Abstract
Purpose:
To determine whether tuberculosis (TB) preventive therapies alter the rate of disease progression to AIDS or death and to identify significant prognostic factors for HIV disease progression to AIDS.
Method:
In a randomized placebo-controlled trial in Kampala, Uganda, 2,736 purified protein derivative (PPD)-positive and anergic HIV-infected adults were randomly assigned to four and two regimens, respectively. PPD-positive patients were treated with isoniazid (INH) for 6 months (6H; n = 536), INH plus rifampicin for 3 months (3HR; n = 556), INH plus rifampicin plus pyrazinamide for 3 months (3HRZ; n = 462), or placebo for 6 months (n = 464). Anergic participants were treated with 6H (n = 395) or placebo (n = 323).
Results:
During follow-up, 404 cases progressed to AIDS and 577 deaths occurred. The cumulative incidence of the AIDS progression was greater in the anergic cohort compared to the PPD-positive cohort (p < .0001). Among PPD-positive patients, the relative risk of the AIDS progression with INH alone was 0.95 (95% CI 0.68–1.32); with 3HR it was 0.83 (95% CI 0.59–1.17); and with 3HRZ it was 0.76 (95% CI 0.52–1.08), controlling for significant baseline predictors. Among anergic patients, the relative risk of the AIDS progression was 0.81 (95% CI 0.56–1.15). Survival was greater in the PPD-positive cohort compared to the anergic cohort (p = .0001).
Conclusion:
The number of signs or symptoms at baseline and anergic status are associated with increasing morbidity and mortality. Even though the tuberculosis preventive therapies were effective in reducing the incidence of TB for HIV-infected adults, their benefit of delaying HIV disease progression to AIDS was not observed.
Keywords: disease progression, HIV/AIDS, natural history, prevention, survival, tuberculosis
The human immunodeficiency virus-1 (HIV) is spread throughout the world, especially affecting most of the countries of subSaharan Africa. Since 1996, the use of protease inhibitors has reduced the incidence of opportunistic infection and death.1-4 However, access to these medications is limited in Africa because of their cost. HIV-infected patients living in developing countries are particularly vulnerable, because they cannot afford antiretroviral medications even when subsidized by governments. Recent political developments have led to the global funding for HIV/TB/ malaria, with recent awards made to many developing countries.5,6 President Bush's Emergency Plan for AIDS Relief (PEPFAR) provides $15 billion, including nearly $10 billion in new funding, to fight the HIV/AIDS pandemic over 5 years, focusing on 15 of the hardest hit countries.6 About 55% of this fund is planned for the treatment of individuals with HIV/AIDS.
There is now the promise for greater access to antiretroviral medications. These medications must be properly used to maximize benefit and minimize untoward, adverse effects. Although recommendations have been developed and the availability of new antiretroviral drugs has expanded treatment choices,7 this does not take into account the technology void in many developing countries. To make informed decisions about when to start treatment, what therapy to start with, and how to monitor the response to treatment, health care practitioners need more details about the natural history of HIV infection. Few studies have addressed disease progression in sub-Saharan Africa, where over 65% of the world's 39.4 million infected adults and children live.7 In Uganda, the prevalence of HIV infection in adults is estimated at 8% in rural areas and up to 40% in some rural areas and trading centers.8,9 The cumulative progression to AIDS at 1, 3, and 5 years after seroconversion is 2%, 6%, and 22%, respectively. The median survival time after the onset of AIDS is 9.3 months.10 Although the recent declines in morbidity and mortality due to HIV/AIDS are attributable to the use of more intensive antiretroviral therapies,3,4 the availability of antiretroviral drugs is limited in developing countries. Even as the antiretroviral therapy rolls out in the developing countries, tuberculosis (TB) is still an important coinfection and rates of TB are likely to remain high.11,12 The clinical course of HIV infection with respect to factors that may predispose to and promote the development of HIV-related signs is still unclear. The identification of such significant prognostic factors for the HIV disease progression contributes substantially to clinical monitoring and the therapeutic decision-making process.
The main aim of this analysis was to investigate the effect of the tuberculosis preventive intervention on HIV disease progression to AIDS and survival. Furthermore, significant prognostic factors for HIV disease progression to AIDS were identified, and the independent effect of significant demographic, laboratory, and clinical predictors of the outcome was also estimated in this study.
METHOD
Study Population
The study population comprised participants enrolled in a randomized, double-blinded, placebo-controlled clinical trial to assess the efficacy of three daily self-administrated drug regimens for the prevention of TB in HIV-infected adults in Uganda. More detailed descriptions of the study design, interventions, measurements, and the study profile have been published elsewhere.13,14 Briefly, between March 1993 and April 1995, 2,736 HIV-infected adults with no history of previous TB or TB treatment were recruited from five sites in Kampala, Uganda. Patients with tuberculin skin tests 5 mm or greater were randomly assigned to receive either placebo daily for 6 months (n = 464), isoniazid 300 mg daily for 6 months (6H; n = 536), isoniazid 300 mg plus rifampicine 600 mg daily for 3 months (3HR; n = 556), or isoniazid 300 mg plus rifampicine 600 mg and pyrazinamide 2 g daily for 3 months (3HRZ; n = 462). Patients with cutaneous anergy were randomly assigned to receive either placebo daily for 6 months (n = 323) or isoniazid 300 mg daily for 6 months (6H; n = 395) by a separate but identical process.
