Abstract
Rationale
Dopamine beta-hydroxylase (DBH) plays an essential role in catecholamine synthesis by converting dopamine into norepinephrine. Here we systematically investigated DBH polymorphisms associated with enzymatic activity as well as autonomic and BP/disease phenotypes in vivo.
Methods and Results
70 genetic variants were discovered at the locus; across ethnicities, much of the promoter was spanned by a 5’ haplotype block, with a larger block spanning the promoter in whites than blacks. DBH secretion was predicted by genetic variants in the DBH promoter, rather than the amino acid coding region. The C allele of common promoter variant C-970T increased plasma DBH activity, epinephrine excretion, the heritable change in BP during environmental stress in twin pairs, and also predicted higher basal BP in three independent populations. Mutagenesis and expression studies with isolated/transfected DBH promoter/luciferase reporters in chromaffin cells indicated that variant C-970T was functional. C-970T partially disrupted consensus transcriptional motifs for n-MYC and MEF-2, and this variant affected not only basal expression, but also the response to exogenous/co-transfected n-MYC or MEF-2; during ChIP, these two endogenous factors interacted with the motif.
Conclusions
These results suggest that common DBH promoter variant C-970T plays a role in the pathogenesis of human essential hypertension: common genetic variation in the DBH promoter region seems to initiate a cascade of biochemical and physiological changes eventuating in alterations of basal BP. These observations suggest new molecular strategies for probing the pathophysiology, risk, and rational treatment of systemic hypertension.
Keywords: Dopamine beta-hydroxylase, polymorphism, hypertension
INTRODUCTION
Dopamine beta-hydroxylase (DBH) catalyzes the oxidative hydroxylation of dopamine to norepinephrine. In the periphery, DBH is preferentially located in the adrenal medulla and synaptic vesicles of postganglionic sympathetic neurons(1). As a result of its secretory vesicular localization, DBH is released together with norepinephrine during synaptic transmitter release (2, 3). Because of its secretion into the extracellular space, DBH occurs in the cerebrospinal fluid (CSF) and plasma. CSF DBH is released mainly from central noradrenergic neurons, while plasma DBH emerges from the sympathochromaffin system. The enzymatic activity of DBH in plasma or CSF corresponds to the level of DBH protein (4, 5); such activity is relatively stable over time in same person, and family studies indicate that the effects of environmental factors such as stress and medications on DBH activity are small compared to genetic factors (6): variation in DBH activity in both plasma and CSF is highly heritable, and heredity accounts for over ~90% of human plasma DBH activity variation, or ~80% in central DBH activity variation(7). The ABO blood-group locus adjacent to DBH on chromosome 9q34 cosegregates with DBH activity(8, 9), indicating that genetic variation in the local DBH region regulates DBH expression. Indeed, several polymorphisms at DBH associate with either plasma or CSF DBH activity (10-12); for example, a polymorphism in the promoter region of DBH (C-970T) statistically accounts for ~35-52% of variation in plasma DBH activity(12).
Norepinephrine is the pivotal transmitter in the sympathetic nervous system, maintaining such functions as heart rate and BP. Excessive sympathoadrenal activity is implicated in the pathogenesis of hypertension, both primary (genetic, essential) and secondary (acquired), in both humans and experimental animals (13, 14). Given its necessary role in converting dopamine to norepinephrine, as well as its hereditary determination, the potential role of DBH in hypertension attracts substantial attention. Several lines of evidence suggest that DBH may play a role in the pathogenesis of hypertension. Genetic deficiency of DBH manifests as hypotension in both humans and knockout mice(15, 16). Of note for more common varieties of BP elevation, DBH inhibition attenuates development of hypertension in the spontaneously hypertensive rat (17, 18).
However it is not established whether common human genetic variation at DBH can be translated into physiological significance, in particular for susceptibility to hypertension. We therefore conducted systematic polymorphism discovery at human DBH, and not only tested associations with human autonomic traits but also probed the functional consequences of key genetic variants in cella.
METHODS
Populations and phenotypic characterization
UCSD twin pairs
432 white (European ancestry) twin subjects, aged 15-84 years (median, 40 years), were studied: 69% monozygotic (MZ) and 31% dizygotic (DZ). Twin zygosity was confirmed by single nucleotide and microsatellite polymorphisms, as previously described (19-21). 9.9% of the twins are hypertensive (8.8% treated with antihypertensive medications). Subject characteristics (Suppl. Table 1) are defined as in previous reports (22). In the twin subjects, BP (in mmHg) and pulse interval (R-R interval or heart period, in ms/beat) were recorded continuously and non-invasively for 5 min in seated subjects with a radial artery applanation device and dedicated sensor hardware (Colin Pilot; Colin Instruments, San Antonio, TX) and software [ATLAS from WR Medical, Stillwater, MN; and Autonomic Nervous System/Tonometric Data Analysis (ANS/TDA) from Colin Instruments], calibrated every 5 min against ipsilateral brachial arterial pressure with a cuff sphygmomanometer. Heart rate was recorded continuously with thoracic EKG electrodes to the Colin Pilot. Vital signs were also recorded with the same devices during environmental stress, in the form of the cold pressor test (CPT; immersion of the left hand in ice water for 60 sec after a preceding 10-min rest), as previously described (23). We identified at least three beats with stable BP and heart rate (each beat within 10% of the mean value) just before and at the end of the cold stress. Baroreceptor slope in the time domain was measured in response to both low pressure and high pressure spontaneous stimuli, as described (24).
Black BP extremes (Nigeria)
Samples from African subjects with the most extreme (upper and lower quartile) BPs in the population were kindly provided by Richard Cooper from Loyola University, Chicago. The subjects are from Nigeria, Africa, one of the cohorts in the International Collaborative Study on Hypertension in Blacks, as described (Suppl. Table 2) (25). The samples consist of men and women in the most extreme (upper and lower) quartiles (25th %iles) for systolic BP (SBP), adjusted by age and sex. SBP in the higher BP group was 177.2±1.7, and 101.2±0.7 in the lower BP group.
San Diego BP unrelated individuals
978 individuals of several biogeographic ancestries were studied: 611 normotensive and 367 hypertensive. 62% of individuals with hypertension took antihypertensive drugs: 43.6% on one antihypertensive medicine, with the remaining 18.4% on 2-6 types of antihypertensive medicines. Since epidemiologic data on blood pressure (BP) may be compromised by the effects of antihypertensive medications (26, 27), we accounted for the number of such drugs in the analysis; stepped increments of 8/4 mmHg, 14/10 mm Hg, or 20/16 mm Hg were added to measured SBP/DBP of treated subjects taking 1, 2, or 3 drug classes, respectively (26). Subject characteristics are defined in Suppl. Table 3.
Molecular genetics
Polymorphism discovery across the DBH locus in 4 population samples
We resequenced the DBH locus (exons, intron/exon borders, UTRs, and proximal promoter) for exhaustive variant discovery in n=88 subjects (2n=176 chromosomes). These individuals’ ancestries were n=25 white (European ancestry), n=25 black (sub-Saharan African ancestry), n=22 east Asian and n=16 Hispanic (Mexican-American). Reference (RefSeq) sequences were obtained from the UCSC Genome Browser (http://genome.ucsc.edu). Promoter positions were numbered with respect to the mRNA cap (transcriptional initiation) site. Segments of DBH were amplified by PCR using primers designed by Primer 3.0(28) and the nucleotide sequence determined using standard reagents and a capillary sequencing instrument as described(29, 30). Sequence was determined on an ABI 3100 automated sequencer and analyzed by Phred/Phrap/Consed (31, 32). Polymorphism and heterozygosity were identified using PolyPhred(33) and manually confirmed as described (30). Rare variants were confirmed by re-sequencing in multiple individuals and from the reverse direction.
