Abstract
Mesothelioma is an uncommon malignancy whose global incidence continues to rise. The therapeutic standard for advanced disease is intravenous pemetrexed and cisplatin. The antifolate capecitabine is significantly less effective than pemetrexed. The balance between thymidylate synthase (TS), dihydropyrimidine dehydrogenase (DPD), and Thymidine Phosphorylase (TP) is critical to the efficacy of capecitabine. DNA from mesothelioma cell lines was bisulfite treated and examined by MS-PCR, RNA was obtained for real time PCR analysis, and protein lysates were obtained for Western immunoblot analysis. Cytotoxicity was assessed by MTT assay, comparing 5-aza-CdR pretreated or untreated cells with 5′-Deoxy-5-fluorouridine (DFUR), 5-FU, and pemetrexed. Finally bisulfite sequencing of the extracellular growth factor-1 (ECGF-1) gene was performed on 4 mesothelioma samples and pericardial tissue. One of the four cell lines tested (H290) was methylated for ECGF-1. This corresponded to a lack of TP expression by real time PCR and Western immunoblot. Treatment with 1 μM 5-aza-CdR increased TP mRNA and protein expression in H290. DFUR, the substrate for TP, showed increased cytotoxicity when delivered after 5-aza-CdR exposure in the methylated cell line. There was no difference in any of the unmethylated cell lines when cells were exposed to 5-FU or pemetrexed with or without 5-aza-CdR. Patient tumor samples revealed an increased number of methylated CpG sites in ECGF-1 compared to normal pericardium. Methylation of ECGF-1, leads to transcriptional silencing of TP and may explain the lack of any effect of capecitabine, especially when compared to pemetrexed.
Keywords: Thymidine phosphorylase, capecitabine, mesothelioma, pemetrexed, methylation
Introduction
Malignant mesothelioma is an uncommon thoracic tumor with 2,000–3,000 new cases in the US annually. It is highly associated with asbestos exposure. Its incidence worldwide is expected to continue to increase until 2020 [1; 2]. In the U.S. it seems to be on the decline due to aggressive public heath interventions. There are three histologic subtypes of mesothelioma: epithelioid, sarcomatoid, and mixed/biphasic. The epithelioid variant tends to have a more indolent clinical course and is characterized by more polygonal cells. The sarcomatoid and mixed variants portend a worse clinical prognosis and are pathologically characterized by elongated nuclei and more spindle cells [3; 4]. The SV40 DNA tumor virus has been implicated as a risk factor or co-factor in the development of this malignancy [5; 6; 7; 8] though its precise role in the pathogenesis of mesothelioma has yet to be clarified.
The clinical course of mesothelioma is aggressive with only a subset of patients being suitable for potentially curative resection, and for those not able to undergo resection, a median survival of 7 months with standard chemotherapeutic approaches can be expected [9]. Multiple single agent and combination chemotherapy regimens have been evaluated [10; 11]. No clearly effective therapy emerged until recently, with response rates ranging from 11 to 20% [12; 13; 14], but overall survival remaining less than 12 months. In reviewing the data, there is a suggestion that antimetabolite drugs have better efficacy than other cytotoxic agents [15]. The two most active drugs that have demonstrable activity in mesothelioma are raltitrexed (Tomudex®) and pemetrexed (Alimta®) which has highlighted the role of folic acid and folate metabolism plays in this tumor. Folate is an integral metabolite used in the cellular processes of DNA replication, RNA synthesis, as well as other normal cellular functions. 5-FU, a fluoropyrimidine, is an analogue of uracil which is converted intracellularly to FdUMP, FdUTP, and FUTP [16]. While it can be incorporated into DNA and RNA, its main mechanism of action appears to be inhibition of the enzyme, thymidylate synthase (TS), an enzyme critical for DNA synthesis. The rate limiting step in the catabolism of 5-FU is determined by the enzyme, dihydropyrimidine dehydrogenase (DPD).
The antimetabolite pemetrexed targets three enzymes within the DNA synthetic pathway; thymidylate synthase (TS), glycinamide ribonucleotide formlytransferase (GARFT), and dihydrofolate reductase (DHFR). Pemetrexed requires no enzymatic activation before directly affecting its targets. The use of pemetrexed in mesothelioma was based on initial Phase I data where activity was noted [17; 18]. Subsequent Phase II studies confirmed this activity [19], and a large Phase III study (comparing pemetrexed with cisplatin was compared to cisplatin alone in nearly 500 patients) led to its approval as first line treatment [20]. A recently completed Phase III study with raltitrexed, also showed activity compared to cisplatin alone [21].
