Abstract
Background
In many cohort studies, biological specimens are being stored without specific plans for analyses. In the Norwegian Mother and Child Cohort Study biological specimens (DNA, plasma, and whole blood) are stored on 96-well plates and as a result may undergo multiple freeze-thaw cycles.
Methods
To explore the impact of multiple freeze-thaw cycles on chemical constituents, we conducted a quality control study using pooled EDTA-plasma. Over a two-year period, samples stored at −80 °C were subjected up to 100 freeze-thaw cycles. Specimens were analyzed in triplicate for sodium, cholesterol, triglycerides, vitamin E, aspartate aminotransferase (AST), and free fatty acids. We assessed the percent change of analyte concentration from the values for the first freeze-thaw cycle, because this is the baseline for all stored specimens.
Results
With the exception of free fatty acids, there was little change over the first 10 freeze-thaw cycles. A majority of analytes showed no significant changes until 30 freeze-thaw cycles. After 30 freeze-thaw cycles, the largest percent change was observed for free fatty acids (+32%), AST (+21%), and triglycerides (−19%).
Conclusions
Human plasma can go through several freeze-thaw cycles before analysis without influencing sample integrity for the selected analytes.
Keywords: biological samples, blood, plasma, long-term storage, specimen banking
Introduction
More and more prospective epidemiological studies collect and store biological specimens for future use. In many of these studies, the specific analyses of this material have not been determined at study start and such decisions may not be made for many years into the future. Therefore it is important to establish a quality assurance program that follows the sample’s behavior in the freezer. It is recommended to store the biological material in small aliquots in single vials [1, 2]; however, for many large studies this is not possible. Thus it is important to know how different components behave during repeated freeze-thaw cycles to be able to use the limited biological material from one individual to its maximum potential. Information on stability during storage is critical in evaluating for what the different biological materials can be used [3].
The Norwegian Mother and Child Cohort Study (MoBa) is a prospective study with the purpose to find causes of diseases among mothers and children. Possible causal factors will be linked to information obtained from questionnaires, blood samples from mother, father and child, urine sample from mother and medical registries [4, 5]. We plan to collect and store biological material from 100,000 pregnancies. Pregnant women are recruited from all parts of Norway; as a result, specimens are collected at a number of hospitals and are stored in a central repository. Most of the biological material in MoBa, including EDTA-plasma, is stored in 96-well plates sealed with heat sealing foil. The 96-well format was selected to allow for rapid, semi-automatic handling for specimen storage [4]. This means that each sample stored on a plate undergoes a freeze-thaw cycle whenever a sample on this plate is retrieved. Given these logistics, we wanted to assess the impact both of multiple freeze-thaw cycles and longterm freezer storage on common analytes as surrogates for broad classes of agents.
We designed a quality control study to assess the impact of multiple freeze-thaw cycles on sample integrity over a two year period. We used a suite of common clinical analytes and analyzed specimens after repeated freeze-thaw cycles, up to 100 freeze-thaw cycles. Results for sodium, cholesterol, triglycerides, free fatty acids, vitamin E and aspartate aminotransferase (AST) are presented here.
Materials and Methods
To assess the effects of long-term storage and freeze-thaw cycles among samples collected in MoBa we designed a quality control study to mimic our study conditions. The MoBa study collects whole blood with EDTA as anti-coagulant from pregnant women throughout Norway [4, 5]. After collection, the specimens for plasma are spun within 4 hours of collection and shipped overnight at room temperature to a central Biobank in Oslo [4]. At receipt in the Biobank, samples are aliquoted and placed in long-term freezer storage. A majority of plasma samples are placed on 96-well plates, with up to 95 individuals on one plate; thus specimens may undergo many freeze-thaw cycles until a specific aliquot is pulled.
