Abstract
Neurturin (NTN) is a member of the glial cell line-derived neurotrophic factor (GDNF) family; and, while GDNF has been shown to increase dopamine (DA) release in normal animals, the ability of NTN to alter DA release has not been previously reported. The purpose of the present study was to determine if NTN could alter striatal DA release, and to compare the effects of NTN to GDNF. Male Fischer-344 rats were given a single injection of vehicle or 5 μg NTN or GDNF into the right substantia nigra. Three weeks later microdialysis experiments were conducted to assess striatal DA release. Basal extracellular levels of striatal DA were not affected by either NTN or GDNF. However, both NTN and GDNF led to increases in amphetamine-evoked overflow of DA from the ipsilateral striatum, and there was a trend for potassium-evoked overflow to be augmented. Postmortem tissue levels of DA were decreased by approximately 20% in the striatum, and increased by approximately 100% in the substantia nigra, on the ipsilateral side of the brain compared to the contralateral side following both NTN and GDNF injection. Thus, NTN, like GDNF, can augment striatal DA release, and the magnitude of the NTN effects are similar to those of GDNF.
Keywords: Neurturin, GDNF, Striatum, Substantia nigra, Dopamine, Microdialysis
Introduction
Glial cell line-derived neurotrophic factor (GDNF) has been shown to have potent effects on brain dopamine (DA) systems of normal animals. In young adult animals these effects include increases in DA levels in the substantia nigra [1, 2], increases in evoked overflow of DA from the striatum [3, 4], changes in expression and phosphorylation of tyrosine hydroxylase (TH) [5, 6] and changes in behaviors thought to involve dopaminergic activation [1, 2, 7]. Similar neurochemical and behavioral effects have been reported in aged animals [8–11].
Neurturin (NTN), another member of the GDNF family of trophic factors, shares approximately 42% sequence homology with mature GDNF [12]. Similar to GDNF, NTN has potent effects on normal DA neurons both in vitro [13–15] and in vivo [13, 16, 17]. However, while the intracerebral administration of GDNF leads to increases in stimulus evoked DA overflow in the striatum of normal animals [3, 4], the ability of NTN to produce functional effects on DA release has not been previously reported. While some studies have reported similarities between the effects of NTN and GDNF on the nigrostriatal DA system [14], there are reports of differences as well [16]. Hence, it is not clear if NTN can augment striatal DA release in a manner comparable to GDNF. However, understanding how NTN affects DA release in intact animals may provide insight into its potential as a possible therapeutic agent for conditions associated with dopaminergic dysfunction. Thus, the purpose of the present study was to determine if a single intranigral injection of NTN could alter striatal DA release, and to compare the effects of NTN to a similar injection of GDNF. In vivo microdialysis was used to evaluate potassium- and amphetamine-evoked overflow of DA, and to monitor basal extracellular levels of DA and its primary metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) from the striatum of rats treated with NTN or GDNF. Postmortem tissue levels of DA and metabolites in the striatum and substantia nigra were determined at the conclusion of each experiment to further evaluate the effects of the two trophic factors.
Experimental Procedure
Animals
Male Fischer-344 rats of approximately 4 months of age were used for all experiments. The animals were obtained from Harlan Sprague–Dawley (Indianapolis, IN, USA) and were housed in groups of two under a 12-h light–dark cycle with food and water freely available. All animal use procedures were in strict accordance with National Institutes of Health guidelines and were approved by the Animal Care and Use Committee at the University of Kentucky. All efforts were made to minimize the number of animals used and to minimize their pain and discomfort.
Trophic Factor Injections
Surgery was performed using sterile instruments and conditions. The rats were anesthetized with isoflurane (2.0–2.5% as needed) and placed into a stereotaxic frame. The skull was exposed and a small hole drilled in the skull over the right substantia nigra (5.4 mm posterior to bregma, 2.2 mm lateral from midline). NTN (5 μg in 5 μl vehicle), GDNF (5 μg in 5 μl vehicle), or 5 μl vehicle (10 mM citrate buffer with 150 mM NaCl, pH 5.0) was injected just dorsal to the substantia nigra (7.0 mm below the surface of the cortex) using an SGE syringe with a 26 gauge blunt tapered needle. The rate of injection was 0.5 μl/min and the needle was left in place for an additional 5 min following the injection and then slowly withdrawn. Both the NTN and GDNF were purchased from PeproTech Inc. (Rocky Hill, NJ, USA). Gelfoam was placed in the burr hole and the incision closed with wound clips. The animals were placed in a heated small animal cage until they recovered from the anesthetic. They were then returned to their home cages.
