D., et al. (2005). Inhibitor of apoptosis 2 and TAK1-binding protein are components of the Drosophila Imd pathway. EMBO J. 24, 3423-3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth S., White K. (2005). Apoptosis in Drosophila: neither fish nor fowl (nor man, nor worm). J. Cell Sci. 118, 1779-1787 [DOI] [PubMed] [Google Scholar]
- Kuo C. T., Zhu S., Younger S., Jan L. Y., Jan Y. N. (2006). Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron 51, 283-290 [DOI] [PubMed] [Google Scholar]
- Kuranaga E., Miura M. (2007). Nonapoptotic functions of caspases: caspases as regulatory molecules for immunity and cell-fate determination. Trends Cell Biol. 17, 135-144 [DOI] [PubMed] [Google Scholar]
- Lee T. V., Ding T., Chen Z., Rajendran V., Scherr H., Lackey M., Bolduc C., Bergmann A. (2008). The E1 ubiquitin-activating enzyme Uba1 in Drosophila controls apoptosis autonomously and tissue growth non-autonomously. Development 135, 43-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann R., Nusslein-Volhard C. (1986). Abdominal segmentation, pole cell formation, and embryonic polarity require the localized activity of oskar, a maternal gene in Drosophila. Cell 47, 141-152 [DOI] [PubMed] [Google Scholar]
- Lisi S., Mazzon I., White K. (2000). Diverse domains of THREAD/DIAP1 are required to inhibit apoptosis induced by REAPER and HID in Drosophila. Genetics 154, 669-678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz K., Pyrowolakis G., Jentsch S. (2004). BRUCE, a giant E2/E3 ubiquitin ligase and inhibitor of apoptosis protein of the trans-Golgi network, is required for normal placenta development and mouse survival. Mol. Cell. Biol. 24, 9339-9350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro I., Berry D. L., Huh J. R., Chen C. H., Huang H., Yoo S. J., Guo M., Baehrecke E. H., Hay B. A. (2006). The Drosophila caspase Ice is important for many apoptotic cell deaths and for spermatid individualization, a nonapoptotic process. Development 133, 3305-3315 [DOI] [PubMed] [Google Scholar]
- Nikolaev A., McLaughlin T., O'Leary D. D., Tessier-Lavigne M. (2009). APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature 457, 981-989 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Noguchi T., Miller K. G. (2003). A role for actin dynamics in individualization during spermatogenesis in Drosophila melanogaster. Development 130, 1805-1816 [DOI] [PubMed] [Google Scholar]
- Ou C. Y., Lin Y. F., Chen Y. J., Chien C. T. (2002). Distinct protein degradation mechanisms mediated by Cul1 and Cul3 controlling Ci stability in Drosophila eye development. Genes Dev. 16, 2403-2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C. (2003). Apoptosis-targeted therapies for cancer. Cancer Cell 3, 17-22 [DOI] [PubMed] [Google Scholar]
- Ribeiro P. S., Kuranaga E., Tenev T., Leulier F., Miura M., Meier P. (2007). DIAP2 functions as a mechanism-based regulator of drICE that contributes to the caspase activity threshold in living cells. J. Cell Biol. 179, 1467-1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogowski K., Juge F., van Dijk J., Wloga D., Strub J. M., Levilliers N., Thomas D., Bre M. H., Van Dorsselaer A., Gaertig J., et al. (2009). Evolutionary divergence of enzymatic mechanisms for posttranslational polyglycylation. Cell 137, 1076-1087 [DOI] [PubMed] [Google Scholar]
- Ryoo H. D., Bergmann A., Gonen H., Ciechanover A., Steller H. (2002). Regulation of Drosophila IAP1 degradation and apoptosis by reaper and ubcD1. Nat. Cell Biol. 4, 432-438 [DOI] [PubMed] [Google Scholar]
- Salvesen G. S., Duckett C. S. (2002). IAP proteins: blocking the road to death's door. Nat. Rev. Mol. Cell Biol. 3, 401-410 [DOI] [PubMed] [Google Scholar]