At enrollment, all participants had a complete medical history and physical examination by trained medical officers. During follow-up, the participants were evaluated monthly during the first 6 months of the study and every 3 months thereafter or at the time of illness. At each visit, participants were interviewed regarding symptoms since the last visit, physical and clinical examinations were performed by clinicians, and current signs and symptoms related to HIV infection were recorded. Laboratory tests were performed, including hemoglobin, platelet counts, absolute lymphocyte counts, and aspartate aminotransferase (AST). In cases of mortality, the cause and date of death were determined through interviews with family members or review of hospital records when available. The last date of follow-up was August 8, 1998.
Study Outcomes
In this analysis, the primary outcome (or event) was HIV disease progression to AIDS.
The current study is a secondary analysis, and criteria for disease progression to AIDS in our study were chosen after the data were collected and are based mainly on the 1990 WHO classification system for HIV/AIDS.15 In our study, AIDS progression event was defined when a patient developed any one major sign or three or more minor signs at any given time during observation. Major signs were defined as Karnofsky performance score less than or equal to 60, Kaposi's sarcoma, and esophageal candidiasis. The minor signs were defined as pruritic papules, oral candidiasis, varicella-zoster virus, genital herpes simplex, oral herpes simplex, and TB. This classification was obtained by a severity order of HIV-related signs based on CD4 lymphocyte counts at which they occur. Rationale behind the classification was based on many studies showing that HIV-infected persons present many clinical signs as the disease progresses15-17 and there is a strong association between HIV-related signs and CD4 lymphocyte counts at which they occur.18,19
The primary outcome was extended to combine the AIDS progression and death, whichever came first. For the AIDS progression outcome, individual failure times were defined as the period between enrollment in the study and the incidence date of the AIDS progression or censoring. Patients were censored at the last clinic visit before the end of the study (August 8, 1998). The individual survival times for death outcome were calculated up to the date of death or to the date of the last clinic visit before the end of the study.
Statistical Analysis
Descriptive statistics were utilized initially, starting with the examination of frequency distributions. When continuous variables were not normally distributed, a transformation was used to normalize them. Cumulative incidence of each HIV-related sign and the AIDS progression event (defined above specifically for this analysis) was estimated as the number of cases per population at risk at baseline. Rates of each HIV-related sign, the AIDS progression event, and mortality were calculated per 100 person-years observation (PYO). PYO for participants was calculated from the study enrollment to the first date of the incidence of each HIV-related sign, the AIDS progression event or death event, or the last clinic visit before the end of the study follow-up.
The Kaplan-Meier method20 was used to estimate distribution of time to the occurrence of the events. The log-rank test was used to compare failure time distributions among treatment arms. The Cox proportional hazards regression model21 was used to examine the risk of the AIDS progression (or death) with unadjusted and adjusted analyses. The adjusted analyses used the baseline demographic and clinical variables. Univariate and multivariate Cox proportional hazards regression models were fitted after testing for the proportional hazard assumption.
To identify potential predictors for the final model, the variables were first examined individually using univariate analysis. Variables were identified as significant using a 0.1 alpha level in the univariate model, and only these were included in a stepwise method to determine a final model. For the final multivariate Cox proportional hazards regression model, a 0.05 alpha level was used.
The following variables were studied as potential risk factors: sex, age (years), education, marital status, anergic status (PPD < 5 mm), hemoglobin (g/L), platelet counts (/L), body mass index (kg/m2), AST/SGOT (SGOT > 40 U/L), creatinine level (mg/dL), absolute lymphocytes count (per mm3), and number of HIV-related clinical signs at baseline. Important baseline predictors of the AIDS progression and mortality were evaluated using univariate and multivariate Cox proportional hazard models to determine relative hazards and 95% confidence intervals (CI). All data analyses were performed using SAS version 8 (SAS Institute, Inc., Cary, North Carolina, USA).22
RESULTS
Between March 1993 and April 1995, 2,736 purified protein derivative (PPD)-positive and anergic HIV-infected adults were enrolled into the randomized placebo-controlled TB preventive study in Kampala, Uganda. PPD-positive patients were treated with isoniazid (INH) for 6 months (6H; n = 536), INH plus rifampicin for 3 months (3HR; n = 556), INH plus rifampicin plus pyrazinamide for 3 months (3HRZ; n = 462), or placebo for 6 months (n = 464). Anergic participants were treated with 6H (n = 395) or placebo (n = 323).
Information regarding compliance, adverse drug reactions, and short- and long-term efficacy of the study regimens has been published elsewhere.13,14 At study enrollment, the mean age of 2,736 participants was 30 years and 862 (32%) were male. Overall, about 99% of all patients had good performance status (Karnofsky score ≥ 80) and appeared only mildly ill at enrollment. At baseline, 2,158 participants had no HIV-related signs, and 491 participants had one and 81 participants had two HIV-related signs. The most common HIV-related signs at baseline were pruritic papules, history of varicella-zoster virus, and oral candidiasis (Table 1).
Table 1.