In Vivo Microdialysis
Three weeks after the vehicle or trophic factor injections the animals were anesthetized with urethane (1.25–1.50 g/kg, i.p.) and positioned in a stereotaxic frame. Microdialysis probes (CMA/11 probes, 3.0 mm length of dialysis membrane; CMA/Microdialysis, Acton, MA) were slowly lowered into both the left and right striata (0.0 mm anterior to bregma, 3.0 mm lateral from midline, tip of probe 6.3 mm below the surface of the brain). The probes were perfused continuously at a rate of 1.2 μl/min with artificial cerebrospinal fluid containing 145 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2, 0.2 mM ascorbic acid, and 2.0 mM NaH2PO4 (pH 7.4) [18]. Dialysate fractions were collected at 20-min intervals. Following a 2-h equilibration period and the collection of 3 baseline fractions, DA overflow was stimulated first by increasing the potassium concentration in the perfusate to 100 mM (NaCl reduced to 47.7 mM) for a single 20-min fraction, and then 2 h later by adding 100 μM d-amphetamine to the perfusate for a single 20-min fraction. Following the d-amphetamine five final fractions with normal artificial cerebrospinal fluid were collected. Dialysate samples were frozen on dry ice and stored at −80°C until assayed for DA, DOPAC and HVA.
Tissue Collection and HPLC Analysis
At the end of the experiments the urethane-anesthetized animals were killed by decapitation. The brains were rapidly removed and chilled in ice-cold saline. A coronal slice of brain approximately 2 mm thick at the level of the dialysis probes was made with the aid of an ice-chilled brain mold (Rodent Brain Matrix, ASI Instruments, Warren, MI, USA). The location of all dialysis probes was confirmed to be centered in the dorsal striatum at the level of, or just rostral to, the crossing of the anterior commissure. The striatum was then dissected from each half of the slice as a single piece. The substantia nigra was dissected from both sides of a 2 mm thick coronal slice through the midbrain. The location of the trophic factor injection sites was verified during dissection of the slice containing the nigra. Following dissection the tissue pieces were placed in preweighed vials, weighed, and frozen on dry ice. Samples were stored at −80°C until assayed by high performance liquid chromatography (HPLC) as previously described [19]. For dialysis samples, 20 μl of the dialysate was injected directly onto the column.
Data Analysis
Basal levels of DA and metabolites were defined as the average value in the three fractions preceding stimulation by excess potassium. All probes were calibrated in vitro prior to use to determine acceptable probes (recovery of DA at least 15%). However, values were not corrected for in vitro recoveries as uncorrected values may be better correlated to true values [20]. Dialysis data were expressed as nM concentration of DA or metabolite in the dialysate and, for evoked overflow, as the total amount of DA in the dialysate above baseline following stimulation with potassium or amphetamine. Tissue levels of DA and metabolites were expressed as ng/g wet weight of tissue. Results were analyzed statistically using analysis of variance (ANOVA) as indicated in the results. Newman-Keuls tests were used for post hoc comparisons.
Results
Effects of NTN and GDNF on Dialysate Levels of DA and Metabolites