For our quality assurance specimens, we created an EDTA plasma pool by collecting blood from 40 healthy adult donors in Oslo. Both males and non-pregnant females were included. The blood was collected venously in EDTA tubes (BD Vacutainer). Our goal was to obtain sufficient sample volume to allow repeated measures over time without the need for a new quality assurance pool. To simulate methods used in the MoBa study, the blood stood on the bench for up to 2 hours before plasma was centrifuged at 1800g for 10 minutes at room temperature, and plasma separated. The samples were pooled and stored at 4 °C overnight before aliquoting onto microtitre plates (AB Gene, Epsom, UK), 300 μl per well and sealed with Easy pierce heat sealing foils (AB gene, Epsom, UK). After aliquoting, all plasma samples were stored in the same manner as other MoBa samples, in electric freezers at −80 °C.
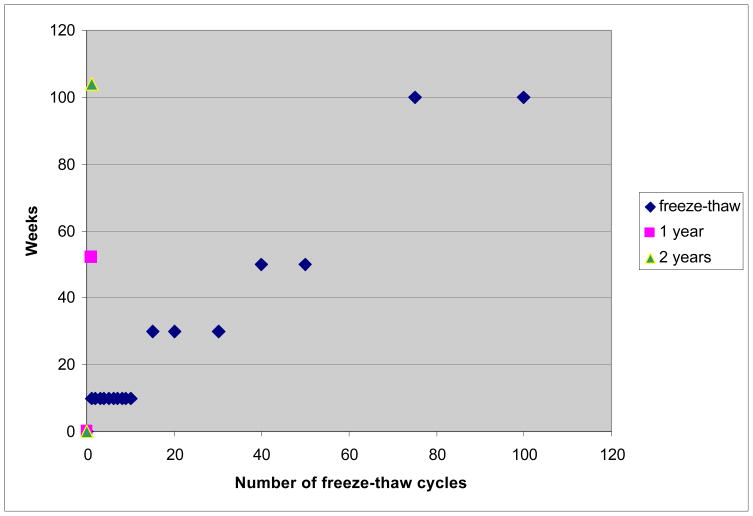
To evaluate the impact of freeze-thaw events on specimen quality, we thawed plates weekly for up to 100 weeks. For each time point, three samples, representing three different plates, were sent to the laboratory and were analyzed as three independent samples. Figure 1 shows the schedule of analyte testing following multiple freeze-thaw cycles over time. The EDTA plasma was thawed once a week. The thawing was done at room temperature (1 hour). The samples were retrieved using a pipette to pierce through the foil. When the retrieval of all selected wells on a plate was completed, the holes in the foil were re-sealed with small pieces of a re-sealing heat foil (AB gene, Epsom, UK). For freeze-thaw cycles where no retrievals were done, the samples were homogenized and left on the bench for another 1 hour before freezing again to mimic the approximate retrieval time. We drew an aliquot for chemical analysis after 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 30, 40, 50, 75 and 100 freeze-thaw cycles; t1,t2,…,t100. A time zero sample (t0) was analyzed on fresh plasma 24 hours after collection and simultaneously with freezing the rest of the plasma.
Figure 1. Experimental design. The figure shows the timeline for the analysis.
Study design
To assess that possible change in marker concentrations were the result of the freeze-thaw process and not storage time in the freezer, we also sent samples that had never been thawed before to analysis after 12 and 24 months, t12m, t24m.
The EDTA plasma was analysed for sodium, cholesterol, triglycerides, free fatty acids, vitamin E and AST and are presented in Table 1. We chose this suite of analytes to represent different aspects of sample integrity and as surrogates for other chemical agents which might behave similarly. Sodium was chosen as a marker of volume; if the sample was evaporating, the sodium concentration would be anticipated to increase [6]. We chose cholesterol and triglycerides as measures of lipid degradation as other investigators have reported changes over time in these analytes [7–10]. For sample analysis, we chose laboratories where the analyses were conducted on routine basis. When possible we also chose clinical laboratories where the analyses were ISO 17025 accredited. The samples for sodium, cholesterol, triglycerides, vitamin E and AST were sent at room temperature and analyzed the same day. Samples for free fatty acids, were sent frozen (−80 °C) to analysis and have therefore undergone an additional freeze-thaw cycle.