SNPs
Genotypes for twin samples and two cohorts with extreme subjects were scored on amplified DNA by extension based methods: mass spectrometry (Sequenom, La Jolla, CA), or Pyrosequencing (Uppsala, Sweden). To ensure accurate assignment, genotypes were verified by visual inspection and artifacts were excluded from further analysis.
Biochemical assays
Blood and urine samples were obtained from individuals after at least 3 hours of fasting. Blood was collected from seated individuals, with a heparin-lock IV in place, into either heparin tubes (to prepare plasma for DBH assay) or EDTA tubes (to prepare plasma for catecholamine assay). Anticoagulated blood was promptly chilled on ice (at 0°C) prior to centrifugation within one hour for preparation of plasma. In untimed urine specimens, analytes were normalized to endogenous creatinine concentration in the same sample. Plasma DBH activity was measured by the spectrophotometric method in heparinized plasma (34). The principle of the method is as follows: the synthetic DBH substrate tyramine is converted by DBH (in the presence of Cu2+, N-ethylmaleimide, and fumarate) to octopamine, which is then oxidized to parahydroxybenzaldehyde by sodium periodate. The oxidation is terminated by sodium metabisulfite, and parahydroxybenzaldehyde is then quantified by its absorbance at 330 nm in the ultraviolet. The DBH activity inter-assay coefficient of variation was 4.5%. Plasma and urine catecholamines were assayed by the radioenzymatic method (35). The catecholamine assay involved transfer of a 3H label to catecholamines from S-adenosylmethionine during O-methylation, mediated by the enzyme catechol-O-methyltransferase (COMT). Prior to O-methylation, plasma catecholamines were extracted into dilute acetic acid to remove COMT inhibitors in plasma. Assay sensitivities (lower limits of detection) were 10 pg for norepinephrine and 6 pg for epinephrine. Intra-assay coefficients of variation were 4% for norepinephrine and 13% for epinephrine. Inter-assay coefficients of variation were 10% for norepinephrine and 13% for epinephrine.
Function of DBH promoter variants
Promoter/luciferase reporter activity assays
Human DBH promoter/reporter plasmids were constructed essentially as described (30). We obtained a human BAC genomic clone (RP11-317B10) spanning the entire DBH gene sequence from CHORI (http://bacpac.chori.org), from which we excised 3,051 bp of DBH promoter (from –3000 to +51 bp), containing common polymorphic sites, for insertion into the upstream region polylinker of the firefly luciferase reporter plasmid pGL3-Basic (Promega; Madison, WI). Synthetic replacements (at polymorphic sites) were made by site-directed mutagenesis (QuikChange; Stratagene) to produce the common haplotypes showed in Table 1. All inserts were sequence-verified before use. PC12 pheochromocytoma cells were transfected (at ~50-60% confluence, 1 day after 1:4 splitting) with 1 μg of supercoiled promoter haplotype/firefly luciferase reporter plasmid and 10 ng of the Renilla luciferase expression plasmid pRL-TK (Herpes simplex virus thymidine kinase promoter driving Renilla luciferase; Promega; Madison, WI) as an internal control per well, by the liposome method (Superfect; Qiagen, Valencia, CA). The firefly and Renilla luciferase activities in the cell lysates were measured 16 hours after transfection, and results were expressed as the ratio of firefly/Renilla luciferase activities (“Stop & Glo®”; Promega, Madison, WI). Each experiment was repeated a minimum of three times.
Trans-activation of the DBH promoter by co-transfected MEF2, or n-MYC
The pCMV (pcDNA3.1) eukaryotic expression plasmid for human MEF2 (Myocyte Enhancer Factor 2) were obtained from Open Biosystems (http://www.openbiosystems.com), while the expression plasmid for n-MYC (oncogene) was obtained from Charles L. Sawyers (UCLA). 50 ng of each expression plasmid were co-transfected with DBH promoter/luciferase reporter plasmids (at 1 μg). In control (“Mock”) experiments, the empty expression vector was co-transfected with DBH promoter/luciferase reporter plasmids. Firefly luciferase activities in the cell lysates were measured 16 hours after transfection, and the results were expressed as firefly luciferase activity/cell protein, rather than to Renilla luciferase, since the co-transfected trans-activator plasmids differentially activated the DBH and TK promoters.
Chromatin ImmunoPrecipitation (ChIP) assays
ChIP assays were performed as described (36) with modifications, using a Millipore ChIP assay kit (catalog #17-295; Millipore, Temecula, CA). Nuclei from PC12 cells (5-10×106) transfected with wild-type (C-970) versus variant (-970T) DBH promoter/reporter plasmids were crosslinked after 24 hours in 1% formaldehyde for 10 min and resuspended in lysis buffer. Chromatin was sonicated to nucleosomes in a Branson Digital Sonifier (Branson Ultrasonics; Danbury, CT), with 15 repeated cycles of 20 sec sonication (on power setting 6/10), separated by 1 min on ice. At the conclusion of sonication, a 1% agarose-TBE (tris-borate-EDTA) gel, stained by ethidium bromide, documented fragmentation of genomic DNA into the 300-1000 bp range. After chromatin sonication and centrifugation, samples were pre-cleared with salmon sperm DNA and protein A/agarose slurry, then incubated with anti-MEF2, anti-n-MYC or control (IgG) antibodies (Santa Cruz Biotechnology) at 4°C overnight. Immune complexes were captured with 40 μl of protein A and salmon sperm DNA slurry, washed extensively and eluted with 100 μl of buffer at room temperature. Eluates were pooled with 20 μl of 5 M NaCl and heated at 65°C overnight to reverse cross-links. DNA fragments were purified with QIAquick PCR purification kits (Qiagen). Human-specific DBH promoter primers surrounding C-970T were used for PCR amplification with 25-30 cycles (linear range); amplicons did not plateau in yield.
Statistical analyses
Descriptive statistics are reported as mean value ± one SEM, unless otherwise noted. Haplotypes were estimated from unphased diploid genotypes by PHASE (37) or HAP (38), and coded in each individual by copy number of a particular haplotype per diploid genome (0, 1, or 2 copies). Pair-wise linkage disequilibrium (LD) between each common SNP and polymorphism was quantified as D’ or r2 by the GOLD (39) or Haploview (40) algorithms. One-way ANOVA (using post-hoc correction for multiple comparisons) was performed in SPSS to test the significance of in vivo association between SNPs or haplotypes with plasma DBH activity, BP, or in vitro reporter activity assays. Generalized estimating equations (GEE) in SAS (Statistical Analysis System) evaluated genetic effects on autonomic biochemistry and physiology traits in the twin study, taking into account intra-pair correlations (22). Genotypes and haplotypes were grouped by the number of copies of the particular allele or haplotype (0,1,2 copies). Univariate analysis within general linear models (i.e., ANOVA) in SPSS were conducted to evaluate the effects of genotype or haplotype on plasma DBH activity, BP or disease status. All analyses were adjusted by age and sex (as covariates). In studies including subjects with hypertension, analyses were adjusted for the effects of antihypertensive medications, as previously described (26). In the single study of subjects with multiple biogeographic ancestries (San Diego blood pressure unrelated individuals), analyses were adjusted by ethnicity (gauged by self-report). Stepwise linear regression was performed in SPSS (version 17.0) on individuals with both promoter sequence and plasma DBH information. Within a haplotype block, SNPSpD (SNP SPectral Decomposition) (41) was used to estimate the experiment-wide significance threshold required to maintain the Type I error rate at 5% or less.
Bioinformatics of variant promoter motifs: Phylogenetic footprinting
Human and other mammalian promoter reference sequences were obtained from ENSEMBL and aligned pair-wise using the Smith-Waterman Algorithm (42). Regions of the alignment with >50% human-rat conservation were scored on position-weight-matrices (PWMs) from JASPAR (43) and TRANSFAC (44) databases, using the ConSite interface <www.phylofoot.org/consite> (45). Motifs from the consensus sequences scoring >80% of maximum for the PWM were considered binding candidates. Details of the scoring function are described elsewhere (46). We then ranked binding candidates in decreasing order, at the top those with maximum difference in PWM score between wild-type and variant human motifs (with at least one allele-score meeting the above criteria). Top hits represent transcription factors that bind to motifs containing at least one allele, are conserved between human and rat, and whose predicted binding motif is altered by mutation and hence likely of functional consequence.