Basal dialysate levels of DA, DOPAC, and HVA are shown in Table 1. The data were statistically analyzed using a mixed ANOVA with treatment as a between factor and side of brain as a within factor. There were no significant effects of either NTN or GDNF on basal extracellular levels of DA. However, basal levels of metabolites were affected on the injected side following both the NTN and GDNF treatments (main effect of side of brain for both DOPAC and HVA, P < 0.005; treatment and side of brain interaction for both DOPAC and HVA, P < 0.01). Basal levels of DOPAC were reduced by 28% in the NTN treated rats (P < 0.01), and by 21% following GDNF injection (P < 0.05). For basal levels of HVA, the NTN treatment led to a reduction of 19% on the treated side compared to the contralateral side (P < 0.01), and the GDNF treatment led to a decrease of 11% on the treated side (P < 0.05).
Table 1.
Basal dialysate levels of DA, DOPAC and HVA from the striatum of animals injected in the right substantia nigra 3 weeks earlier with vehicle,NTN or GDNF
| Group | Dialysate level (nM) |
||
|---|---|---|---|
| DA | DOPAC | HVA | |
| Vehicle | |||
| Left striatum | 4.74 ± 0.44 | 1,731 ± 40 | 940 ± 33 |
| Right striatum | 5.11 ± 0.47 | 1,749 ± 102 | 1,006 ± 51 |
| NTN | |||
| Left striatum | 4.58 ± 0.38 | 1,850 ± 126 | 1,057 ± 58 |
| Right striatum | 4.71 ± 0.26 | 1,317 ± 154* | 847 ± 69* |
| GDNF | |||
| Left striatum | 5.10 ± 0.41 | 1,815 ± 65 | 1,044 ± 28 |
| Right striatum | 4.81 ± 0.30 | 1,437 ± 128* | 926 ± 47* |
Values are mean ± SEM from 7–8 animals per group
P < 0.05 versus left striatum (mixed ANOVA followed by New-man-Keuls post hoc comparisons)
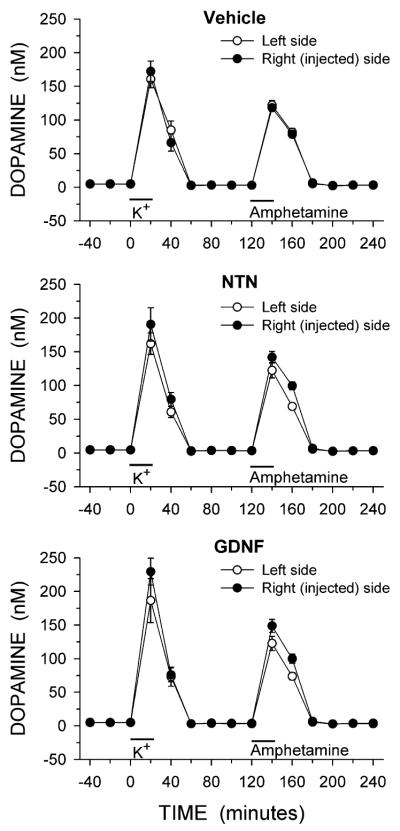
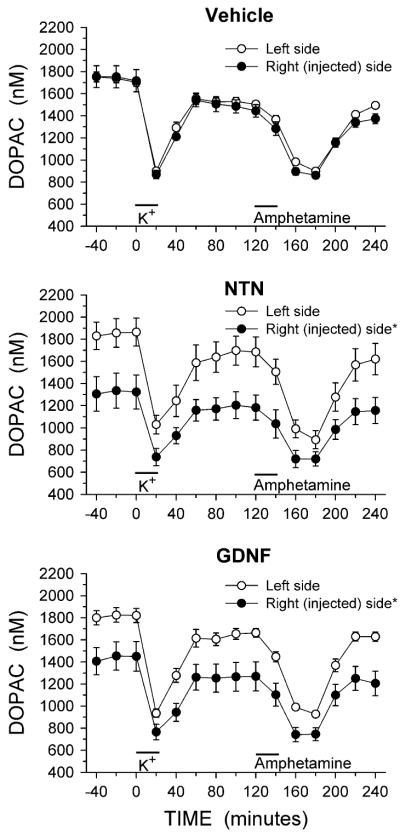
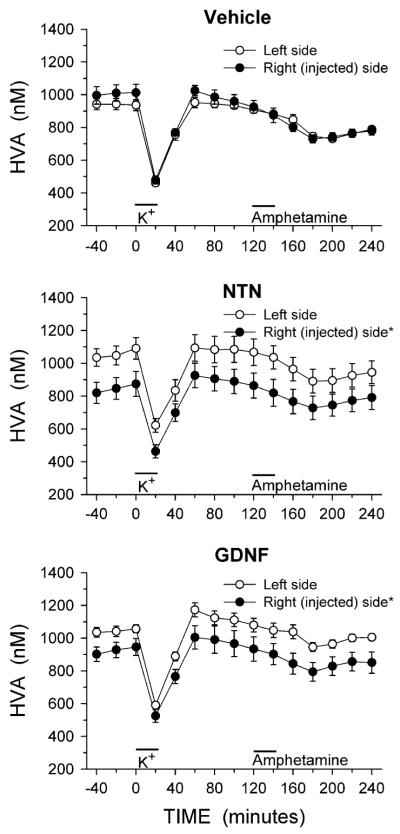
The complete time course from the in vivo microdialysis experiments for DA, DOPAC and HVA are shown in Figs. 1, 2, and 3, respectively. The relative lack of effect of NTN and GDNF on basal levels of DA (Fig. 1) is emphasized by the overlapping symbols prior to potassium stimulation, while the NTN and GDNF induced reductions in basal levels of DOPAC and HVA (Figs. 2, 3) are clear. For both DOPAC and HVA, a repeated measures ANOVA with side of brain and time of sample collection as within factors found a main effect of side of brain for both DOPAC and HVA in animals treated with NTN or GDNF (main effect of side: DOPAC, NTN P < 0.02, GDNF P < 0.02; HVA, NTN P < 0.01, GDNF P < 0.02).
Fig. 1.
Dialysate levels of DA from the striatum of animals injected into the right substantia nigra with vehicle, NTN or GDNF. Microdialysis experiments were carried out 3 weeks after the injections. Excess potassium (100 mM) was included in the perfusate for 20-min starting at 0 min (horizontal bar above K+), and 100 μM amphetamine was included in the perfusate for 20-min starting at 120 min (horizontal bar above Amphetamine). Values shown are mean ± SEM from 7–8 animals per group
Fig. 2.
Dialysate levels of DOPAC from the striatum of animals injected into the right substantia nigra with vehicle, NTN or GDNF. Microdialysis experiments were carried out 3 weeks after the injections. Excess potassium (100 mM) was included in the perfusate for 20-min starting at 0 min (horizontal bar above K+), and 100 μM amphetamine was included in the perfusate for 20-min starting at 120 min (horizontal bar above Amphetamine). Values shown are mean ± SEM from 7–8 animals per group. * P < 0.05 versus left side (repeated measures ANOVA; Newman-Keuls post-hoc comparisons indicated a significant difference (P < 0.01) between the left and right sides at each individual time point for both the NTN and GDNF treated groups)
Fig. 3.