Capecitabine is an oral fluorouracil prodrug that is converted to DFUR in the liver by the enzymes carboxylesterase and cytidine deaminase. DFUR is then transported to the tumor cell where the enzyme Thymidine Phosphorylase (TP) catalyses the reaction that then converts it to active 5-FU. One of the potential advantages of capecitabine is the relatively preferential expression of TP in tumor tissue compared to normal tissue [22]. This finding in addition to the evidence of anti-metabolite therapy as amongst the most active of the agents used to date against mesothelioma led to further exploration of oral capecitabine. After a Phase I study reported a mesothelioma patient treated with capecitabine orally at 1657 mg/M2 who had stable disease for 18 months [23], a Phase II study by the Cancer and Leukemia Group B evaluated capecitabine [24]. The 27 patient study showed a 4% partial response rate with 38% stable disease and a poor 1 year survival.
Methylation is an epigenetic phenomenon by which the nucleotide cytosine has a hydrogen replaced by a methyl group by the enzyme, DNA methyltransferase. The promoter related CG islands in tumor DNA are frequently methylated, leading to transcriptional silencing. The mechanism of this transcriptional silencing is hypothesized to be related to the inability of key transcriptional elements to access DNA in the presence of the bulky methyl groups or through the recruitment of key transcriptional repressors.
We hypothesized that the reason that capecitabine is ineffective in the treatment of mesothelioma as opposed to other anti-folate therapy like pemetrexed and raltitrexed, is through methylation induced transcriptional silencing of TP which is critical to the activation of capecitabine but plays no role in the metabolism or catabolism of other anti-folate agents.
Materials and Methods
Cell Culture
Four mesothelioma cell lines were obtained from ATCC and cultured in RPMI with 10% fetal bovine serum (FBS). Cells treated with 1 μM 5-aza-CdR had fresh media and drug added every 24 hours.
DNA isolation
DNA from both 5-aza-CdR treated and untreated cells was isolated by means of Qiagen Mini Kit per manufacturer’s directions.
Bisulfite modification
DNA was purified and treated with sodium bisulfite as described [25; 26]. The DNA was first denatured and then incubated with bisulfite overnight in a volume of 50 μl, then isolated and purified using the Qiaquick gel extraction kit as per manufacturer’s directions. The bisulfite reaction was completed by incubating the DNA with 5 μl fresh 3M NaOH at room temperature for 20 minutes. The DNA was again purified as above.
MS-PCR
Methylation specific primers for each gene were designed (Table 1). PCR conditions were as follows: 94°C for 20 sec, 62°C for 30 sec, and 72°C for 30 sec, for 35 cycles. SSS methylated treated DNA served as a positive control and the hypomethylated cell line, A549, was used as a negative control.
Table 1.
Methylated and Unmethylated primers for Key enzymes involved in the anti-tumor effects of anti-folate therapies:
| Thymidylate Synthase (TS) | |
|
Methylated-Forward 5′ AAG GCG CGG TCG ATT AGA C |
Methylated-Reverse 5′ AAA ACC ACG AAT ATA ACA CAA AAC G |
|
Unmethylated – Forward 5′ GGT TTA GAG AAG GTG TGG TTG ATT AGA T |
Unmethylated-Reverse 5′ CAC AAA ACC ACA AAT ATA ACA CAA AAC A |
| Dihydropyrimidine Dehydrogenase (DPD) | |
|
Methylated-Forward 5′ GGT TGT CGT GTT TGG CGC |
Methylated-Reverse 5′ ATC TAC CAA TAA CAA ACC CTC CTT ACG |
|
Unmethylated – Forward 5′ GTT GTG GTT GTT GTG TTT GGT GT |
Unmethylated-Reverse 5′ ATC TAC CAA TAA CAA ACC CTC CTT ACA |
| Thymidylate Phosphatase (TP) | |
|
Methylated-Forward 5′ TTA GCG TTG CGT CGC GTT C |
Methylated-Reverse 5′ CCG ACC AAT CCC CCG ATA C |
|
Unmethylated – Forward 5′ TGG GAT TTT AGT GTT GTG TTG TGT TT |
Unmethylated-Reverse 5′ CCC AAC CAA TCC CCC AAT AC |
RNA isolation and cDNA synthesis
The semi-quantitative real-time PCR was performed with optimized conditions for both the target gene and β-actin in a single reaction tube. Amplification was stopped in the exponential range for both genes. The exponential range was determined by phosphoimager quantification of the PCR product band intensities from different amplification cycles.
