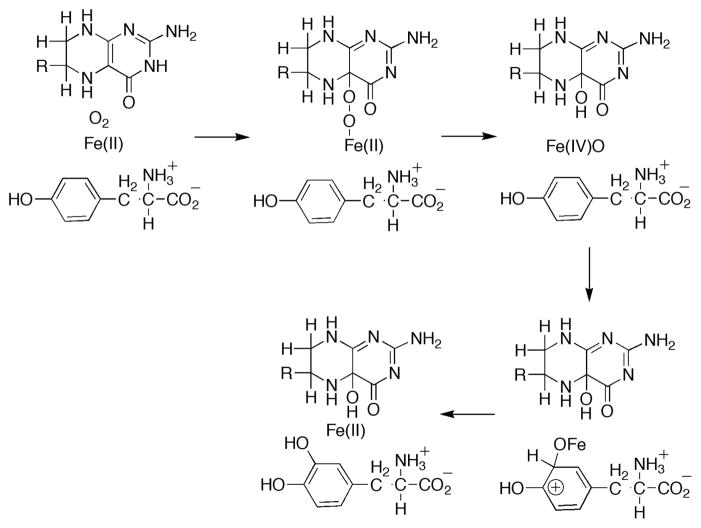
Tyrosine hydroxylase (TyrH1), the key enzyme in the biosynthesis of catecholamine neurotransmitters, is one of three members of the aromatic amino acid hydroxylase enzyme family.2,3 The enzyme is found in the brain and adrenal gland where it catalyses the conversion of L-tyrosine to L-DOPA. The other members of the family are phenylalanine hydroxylase, which catabolizes excess phenylalanine to tyrosine, and tryptophan hydroxylase, which catalyzes the rate limiting step in the biosynthesis of the neurotransmitter serotonin. All three enzymes have a mononuclear non-heme iron, coordinated by the common His2-Glu facial triad motif,4,5 and use a tetrahydropterin to activate dioxygen for hydroxylation of the aromatic side chains of their corresponding amino acid substrates.2,3 In the proposed mechanism6–8 (Scheme 1), oxygen reacts with ferrous iron and tetrahydropterin to produce a Fe(IV)O (ferryl) hydroxylating intermediate and 4a-hydroxypterin (4a-HOPH3). Then, through an electrophilic aromatic substitution, the ferryl species reacts with the aromatic side chain of the tyrosine substrate (Tyr) to form the product dihydroxyphenylalanine (DOPA). To date there has been no direct evidence for this ferryl species. Here, we report the detection of an Fe(IV) intermediate, which is likely to be the proposed ferryl species, in the TyrH reaction by the use of rapid reaction methods.
Scheme 1.
The anaerobic TyrH•Fe(II) •6-MePH4•Tyr complex9 was reacted with oxygen and quenched by rapid-freeze at time points from 20 ms to 390 ms.10 Figure 1 (left panel) shows representative Mössbauer spectra of the samples from such a time course. The spectrum of the reactant complex reveals the presence of two broad lines with parameters typical of high-spin Fe(II). The asymmetry suggests the presence of at least two distinct Fe(II) complexes. A new line at ~0.9 mm/s is observed in the spectra of samples in which the reactant complex was exposed to oxygen for either 20 ms or 100 ms, but it is not detected in the spectrum of a sample reacted for 390 ms. Thus, this peak is associated with a reaction intermediate which exhibits a quadrupole doublet in a weak external magnetic field. The low-energy line of this quadrupole doublet overlaps with the low-energy line of the Fe(II). The features of the intermediate are similar to those observed for Fe(IV) intermediates in other mononuclear non-heme enzymes.11,12
Figure 1.
4.2-K Mössbauer spectra of the reaction at 5 °C of the TyrH•Fe(II) •6-MePH4•Tyr complex (2.15 mM TyrH, 1.95 mM Fe(II), 3.7 mM 6-MePH4 and 3.7 mM Tyr in 200 mM Hepes, 10% glycerol, 0.1 M KCl at pH 7.5) with 1.9 mM oxygen-containing buffer in a ratio of 1:2. Reaction times and magnetic field strengths are as indicated. Left panel: Spectra (hashed marks) at various reaction times. The solid lines are quadrupole doublet simulations of the Fe(IV) intermediate (δ = 0.25 mm/s and ΔEQ = 1.27 mm/s). Right panel: Deconvolution of the spectrum of the 20-ms sample in zero-field (top panel) and an 8-T field (bottom panel). The spectrum of the anaerobic control scaled to 60% of the total intensity is shown as a solid line overlaid with the raw data. The resulting difference spectra (hashed marks) can be simulated with spin Hamiltonian simulations of the Fe(IV) intermediate (24% intensity) with the following parameters: S = 2, D = 12.5 cm−1, E/D = 0.05, δ = 0.25 mm/s, ΔEQ = −1.27 mm/s, η = −0.5, A/gNβN = (−18.0, −18.0, −31.0) T.