Dialysate levels of HVA from the striatum of animals injected into the right substantia nigra with vehicle, NTN or GDNF. Microdialysis experiments were carried out 3 weeks after the injections. Excess potassium (100 mM) was included in the perfusate for 20-min starting at 0 min (horizontal bar above K+), and 100 μM amphetamine was included in the perfusate for 20-min starting at 120 min (horizontal bar above Amphetamine). Values shown are mean ± SEM from 7–8 animals per group. * P < 0.05 versus left side (repeated measures ANOVA; Newman-Keuls post-hoc comparisons indicated a significant difference (P < 0.01) between the left and right sides at each individual time point for both the NTN and GDNF treated groups)
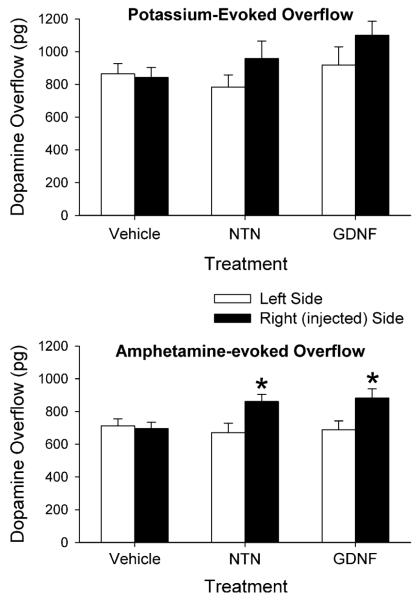
In order to facilitate comparisons of the evoked overflow of DA between the groups and sides of the brain the data were expressed as total amount of DA in the dialysate fractions above basal levels following stimulation by excess potassium or d-amphetamine (Fig. 4). The results were then statistically analyzed using a mixed ANOVA with treatment as a between factor and side of brain as a within factor. For potassium-evoked overflow of DA there was a main effect of side (P < 0.05), but not of treatment; and there was not a significant interaction. Thus, while the NTN and GDNF treatments tended to increase potassium-evoked overflow (by approximately 20%, Fig. 4, the increase did not reach statistical significance. For amphetamine-evoked overflow of DA, the mixed two-factor ANOVA revealed a main effect of side (P < 0.01) and a significant interaction of treatment with side (P < 0.05), but not a main effect of treatment. Post hoc comparisons indicated that both NTN and GDNF treatments led to increases in amphetamine-evoked overflow of DA by approximately 28% on the injected side.
Fig. 4.
Potassium-evoked (top panel) and amphetamine-evoked (bottom panel) overflow of DA from the striatum of animals injected into the right substantia nigra with vehicle, NTN or GDNF. Microdialysis experiments were carried out 3 weeks after the injections. Values shown are mean ± SEM from 7–8 animals per group. * P < 0.05 versus left side (mixed ANOVA followed by Newman-Keuls post-hoc comparisons)
Effects of NTN and GDNF on Tissue Levels of DA and Metabolites
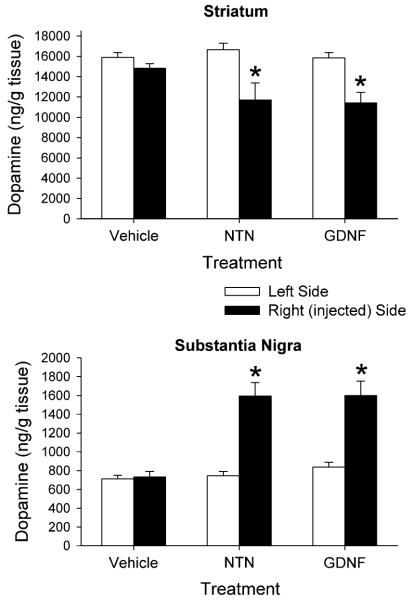
The intranigral injection of NTN and GDNF affected postmortem levels of DA in both the striatum and substantia nigra (Fig. 5). The data were compared statistically using a mixed ANOVA with treatment as a between factor and side of brain as a within factor. In the striatum, the ANOVA indicated a main effect of side (P < 0.001) and an interaction of treatment with side (P < 0.02), but not a main effect of drug. On the injected side, the NTN led to a 20% decrease in striatal DA content, and the GDNF led to an 18% decrease in striatal DA, compared to the contralateral sides. In the substantia nigra there was a main effect of treatment (P < 0.001), a main effect of side (P < 0.001), and a significant interaction (P < 0.001). The NTN led to a 114% increase in nigral DA content, and the GDNF led to a 91% increase in nigral DA, on the injected side compared to the contralateral side. The trophic factor injections also altered DA metabolite levels in both the striatum and substantia nigra. In the striatum NTN led to a 29% decrease in DOPAC, and GDNF led to a 25% decrease in DOPAC on the injected side of the brain (Table 2). Striatal HVA levels were decreased by 23 and 16%, respectively, following NTN and GDNF administration. In the substantia nigra both trophic factors increased tissue DOPAC levels by 27–28%, and increased tissue HVA levels by 17–20% on the injected side compared to the contralateral side (Table 2).
Fig. 5.
Postmortem tissue levels of DA from the striatum and substantia nigra of animals injected into the right substantia nigra with vehicle, NTN or GDNF. Tissue was harvested 3 weeks after the injections. Values shown are mean ± SEM from 7–8 animals per group. * P < 0.001 versus left side (mixed ANOVA followed by Newman-Keuls post-hoc comparisons)
Table 2.