RESULTS
DBH systematic polymorphism discovery
We resequenced 8,971 base pairs of DBH, including all coding and evolutionarily conserved regions, as well as ~3 kbp of proximal promoter, from 80 human subjects (2n=160 chromosomes) of diverse biogeographic ancestries (Suppl. Figure 1). To infer the ancestral alleles for each polymorphism, we also resequenced DBH from two chimpanzees and one bonabo. We identified 71 polymorphisms across the locus: 68 single nucleotide polymorphisms (SNPs), 2 insertion/deletions and 1 repeat (Suppl. Table 4). Eighteen DBH SNPs had minor allele frequencies greater than 5%. In the coding region, we found 8 non-synonymous SNPs (cSNPs), but only one (Ala172Thr) was common (minor allele frequency >5%), and none were in likely functional domains, e.g., Cys residues (that mediate inter-subunit disulfide bonds) (47), or Cu2+ cofactor-binding His-His or His-Xxx-His domains (48) (Suppl. Figure 2).
Minor alleles of the non-coding Ins/Del and repeat polymorphisms were also quite rare. We used all 18 common variants (minor allele frequency >5%) to construct haplotype blocks in Haploview, separately for resequenced white and black subjects. Haplotype “block 1” (containing the promoter) spanned a far longer range in whites than blacks, extending into exon-3 in whites (Figure 1A).
Figure 1. DBH polymorphism discovery.
1A. Haplotype blocks at the DBH locus for American populations of European and African ancestry. Haplotype blocks in the DBH region for European American population (left) and African population (right). LD map and haplotype blocks were constructed by Haploview, using our resequencing data. The “solid spine” method was used to position haplotype block boundaries. The four-color scheme (from white, blue, pink, red) represents the increased value of LD with maximum D’ as one in bright red. Block-1 is longer in Whites than in Blacks, extending further into the coding region.
1B. Human DBH proximal promoter elements, common polymorphisms, and motifs altered by common genetic variants. Motifs altered by common variant C-970T were predicted by phylogenetic footprinting, as detailed in Methods. Elements within the core proximal promoter are assembled from several previous reports in the literature, rather than being identified in this report; of note, this very proximal region (-210/-1 bp) was invariant during our resequencing of n=88 subjects (2n=176 chromosomes). The short homeodomain 4-bp motif (ATTA) indicates potential homeodomain sites. Regions are not drawn to scale.
Figure 1B positions DBH variants in the promoter, with respect to known promoter elements; of note, the core proximal promoter (-210/-1 bp), containing several elements of documented importance (e.g., TATA, CRE, TPA response, AP1, E-boxes, homeodomains), was devoid of common variation.
DBH common genetic variants: Effects on plasma DBH enzymatic activity
(Suppl. Figure 3). To determine which polymorphisms in DBH predict release of the enzyme into plasma, we studied 95 healthy subjects from two populations (41 African American [A-A] and 54 European American [E-A]), coupled with genotypes at 18 common polymorphic sites by direct sequencing. DBH activity in E-A was nearly two-fold higher than in A-A (P<0.001), consistent with previous reports stretching back for nearly 3 decades( 34, 49). Several polymorphisms, including C-970T (suppl. Figure 3), showed significant association with DBH activity in both A-A and E-A by ANOVA. These SNPs are located in promoter region, except one in intron-1 (G+457C) and one in exon-2 (A+3628G) in strong linkage disequilibrium (LD) with promoter SNPs T-2734C, C-2073T and G+457C in E-A. All promoter SNPs lay within the most 5’ haplotype block, both in Whites and Blacks (Figure 1A).
We tested whether DBH genetic variants can account for DBH activity differences between E-A and A-A; by linear regression, a model containing C-2073T and C-970T accounted for ~59.5% of trait variance, while addition of ethnicity increased R2 only to ~67.6%.
DBH common promoter variants: Prediction of BP in several populations
DBH resequencing identified common variants in the promoter region that formed a tightly linked block extending into intron-3 in white individuals, but ending within the promoter in blacks. Thus we began DBH marker-on-trait studies with a 7-SNP haplotype (6 in the promoter, one in intron-1) in white twins, while in blacks only 4 SNPs in the promoter region were used (Suppl. Table 5).
DBH C-970T in San Diego twin pairs (white)
We tested the association of promoter SNPs with biochemical and physiological autonomic phenotypes in a series of twins for which we have previously shown high heritability of such traits ( 22). Within the promoter haplotype block, C-970T was significantly associated with both pre- and post-SBP: the minor allele (T) that predicts lower DBH activity (Suppl. Figure 3) is associated with lower SBP both before and after environmental (cold) stress (Figure 2B). The T allele at C-970T also predicted decreased catecholamine excretion (Figure 2C). By SNPSpD (41) within the promoter block, the experiment-wide significance threshold required to maintain the Type I error rate at <5% remained 0.05 (reflecting the high degree of LD within this block); this threshold was exceeded by C-970T.
Figure 2. DBH promoter variants: Effects on BP and catecholamines in San Diego twin pairs.
Mean values ± SEM are shown, calculated from all individuals as indicated in each bar (both members of each twin pairs, MZ and DZ) in that group, by GEE. Analyses were adjusted by age and sex. BP results were also adjusted for the effects of anthypertensive medications in the 8.8% treated for hypertension.
2A: Haplotype effects on basal/resting BP. 7-SNP haplotype-1 (suppl. Table 5B) is significantly associated with basal SBP.
2B: Stress-induced BP changes. Effects of C-970T genotype on both pre- and post-cold stress SBP in twins.
2C: Catecholamines. Joint effects of C-970T genotype on both urine epinephrine excretion and blood pressure.
DBH C-970T in black (Nigeria) population BP extremes: Replication #1
Since the promoter haplotype block is shorter in blacks than whites (Figure 1A), we genotyped four common promoter SNPs to identify common haplotypes in this sample. In line with other populations, four common haplotypes are present in this extreme sample (Suppl. Table 5). We did not find an overall haplotype effect on BP, but when each SNP was analyzed separately, the T allele at C-970T predicted lower SBP and DBP (Suppl. Figure 4).
DBH C-970T in San Diego unrelated individuals: Replication #2
We typed C-970T in a mixed population of hypertensives and normotensives from San Diego (Suppl. Table 3), and once again found that the T allele predicted lower BP. Since subjects of 5 different biogeographic ancestries were included in this sample, we performed analyses with or without ethnicity as covariate; in each case, the T allele of C-970T predicted lower BP (Suppl. Figure 5).
Baroreceptor sensitivity as a function of DBH genotype
Since biochemical/physiological counter-regulatory responses to hypertension are mediated by baroreceptor/mechanoreceptor systems, we tested the effectiveness of the response to both low pressure and high-pressure stimuli as a function of DBH promoter genotype at C-970T (Suppl. Figure 6). Baroreceptor function was intact (at ~12-15 msec/mmHg), but did not differ as a function of DBH promoter genotype.
Function of DBH promoter variants (common haplotypes and C-970T)
Basal activity in chromaffin cells
We constructed luciferase reporter plasmids for the 4 most common naturally occurring DBH haplotypes (Suppl. Table 5), and then created 4 mutant haplotypes by altering position -970, from C→T or T→C, forming 4 pairs of haplotypes in which only difference occurs at -970. Since variant C-970T appeared to be crucial in influencing BP (Figure 2) and plasma DBH activity (Suppl. Figure 3), we then took advantage of mutant haplotypes to isolate the function of individual variants in cella. Higher gene expression resulted from the T allele at C-970T (Figure 3A), when tested upon balanced/matched haplotype backgrounds.