Western blot
Western immunoblot analysis was performed in standard fashion [27]. Cells were harvested and protein extracted with lysis buffer. Equivalent amounts of protein, 20 μg, were fractionated on a 9% SDS-PAGE, then transferred onto nylon membranes. The membranes were incubated with primary antibody at 4°C overnight while rocking. After washing with TBS-T four X 5 minutes, membranes are incubated with the appropriate secondary antibody for 1–2 hours. Protein bands were detected using chemiluminescent detection (Hy Glo®, Denville Scientific). The nylon membranes were then stripped in 0.2 M BME buffer at 55°C and then blocked and incubated with anti-tubulin antibody, secondary antibody with chemiluminescent detection performed as above.
MTT assay
Cytotoxicity was assayed by MTT assay and Trypan blue exclusion of treated cells. Two cell lines were chosen, H290 (TP methylated) and H1977 (TP unmethylated), to compare cytotoxicity following exposure to anti-metabolite therapy. For each cell line, cells were exposed to 1 μM 5-aza-CdR for 72 hours or control DMSO. An equal number of cells (2000) from each group were seeded into a 96-well plate 24 hr prior to experiments. Then all the cells were treated with varying concentrations of DFUR, 5-FU, or pemetrexed for an additional 48 hours. At 120 hrs after treatments began, dimethylthiazolyl-2–5-diphenyltetrazolium bromide (MTT) dye solution (20μl/100μl RPMI) (Sigma, St. Louis, MO) was added for 3 hours at 37°C. The absorbance at 490 nm wavelength was recorded using an ELISA microplate reader (Dynatech MR7000). Each experiment was performed in triplicate. The corrected absorbance of each sample was calculated by comparing with untreated control.
Bisulfite treatment of genomic DNA and sequencing
Mesothelioma samples were procured from the Cooperative Human Tissue Network through an IRB approved protocol. Genomic DNA from the mesothelioma tumor was isolated using TRIzol (Invitrogen). Bisulfite treatment of genomic DNA was performed with modifications using the previously published protocol [25]. Briefly, 1 μg genomic DNA was denatured by 3M NaOH for 10 min at 37°C in a volume of 50 μl and then incubated at 50°C overnight following the addition of 10 μl 10mM hydroquinone and 520 μl 3M sodium bisulfite. The DNA was then purified using the Qiaquick gel extraction kit (Qiagen, Valencia, CA, USA) and eluted into 50μl elution buffer. After adding 5 μl fresh 3M NaOH, the reaction was incubated at room temperature for 5 min. A measure of 10 μl 3M NaOAc (pH 5.0) was added to adjust the pH. The treated DNA was again purified using the Qiaquick gel extraction kit and eluted into 30 μl elution buffer.
One μg bisulfite converted DNA was amplified in a 50 μl volume for 95°C for 10 min to activate Taq DNA polymerase, followed by 35 cycles of amplification (denaturing at 94°C for 30 s, annealing at 58°C for 45 s and extension at 72°C for 45 s). The PCR product was then gel purified using the Qiaquick gel extraction kit and cloned into the PCR XL-TOPO vector (Invitrogen, Carlsbad, CA, USA). The cloning process followed the instructions from the TOPO TA Cloning Kits. 5 clones of each patient’s ECGF-1 gene were picked for plasmid isolation using the QIAprep® Spin Miniprep kit (Qiagen) and sequencing. Analysis of each single colony’s ECGF-1 gene was performed with web-based ClustalW2 software available from the European Bioinformatics Institute.