Tissue levels of DOPAC and HVA from the striatum and substantia nigra of animals injected in the right substantia nigra 3 weeks earlier with vehicle,NTN or GDNF
| Region and group | Tissue content (ng/g tissue) |
||
|---|---|---|---|
| Side | DOPAC | HVA | |
| Striatum | |||
| Vehicle | Left | 2,767 ± 129 | 1,713 ± 91 |
| Right | 2,593 ± 155 | 1,594 ± 124 | |
| NTN | Left | 2,756 ± 125 | 1,778 ± 125 |
| Right | 1,965 ± 299* | 1,376 ± 191* | |
| GDNF | Left | 2,882 ± 103 | 1,866 ± 78 |
| Right | 2,164 ± 235* | 1,559 ± 137* | |
| Substantia Nigra | |||
| Vehicle | Left | 149 ± 8 | 104 ± 8 |
| Right | 154 ± 11 | 107 ± 9 | |
| NTN | Left | 151 ± 13 | 100 ± 7 |
| Right | 194 ± 14* | 120 ± 14* | |
| GDNF | Left | 172 ± 10 | 110 ± 8 |
| Right | 219 ± 15* | 129 ± 7* | |
Values are mean ± SEM from 7–8 animals per group
P < 0.05 versus left side (mixed ANOVA followed by Newman-Keuls post hoccomparisons)
Discussion
The present study compared the effects of NTN and GDNF on striatal DA overflow and striatal and substantia nigra DA content. The results indicate that both trophic factors, when administered as a single injection into the substantia nigra, lead to almost identical changes in evoked overflow of DA from the striatum and in changes in tissue levels of DA and metabolites in the nigrostriatal pathway.
The changes in DA release and tissue content of DA after NTN or GDNF injection are similar to those reported in the literature for animals administered a single injection of GDNF. Previous in vivo studies in rats have reported increases in both potassium- and amphetamine-evoked overflow of striatal DA following intranigral GDNF [3, 9], a trend for an increase in potassium-evoked overflow following intranigral GDNF [21], and an increase in potassium-evoked overflow following intrastriatal GDNF [4]. The present study found a significant increase in amphetamine-evoked overflow of DA in the striatum, and a trend for an increase in potassium-evoked overflow of DA. The magnitude of the effects observed in the present study was generally smaller than that reported in other papers. The smaller effect we observed on stimulus evoked overflow may be due to the lower dose of trophic factor we used (5 μg) compared to that in the other studies (10 μg) [3, 9].
Numerous studies have reported changes in tissue levels of DA following intranigral GDNF administration. In the striatum these changes include a decrease, or a trend for a decrease, in tissue levels of DA [1–3, 21]. In the substantia nigra these changes include a significant increase in tissue content of DA [1–3, 21]. Both these effects were observed in the present study following either NTN or GDNF injection into the substantia nigra.
Taken together, the increases in striatal DA release, combined with decreases in striatal tissue levels of DA and decreases in dialysate and tissue levels of DOPAC and HVA, suggest a redistribution of DA in the terminal towards a more readily releasable pool of DA. This would help explain an increase in release of DA with a decrease in total tissue content. Pothos et al. [22] demonstrated that GDNF leads to more DA being released per synaptic vesicle, supporting a change in DA packaging within vesicles. It has also been suggested that the level of DA metabolites is primarily a result of metabolism in the terminal during storage of newly synthesized DA as well as to breakdown following leakage from synaptic vesicle [23–25]. Thus, the decreases in striatal DA metabolite levels observed in the present study following NTN and GDNF administration may indicate more efficient storage or vesicular sequestration of DA so that there is less unprotected DA in the terminal available for degradation. Another factor that may be involved in the observed increase in dialysate levels of DA is a change in the functioning of the DA transporter (DAT). DAT activity plays a major role in regulating synaptic levels of DA, and GDNF has been shown to reduce DAT protein levels [26]. Thus, potential NTN and GDNF induced decreases in striatal DAT activity could also help explain our results of an increase in stimulus-evoked overflow of DA.
In the substantia nigra the trophic factor-induced increases in both DA and its metabolites suggest an upregulation of synthesis and storage in the cell bodies and dendrites. The relatively greater increase in nigral DA levels (91–114%) than metabolite levels (17–28%) is also consistent with more efficient storage and sequestration of DA. The differences between striatal and nigral DA and metabolite levels following trophic factor administration may be due in part to differences in how TH activity is affected, as previous work has shown that a single injection of GDNF leads to a greater biosynthesis capacity in the substantia nigra compared to the striatum [6]. Several previous studies have concluded that changes in DA release and content in the nigrostriatal DA system following GDNF administration are due to altered synthesis, storage and/or uptake of DA [3, 4, 21, 22]. The present data supports this, and the parallel changes found with NTN and GDNF suggest that both trophic factors are affecting these processes in similar ways.