Baseline characteristics in 2,736 participants
| PPD-positive |
Anergy |
|||||
|---|---|---|---|---|---|---|
| Characteristics | Placebo (n = 464) |
6H (n = 536) |
3HR (n = 556) |
3HRZ (n = 462) |
Placebo (n = 323) |
6H (n = 395) |
| Male, % | 31 | 31 | 29 | 34 | 31 | 32 |
| Mean age, years | 30 | 29 | 29 | 29 | 30 | 30 |
| Body mass indexa | 22.2 | 22.1 | 22.6 | 22.3 | 22.9 | 21.9 |
| Karnofsky score | 91 | 91 | 91 | 91 | 90 | 90 |
| PPD skin test | 14 | 14 | 13 | 14 | 0 | 0 |
| Hemoglobin, g/L | 126 | 125 | 127 | 126 | 125 | 123 |
| Platelet count, /L | 264 | 258 | 264 | 262 | 267 | 264 |
| Creatinine, mg/dL | 0.89 | 0.88 | 0.89 | 0.9 | 0.94 | 0.84 |
| AST/SGOT, U/L | 27.1 | 27.7 | 26.3 | 26.4 | 28.1 | 27.9 |
| Absolute lymphocyte count per mm3 |
2245 | 2327 | 2309 | 2223 | 1968 | 2116 |
| Pruritic papules, % | 8 | 12 | 10 | 8 | 20 | 19 |
| Previous herpes zoster or thrush, % | 25 | 25 | 25 | 27 | 33 | 35 |
Note: PPD = purified protein derivative; 6H = isoniazid (INH) for 6 months; 3HR = INH plus rifampicin for 3 months; 3HRZ = INH plus rifampicin plus pyrazinamide for 3 months.
The body mass index was calculated as the weight in kilograms divided by the square of the height in meters.
Natural History
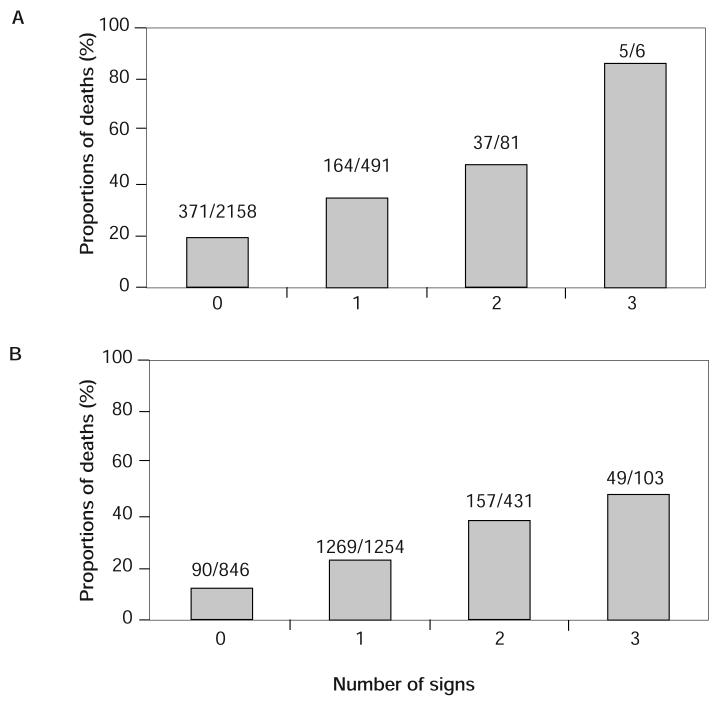
During follow-up (mean = 1.9 years), a total of 1,788 (65%) patients experienced one or more HIV-related signs at any given time. Of these patients, 1,254 (70%) had one sign, 431 (24%) had two signs, and 103 (6%) had three or four signs (Figure 1). Occurring as a single HIV-related sign during follow-up, pruritic papules, varicella-zoster virus, and oral candidiasis were the most common (Table 2). During follow-up, a total of 1,360 episodes of pruritic papules were recorded (overall incidence rate = 38.8 per 100 PYO). The incidence rates of pruritic papules in years 1, 2, 3, and 4 were 52.1, 25.3, 25.2, and 21.4 per 100 PYO, respectively (Table 2). Less frequent signs were oral herpes simplex and Kaposi's sarcoma with overall incidence rates 1.79 and 0.87 per 100 PYO, respectively.
Figure 1.
Proportion of deaths according to the number of HIV-related signs (A) at baseline and (B) during follow-up.
Table 2.
Incidence rates of HIV-related signs according to year of study follow-up
| Year 1 |
Year 2 |
Year 3 |
Year 4 |
Overall |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case (n) | Ratea | Case (n) | Ratea | Case (n) | Ratea | Case (n) | Ratea | Case (n) | Ratea | |
| Pruritic papules | 938 | 52.1 | 256 | 25.3 | 135 | 25.2 | 31 | 21.4 | 1360 | 38.8 |
| VZV | 223 | 10.1 | 112 | 4.17 | 57 | 3.19 | 10 | 2.19 | 402 | 8.43 |
| Oral candidiasis | 308 | 14.1 | 109 | 7.05 | 60 | 6.68 | 20 | 7.14 | 497 | 10.1 |
| Herpes simplex | 101 | 4.44 | 47 | 2.92 | 32 | 3.42 | 7 | 2.46 | 187 | 3.65 |
| Oral herpes simplex | 50 | 2.17 | 22 | 1.33 | 15 | 1.54 | 7 | 2.38 | 94 | 1.79 |
| TB | 53 | 2.29 | 54 | 3.22 | 33 | 3.33 | 12 | 3.75 | 152 | 2.86 |
| Esophageal candidiasis | 109 | 4.76 | 40 | 2.43 | 26 | 2.69 | 18 | 5.88 | 193 | 3.71 |
| Kaposi's sarcoma | 24 | 1.04 | 13 | 0.78 | 6 | 0.61 | 3 | 1.00 | 46 | 0.87 |
| Karnofsky score ≤ 60 | 123 | 5.36 | 52 | 3.14 | 22 | 2.26 | 5 | 1.60 | 202 | 3.86 |
| Major signb or no. of minor signsc≥ 3 | 240 | 10.7 | 92 | 5.79 | 49 | 5.30 | 23 | 8.06 | 404 | 8.02 |
| Death | 282 | 12.1 | 172 | 10.2 | 99 | 9.88 | 24 | 7.43 | 577 | 10.8 |
Note: VZV = varicella-zoster virus; TB = tuberculosis.