Results
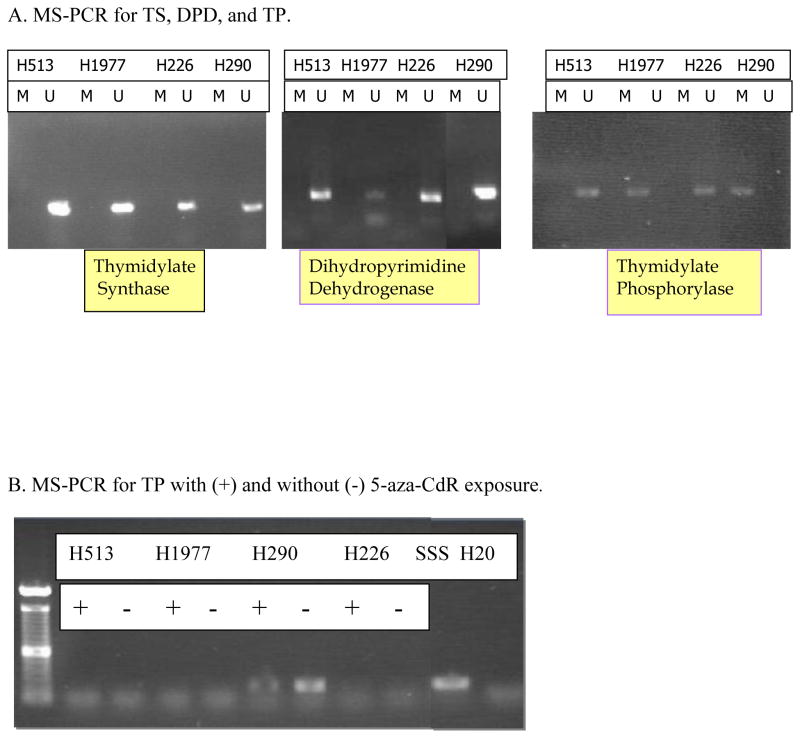
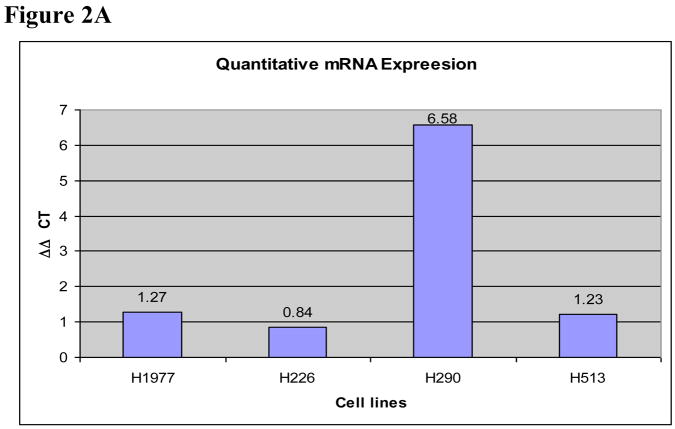
The four cell lines (H226, H513, H290, and H1977) were screened for the presence of methylation of three genes involved in folate and anti-folate metabolism: thymidylate synthase, dihydropyrimidine dehydrogenase, and thymidine phosphorylase with MS-PCR. We found no evidence of methylation in TS or DPD in the 4 mesothelioma cell lines. There was, however, evidence for methylation in the H290 cell line for the gene encoding TP, ECGF-1 (Figure 1). Based on the methylation of ECGF-1 in the H290 cell line, we confirmed that this epigenetic phenomenon correlated to mRNA expression as well as protein expression. Real time PCR results confirmed the greater than six-fold increase in TP mRNA in the H290 cell line after treatment with the DNMT inhibitor, 5-aza-CdR, compared to the same cell line that was not treated with the 5-aza-CdR. The H513, H1977, and H226 cell lines did not exhibit this upregulation of mRNA expression when exposed to 5-aza-CdR. Appropriate β-actin controls were performed to ensure specimen integrity (Figure 2A).
Figure 1.
A. MS-PCR for TS, DPD, TP. 4 mesothelioma cell lines were screened with methylated (M) and unmethylated (U) primer sets for the evidence of methylation by methylation-specific PCR. There was no such evidence for the methylation of TS or DPD in any of the four cell lines. There is evidence of methylation of TP in the H290 cell line. The experiment included a SSS treated positive control as well as water.
B. MS-PCR for TP. Cell lines were treated (+) or untreated (−) with 5-aza-CdR. After bisulfite treatment, MSPCR was performed with methylated sequence primers set. Significant amplification of H290 untreated methylated DNA is seen. + is SSS treated control and − is water.
Figure 2.