In order to characterize this intermediate further, we recorded spectra of the 20-ms sample (this sample contains a maximum amount of the intermediate, 24% of total Fe) without an applied field (Figure 1, top right panel) and with an 8-T applied field (bottom right panel). The spectrum of the 20-ms sample is shown as the top spectrum in each panel (vertical bars). Removal of the spectral contribution of the starting material (55% of the total intensity, shown as a solid line in the top spectra) results in the spectra depicted as vertical bars (lower spectrum in each panel). The zero-field spectrum reveals the position of the low-energy line and allows the isomer shift (δ) and quadrupole splitting (ΔEQ) of the intermediate to be determined: δ = 0.25 mm/s and ΔEQ = 1.27 mm/s. These parameters are similar to those experimentally observed10–14 and theoretically predicted15,16 for non-heme ferryl intermediates and strongly suggest the presence of such a complex in TyrH.17 In addition, the spectrum reveals two broad lines at 0 mm/s and 2.4 mm/s. These features are associated with a high-spin Fe(II) complex formed during the reaction.
The 8-T spectrum provides further insight into the electronic structure of the Fe(IV) intermediate. In particular, the sharp line at 4 mm/s is associated with the intermediate and not the reactant complex. The spectrum resulting after removal of the 55%-contribution of the reactant complex reflects the Fe(IV) intermediate (24%) and the new high-spin Fe(II) complex (20%). The contribution of the Fe(IV) complex was simulated according to the spin Hamiltonian formalism with parameters typical of high-spin Fe(IV) complexes.10–15,18
The presence of a Fe(IV) complex is also supported by the EPR spectra of a 20-ms sample (prepared under identical conditions) recorded before and after exposure to γ-radiation at 77 K (cryoreduction). The hallmark features of a high-spin Fe(III) complex at g = 4.3 become much more intense, suggesting that a Fe(III) complex is formed during cryoreduction (Figure S1). Similar results have been observed for a non-heme Fe(IV)O intermediate.11
An additional rapid-reaction experiment was performed to establish the kinetic competency as the hydroxylating intermediate of the Fe(IV) complex detected by Mössbauer spectroscopy. The TyrH•Fe(II) •6-MePH4•Tyr complex was reacted with oxygen in the same way as for the Mössbauer study, except that the reaction was quenched with acid and the amount of DOPA quantified (Figure 2).19 The data could be fit reasonably well as a single exponential increase with a rate constant of 15 ± 2 s−1. This is significantly faster than the kcat value at this temperature. The kinetics of formation of both DOPA and the Fe(IV) species were then analyzed20 according to the mechanism of Scheme 2, with the single rate constant for DOPA formation as an initial estimate. In this kinetic mechanism, the first step is the concomitant formation of Fe(IV)O and 4a-hydroxypterin. Fe(IV)O and tyrosine then react to form the product DOPA. Both time courses were well fit with values for the rate constants k1 and k2 of 24 and 35 s−1, respectively, consistent with the Fe(IV) intermediate being the hydroxylating species.
Figure 2.
Comparison of time courses for Fe(IV)O formation and decay (diamonds) and for DOPA formation (circles). DOPA was quantified by rapid-quench of the reaction at 5 °C of the complex of 500 μM TyrH, 480 μM Fe(II), 1 mM Tyr and 2 mM 6-MePH4 with an equal volume of 1.9 mM oxygen-containing buffer. The lines are simulations using the mechanism of Scheme 2 and values of k1 and k2 of 24 and 35 s−1, respectively, assuming that 80% of the enzyme complex is active.
Scheme 2.
To conclude, this work shows direct spectroscopic evidence for a high spin Fe(IV) species, presumably the postulated Fe(IV)O, as the hydroxylating intermediate in the reaction catalyzed by TyrH. This is the first example for this family of enzymes as well as the first for a mononuclear nonheme enzyme which catalyzes aromatic hydroxylation.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (GM-47291 to PFF), The Welch Foundation (A1245 to PFF), the donors of the Petroleum Research Fund (41170-G3 to CK), the National Science Foundation (NSF-642058 to JMB and CK), the Beckman Foundation (Young Investigator Award to CK), and the Dreyfus Foundation (Teacher Scholar Award to CK). We thank Candace C. Davison for help with the cryoreduction experiments.