Figure 3. DBH promoter activity: Role of common variant C-970T.
3A. Site-directed mutagenesis at C-970T. On 4 common natural haplotype backgrounds, C-970 or -970T were point-mutated to -970T or C-970, creating 4 non-natural/artificial haplotypes. Results are compared by ANOVA followed by post-hoc T-tests. These basal (unstimulated) activity results are presented as firefly luciferase activity normalized to Renilla activity in the same cell lysate. As a negative control, the ratio of firefly/renilla luciferase activities for the promoterless luciferase reporter vector (empty vector pGL3-Basic) co-transfected with transfection efficiency control plasmid Renilla plasmid (pTK-RL) was 0.008±0.001. The absolute firefly luminescence for haplotype-1→firefly luciferase transfection was 1334.7±198.2 RLU/sec; 50 μl from each 500 μl cell lysate were used for luciferase assay. Each experiment was performed in triplicate, and such experiments were repeated at least three times.
3B. Trans-activation of C-970T by n-MYC. The n-MYC motif in the sequence is shown. Results are presented as fold-stimulation by exogenous/co-transfected n-MYC for each haplotype. Mock: Co-transfected n-MYC empty expression vector. The RLU/protein for haplotype-1→firefly luciferase transfection (without co-transfected n-MYC) was 9.98±0.31 RLU/μg, with an absolute firefly RLU/sec of 346±19.2. 50 μl of each 500 μl cell lysate was used for luciferase assay.
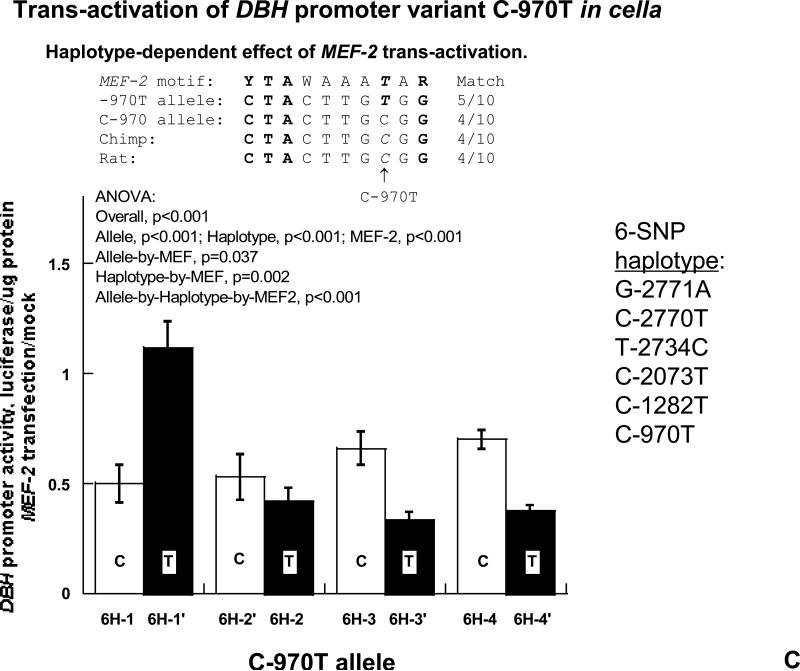
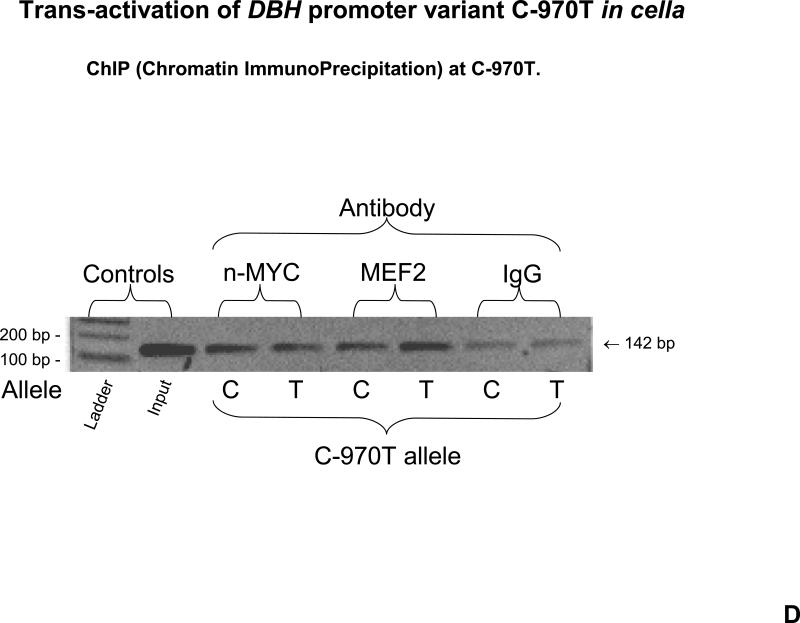
3C, Trans-activation of C-970T by MEF-2. The MEF-2 motif in the sequence is shown. Results are presented as fold over basal activity for each haplotype. Mock: Co-transfected MEF-2 empty expression vector. Basal RLU values were as reported in Figure 3A. 3D. ChIP (Chromatin ImmunoPrecipitation) at C-970T. Results of nucleosomal immunoprecipitations from PC12 cells. “Input” DNA derives from the nucleosomal preparation prior to immunoprecipitation. Antibodies directed against MYC or MEF-2 (or pre-immune serum) were used to precipitate nucleosomes derived from PC12 cells transfected with either the C-970 or -970T alleles, on promoter/reporter plasmids. The nucleosomal immunoprecipitates were interrogated with PCR primers spanning a 142 bp amplicon centered upon C-970T.
Role of promoter variant C-970T: Differential trans-activation
Analysis of the sequence immediately surrounding C-970T revealed partial matches to consensus n-MYC (4/6 bases; Figures 1B & 3B) and MEF2 (5/10 bases; Figures 1B & 3C) motifs. Other sequenced mammals (chimp, cow, rat, mouse) had the C-970 allele at the equivalent position of human C-970T, while a subset of humans displayed transition to -970T. To probe the significance of the putative n-MYC and MEF2 motifs, we tested the effects of n-MYC or MEF2 by co-transfection with DBH promoter/reporters bearing either the C-970 or the -970T allele. In each case, the effects were dependent upon haplotype context, with significant factor-by-haplotype interactions. As expected from the n-MYC motif match, transfected n-MYC preferentially activated the T allele on 6H-1, 6H-3, and 6H-4 backgrounds, though not the 6H-2 background. As expected from the MEF2 motif match, transfected/expressed MEF2 preferentially activated the T allele on a 6H-1 background, but not on 6H-3 or 6H-4 backgrounds (Figure 3C).
Endogenous transcription factor binding to C-970T: ChIP (Chromatin ImmunoPrecipitation)
After nucleosomal immunoisolation (Figure 3D), both anti-MEF2 and anti-n-MYC yielded specific DNA amplicons spanning C-970T; at MEF2, the semiquantitative pattern (T allele > C allele) was consistent with the functional results for the most common DBH haplotype (6H-1; Figure 3C), while for n-MYC the C>T pattern was consistent with luciferase results for DBH common haplotype 6H-2 (Figure 3B).
DISCUSSION
Overview
DBH encodes an indispensable enzyme in the catecholamine biosynthetic chain, resulting in the formation of norepinephrine. In this report we approach the effect of common variation in the DBH promoter region for DBH expression and activity, catecholamine secretion, autonomic physiology, and finally for cardiovascular disease (hypertension) risk.
We began with systematic variant discovery across the locus in 4 biogeographic ancestry groups, and found that DBH promoter variants lie within a linkage disequilibrium (LD) block, whose extent varied by ethnicity. Promoter variants predicted the very proximate trait of plasma DBH activity. In twin pairs, DBH promoter variant C-970T influenced heritable “intermediate phenotypes” for later development of hypertension: the proximate biochemical trait of urine epinephrine excretion, and the more distant physiological risk trait of BP response to environmental (cold) stress. Finally, DBH promoter variant C-970T associated with BP in three different populations.