A. Thymidine phosphorylase mRNA expression. Chart with relative expression of mRNA by real time PCR. Each cell line was treated or untreated with 5-aza-CdR for 72 hours. By Syber Green analysis of the real-time PCR products, there was a substantial increase (>6 fold) in mRNA expression of cell line TP in H290 5-aza-CdR pretreated cells relative to H290 cells that were not treated. This increase was not seen in the unmethylated cell lines, H1977, H226, and H513. The control β-Actin (BA) RNA expression was consistent for all samples.
B. Thymidine phosphorylase western immunoblot: The H290 cell line when pretreated with 5-aza-CdR (+) increased expression of the protein TP compared to when not exposed to 5-aza-CdR (−). The unmethylated cell line, H1977, did not demonstrate a significant change in protein quantity despite 5-aza-CdR exposure. Tubulin controls, as seen below the TP assay, for the samples indicate an equal amount of protein was loaded in each lane.
Western immunoblot was performed to assess the relative abundance of TP protein. Analogous to the results seen in the RNA analysis, there was up-regulation of TP protein expression in H290 cells exposed to 5-aza-CdR compared to those which were not (Figure 2B). The H1977 cells showed no significant change in protein expression after 5-aza-CdR exposure.
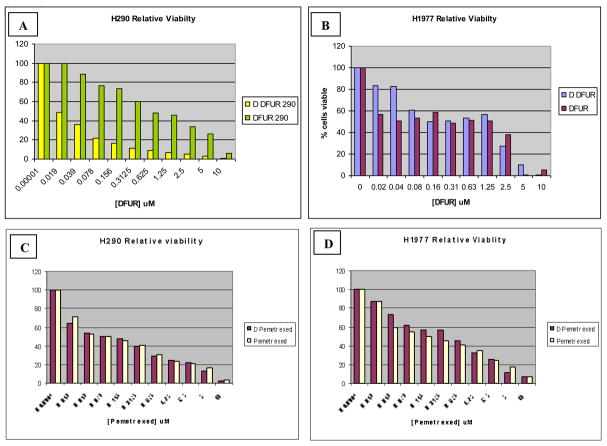
To assess the functional consequences of this protein up-regulation, MTT cytotoxicity assays were performed. We performed a series of cytotoxicity assays with various drugs that depend upon TS, DPD, and TP. When utilizing 5-FU or pemetrexed, (drugs that do not require processing to target TS directly in order to have cytotoxic effects), we predicted that the exposure to 5-aza-CdR would have no influence on the cytotoxicity relative to those that were not exposed. With DFUR, however, which requires TP in order to be converted to the cytotoxic 5-FU (Figure 1), we predicted that the presence of 5-aza-CdR in the methylated H290 cell line would enhance the cytotoxic effect of the drug compared to the methylated cells that were not exposed to the DNMT inhibitor. In the first assay, 5-aza-CdR pretreated and untreated H290 cells were exposed to increasing concentrations of DFUR, the active capecitabine metabolite. There was a relatively enhanced cytotoxicity of the H290 pretreated cells compared to those that were not pretreated Figure 3, Panel A. This same experiment was then performed on H1977 cells to see if such a change in cell viability would be seen in this unmethylated cell line. H1977 showed no difference in DFUR cytotoxicity despite prior exposure to 5-aza-CdR (Figure 3, Panel B. H290 and H1977 5-aza-CdR pretreated and untreated cells were then exposed to varying concentrations of 5-FU (Figure 3, Panels C and D) or pemetrexed (Figure 3 Panels E and F).
Figure 3.
MTT assay Curves. Panel A shows the results of the MTT cell viability assay (performed in triplicate) with the methylated H290 cell line treated with 5-aza-CdR in combination with DFUR as well as DFUR alone (D DFUR = 5-aza-CdR plus DFUR). There is a significant difference in viability with the addition of 5-aza-CdR to the H290 (methylated) cell line (panel A), but not in the H1977 (unmethylated) cell line (panel B). Also this difference cannot be seen in H290 and H1977 cells treated with pemetrexed (panels C and D) with or without 5-aza-CdR.