Per 100 person-years observation.
Major sign is an indicator of Karnofsky score ≤60, Kaposi's sarcoma, esophageal candidiasis.
Minor signs denote TB, varicella-zoster virus, oral candidiasis, pruritic papules, herpes simplex, oral herpes simplex.
During follow-up, 577 (21%) deaths occurred for an overall mortality rate of 10.8 per 100 PYO (Table 2). Mortality showed a monotone increasing trend as the number of HIV-related signs that patients had at baseline and developed during follow-up increased (Figure 1). Of participants who had 0, 1, and 2 HIV-related signs at baseline, 17%, 33%, and 46% of them died during follow-up, respectively. Although only 11% of patients who did not develop any sign died during follow-up, the mortality among the patients who developed three or more signs was 48%. A higher proportion of patients who developed Karnofsky score ≤ 60, esophageal candidiasis, oral candidiasis, or Kaposi's sarcoma during follow-up were dead than patients who developed any other signs.
A total of 779 (28%) patients developed either AIDS progression or death, and the overall incidence rate was 15.5 per 100 PYO. Among them, 404 patients progressed to AIDS with an incidence rate of 8.02 per 100 PYO, and 202 (50%) of the 404 cases died. A total of 375 patients died without AIDS progression.
Predictors of AIDS Progression and Survival
In both univariate and multivariate proportional hazards models, age, anergic status, hemoglobin, body mass index, AST, absolute lymphocyte count, and number of HIV-related signs at baseline were associated with disease progression to AIDS (Table 3). However, other characteristics such as sex, education, marital status, platelet counts, and creatinine level did not show any association with the AIDS progression. The risk of the AIDS progression or death increased with age, anergy, elevated AST, and the number of HIV-related signs at baseline, but the risk decreased with hemoglobin, body mass index, and absolute lymphocyte count.
Table 3.
Multivariate Cox proportional hazard model for the relative hazard of AIDS progression, death, and combination of AIDS progression or death, whichever comes first
| AIDS progression |
Death |
AIDS progression or death |
||||
|---|---|---|---|---|---|---|
| RH | 95% CI | RH | 95% CI | RH | 95% CI | |
| Sex | — | — | 0.71 | 0.58–0.86 | 0.72 | 0.61–0.86 |
| Age, years | 1.02 | 1.01–1.04 | 1.02 | 1.01–1.03 | 1.02 | 1.01–1.03 |
| Anergica | 1.36 | 1.09–1.70 | 1.63 | 1.3 –1.95 | 1.45 | 1.24–1.69 |
| Hemoglobin, g/L | 0.80 | 0.76–0.84 | 0.79 | 0.75–0.83 | 0.80 | 0.76–0.83 |
| Body mass indexb | 0.93 | 0.90–0.96 | 0.88 | 0.85–0.91 | 0.90 | 0.88–0.92 |
| SGOTc | 1.54 | 1.17–2.03 | 1.56 | 1.38–2.15 | 1.56 | 1.28–1.90 |
| Lymphocyte count per mm3 | 0.77 | 0.67–0.89 | 0.56 | 0.49–0.64 | 0.65 | 0.59–0.72 |
| Baseline signd | 1.71 | 1.45–2.03 | 1.62 | 1.42–1.86 | 1.76 | 1.56–1.99 |
Note: AIDS progression is defined by the presence of a major sign or ≥3 minor signs. RH = relative hazard; CI = confidence interval.
Anergic is an indicator of TB skin test induration <5 mm.
The body mass index was calculated as the weight in kilograms divided by the square of the height in meters.
SGOT is an indicator of SGOT > 40 U/L.
Baseline sign is the number of HIV-related signs at baseline: Karnofsky score ≤ 60, TB, esophageal candidiasis, varicella-zoaster virus, Kaposi's sarcoma, oral candidiasis, pruritic papules, herpes simplex, oral herpes simplex.
Effect of TB Preventive Therapy on AIDS Progression and Survival
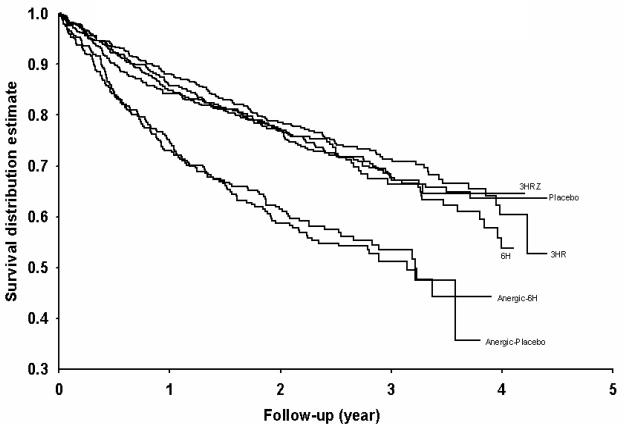
During follow-up, 404 AIDS progression cases (267 PPD-positive and 137 anergic) and 577 deaths (351 PPD-positive and 226 anergic) occurred. The cumulative incidence of the AIDS progression and mortality was greater in the anergic cohort compared to the PPD-positive cohort (p < .0001, log-rank test). When stratified by anergic status, there was no difference in the cumulative incidence rates of AIDS progression and mortality among TB preventive treatment arms for both the PPD-positive and anergic cohorts (Figure 2). Among the PPD-positive patients, overall AIDS progression incidence rates of the placebo, 6H, 3HR, and 3HRZ arms were 7.02, 7.49, 6.10, and 6.68 per 100 PYO, respectively. Among the anergic patients, overall AIDS progression incidence rates of the placebo and 6H were 13.7 and 11.7 per 100 PYO, respectively. The results were unchanged even after adjusting for sex, age, hemoglobin, body mass index, AST, absolute lymphocyte count, and number of HIV-related clinical signs at baseline in a multivariate Cox proportional hazards model (Table 4). Similar results were observed for the combined event of AIDS progression and death.