Transfected DBH promoter haplotypes indicated a functional role for variant C-970T, which disrupted known transcriptional regulatory motifs, and trans-activation of the variant by the known factors resulted in differential activation of the two alleles.
DBH C-970T contribution to blood pressure: Multiple lines of evidence
First, we used both haplotypes and individual variants in marker-on-trait association analyses. Haplotypes are a useful tool for scanning large genomic regions in the search for disease-predisposition variants (50). Once a trait-contributory genetic locus (or block) has been identified, systematic variant discovery may then yield the responsible polymorphism. During the initial hypertension associations, the haplotypes as well as single SNP C-970T were associated with basal systolic BP in the twin group. However in the replicate population groups, only SNP C-970T (though not more extended promoter haplotypes) predicted BP, perhaps reflecting “dilution” of the effects of the functional variant (C-970T) by the larger genomic region captured by LD.
Second, we investigated the hemodynamic response to environmental (cold) stress as an “Intermediate phenotype” in the analyses. In a complex trait (51) such as hypertension, where there are heritable effects but the genetic architecture of the trait is largely unknown, genetic approaches focused only on the ultimate disease trait (either family/pedigree linkage or case/control association) might have limited statistical power. In a setting of late/delayed penetrance of the ultimate disease trait (such as hypertension), as well as likely genetic heterogeneity, the “intermediate” phenotype (52) strategy may be a useful in the search for disease predisposition loci, where such traits may be “intermediate” in both a temporal and a mechanistic sense. The hemodynamic response to environmental (cold) stress is a predictor of the development of later cardiovascular events, such as hypertension (53-57), rendering it useful even prior to the onset of overt disease (52, 58, 59). Here we found that DBH C-970T predicted two such heritable traits in predominantly normotensive twin pairs: catecholamine secretion and environmental stress BP change (22).
Third, we conducted in cella functional studies to confirm differential effects of the C-970T alleles on DBH expression. Expression of DBH promoter variants on a variety of haplotypic backgrounds, both natural and artificial, indicated that C-970T exerted functional changes upon reporter activity. Follow-up experiments indicated that this variant harbored partial consensus matches for two transcriptional control motifs: MYC and MEF-2. Context/haplotype background-dependence was observed for trans-activation by either n-MYC or MEF-2 at C-970T. Finally, ChIP demonstrated interactions of endogenous MYC and MEF-2 with the C-970T region in nucleosomes. Taken together, these complementary results indicate that particular transcriptional motifs function differentially at C-970T.
Mechanisms in cella versus those in vivo
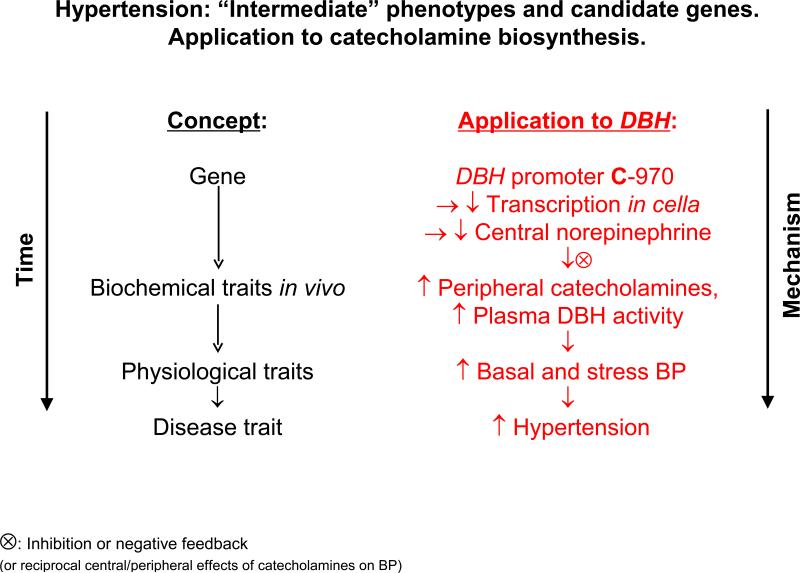
We were initially surprised to note that the DBH haplotypes and individual variants tested had directionally opposite effects on transcription in cella (reporter gene activity in transfected cells) and plasma DBH activity in vivo. However, norepinephrine has very different actions on BP centrally and peripherally. Indeed, such actions are reciprocal: while peripherally (e.g., intravenously) administered norepinephrine elevates BP, centrally administered norepinephrine acts in the vasomotor center of the brainstem to decrease sympathetic outflow and hence BP (Figure 4).
Figure 4. Human DBH genetic variation: Schema for effects on autonomic and disease traits Intermediate phenotype hypothesis for DBH.
Framework for integration of the experimental results on DBH genetic variation, early/proximate autonomic traits, later disease traits (such as hypertension), and changes in DBH secretion.
Alternatively, negative feedback mechanisms can be proposed to explain the contrasting results in vivo and in cella. After exocytosis, norepinephrine acts via presynaptic α2-receptors on sympathetic termini, both decreasing catecholamine biosynthesis and inhibiting vesicle exocytosis. Blood pressure elevation by catecholamines also activates the baroreceptor mechanism, thereby diminishing efferent sympathetic activity and consequently decreasing vesicle exocytosis. We have previously reported a similar effect for the catecholamine release-inhibitory catestatin region of CHGA, for which we proposed reciprocal central and peripheral actions on BP (30).
Conclusions and perspectives
Our data suggest that common genetic variation in the promoter region of DBH, a necessary enzyme in catecholamine biosynthesis, confers a series of changes in autonomic traits (Figure 4): DBH expression and catecholamine secretion, heritable environmental stress-induced BP increments, and ultimately risk for hypertension. Studies with the isolated DBH promoter established the role of particular cis-/trans- interactions in the nucleus, especially at promoter position C-970T. Since this promoter variant is so common (indeed, trait-elevating C allele is the major/most frequent allele in each population studied), our observations are consistent with the “common disease/common allele” hypothesis for frequent traits in the population (60), and suggest new molecular strategies for probing the pathophysiology, risk, and rational treatment of hypertension.
Since catecholamine biosynthesis proceeds by a sequence of reactions in series ( 61, 62). Further analysis of genetic interactions between variants of DBH and other genes in the biosynthetic pathway may assist in defining contributions of such genes to susceptibility for hypertension.
Condensed abstract.
We systematically probed dopamine beta-hydroxylase polymorphisms associated with enzymatic activity as well as autonomic and BP/disease phenotypes in vivo. DBH secretion was predicted by genetic variants in the DBH promoter, rather than the coding region. The C allele of variant C-970T increased plasma DBH activity, epinephrine excretion, the heritable change in BP during environmental stress in twin pairs, and also predicted higher basal BP in three independent populations. Mutagenesis and expression studies with isolated/transfected DBH promoter/luciferase reporters in chromaffin cells indicated that variant C-970T was functional. C-970T partially disrupted consensus transcriptional motifs for n-MYC and MEF-2, and this variant affected not only basal expression, but also the response to exogenous/co-transfected n-MYC or MEF-2; during ChIP, these two endogenous factors interacted with the motif. These results suggest that DBH variant C-970T may play a role in the pathogenesis of human essential hypertension.
Supplementary Material
On-line/Supplementary Figure legends.
Supplementary Figure 1. Human DBH polymorphism discovery: Re-sequencing strategy. Sequences conserved between mouse and human DBH were visualized in VISTA. Location of common SNPs (solid rods) relative to exons and conserved non-coding sequences is indicated by position. Common haplotypes in each haplotype block are shown and connections between blocks are indicated by solid lines. Common haplotype blocks were constructed using HaploBlockFinder (63) with minimal haplotype coverage of 80% in each block. The chimp haplotype for the common SNPs is also shown.