Table 1. Analysis performed.
Analytes selected for the freeze-thaw quality control study
| Analytes in EDTA plasma | Indicator for |
|---|---|
| Sodium | Volume changes |
| Cholesterol | Lipid degradation |
| Triglycerides | Lipid degradation |
| Free fatty acids | Degradation of fatty acids |
| Vitamin E | Anti-oxidative capacity |
| Aspartate aminotransferase (AST) | Enzyme activity |
For each time period and each analyte, we calculated the mean and standard deviation and compared the means after each time point. We compared all sample results to the values reported in the baseline sample (t1). The t0 sample provides a “fresh” level, but because all specimens in the MoBa will undergo one freeze-thaw cycle at a minimum, this sample was not used to explore changes as a result of freeze-thaw events. Only one of the 360 results was excluded as an outlier using Grubb’s test [11]. We calculated the percent change from baseline (t1) as follows: ((tx−t1)/t1)*100. Changes in mean component concentrations were evaluated using paired t-tests. All statistical analyses were conducted in SPSS (version 11, Chicago, Illinois). We used p<0.05 as a measure of statistical significance.
Results
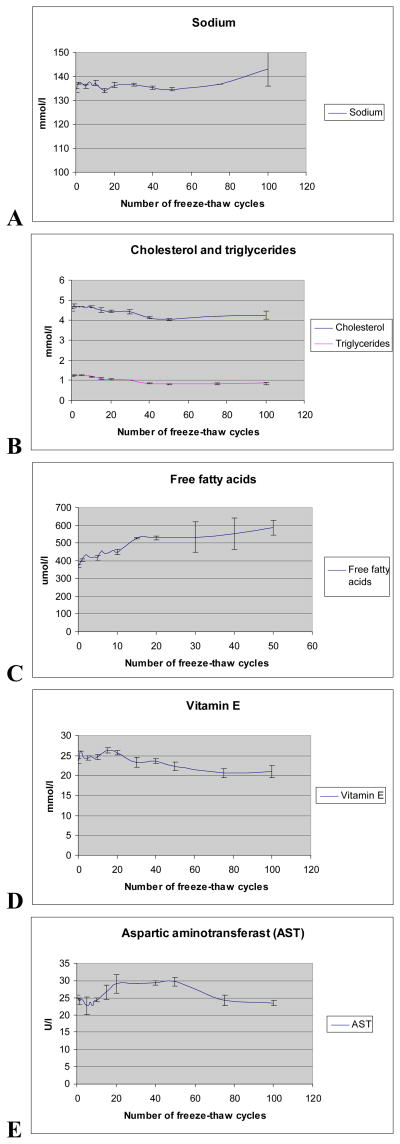
The concentrations of sodium, cholesterol, triglycerides, free fatty acids, vitamin E and AST in EDTA plasma over the 100 freeze-thaw cycles are presented in Figure 2. Sodium was the only analyte that did not have big changes in concentration over 100 freeze-thaw cycles. Cholesterol, triglycerides, and vitamin E were stable for the first 10–20 cycles, and then the concentrations dropped. Free fatty acid levels increased with the number of freeze-thaw cycles, with a large increase after 10 freeze-thaw cycles. As a result of these data, we stopped analysis of free fatty acids after 50 cycles. After the first 15 cycles, AST levels increased and then after 50 cycles concentrations decreased.
Figure 2.