Although we did not measure locomotor activity in the present study, previous reports have demonstrated that intranigral GDNF increases spontaneous locomotor activity [1–3, 9]. Thus, due to the similarities we found regarding changes in DA levels following NTN and GDNF administration, it is possible that intranigral NTN would produce behavioral changes analogous to those of GDNF. While striatal DA release is an important modulator of motor activity, somatodendritic DA release in the substantia nigra also appears to be important for regulating behavior [27–29]. We found that, in addition to increasing stimulus-evoked overflow of DA in the striatum, both NTN and GDNF produced substantial increases in nigral DA levels. It has been reported that intracerebral administration of GDNF can augment DA release in the substantia nigra [11, 30], suggesting that GDNF-induced increases in tissue levels of DA in the nigra may lead to enhanced somatodendritic release of DA. Thus, it will be important in future studies to define the behavioral effects of NTN and the role of striatal versus nigral release of DA on any observed behavioral changes.
Trophic factors of the GDNF family signal through a multicomponent receptor complex. The ligands initially bind to a cell surface-bound GDNF family receptor (GFR). Both GDNF and NTN can interact with either GFRα-1 or GFRα-2 receptors; however, GDNF preferentially binds to the GFRα-1 receptors, while NTN preferentially binds to GFRα-2 receptors [31, 32]. Both types of receptors are present in the substantia nigra of the adult rat, with GFRα-1 expression reported as higher [33–35] or approximately equal [36] to expression of GFRα-2. In the present study the analogous results following either exogenous NTN or GDNF administration may indicate that both trophic factors are activating a similar pattern of GFRα-1 or GFRα-2 receptors. However, more detailed studies would be necessary to confirm this.
Previous studies that have compared the effects of NTN and GDNF have reported both differences and similarities in their effects on the nigrostriatal DA system. Hoane et al. [16], using osmotic minipumps to deliver NTN or GDNF into the lateral ventricles for 30 days, found transient differences in amphetamine-induced activity, and differences in DA levels and utilization between the ventrolateral and mediodorsal striatum, between the two trophic factors. Rosenblad et al. [37] found that multiple intraventricular or intrastriatal injections of GDNF were more effective than similar injections of NTN at protecting against 6-hydroxydopamine (6-OHDA)-induced nigral cell death; and they suggest that the lower efficacy of NTN may be due in part to its reduced solubility and diffusion compared to GDNF. Akerud et al. [14], using fibroblasts genetically engineered to deliver NTN or GDNF into the substantia nigra, found both trophic factors equally protected against 6-OHDA-induced degeneration of nigral TH + neurons, but that only GDNF induced sprouting and soma hypertrophy of these neurons. Horger et al. [13] reported that injection of NTN into the nigra was as effective as a similar injection of GDNF at promoting survival of DA neurons after 6-OHDA exposure, and that intrastriatal injection of either NTN or GDNF in non-lesioned rats led to similar increases in amphetamine-induced locomotor activity. The authors of both these last two studies [13, 14], due to the equivalent effects they found with both trophic factors, suggest that NTN and GDNF may be acting through similar receptor components. The comparable results between NTN and GDNF noted in the last two studies mentioned above, as well as in the current study, were observed following intranigral administration of the trophic factors. Thus, it is likely that site of administration, as well as other factors such as dose and schedule of administration, will affect how equivalent the results are when comparing NTN with GDNF.
For the present study equal amounts of NTN and GDNF (5 μg) were injected into the substantia nigra. Similarly, in other studies that have compared NTN with GDNF, equal weights of the two trophic factors were used [13, 14, 16, 37]. However, it should be noted that the molecular weights of NTN (23.6 kDa) and GDNF (30.4 kDa) are different. Thus, although equal weights of trophic factors were injected into the animals, the molar concentration of NTN was higher by approximately 28%. The lack of a difference between the results for NTN and GDNF indicates that the slightly greater NTN dose did not have a significance effect on the outcome. In addition, as mentioned above, it has been reported that NTN does not diffuse as far from the injection site as GDNF does [37]. However, considering the relatively small size of the rat substantia nigra, the possible reduced diffusion distance with NTN does not appear to have been a factor in the present study.
In conclusion, the intranigral administration of similar doses of NTN and GDNF led to equivalent changes in potassium- and amphetamine-evoked overflow of DA from the striatum, to equivalent changes in extracellular levels of metabolites, and to equivalent changes in striatal and nigral tissue levels of DA and metabolites. These results indicate that NTN can upregulate dopaminergic release processes in the terminals of nigrostriatal neurons, and further support that NTN, like GDNF, may have therapeutic potential for treatment of diseases involving dopaminergic dysfunction.
Acknowledgments
This study was supported in part by United States Public Health Service Grant AG17963.