Supplementary Figure 2. Human dopamine beta-hydroxylase (DBH): Functional domains and coding region (open reading frame) cSNPs.
Supplementary Figure 3. DBH promoter variant C-970T: Effects on plasma DBH activity in African Americans (A-A) and European American (E-A) subjects. Analyses were adjusted by age and sex. Error bars indicate mean ± SEM in each panel.
Left: Plasma DBH activity variation between European-American (E-A) and African-American (A-A) subjects.
Right. Plasma DBH activity in E-A and A-A subjects, as a function of promoter C-970T genotype.
Supplementary Figure 4. Replication: DBH promoter variant C-970T effects on BP in an independent population of Nigeria BP extreme groups. Variant -970T is associated with reduced systolic and diastolic BP in Nigerian subjects from population BP extremes (upper and lower quartiles of BP). Mean BP values ± SEM are shown, calculated from all individuals as indicated in each bar. Effects of genotypes on BP were evaluated using a general linear model (ANOVA), adjusted by age and sex (BP was untreated). Significant p values are shown.
Supplementary Figure 5. Replication: DBH promoter variant C-970T effects on BP in an independent population of San Diego unrelated individuals. Variant -970T is associated with reduced systolic BP in San Diego unrelated subjects. Analyses were adjusted by age, sex, self-identified ethnicity (because of the inclusion of more than one biogeographic ancestry group), and effect of antihypertensive medications (in treated hypertensives). Mean SBP values ± SEM are shown, calculated from all individuals as indicated in each bar.
Supplementary Figure 6: DBH promoter functional polymorphism C-970T: Baroreceptor function is intact in twin pairs. Downward and upward deflections measured in San Diego white twins are shown. Analyses were adjusted for age and sex. There were no trait differences among individuals stratified by C-970T genotype.
Supplementary Table 1. Descriptive statistics for the San Diego twin study.
Supplementary Table 2. Descriptive statistics for the Nigeria (black) population BP extreme study (upper and lower 25th %iles).
Supplementary Table 3. Descriptive statistics for the San Diego BP unrelated individuals.
Supplementary Table 4. Summary of naturally occurring genetic variation at the human DBH locus in 4 populations.
Supplementary Table 5. Promoter region haplotypes (based on different numbers of SNPs) in population analyses and in cella studies.
ACKNOWLEDEMENTS
Supported by grants from the National Institutes of Health and the Department of Veterans Affairs. We appreciate the assistance of the NIH/NCRR-supported General Clinical Research Center at UCSD (RR00827) and the NIH/NCMHD EXPORT minority health center (MD000220). We appreciate scientific discussions on DBH with David Weinshenker, Emory University.
Support: National Institutes of Health, Department of Veterans Affairs.
Abbreviations (alphabetical)
- A-A
African American
- BP
Blood Pressure
- ChIP
CHromatin ImmunoPrecipitation
- CPT
Cold Pressor Test
- DBH
Dopamine Beta-Hydroxylase
- DZ
Dizygotic twin
- E-A
European American
- GEE
Generalized Estimating Equations (in SAS)
- LD
Linkage Disequilibrium
- MZ
Monozytotic twin
- ORF
Open Reading Frame
- PCR
Polymerase Chain Reaction
- SNP
Single Nucleotide Polymorphism
- SNPSpD
SNP SPectral Decomposition
- QTL
Quantitative Trait Locus
REFERENCES
- 1.Kim CH, Zabetian CP, Cubells JF, Cho S, Biaggioni I, Cohen BM, Robertson D, et al. Mutations in the dopamine beta-hydroxylase gene are associated with human norepinephrine deficiency. Am J Med Genet. 2002;108:140–147. [PubMed] [Google Scholar]
- 2.De Potter WP, De Schaepdryver AF, Smith AD. Release of chromogranin A and dopamine-beta-hydroxylase from adrenergic nerves during nerve stimulation. Acta Physiol Scand Suppl. 1970;357:8. [PubMed] [Google Scholar]
- 3.Weinshilboum RM, Thoa NB, Johnson DG, Kopin IJ, Axelrod J. Proportional release of norepinephrine and dopamine-beta-hydroxylase from sympathetic nerves. Science. 1971;174:1349–1351. doi: 10.1126/science.174.4016.1349. [DOI] [PubMed] [Google Scholar]
- 4.O'Connor DT, Cervenka JH, Stone RA, Levine GL, Parmer RJ, Franco-Bourland RE, Madrazo I, et al. Dopamine beta-hydroxylase immunoreactivity in human cerebrospinal fluid: properties, relationship to central noradrenergic neuronal activity and variation in Parkinson's disease and congenital dopamine beta-hydroxylase deficiency. Clin Sci (Lond) 1994;86:149–158. doi: 10.1042/cs0860149. [DOI] [PubMed] [Google Scholar]
- 5.Weinshilboum RM, Raymond FA, Elveback LR, Weidman WH. Serum dopamine-beta-hydroxylase activity: sibling-sibling correlation. Science. 1973;181:943–945. doi: 10.1126/science.181.4103.943. [DOI] [PubMed] [Google Scholar]
- 6.Weinshilboum RM, Schorott HG, Raymond FA, Weidman WH, Elveback LR. Inheritance of very low serum dopamine-beta-hydroxylase activity. Am J Hum Genet. 1975;27:573–585. [PMC free article] [PubMed] [Google Scholar]
- 7.Oxenstierna G, Edman G, Iselius L, Oreland L, Ross SB, Sedvall G. Concentrations of monoamine metabolites in the cerebrospinal fluid of twins and unrelated individuals--a genetic study. J Psychiatr Res. 1986;20:19–29. doi: 10.1016/0022-3956(86)90020-8. [DOI] [PubMed] [Google Scholar]
- 8.Craig SP, Buckle VJ, Lamouroux A, Mallet J, Craig IW. Localization of the human dopamine beta hydroxylase (DBH) gene to chromosome 9q34. Cytogenet Cell Genet. 1988;48:48–50. doi: 10.1159/000132584. [DOI] [PubMed] [Google Scholar]
- 9.Perry SE, Summar ML, Phillips JA, 3rd, Robertson D. Linkage analysis of the human dopamine beta-hydroxylase gene. Genomics. 1991;10:493–495. doi: 10.1016/0888-7543(91)90339-g. [DOI] [PubMed] [Google Scholar]
- 10.Cubells JF, van Kammen DP, Kelley ME, Anderson GM, O'Connor DT, Price LH, Malison R, et al. Dopamine beta-hydroxylase: two polymorphisms in linkage disequilibrium at the structural gene DBH associate with biochemical phenotypic variation. Hum Genet. 1998;102:533–540. doi: 10.1007/s004390050736. [DOI] [PubMed] [Google Scholar]
- 11.Wei J, Ramchand CN, Hemmings GP. Possible control of dopamine beta-hydroxylase via a codominant mechanism associated with the polymorphic (GT)n repeat at its gene locus in healthy individuals. Hum Genet. 1997;99:52–55. doi: 10.1007/s004390050310. [DOI] [PubMed] [Google Scholar]
- 12.Zabetian CP, Anderson GM, Buxbaum SG, Elston RC, Ichinose H, Nagatsu T, Kim KS, et al. A quantitative-trait analysis of human plasma-dopamine beta-hydroxylase activity: evidence for a major functional polymorphism at the DBH locus. Am J Hum Genet. 2001;68:515–522. doi: 10.1086/318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esler M, Ferrier C, Lambert G, Eisenhofer G, Cox H, Jennings G. Biochemical evidence of sympathetic hyperactivity in human hypertension. Hypertension. 1991;17:III29–35. doi: 10.1161/01.hyp.17.4_suppl.iii29. [DOI] [PubMed] [Google Scholar]
- 14.Ferrier C, Cox H, Esler M. Elevated total body noradrenaline spillover in normotensive members of hypertensive families. Clin Sci (Lond) 1993;84:225–230. doi: 10.1042/cs0840225. [DOI] [PubMed] [Google Scholar]