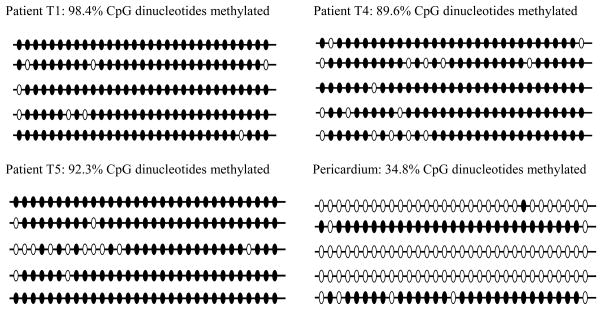
The analysis of bisulfite sequencing of the PCR amplified clones of ECGF-1 gene (encoding for TP protein) from patient tumor specimens was completed as well as the “normal” control of pericardial tissue purchased from ATCC. The methylation analysis revealed an increased number of methylated CpG islands in the tumor samples, averaging 93.45% methylated in the 4 patient samples compared to the pericardial tissue whose clones averaged only a 34.8 % methylated CpG islands in the same gene (Figure 4).
Figure 4.
Bisulfite sequencing of ECGF-1 promoter region containing 31 CpG dinucleotides for matched patient samples T1, T4, T5, and pericardium. Each line represents each of the 31 CpG islands from a specific clone. Open circles: no methylation of CpG islands. Filled circles: methylated CpG islands.
Discussion
In this study, we found that promoter methylation of CpG dinucleotides within the ECGF-1 gene (responsible for TP protein expression) led to transcriptional silencing of this gene product in one cell line as well as in patient specimens. Furthermore, treatment of the methylated cell lines with the DNMT inhibitor, 5-aza-CdR, led to mRNA and protein re-expression as predicted. Critical to the understanding of the clinical implications of this finding, is the cytotoxicity assay using 5-FU, pemetrexed, and DFUR. When the methylated cell line was pretreated with a DNMT inhibiting agent, 5-aza-CdR, it enhanced the response to DFUR compared to its untreated counterpart. Demethylation of a specific gene reversed a mechanism of chemo-resistance. The demonstration of hypermethylation of the ECGF-1 gene in tumor relative to pericardial tissue, as demonstrated in the bisulfite sequencing analysis suggests that this finding may be a relevant mechanism of drug resistance.
While DFUR is not a clinically utilized drug, it is an important penultimate metabolite of capecitabine on its path to active fluorouracil. The cytotoxic mechanisms of both capecitabine and DFUR should consequently be very similar. In fact, since the major target of raltitrexed and pemetrexed is TS, one would expect that clinical use of capecitabine should yield comparable results as these two drugs. Thus, the experiments presented in this paper represent a plausible and likely explanation for the lack of efficacy of the oral anti-folate, capecitabine in this disease. Orally delivered chemotherapeutic agents are often preferred by patients, from a tolerability stand point as well as a quality of life stand point. In the face of paucity of active agents in this malignancy, chemo-resistance mechanisms to existing agents should be examined. Improved utilization of existing, safe agents whose mechanisms of resistance can be reversed are as important to the treatment of this malignancy as are, yet- unproven, novel agents. In the era where targeted agents and molecular signatures of an individual’s tumor are being uncovered, even infrequent patterns become relevant discoveries.
The role of epigenetics in tumor biology has been well established. Recent studies have demonstrated that methylation of the O6MGMT gene leads to enhanced clinical activity of the oral alkylating temozolomide in patients with glioblastoma multiforme [28]. In this instance, MGMT enzymatically reverses the DNA damaging activity of temozolomide, therefore, transcriptional silencing of this enzyme adds to the efficacy of the drug. In contrast, the situation we describe of ECGF-1 methylation would be predicted to lead to reduced activation of the prodrug capecitabine. These modifications can have a tremendous impact on which therapies are active in a malignancy. If methylation can impart chemo-resistance to agents that have a reasonable to work then demethylation may increase the number of active agents to treat malignant mesothelioma. Demethylating agents in combination with cytotoxic chemotherapy may offer alternative treatment modalities to the few know actives ones that are presently used. While the clinical strategy of adding the demethylating agent 5-aza-CdR to capecitabine in mesothelioma is not an attractive option (given the ease of administration and activity of pemetrexed), we would predict, based upon our data, that this combination would have activity. Thus the significance of epigenetic modification in tumor DNA may affect both tumorigenesis as well as efficacy of treatment agents. Further experiments to determine epigenetic alterations in mesothelioma cell lines as well as in primary tumors are needed to further explore the other mechanisms of chemo-resistance as well as explanations of pathogenesis of tumor biology in this malignancy.
Acknowledgments
Mesothelioma Applied Research Foundation (GAO), 5T32CA009338-27 (KVK), and 5P30CA016058-31 (OSU-Comprehensive Cancer Center).