Figure 2.
Time to AIDS progression or death, whichever come first, among the TB preventive treatment regimens.
Table 4.
Multivariate Cox proportional hazard model for the relative hazard of AIDS progression and the combination of AIDS progression or death
| AIDS progression |
AIDS progression or death |
|||
|---|---|---|---|---|
| RHa | 95% CI | RH | 95% CI | |
| PPD-positiveb | ||||
| Placebo (n = 464) | 1.0 | — | 1.0 | — |
| 6H (n = 536) | 0.95 | 0.68–1.32 | 1.02 | 0.80–1.30 |
| 3HR (n = 556) | 0.83 | 0.59–1.17 | 0.91 | 0.71–1.17 |
| 3HRZ (n = 462) | 0.76 | 0.52–1.10 | 0.87 | 0.67–1.14 |
| 3HR & 3HRZ (n = 1,018) | 0.80 | 0.59–1.08 | 0.90 | 0.72–1.12 |
| PPD-positivec | ||||
| 6H (n = 536) | 1.0 | — | 1.0 | — |
| 3HR (n = 556) | 0.88 | 0.63–1.23 | 0.89 | 0.70–1.14 |
| 3HRZ (n = 462) | 0.81 | 0.56–1.17 | 0.86 | 0.66–1.12 |
| 3HR & 3HRZ (n = 1,018) | 0.85 | 0.63–1.14 | 0.88 | 0.71–1.09 |
| Anergic | ||||
| Placebo (n = 323) | 1.0 | — | 1.0 | — |
| 6H (n = 395) | 0.81 | 0.56–1.15 | 1.00 | 0.78 – 1.28 |
Note: Event = major sign or ≥3 minor signs or death, whichever comes first. RH = relative hazard; CI = confidence interval.
The relative hazard is adjusted for sex, age, hemoglobin, body mass index, SGOT, absolute lymphocyte count and the number of HIV-related signs at baseline in a Cox proportional hazards regression model; Baseline sign is the number of HIV-related signs at baseline: {Karnofsky score ≤ 60, TB, esophageal candidiasis, VZV, Kaposi's sarcoma, oral candidiasis, pruritic papules, herpes simplex, oral herpes simplex (oral)}.
Placebo is the reference group.
6H is the reference group.
DISCUSSION
The data from this randomized, placebo-controlled study showed that even though the TB preventive therapies were effective in reducing the incidence of TB for HIV-infected adults, their benefit of delaying HIV disease progression to AIDS was not observed.
In this analysis, the association of the number of HIV-related signs/symptoms at baseline and during follow-up with morbidity and mortality was seen after controlling for sex, age, hemoglobin, body mass index, AST, and absolute lymphocyte count. Association with the disease progression to AIDS and survival was made through the linkage of the signs and symptoms to replication of HIV virus and to decline of CD4 lymphocyte counts.
The progression of HIV infection is the result of a decline in immune competence that occurs due to increased replication of HIV virus. As the disease progresses, HIV-infected persons suffer and present with many clinical signs and symptoms, which may be systemically classified in severity from stages I to IV according to the WHO.15-17 A severity order of HIV-related signs based on CD4 lymphocyte counts at which they occur has been suggested from studies in developed countries.18,19 HIV disease progression is known to be similar for patients in Africa and in developed countries.23-25 In Uganda, HIV-infected patients had less than 3.5 months of the median survival and less than 200 CD4 count around the time of developing Kaposi's sarcoma, esophageal candidiasis, and wasting syndrome.26,27 On the other hand, the Karnofsky performance status is a measure of global health status that is widely used for HIV-infected patients. Studies have shown a strong effect of the stage of the disease on the Karnofsky performance status for HIV-infected patients.28-30 The classification of AIDS progression in this analysis was made through a severity order of HIV-related signs based on CD4 lymphocyte counts at which the HIV-related signs occur.
This study was based on a large number of participants from an area with a high prevalence of HIV infection and TB. In our cohort, age, anergic status, hemoglobin, body mass index, AST, absolute lymphocyte count, and the number of clinical signs of HIV infection at baseline were associated with the AIDS progression. The older age in an HIV-infected individual has been found in several studies to be correlated with rapid disease progression.31,32 Our analysis also showed that older age was associated with shorter time to the AIDS progression and survival. Survival was shorter among men than women but did not differ in the time to AIDS progression. The risks of AIDS progression and death were increased among anergic participants in our cohort, consistent with the previous studies.33,34 Another important finding of the current study was that AST is associated with the AIDS progression and mortality. Our study showed that AST higher than 40 U/L in an HIV-infected participant was associated with rapid AIDS disease progression and high mortality.
In our study, the number of initial HIV-related signs was highly predictive of the outcomes – risks of AIDS progression and death. Significant associations between the number of HIV-related signs at baseline and the AIDS progression or mortality were observed. Significant associations were also observed between the number of HIV-related signs during follow-up and mortality. This showed a dose-response relationship in which the general trend in risk of mortality was upward in more signs at baseline and during follow-up. There was no evidence of confounding by the number of HIV-related signs at baseline. Without the baseline signs/symptoms variable, the model was stable and presents similar estimates of risk for other independent variables. We have retained the analysis including the baseline signs/symptoms because it will tell us whether a person with one or two of the minor symptoms/signs is more likely to develop other signs, symptoms, or conditions that represent advanced HIV disease or AIDS. Moreover, from a clinical perspective, especially in a resource-limited setting such as Africa, it is useful to know that when a person presents with one of the minor signs he/she will develop AIDS sooner than a person without any symptoms or signs.