- 15.Robertson D, Haile V, Perry SE, Robertson RM, Phillips JA, 3rd, Biaggioni I. Dopamine beta-hydroxylase deficiency. A genetic disorder of cardiovascular regulation. Hypertension. 1991;18:1–8. doi: 10.1161/01.hyp.18.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Swoap SJ, Weinshenker D, Palmiter RD, Garber G. Dbh(-/-) mice are hypotensive, have altered circadian rhythms, and have abnormal responses to dieting and stress. Am J Physiol Regul Integr Comp Physiol. 2004;286:R108–113. doi: 10.1152/ajpregu.00405.2003. [DOI] [PubMed] [Google Scholar]
- 17.Ohlstein EH, Kruse LI, Ezekiel M, Sherman SS, Erickson R, DeWolf WE, Jr., Berkowitz BA. Cardiovascular effects of a new potent dopamine beta-hydroxylase inhibitor in spontaneously hypertensive rats. J Pharmacol Exp Ther. 1987;241:554–559. [PubMed] [Google Scholar]
- 18.Ross ST, Kruse LI, Ohlstein EH, Erickson RW, Ezekiel M, Flaim KE, Sawyer JL, et al. Inhibitors of dopamine beta-hydroxylase. 3. Some 1-(pyridylmethyl)imidazole-2-thiones. J Med Chem. 1987;30:1309–13. doi: 10.1021/jm00391a008. [DOI] [PubMed] [Google Scholar]
- 19.Greenwood TA, Rao F, Stridsberg M, Mahapatra NR, Mahata M, Lillie EO, Mahata SK, et al. Pleiotropic effects of novel trans-acting loci influencing human sympathochromaffin secretion. Physiol Genomics. 2006;25:470–479. doi: 10.1152/physiolgenomics.00295.2005. [DOI] [PubMed] [Google Scholar]
- 20.Seasholtz TM, Wessel J, Rao F, et al. Rho kinase polymorphism influences blood pressure and systemic vascular resistance in human twins: role of heredity. Hypertension. 2006;47:937–47. doi: 10.1161/01.HYP.0000217364.45622.f0. [DOI] [PubMed] [Google Scholar]
- 21.Wessel J, Moratorio G, Rao F, Rana BK, Khandrika S, Kennedy BP, Lillie EO, et al. C-reactive protein, an ‘intermediate phenotype’ for inflammation: human twin studies reveal heritability, association with blood pressure and the metabolic syndrome, and the influence of common polymorphism at catecholaminergic/beta-adrenergic pathway loci. J Hypertens. 2007;25:329–343. doi: 10.1097/HJH.0b013e328011753e. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Rao F, Wessel J, Kennedy BP, Rana BK, Taupenot L, Lillie EO, et al. Functional allelic heterogeneity and pleiotropy of a repeat polymorphism in tyrosine hydroxylase: prediction of catecholamines and response to stress in twins. Physiol Genomics. 2004;19:277–291. doi: 10.1152/physiolgenomics.00151.2004. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy BP, Rao F, Botiglieri T, Sharma S, Lillie EO, Ziegler MG, O'Connor DT. Contributions of the sympathetic nervous system, glutathione, body mass and gender to blood pressure increase with normal aging: influence of heredity. J Hum Hypertens. 2005;19:951–969. doi: 10.1038/sj.jhh.1001912. [DOI] [PubMed] [Google Scholar]
- 24.Rao F, Wen G, Gayen JR, Das M, Vaingankar SM, Rana BK, Mahata M, et al. Catecholamine release-inhibitory peptide catestatin (chromogranin A(352-372)): naturally occurring amino acid variant Gly364Ser causes profound changes in human autonomic activity and alters risk for hypertension. Circulation. 2007;115:2271–2281. doi: 10.1161/CIRCULATIONAHA.106.628859. [DOI] [PubMed] [Google Scholar]
- 25.Cooper R, Rotimi C, Ataman S, McGee D, Osotimehin B, Kadiri S, Muna W, et al. The prevalence of hypertension in seven populations of west African origin. Am J Public Health. 1997;87:160–168. doi: 10.2105/ajph.87.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui JS, Hopper JL, Harrap SB. Antihypertensive treatments obscure familial contributions to blood pressure variation. Hypertension. 2003;41:207–210. doi: 10.1161/01.hyp.0000044938.94050.e3. [DOI] [PubMed] [Google Scholar]
- 27.Wu J, Kraja AT, Oberman A, Lewis CE, Ellison RC, Arnett DK, Heiss G, et al. A summary of the effects of antihypertensive medications on measured blood pressure. Am J Hypertens. 2005;18:935–942. doi: 10.1016/j.amjhyper.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 29.Concepcion D, Seburn KL, Wen G, Frankel WN, Hamilton BA. Mutation rate and predicted phenotypic target sizes in ethylnitrosourea-treated mice. Genetics. 2004;168:953–959. doi: 10.1534/genetics.104.029843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen G, Mahata SK, Cadman P, Mahata M, Ghosh S, Mahapatra NR, Rao F, et al. Both rare and common polymorphisms contribute functional variation at CHGA, a regulator of catecholamine physiology. Am J Hum Genet. 2004;74:197–207. doi: 10.1086/381399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ewing B, Green P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998;8:186–194. [PubMed] [Google Scholar]
- 32.Gordon D, Abajian C, Green P. Consed: a graphical tool for sequence finishing. Genome Res. 1998;8:195–202. doi: 10.1101/gr.8.3.195. [DOI] [PubMed] [Google Scholar]
- 33.Nickerson DA, Tobe VO, Taylor SL. PolyPhred: automating the detection and genotyping of single nucleotide substitutions using fluorescence-based resequencing. Nucleic Acids Res. 1997;25:2745–2751. doi: 10.1093/nar/25.14.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Connor DT, Levine GL, Frigon RP. Homologous radio-immunoassay of human plasma dopamine-beta-hydroxylase: analysis of homospecific activity, circulating plasma pool and intergroup differences based on race, blood pressure and cardiac function. J Hypertens. 1983;1:227–233. doi: 10.1097/00004872-198310000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Kennedy B, Ziegler MG. A more sensitive and specific radioenzymatic assay for catecholamines. Life Sci. 1990;47:2143–2153. doi: 10.1016/0024-3205(90)90314-h. [DOI] [PubMed] [Google Scholar]
- 36.Wen G, Wessel J, Zhou W, Ehret GB, Rao F, Stridsberg M, Mahata SK, Gent PM, Das M, Cooper RS, Chakravarti A, Zhou H, Schork NJ, O'Connor DT, Hamilton BA. An ancestral variant of Secretogranin II confers regulation by PHOX2 transcription factors and association with hypertension. Hum Mol Genet. 2007;16:1752–1764. doi: 10.1093/hmg/ddm123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eskin E, Sharan R, Halperin E. A note on phasing long genomic regions using local haplotype predictions. J Bioinform Comput Biol. 2006;4:639–647. doi: 10.1142/s0219720006002272. [DOI] [PubMed] [Google Scholar]
- 39.Abecasis GR, Cookson WO. GOLD--graphical overview of linkage disequilibrium. Bioinformatics. 2000;16:182–183. doi: 10.1093/bioinformatics/16.2.182. [DOI] [PubMed] [Google Scholar]
- 40.Barrett J, Fry B, Maller J, Daly M. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 41.Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74:765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith TF, Waterman MS. Identification of common molecular subsequences. J Mol Biol. 1981;147:195–197. doi: 10.1016/0022-2836(81)90087-5. [DOI] [PubMed] [Google Scholar]
- 43.Vlieghe D, Sandelin A, De Bleser PJ, et al. A new generation of JASPAR, the open-access repository for transcription factor binding site profiles. Nucleic Acids Res. 2006;34:D95–7. doi: 10.1093/nar/gkj115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wingender E, Dietze P, Karas H, Knuppel R. TRANSFAC: a database on transcription factors and their DNA binding sites. Nucleic Acids Res. 1996;24:238–241. doi: 10.1093/nar/24.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandelin A, Wasserman WW, Lenhard B. ConSite: web-based prediction of regulatory elements using cross-species comparison. Nucleic Acids Res. 2004;32:W249–252. doi: 10.1093/nar/gkh372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wasserman WW, Sandelin A. Applied bioinformatics for the identification of regulatory elements. Nat Rev Genet. 2004;5:276–287. doi: 10.1038/nrg1315. [DOI] [PubMed] [Google Scholar]