Abbreviations
- TS
thymidylate synthase
- TP
thymidine phosphorylase
- DPD
dihydropyrimidine dehydrogenase
- DNMT
DNA methyltransferase
- DFUR
5′-Deoxy-5-fluorouridine
- ECGF-1
extracellular growth factor-1
- 5-aza-CdR
5-aza-2′-deoxycytidine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peto J, Hodgson JT, Matthews FE, Jones JR. Continuing increase in mesothelioma mortality in Britain. Lancet. 1995;345:535–9. doi: 10.1016/s0140-6736(95)90462-x. [DOI] [PubMed] [Google Scholar]
- 2.Price B. Analysis of current trends in United States mesothelioma incidence. Am J Epidemiol. 1997;145:211–8. doi: 10.1093/oxfordjournals.aje.a009093. [DOI] [PubMed] [Google Scholar]
- 3.Curran D, Sahmoud T, Therasse P, van Meerbeeck J, Postmus PE, Giaccone G. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer experience. J Clin Oncol. 1998;16:145–52. doi: 10.1200/JCO.1998.16.1.145. [DOI] [PubMed] [Google Scholar]
- 4.Steele JP. Prognostic factors in mesothelioma. Semin Oncol. 2002;29:36–40. doi: 10.1053/sonc.2002.30299. [DOI] [PubMed] [Google Scholar]
- 5.Carbone M, Rdzanek MA. Pathogenesis of malignant mesothelioma. Clin Lung Cancer. 2004;5(Suppl 2):S46–50. doi: 10.3816/clc.2004.s.002. [DOI] [PubMed] [Google Scholar]
- 6.Bocchetta M, Di Resta I, Powers A, Fresco R, Tosolini A, Testa JR, Pass HI, Rizzo P, Carbone M. Human mesothelial cells are unusually susceptible to simian virus 40-mediated transformation and asbestos cocarcinogenicity. Proc Natl Acad Sci U S A. 2000;97:10214–9. doi: 10.1073/pnas.170207097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Procopio A, Strizzi L, Vianale G, Betta P, Puntoni R, Fontana V, Tassi G, Gareri F, Mutti L. Simian virus-40 sequences are a negative prognostic cofactor in patients with malignant pleural mesothelioma. Genes Chromosomes Cancer. 2000;29:173–9. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1019>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.De Luca A, Baldi A, Esposito V, Howard CM, Bagella L, Rizzo P, Caputi M, Pass HI, Giordano GG, Baldi F, Carbone M, Giordano A. The retinoblastoma gene family pRb/p105, p107, pRb2/p130 and simian virus-40 large T-antigen in human mesotheliomas. Nat Med. 1997;3:913–6. doi: 10.1038/nm0897-913. [DOI] [PubMed] [Google Scholar]
- 9.Herndon JE, Green MR, Chahinian AP, Corson JM, Suzuki Y, Vogelzang NJ. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest. 1998;113:723–31. doi: 10.1378/chest.113.3.723. [DOI] [PubMed] [Google Scholar]
- 10.Baas P, Ardizzoni A, Grossi F, Nackaerts K, Numico G, Van Marck E, van de Vijver M, Monetti F, Smid-Geirnaerdt MJ, van Zandwijk N, Debruyne C, Legrand C, Giaccone G. The activity of raltitrexed (Tomudex(R)) in malignant pleural mesothelioma. an EORTC phase II study (08992) Eur J Cancer. 2003;39:353–7. doi: 10.1016/s0959-8049(02)00668-8. [DOI] [PubMed] [Google Scholar]
- 11.Baas P. Chemotherapy for malignant mesothelioma: from doxorubicin to vinorelbine. Semin Oncol. 2002;29:62–9. doi: 10.1053/sonc.2002.30231. [DOI] [PubMed] [Google Scholar]
- 12.Steele JP, O’Doherty CA, Shamash J, Evans MT, Gower NH, Tischkowitz MD, Rudd RM. Phase II trial of liposomal daunorubicin in malignant pleural mesothelioma. Ann Oncol. 2001;12:497–9. doi: 10.1023/a:1011139918558. [DOI] [PubMed] [Google Scholar]
- 13.Zidar BL, Green S, Pierce HI, Roach RW, Balcerzak SP, Militello L. A phase II evaluation of cisplatin in unresectable diffuse malignant mesothelioma: a Southwest Oncology Group Study. Invest New Drugs. 1988;6:223–6. doi: 10.1007/BF00175403. [DOI] [PubMed] [Google Scholar]