- Santel A., Winhauer T., Blümer N., Renkawitz-Pohl R. (1997). The Drosophila don juan (dj) gene encodes a novel sperm specific protein component characterized by an unusual domain of a repetitive amino acid motif. Mech. Dev. 64, 19-30 [DOI] [PubMed] [Google Scholar]
- Santel A., Blumer N., Kampfer M., Renkawitz-Pohl R. (1998). Flagellar mitochondrial association of the male-specific Don Juan protein in Drosophila spermatozoa. J. Cell Sci. 111, 3299-3309 [DOI] [PubMed] [Google Scholar]
- Schile A. J., Garcia-Fernandez M., Steller H. (2008). Regulation of apoptosis by XIAP ubiquitin-ligase activity. Genes Dev. 22, 2256-2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreader B. A., Wang Y., Nambu J. R. (2003). Drosophila morgue and the intersection between protein ubiquitination and programmed cell death. Apoptosis 8, 129-139 [DOI] [PubMed] [Google Scholar]
- Schumacher B., Hofmann K., Boulton S., Gartner A. (2001). The C. elegans homolog of the p53 tumor suppressor is required for DNA damage-induced apoptosis. Curr. Biol. 11, 1722-1727 [DOI] [PubMed] [Google Scholar]
- Steller H. (1995). Mechanisms and genes of cellular suicide. Science 267, 1445-1449 [DOI] [PubMed] [Google Scholar]
- Steller H. (2008). Regulation of apoptosis in Drosophila. Cell Death Differ. 15, 1132-1138 [DOI] [PubMed] [Google Scholar]
- Thompson C. B. (1995). Apoptosis in the pathogenesis and treatment of disease. Science 267, 1456-1462 [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T., Peacock W. J., Hardy R. W. (1972). Dynamics of spermiogenesis in Drosophila melanogaster. I. Individualization process. Z. Zellforsch. Mikrosk. Anat. 124, 479-506 [DOI] [PubMed] [Google Scholar]
- Vaux D. L., Silke J. (2005). IAPs, RINGs and ubiquitylation. Nat. Rev. Mol. Cell Biol. 6, 287-297 [DOI] [PubMed] [Google Scholar]
- Vernooy S. Y., Chow V., Su J., Verbrugghe K., Yang J., Cole S., Olson M. R., Hay B. A. (2002). Drosophila Bruce can potently suppress Rpr- and Grim-dependent but not Hid-dependent cell death. Curr. Biol. 12, 1164-1168 [DOI] [PubMed] [Google Scholar]
- Vucic D. (2008). Targeting IAP (inhibitor of apoptosis) proteins for therapeutic intervention in tumors. Curr. Cancer Drug Targets 8, 110-117 [DOI] [PubMed] [Google Scholar]
- Wakimoto B. T., Lindsley D. L., Herrera C. (2004). Toward a comprehensive genetic analysis of male fertility in Drosophila melanogaster. Genetics 167, 207-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. W., Kondo S., Krzyzanowska A., Hiromi Y., Truman J. W. (2006). Local caspase activity directs engulfment of dendrites during pruning. Nat. Neurosci. 9, 1234-1236 [DOI] [PubMed] [Google Scholar]
- Wilson R., Goyal L., Ditzel M., Zachariou A., Baker D. A., Agapite J., Steller H., Meier P. (2002). The DIAP1 RING finger mediates ubiquitination of Dronc and is indispensable for regulating apoptosis. Nat. Cell Biol. 4, 445-450 [DOI] [PubMed] [Google Scholar]
- Wing J. P., Schreader B. A., Yokokura T., Wang Y., Andrews P. S., Huseinovic N., Dong C. K., Ogdahl J. L., Schwartz L. M., White K., et al. (2002). Drosophila Morgue is an F box/ubiquitin conjugase domain protein important for grim-reaper mediated apoptosis. Nat. Cell Biol. 4, 451-456 [DOI] [PubMed] [Google Scholar]
- Yang Y., Fang S., Jensen J. P., Weissman A. M., Ashwell J. D. (2000). Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science 288, 874-877 [DOI] [PubMed] [Google Scholar]
- Yi C. H., Yuan J. (2009). The Jekyll and Hyde functions of caspases. Dev. Cell 16, 21-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L., Belote J. M. (2007). The testis-specific proteasome subunit Prosalpha6T of D. melanogaster is required for individualization and nuclear maturation during spermatogenesis. Development 134, 3517-3525 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.