Our finding that the TB preventive therapy had no protective effect on HIV progression and death was consistent with other clinical trials 33,34 and a recent meta-analysis.35 In a randomized controlled study from Zambia, Quigley and colleagues found that protective benefit from treatment of latent TB infection was observed during the first 30 months after beginning treatment, however, there was no effect on HIV progression on mortality.34 Bucher and colleagues found in a meta-analysis of seven randomized controlled trials that prophylaxis with isoniazid reduced the risk of TB in persons with HIV infection (relative risk [RR] = 0.73, 95% CI 0.57–0.95).35 However, two observational studies conducted in Spain36 and Brazil37 and a clinical trial in Haiti38 found that TB preventive therapy improved survival of HIV-infected, PPD-positive patients, which was contrary to our finding. Pinho and colleagues found in their prospective observational cohort study that anti-TB chemoprophylaxis was associated with a substantially prolonged survival after adjusting CD4 counts in Brazil (hazard ratio [HR] = 0.24, 95% CI 0.09–0.65).37 Pape and colleagues found in a randomized clinical trial that isoniazid effectively decreased the incidence of TB (odds ratio [OR] = 5.7, 95% CI 1.2–29.8) and delayed the onset of AIDS (OR = 3.83, 95% CI 1.41–10.4) for PPD-positive patients in symptom-free HIV-seropositive individuals in Haiti.38 The efficacy of isoniazid in our study was similar to the efficacy of 71% found in Pape's study, but our analysis was based on a larger sample size and also addressed some of the methodologic issues raised about the Haitian study.
Although the original data were designed to investigate TB incidence as a primary outcome, our analysis of this study suggested that the TB preventive therapy does not slow AIDS progression. When we analyzed the association between the number of signs and symptoms and morbidity and mortality according to patients' anergic status, it become clear that the association remained true in PPD-positive, anergic, or all participants. However, our study showed that the risks of AIDS progression, death, and the combined event of AIDS progression with death were higher among anergic participants than among PPD-positive participants, consistent with earlier studies.39,40 Significant predictors of AIDS progression and death remained the same in our model, with and without controlling for TB preventive treatments. The findings from our study were also unchanged when the analyses were restricted to data when TB incidence cases were included or excluded or when TB was regarded as a non–HIV-related sign.
Our study had some limitations. This study was based on an established cohort with unknown dates of seroconversion; consequently, estimates of progression to AIDS and death did not provide the complete picture of the incubation periods of AIDS and natural history of HIV-infection in the study groups. The study lacked important surrogate markers such as CD4 cell counts and viral load as well as other clinical signs such as HIV wasting syndrome. The analyses for the study cohort were performed without those covariates, hence our definition of the AIDS progression applied to this study was limited.
Our study has important implications for the care of HIV-infected individuals. In aggregate, the evidence from many studies shows that several signs and symptoms are associated with CD4 lymphocyte counts and viral load measurement, which are used for the classification of the AIDS progression. In developing countries where the CD4 counts and viral load measurement are not widely available, clinicians need to utilize clinical signs or symptoms to make the disease prognosis. Because significant associations between the number of signs at baseline and the AIDS progression or survival are observed, treatment may be warranted if a patient starts to develop three or more HIV-related signs and symptoms. Because anergy is also associated with AIDS progression and poor survival, clinicians need to treat an anergic patient aggressively.
In this study, the TB preventive therapies did not show a benefit in delaying HIV disease progression. However, TB remains an important disease and cause of death in Africa. Even as antiretroviral therapy is rolled out in Africa, rates of TB are likely to remain high. Future independent studies should focus on the dual effect of TB preventive therapy and antiretroviral therapy for HIV-infected persons.
ACKNOWLEDGMENTS
The successful completion of this study required the long-term efforts of many dedicated individuals. We are especially indebted to our study team: administration, L. Gary, M. Kasujja, S. Kibende, M. Manning, A. Nakayiza, P. Nasige; counselors and interviewers, G. Bwamiki, R. Byaruhanga, R. Galiwango; data management, P. Bajeneza, D. Guwatudde, S. Katabalwa, J. Milberg, J.B. Mukasa, M. Odie, C. Opit, G. Olupot, V. Pekovic, A. Turyamureba; dispensers, A. Abenakyo, L. Ndegumu; drivers, E. Kaggwa, H. Kabymera, G. Lumumba, R. Mukasa, L. Oryema; filing, G. Bukenya, A. Mulyabintu, B. Nansubuga; health educators, W. Bagundirire, M. Mwanje; home health visitors, K. Kataliwa, M. Kato, J. Mulabbi; laboratory, K. Edmonds, P. Kataaha, S. Kabengera, J. Okiror, E. Piwowar-Manning, B.S. Tugume, R.S. Wallis; medical officers, W. Bukulu, F. Byekwaso, A. Gasisira, P. Kyambadde, H. Luzze, A. Mateega, F. Mubiru, C. Mukulu, R. Odeke, P. Gitta, A. Wajja; microbiology, T. Aisu, E. Hatanga, M.L. Joloba, A. Morrissey; nurses, J. Kayungirizi, C. Kiramba, M. Mulindwa, G. Nalugwa, A. Rwamucece, G. Tumusiime; radiology, A. Adongo, E. Katende; study coordination, P. Langi, M.J. Vjecha; PPD skin testers, G. Mpalanyi, P. Nasozzi, S. Nyole, G. Wasswa. We are also indebted to S. Okware, Ministry of Health; F. Engwau-Adatu, Head, Ugandan National Tuberculosis and Leprosy Programme; M.G. Alwano-Edyegu, Director, AIDS Information Centre; and the clients and staff of the following organizations: AIDS Information Centre, Joint Clinical Research Centre, the AIDS Support Organization, Mulago Branch; the Post-HIV Test Club, Kisenyi; Good Samaritan Counseling Centre; HIV/AIDS Clinics of St. John's, Rubaga, and Nsambya Hospitals; Uganda Tuberculosis Diagnostic Investigations Unit, Wandegeya, Kampala, Uganda; to Drs. E. Villarino, R. Huebner, P. Smith, R. O'Brien, and L. Geiter of the Centers for Disease Control and Prevention for the contributions to study design and analysis; to Drs. T. M. Daniel and F. van der Kuyp for independent review of incident cases of tuberculosis; and to all of the patients who participated in the trial.