- 14.Kindler HL, Millard F, Herndon JE, 2nd, Vogelzang NJ, Suzuki Y, Green MR. Gemcitabine for malignant mesothelioma: A phase II trial by the Cancer and Leukemia Group B. Lung Cancer. 2001;31:311–7. doi: 10.1016/s0169-5002(00)00166-5. [DOI] [PubMed] [Google Scholar]
- 15.Bueno R, Appasani K, Mercer H, Lester S, Sugarbaker D. The alpha folate receptor is highly activated in malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2001;121:225–33. doi: 10.1067/mtc.2001.111176. [DOI] [PubMed] [Google Scholar]
- 16.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–8. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 17.Curtin NJ, Hughes AN. Pemetrexed disodium, a novel antifolate with multiple targets. Lancet Oncol. 2001;2:298–306. doi: 10.1016/s1470-2045(00)00325-9. [DOI] [PubMed] [Google Scholar]
- 18.Mita AC, Sweeney CJ, Baker SD, Goetz A, Hammond LA, Patnaik A, Tolcher AW, Villalona-Calero M, Sandler A, Chaudhuri T, Molpus K, Latz JE, Simms L, Chaudhary AK, Johnson RD, Rowinsky EK, Takimoto CH. Phase I and pharmacokinetic study of pemetrexed administered every 3 weeks to advanced cancer patients with normal and impaired renal function. J Clin Oncol. 2006;24:552–62. doi: 10.1200/JCO.2004.00.9720. Epub 2006 Jan 3. [DOI] [PubMed] [Google Scholar]
- 19.Scagliotti GV, Shin DM, Kindler HL, Vasconcelles MJ, Keppler U, Manegold C, Burris H, Gatzemeier U, Blatter J, Symanowski JT, Rusthoven JJ. Phase II Study of Pemetrexed With and Without Folic Acid and Vitamin B12 as Front-Line Therapy in Malignant Pleural Mesothelioma. J Clin Oncol. 2003;21:1556–61. doi: 10.1200/JCO.2003.06.122. [DOI] [PubMed] [Google Scholar]
- 20.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, Niyikiza C, Paoletti P. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–44. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 21.van Meerbeeck JP, Gaafar R, Manegold C, Van Klaveren RJ, Van Marck EA, Vincent M, Legrand C, Bottomley A, Debruyne C, Giaccone G. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol. 2005;23:6881–9. doi: 10.1200/JCO.20005.14.589. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama Y, Inoue Y, Nagashima N, Katsuki T, Matsumoto K, Kadowaki K, Shibao K, Tsurudome Y, Hirata K, Sako T, Nagata N, Itoh H. Expression levels of thymidine phosphorylase (TP) and dihydropyrimidine dehydrogenase (DPD) in patients with gastrointestinal cancer. Anticancer Res. 2005;25:3755–61. [PubMed] [Google Scholar]
- 23.Budman DR, Meropol NJ, Reigner B, Creaven PJ, Lichtman SM, Berghorn E, Behr J, Gordon RJ, Osterwalder B, Griffin T. Preliminary studies of a novel oral fluoropyrimidine carbamate: capecitabine. J Clin Oncol. 1998;16:1795–802. doi: 10.1200/JCO.1998.16.5.1795. [DOI] [PubMed] [Google Scholar]
- 24.Otterson GA, Herndon JE, 2nd, Watson D, Green MR, Kindler HL. Capecitabine in malignant mesothelioma: a phase II trial by the Cancer and Leukemia Group B (39807) Lung Cancer. 2004;44:251–9. doi: 10.1016/j.lungcan.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–6. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu WG, Dai Z, Ding H, Srinivasan K, Hall J, Duan W, Villalona-Calero MA, Plass C, Otterson GA. Increased expression of unmethylated CDKN2D by 5-aza-2′-deoxycytidine in human lung cancer cells. Oncogene. 2001;20:7787–7796. doi: 10.1038/sj.onc.1204970. [DOI] [PubMed] [Google Scholar]
- 27.Zhu WG, Lakshmanan RR, Beal MD, Otterson GA. DNA methyltransferase inhibition enhances apoptosis induced by histone deacetylase inhibitors. Cancer Res. 2001;61:1327–33. [PubMed] [Google Scholar]
- 28.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]