References
- 1.Hudson J, Granholm A-C, Gerhardt GA, et al. Glial cell line-derived neurotrophic factor augments midbrain dopaminergic circuits in vivo. Brain Res Bull. 1995;36:425–432. doi: 10.1016/0361-9230(94)00224-o. [DOI] [PubMed] [Google Scholar]
- 2.Martin D, Miller G, Cullen T, et al. Intranigral or intrastriatal injections of GDNF: effects on monoamine levels and behavior in rats. Eur J Pharmacol. 1996;317:247–256. doi: 10.1016/s0014-2999(96)00756-x. [DOI] [PubMed] [Google Scholar]
- 3.Hebert MA, Van Horne CG, Hoffer BJ, et al. Functional effects of GDNF in normal rat striatum: presynaptic studies using in vivo electrochemistry and microdialysis. J Pharmacol Exp Ther. 1996;279:1181–1190. [PubMed] [Google Scholar]
- 4.Xu K, Dluzen DE. The effect of GDNF on nigrostriatal dopaminergic function in response to a two-pulse K+ stimulation. Exp Neurol. 2000;166:450–457. doi: 10.1006/exnr.2000.7515. [DOI] [PubMed] [Google Scholar]
- 5.Rosenblad C, Georgievska B, Kirik D. Long-term striatal overexpression of GDNF selectively downregulates tyrosine hydroxylase in the intact nigrostriatal dopamine system. Eur J Neurosci. 2003;17:260–270. doi: 10.1046/j.1460-9568.2003.02456.x. [DOI] [PubMed] [Google Scholar]
- 6.Salvatore MF, Zhang J-L, Large DM, et al. Striatal GDNF administration increases tyrosine hydroxylase phosphorylation in the rat striatum and substantia nigra. J Neurochem. 2004;90:245–254. doi: 10.1111/j.1471-4159.2004.02496.x. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi S, Ogren SO, Hoffer BJ, et al. Dopamine D1 and D2 receptor-mediated acute and long-lasting behavioral effects of glial cell line-derived neurotrophic factor administered into the striatum. Exp Neurol. 1998;154:302–314. doi: 10.1006/exnr.1998.6952. [DOI] [PubMed] [Google Scholar]
- 8.Emerich DF, Plone M, Francis J, et al. Alleviation of behavioral deficits in aged rodents following implantation of encapsulated GDNF-producing fibroblasts. Brain Res. 1996;736:99–110. [PubMed] [Google Scholar]
- 9.Hebert MA, Gerhardt GA. Behavioral and neurochemical effects of intranigral administration of glial cell line-derived neurotrophic factor on aged Fischer 344 rats. J Pharmacol Exp Ther. 1997;282:760–768. [PubMed] [Google Scholar]
- 10.Lapchak PA, Miller PJ, Jiao S. Glial cell line-derived neurotrophic factor induces the dopaminergic and cholinergic phenotype and increases locomotor activity in aged Fischer 344 rats. Neuroscience. 1997;77:745–752. doi: 10.1016/s0306-4522(96)00492-7. [DOI] [PubMed] [Google Scholar]
- 11.Grondin R, Cass WA, Zhang Z, et al. Glial cell line-derived neurotrophic factor increases stimulus-evoked dopamine release and motor speed in aged rhesus monkeys. J Neurosci. 2003;23:1974–1980. doi: 10.1523/JNEUROSCI.23-05-01974.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotzbauer PT, Lampe PA, Heuckeroth RO, et al. Neurturin, a relative of glial-cell-line-derived neurotrophic factor. Nature. 1996;384:467–470. doi: 10.1038/384467a0. [DOI] [PubMed] [Google Scholar]
- 13.Horger BA, Nishimura MC, Armanini MP, et al. Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. J Neurosci. 1998;18:4929–4937. doi: 10.1523/JNEUROSCI.18-13-04929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akerud P, Alberch J, Eketjall S, et al. Differential effects of glial cell line-derived neurotrophic factor and neurturin on developing and adult substantia nigra dopaminergic neurons. J Neurochem. 1999;73:70–78. doi: 10.1046/j.1471-4159.1999.0730070.x. [DOI] [PubMed] [Google Scholar]
- 15.Zihlmann KB, Ducray AD, Schaller B, et al. The GDNF family members neurturin, artemin and persephin promote the morphological differentiation of cultured ventral mesencephalic dopaminergic neurons. Brain Res Bull. 2005;68:42–53. doi: 10.1016/j.brainresbull.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Hoane MR, Gulwadi AG, Morrison S, et al. Differential in vivo effects of neurturin and glial cell-line-derived neurotrophic factor. Exp Neurol. 1999;160:235–243. doi: 10.1006/exnr.1999.7175. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton JF, Morrison PF, Chen MY, et al. Heparin coinfusion during convection-enhanced delivery (CED) increases the distribution of the glial-derived neurotrophic factor (GDNF) ligand family in rat striatum and enhances the pharmacological activity of neurturin. Exp Neurol. 2001;168:155–161. doi: 10.1006/exnr.2000.7571. [DOI] [PubMed] [Google Scholar]
- 18.Moghaddam B, Bunney BS. Ionic composition of microdialysis perfusing solution alters the pharmacological responsiveness and basal outflow of striatal dopamine. J Neurochem. 1989;53:652–654. doi: 10.1111/j.1471-4159.1989.tb07383.x. [DOI] [PubMed] [Google Scholar]
- 19.Cass WA, Harned ME, Peters LE, et al. HIV-1 protein tat potentiation of methamphetamine-induced decreases in evoked overflow of dopamine in the striatum of the rat. Brain Res. 2003;984:133–142. doi: 10.1016/s0006-8993(03)03122-6. [DOI] [PubMed] [Google Scholar]