REFERENCES
- 1.Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 2.Gulick RM, Mellors JW, Havlir D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human deficiency virus infection. N Engl J Med. 1998;338:853–856. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Hogg RS, O'Shaughnessy MV, Gataric N, et al. Decline in deaths from AIDS due to new antiretrovirals. Lancet. 1997;349:1294. doi: 10.1016/S0140-6736(05)62505-6. [DOI] [PubMed] [Google Scholar]
- 5.UNAIDS Report on the global HIV/AIDS epidemic, December 2004. Available at: http://www.usaids.org/publications/index.html. Accessed September 1, 2005.
- 6.The President's Emergency Plan for AIDS Relief - 5/27/03. Available at: http://www.usaid.gov/our_work/global_health/aids/pepfarfact.html. Accessed September 1, 2005.
- 7.Yeni PG, Hammer SM, Hirsch MS, Saag MS, et al. Treatment for Adult HIV infection. 2004 recommendations of the International AIDS Society-USA Panel. JAMA. 2004;292:251–365. doi: 10.1001/jama.292.2.251. [DOI] [PubMed] [Google Scholar]
- 8.Nunn AJ, Kengeya-Keyondo JF, Malamba SS, Seeley JA, Moulder DW. Risk factors for HIV-1 infection in adults in a rural Ugandan community: a population study. AIDS. 1994;8:81–86. doi: 10.1097/00002030-199401000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Nunn AJ, Wagner HU, Okongo JM, Malamba SS, Kengeya-Keyondo JF, Moulder DW. HIV-1 infection in a Ugandan town on the trans African highway: prevalence and risk factors. Int J STD AIDS. 1996;7:123–130. doi: 10.1258/0956462961917320. [DOI] [PubMed] [Google Scholar]
- 10.Morgan D, Maude GH, Malamba SS, et al. HIV-1 disease progression and AIDS-defining disorder in a rural Uganda cohort. Lancet. 1997;350:245–250. doi: 10.1016/S0140-6736(97)01474-8. [DOI] [PubMed] [Google Scholar]
- 11.Narain JP, Raviglione MC, Kochi A. HIV-associated tuberculosis in developing countries: epidemiology and strategies for prevention. Tuberc Lung Dis. 1992;73:311–321. doi: 10.1016/0962-8479(92)90033-G. [DOI] [PubMed] [Google Scholar]
- 12.Guelar A, Gatell JM, Verdejo J, et al. A prospective study of the risk of tuberculosis among HIV-infected patients. AIDS. 1993;6:1435–1439. doi: 10.1097/00002030-199310000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Whalen CC, Johnson JL, Okwera A, Hom DL, Huebner R, et al. A trial of three regimens to prevent tuberculosis in Ugandan adults infected with the human immunodeficiency virus. New Engl J Med. 1997;337:801–808. doi: 10.1056/NEJM199709183371201. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JL, Okwera A, Hom DL, Mayanja H, Kityo CM, et al. Duration of efficacy of treatment of latent tuberculosis infection in HIV-infected adults. AIDS. 2001;15:1–11. doi: 10.1097/00002030-200111090-00009. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization Acquired immune deficiency syndrome (AIDS): interim proposal for a WHO staging system for HIV-1 infection and disease. Wekly Epidemiol Rec. 1990;65:221–228. [PubMed] [Google Scholar]
- 16.Tuner BJ, Markson LE, McKee L, et al. The AIDS-defining diagnosis and subsequent complications: a survival-based severity index. J Acquir Immune Defic Syndr. 1991;4:1059–1071. [PubMed] [Google Scholar]
- 17.Mocroft AJ, Johnson MA, Sabin CA, Lipman M, Elford J, et al. Staging system for clinical AIDS patients. Lancet. 1995;346:L12–17. doi: 10.1016/s0140-6736(95)92649-6. [DOI] [PubMed] [Google Scholar]
- 18.Hanson DL, Chu SY, Farizo KM, Ward JW, et al. Distribution of CD4 T lymphocytes at diagnosis of acquired immunodeficiency syndrome-defining and other human immunodeficiency virus-related illnesses. Arch Inter Med. 1995;155:1537–1542. [PubMed] [Google Scholar]
- 19.Crowe SM, Carlin JB, Stewart KI, Lucas CR, Hoy JF. Predictive value of CD4 lymphocyte numbers for the development of opportunistic infections and malignancies in HIV-infected persons. J Acquir Immune Defic Syndr. 1991;4:770–776. [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Cox DR. Regression models and life tables (with discussion) J Royal Stat Soc B. 1972;74:187–220. [Google Scholar]
- 22.SAS Institute Inc . SAS/STAT software. SAS Institute Inc; Cary, NC: [Google Scholar]
- 23.Morgan D, Maude GH, Malamba SS, Okongo MJ, Wagner H-U, et al. HIV-1 disease progression and AIDS-defining disorders in rural Uganda. Lancet. 1997;350:245–250. doi: 10.1016/S0140-6736(97)01474-8. [DOI] [PubMed] [Google Scholar]