- 47.O'Connor D, Frigon R, Stone R. Human pheochromocytoma dopamine-beta-hydroxylase: purification and molecular parameters of the tetramer. Mol Pharmacol. 1979;16:529–538. [PubMed] [Google Scholar]
- 48.Wu HJ, Parmer RJ, Koop AH, Rozansky DJ, O'Connor DT. Molecular cloning, structure, and expression of dopamine-beta-hydroxylase from bovine adrenal medulla. J Neurochem. 1990;55:97–105. doi: 10.1111/j.1471-4159.1990.tb08826.x. [DOI] [PubMed] [Google Scholar]
- 49.Voors AW, Berenson GS, Dalferes ER, Webber LS, Shuler SE. Racial differences in blood pressure control. Science. 1979;204:1091–1094. doi: 10.1126/science.451554. [DOI] [PubMed] [Google Scholar]
- 50.Schork NJ, Fallin D, Thiel B, Xu X, Broeckel U, Jacob HJ, Cohen D. The future of genetic case-control studies. Adv Genet. 2001;42:191–212. doi: 10.1016/s0065-2660(01)42023-2. [DOI] [PubMed] [Google Scholar]
- 51.Lander ES, Schork NJ. Genetic dissection of complex traits. Science. 1994;265:2037–2048. doi: 10.1126/science.8091226. [DOI] [PubMed] [Google Scholar]
- 52.Lillie EO, O'Connor DT. Early phenotypic changes in hypertension: a role for the autonomic nervous system and heredity. Hypertension. 2006;47:331–333. doi: 10.1161/01.HYP.0000203980.44717.aa. [DOI] [PubMed] [Google Scholar]
- 53.Kasagi F, Akahoshi M, Shimaoka K. Relation between cold pressor test and development of hypertension based on 28-year follow-up. Hypertension. 1995;25:71–76. doi: 10.1161/01.hyp.25.1.71. [DOI] [PubMed] [Google Scholar]
- 54.Menkes MS, Matthews KA, Krantz DS, Lundberg U, Mead LA, Qaqish B, Liang KY, Thomas CB, Pearson TA. Cardiovascular reactivity to the cold pressor test as a predictor of hypertension. Hypertension. 1989;14:524–530. doi: 10.1161/01.hyp.14.5.524. [DOI] [PubMed] [Google Scholar]
- 55.Schneider GM, Jacobs DW, Gevirtz RN, O'Connor DT. Cardiovascular haemodynamic response to repeated mental stress in normotensive subjects at genetic risk of hypertension: evidence of enhanced reactivity, blunted adaptation, and delayed recovery. J Hum Hypertens. 2003;17:829–840. doi: 10.1038/sj.jhh.1001624. [DOI] [PubMed] [Google Scholar]
- 56.Snieder H, Harshfield GA, Barbeau P, Pollock DM, Pollock JS, Treiber FA. Dissecting the genetic architecture of the cardiovascular and renal stress response. Biol Psychol. 2002;61:73–95. doi: 10.1016/s0301-0511(02)00053-4. [DOI] [PubMed] [Google Scholar]
- 57.Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 58.O'Connor DT, Insel PA, Ziegler MG, Hook VY, Smith DW, Hamilton BA, Taylor PW, et al. Heredity and the autonomic nervous system in human hypertension. Curr Hypertens Rep. 2000;2:16–22. doi: 10.1007/s11906-000-0053-8. [DOI] [PubMed] [Google Scholar]
- 59.O'Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens. 2002;20:1335–1345. doi: 10.1097/00004872-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 60.Reich DE, Lander ES. On the allelic spectrum of human disease. Trends Genet. 2001;17:502–10. doi: 10.1016/s0168-9525(01)02410-6. [DOI] [PubMed] [Google Scholar]
- 61.Carrasquillo MM, McCallion AS, Puffenberger EG, Kashuk CS, Nouri N, Chakravarti A. Genome-wide association study and mouse model identify interaction between RET and EDNRB pathways in Hirschsprung disease. Nat Genet. 2002;32:237–44. doi: 10.1038/ng998. [DOI] [PubMed] [Google Scholar]
- 62.Ma DQ, Whitehead PL, Menold MM, Martin ER, Ashley-Koch AE, Mei H, et al. Identification of significant association and gene-gene interaction of GABA receptor subunit genes in autism. Am J Hum Genet. 2005;77:377–388. doi: 10.1086/433195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang K, Jin L. HaploBlockFinder: haplotype block analyses. Bioinformatics. 2003;19:1300–1301. doi: 10.1093/bioinformatics/btg142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
On-line/Supplementary Figure legends.
Supplementary Figure 1. Human DBH polymorphism discovery: Re-sequencing strategy. Sequences conserved between mouse and human DBH were visualized in VISTA. Location of common SNPs (solid rods) relative to exons and conserved non-coding sequences is indicated by position. Common haplotypes in each haplotype block are shown and connections between blocks are indicated by solid lines. Common haplotype blocks were constructed using HaploBlockFinder (63) with minimal haplotype coverage of 80% in each block. The chimp haplotype for the common SNPs is also shown.
Supplementary Figure 2. Human dopamine beta-hydroxylase (DBH): Functional domains and coding region (open reading frame) cSNPs.
Supplementary Figure 3. DBH promoter variant C-970T: Effects on plasma DBH activity in African Americans (A-A) and European American (E-A) subjects. Analyses were adjusted by age and sex. Error bars indicate mean ± SEM in each panel.
Left: Plasma DBH activity variation between European-American (E-A) and African-American (A-A) subjects.
Right. Plasma DBH activity in E-A and A-A subjects, as a function of promoter C-970T genotype.
Supplementary Figure 4. Replication: DBH promoter variant C-970T effects on BP in an independent population of Nigeria BP extreme groups. Variant -970T is associated with reduced systolic and diastolic BP in Nigerian subjects from population BP extremes (upper and lower quartiles of BP). Mean BP values ± SEM are shown, calculated from all individuals as indicated in each bar. Effects of genotypes on BP were evaluated using a general linear model (ANOVA), adjusted by age and sex (BP was untreated). Significant p values are shown.
Supplementary Figure 5. Replication: DBH promoter variant C-970T effects on BP in an independent population of San Diego unrelated individuals. Variant -970T is associated with reduced systolic BP in San Diego unrelated subjects. Analyses were adjusted by age, sex, self-identified ethnicity (because of the inclusion of more than one biogeographic ancestry group), and effect of antihypertensive medications (in treated hypertensives). Mean SBP values ± SEM are shown, calculated from all individuals as indicated in each bar.
Supplementary Figure 6: DBH promoter functional polymorphism C-970T: Baroreceptor function is intact in twin pairs. Downward and upward deflections measured in San Diego white twins are shown. Analyses were adjusted for age and sex. There were no trait differences among individuals stratified by C-970T genotype.
Supplementary Table 1. Descriptive statistics for the San Diego twin study.
Supplementary Table 2. Descriptive statistics for the Nigeria (black) population BP extreme study (upper and lower 25th %iles).
Supplementary Table 3. Descriptive statistics for the San Diego BP unrelated individuals.
Supplementary Table 4. Summary of naturally occurring genetic variation at the human DBH locus in 4 populations.
Supplementary Table 5. Promoter region haplotypes (based on different numbers of SNPs) in population analyses and in cella studies.