- 20.Glick SD, Dong N, Keller RW, Jr, et al. Estimating extracellular concentrations of dopamine and 3, 4-dihydroxyphenylacetic acid in nucleus accumbens and striatum using microdialysis: relationships between in vitro and in vivo recoveries. J Neurochem. 1994;62:2017–2021. doi: 10.1046/j.1471-4159.1994.62052017.x. [DOI] [PubMed] [Google Scholar]
- 21.Cass WA, Manning MW. GDNF protection against 6-OHDA-induced reductions in potassium-evoked overflow of striatal dopamine. J Neurosci. 1999;19:1416–1423. doi: 10.1523/JNEUROSCI.19-04-01416.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pothos EN, Davila V, Sulzer D. Presynaptic recording of quanta from midbrain dopamine neurons and modulation of the quantal size. J Neurosci. 1998;18:4106–4118. doi: 10.1523/JNEUROSCI.18-11-04106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56:331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- 24.Wood PL, Alter CA. Dopamine release in vivo from nigrostriatal, mesolimbic, and mesocortical neurons: utility of 3-methoxytyramine measurements. Pharmacol Rev. 1988;40:163–187. [PubMed] [Google Scholar]
- 25.Zetterstrom T, Sharp T, Collin AK, et al. In vivo measurement of extracellular dopamine and DOPAC in rat striatum after various dopamine-releasing drugs; implications for the origin of extracellular DOPAC. Eur J Pharmacol. 1988;148:327–334. doi: 10.1016/0014-2999(88)90110-0. [DOI] [PubMed] [Google Scholar]
- 26.Salvatore MF, Gerhardt GA, Dayton RD, et al. Bilateral effects of unilateral GDNF administration on dopamine- and GABA-regulating proteins in the rat nigrostriatal system. Exp Neurol. 2009;219:197–207. doi: 10.1016/j.expneurol.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robertson GS, Robertson HA. Evidence that L-dopainduced rotational behavior is dependent on both striatal and nigral mechanisms. J Neurosci. 1989;9:3326–3331. doi: 10.1523/JNEUROSCI.09-09-03326.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trevitt JT, Carlson BB, Nowend K, et al. Substantia nigra pars reticulata is a highly potent site of action for the behavioral effects of the D1 antagonist SCH 23390 in the rat. Psychopharmacology. 2001;156:32–41. doi: 10.1007/s002130100708. [DOI] [PubMed] [Google Scholar]
- 29.Gerhardt GA, Cass WA, Yi A, et al. Changes in somatodendritic but not terminal dopamine regulation in aged rhesus monkeys. J Neurochem. 2002;80:168–177. doi: 10.1046/j.0022-3042.2001.00684.x. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman AF, Van Horne CG, Eken S, et al. In vivo microdialysis studies of somatodendritic dopamine release in the rat substantia nigra: effects of unilateral 6-OHDA lesions and GDNF. Exp Neurol. 1997;147:130–147. doi: 10.1006/exnr.1997.6571. [DOI] [PubMed] [Google Scholar]
- 31.Baloh RH, Enomoto H, Johnson EM, Jr, et al. The GDNF family ligands and receptors–implications for neural development. Curr Opin Neurobiol. 2000;10:103–110. doi: 10.1016/s0959-4388(99)00048-3. [DOI] [PubMed] [Google Scholar]
- 32.Airaksinen MS, Saarma M. The GDNF family: signaling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 33.Widenfalk J, Nosrat C, Tomac A, et al. Neurturin and glial cell line-derived neurotrophic factor receptor-ß (GDNFR-ß), novel proteins related to GDNF and GDNFR-α with specific cellular patters of expression suggesting roles in the developing and adult nervous system and in peripheral organs. J Neurosci. 1997;17:8506–8519. doi: 10.1523/JNEUROSCI.17-21-08506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golden JP, Baloh RH, Kotzbauer PT, et al. Expression of neurturin, GDNF, and their receptors in the adult mouse CNS. J Comp Neurol. 1998;398:139–150. doi: 10.1002/(sici)1096-9861(19980817)398:1<139::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 35.Burazin TCD, Gundlach AL. Localization of GDNF/neurturin receptor (c-ret, GFRα-1 and α-2) mRNAs in postnatal rat brain: differential regional and temporal expression in hippocampus, cortex and cerebellum. Mol Brain Res. 1999;73:151–171. doi: 10.1016/s0169-328x(99)00217-x. [DOI] [PubMed] [Google Scholar]
- 36.Kozlowski DA, Miljan EA, Bremer EG, et al. Quantitative analysis of GFRα-1 and GFRα-2 mRNAs and tyrosine hydroxylase protein in the nigrostriatal system reveal bilateral compensatory changes following unilateral 6-OHDA lesions in the rat. Brain Res. 2004;1016:170–181. doi: 10.1016/j.brainres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Rosenblad C, Kirik D, Devaux B, et al. Protection and regeneration of nigral dopaminergic neurons by neurturin or GDNF in a partial lesion model of Parkinson’s disease after administration into the striatum or the lateral ventricle. Eur J Neurosci. 1999;11:1554–1566. doi: 10.1046/j.1460-9568.1999.00566.x. [DOI] [PubMed] [Google Scholar]