Footnotes
Supporting Information Available: EPR spectra of a 20-ms sample recorded before and after cryoreduction and the spin Hamiltonian used for analysis of the Mössbauer spectra. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Abbreviations used: TyrH, tyrosine hydroxylase; Tyr, tyrosine; DOPA, dihydroxyphenylalanine; 6-MePH4, 6-methyl tetrahydropterin; 4a-HOPH3, 4a-hydroxypterin.
- 2.Fitzpatrick PF. Ann Rev Biochem. 1999;68:355–381. doi: 10.1146/annurev.biochem.68.1.355. [DOI] [PubMed] [Google Scholar]
- 3.Fitzpatrick PF. In: Advances in Enzymology and Related Areas of Molecular Biology. Purich DL, editor. Vol. 74. John Wiley & Sons; New York: 2000. pp. 235–294. [DOI] [PubMed] [Google Scholar]
- 4.Que L., Jr Nat Struct Biol. 2000;7:182–184. doi: 10.1038/73270. [DOI] [PubMed] [Google Scholar]
- 5.Goodwill KE, Sabatier C, Marks C, Raag R, Fitzpatrick PF, Stevens RC. Nat Struct Biol. 1997;4:578–585. doi: 10.1038/nsb0797-578. [DOI] [PubMed] [Google Scholar]
- 6.Fitzpatrick PF. Biochemistry. 2003;42:14083–14091. doi: 10.1021/bi035656u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillas PJ, Fitzpatrick PF. Biochemistry. 1996;35:6969–6975. doi: 10.1021/bi9606861. [DOI] [PubMed] [Google Scholar]
- 8.Moran GR, Derecskei-Kovacs A, Hillas PJ, Fitzpatrick PF. J Am Chem Soc. 2000;122:4535–4541. [Google Scholar]
- 9.Frantom PA, Seravalli J, Ragsdale SW, Fitzpatrick PF. Biochemistry. 2006;45:2372–2379. doi: 10.1021/bi052283j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krebs C, Price JC, Baldwin J, Saleh L, Green MT, Bollinger JM., Jr Inorg Chem. 2005;44:742–757. doi: 10.1021/ic048523l. [DOI] [PubMed] [Google Scholar]
- 11.Price JC, Barr EW, Tirupati B, Bollinger JM, Jr, Krebs C. Biochemistry. 2003;42:7497–7508. doi: 10.1021/bi030011f. [DOI] [PubMed] [Google Scholar]
- 12.Hoffart LM, Barr EW, Guyer RB, Bollinger JM, Jr, Krebs C. Proc Natl Acad Sci, USA. 2006;103:14738–14743. doi: 10.1073/pnas.0604005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galonic’ DP, Barr EW, Walsh CT, Bollinger JM, Jr, Krebs C. Nature Chem Biol. 2007;3:113–116. doi: 10.1038/nchembio856. [DOI] [PubMed] [Google Scholar]
- 14.Krebs C, Galonic’ Fujimori D, Walsh CT, Bollinger JM., Jr Acc Chem Res. 2007;40:484–492. doi: 10.1021/ar700066p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinnecker S, Svensen N, Barr EW, Ye S, Bollinger JM, Jr, Neese F, Krebs C. J Am Chem Soc. 2007;129:6168–6179. doi: 10.1021/ja067899q. [DOI] [PubMed] [Google Scholar]
- 16.Neese F. J Inorg Biochem. 2006;100:716–727. doi: 10.1016/j.jinorgbio.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 17.While δ = 0.25 mm/s would also be consistent with a low-spin Fe(III) complex, such a complex would not be expected to exhibit a quadrupole doublet in low fields, due to its S = 1/2 ground state.
- 18.Pestovsky O, Stoian S, Bominaar EL, Shan X, Münck E, Que L, Jr, Bakac A. Angew Chem Int Ed. 2005;44:6871–6874. doi: 10.1002/anie.200502686. [DOI] [PubMed] [Google Scholar]
- 19.Fitzpatrick PF. Biochemistry. 1991;30:3658–3662. doi: 10.1021/bi00229a010. [DOI] [PubMed] [Google Scholar]
- 20.Kuzmic P. Anal Biochem. 1996;237:260–273. doi: 10.1006/abio.1996.0238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.