- 24.Morgan D, Whitworth JAG. The natural history of HIV-1 infection in Africa. Nat Med. 2001;7:143–145. doi: 10.1038/84564. [DOI] [PubMed] [Google Scholar]
- 25.Del Amo J, Petruckevitch A, Phillips AN, Johnson AM, Stephenson JM, et al. Spectrum of disease in Africans with AIDS in London. AIDS. 1996;10:1563–1569. doi: 10.1097/00002030-199611000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Del Amo J, Petruckevitch A, Phillips A, Johnson AM, Stephenson J, et al. Disease progression and survival in HIV-1 infected Africans in London. AIDS. 1998;12:1203–1209. doi: 10.1097/00002030-199810000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Morgan D, Malamba SS, Orem J, Mayanja B, Okongo M, Whitworth JAG. Survival by AIDS defining conditions in rural Uganda. Sex Transm Infect. 2000;76:193–197. doi: 10.1136/sti.76.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Dell MW, Lubeck DP, O'Driscoll P, Matsuno S. Validity of the Karnofsky performance status in an HIV-infected sample. J Acquir Defic Syndr Human Retrovirus. 1995;1:350–357. [PubMed] [Google Scholar]
- 29.Wenzel T, Pindur G, Morsdorf S, Giacchi J. Influence of HIV-infection on the Karnofsky score and general social functioning in patients with hemophilia. Haemostasis. 1998;28:106–110. doi: 10.1159/000022420. [DOI] [PubMed] [Google Scholar]
- 30.Fantoni M, Izzi I, Borgo D, Forno D, et al. Inter-rater reliability of a modified Karnofsky scale of performance status for HIV-infected individuals. AIDS Patient Care STDS. 1999;13:23–28. doi: 10.1089/apc.1999.13.23. [DOI] [PubMed] [Google Scholar]
- 31.Pezzotti P, Phillips AN, Dorrucci M, et al. Category of exposure to HIV and age in progression to AIDS: longitudinal study of 1199 people with known dates of seroconversion. BMJ. 1996;313:583–586. doi: 10.1136/bmj.313.7057.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Touloumi G, Karafoulidou A, Gialeraki A, Katsarou O, Milona I, et al. Determinants of progression of HIV infection in a Greek hemophilia cohort followed for up to 16 years after seroconversion. J Acquir Defic Syndr Human Retrovirus. 1998;19:89–97. doi: 10.1097/00042560-199809010-00014. [DOI] [PubMed] [Google Scholar]
- 33.Hawken MP, Meme HK, Elliot LC, et al. Isonizid preventive therapy for tuberculosis in HIV-infected adults: results of a randomized controlled trial. AIDS. 1997;11:875–882. doi: 10.1097/00002030-199707000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Quigley MA, Mwinga A, Hosp M, Lisse I, Fuchs D, et al. Long-term effect of preventive therapy for tuberculosis in a cohort of HIV-infected Zambian adults. AIDS. 2001;15:215–222. doi: 10.1097/00002030-200101260-00011. [DOI] [PubMed] [Google Scholar]
- 35.Bucher HC, Griffith LE, Guyatt GH, et al. Isonizid prophylaxis for tuberculosis in HIV infection: a meta-analysis of randomized controlled trials. AIDS. 1999;13:501–507. doi: 10.1097/00002030-199903110-00009. [DOI] [PubMed] [Google Scholar]
- 36.Moreno S, Miralles P, Diaz MD, et al. Isoniazid preventive therapy in human immunodeficiency virus-infected person. Long-term effect on development of tuberculosis and survival. Arch Intern Med. 1997;157:1729–1734. [PubMed] [Google Scholar]
- 37.Pinho AMF, Santoro-Lopes G, Harrison LH, Schechter M. Chemoprophylaxis for tuberculosis and survival of HIV-infected patient in Brazil. AIDS. 2001;15:2129–2135. doi: 10.1097/00002030-200111090-00008. [DOI] [PubMed] [Google Scholar]
- 38.Pape JW, Jean SS, Ho JL, et al. Effect of Isonizid prophylaxis on incidence of active tuberculosis and progression of HIV infection. Lancet. 1993;342:268–272. doi: 10.1016/0140-6736(93)91817-6. [DOI] [PubMed] [Google Scholar]
- 39.Dolan MJ, Clerici M, Blaatt SP, et al. In vitro T cell function delayed-type hypersensitivity skin testing, and CD4+ T cell subset phenotyping independently predict survival time in patients infected with human immunodeficiency virus. J Infect Dis. 1995;172:79–87. doi: 10.1093/infdis/172.1.79. [DOI] [PubMed] [Google Scholar]
- 40.Gordin FM, Hatigan PM, Klimas NG, et al. Delayed-type hypersensitivity skin tests are an independent predictor of human immunodeficiency virus disease progression. J Infect Dis. 1994;169:893–897. doi: 10.1093/infdis/169.4.893. [DOI] [PubMed] [Google Scholar]