Mean concentrations with standard deviations in plasma-EDTA during repeated freeze-thaw cycles. A - Sodium, B - cholesterol and triglyserides, C - free fatty acids, D - Vitamin E, E - aspartate aminotransferase
Results for the freeze-thaw cycle analyses are presented in Table 2. A majority of the analytes changed between collection and 1 freeze-thaw, but only sodium, cholesterol and vitamin E were significantly different. Because the design of the MoBa study and other biobanks require a minimum of 1 freeze-thaw cycle, we did all further comparisons to t1. All analytes, except free fatty acids, were stable for at least the first 10 freeze-thaw cycles. The triglyceride and cholesterol concentrations dropped after 10 and we saw a significant change after 20 cycles. These changes corresponded to a reduction of 17% for triglycerides and 6% for cholesterol. Vitamin E was stable over the first 20 cycles and after 50 cycles the decrease was statistically significant. Free fatty acids were the most unstable and increased with each freeze-thaw cycle (Figure 2). After 10 cycles they had a significant change corresponding to an 11% increase and they increased by 31 % after 20 freeze-thaw cycles. The AST concentration had, as seen in figure 2, a different type of curve from the other analytes. The increase in concentration was statistically significant after 30 and 50 cycles, before it returned to the level near t1 after 75 cycles. A similar change was seen after 20 cycles, but this change was not statistically significant.
Table 2.
Influence of freeze-thaw cycles on mean concentrations for different plasma components.
| Component | mean conc “fresh” | mean conc 1 cycle | mean change 0 to 1 cycle | mean change after 10 cycles, % | mean change after 20 cycles, % | mean change after 30 cycles, % | mean change after 50 cycles, % |
|---|---|---|---|---|---|---|---|
| Sodium, mmol/l | 134 | 137 | 2a | 0 | −1 | 0 | −2a |
| Cholesterol, mmol/l | 4.5 | 4.7 | 4a | 0 | −6a | −6a | −15a |
| Triglycerides, mmol/l | 1.21 | 1.25 | 3 | −5 | −17a | −19a | −35a |
| Free fatty acids, umol/l | 372 | 404 | 9 | 11a | 31a | 32a | 45a |
| Vitamin E, umol/l | 24 | 26 | 8a | −8 | 0 | −12 | −15a |
| Aspartate aminotransferase, U/L | 25 | 24 | −4 | 0 | 21 | 21a | 25a |
p<0,05
All comparisons are done to one freeze-thaw cycle.
Table 3 presents the results for specimens after their first thawing: 12 and 24 months after storage. Compared to baseline (t1), there were no significant differences in concentrations of sodium, cholesterol, free fatty acids and AST, after 12 and 24 months. Vitamin E had a significant increase after 24 months while triglycerides had a significant decrease already after 12 months.
Table 3.
Influence of long-term storage on mean concentrations for different plasma components.
| Component | mean t1 | mean 12 months | Δmean 12 mo, % | mean 24 months | Δmean 24 mo, % |
|---|---|---|---|---|---|
| Sodium, mmol/l | 137 | 137 | 0 | 137 | 0 |
| Cholesterol, mmol/l | 4.7 | 4.7 | 0 | 4.7 | 0 |
| Triglycerides, mmol/l | 1.25 | 1.17 | −6a | 1.18 | −6a |
| Free fatty acids, umol/l | 404 | 402 | 0 | 403 | 0 |
| Vitamin E, umol/l | 26 | 27 | 4 | 29 | 12a |
| Aspartate aminotransferase, U/L | 24 | 26 | 8 | 22 | −8 |
p<0,05
Discussion
Our study showed that several components in EDTA plasma were stable over multiple freeze-thaw cycles. Over the first 10 cycles, the biggest change was between t0 and t1 for the majority of the analytes, however, following that there was little change in most analytes. This is good news for specimen banks which by definition have a minimum of one freeze-thaw cycle before conducting any analyses. Our suite of analytes provided information on a number of aspects of specimen integrity. Sodium was the most stable component both when it came to repeatedly freeze-thaw cycles and long-term storage. However, it alone can not be used as a marker for sample integrity, but primarily as an indicator of volume changes.
Cholesterol and triglycerides were stable for approximately 10 freeze-thaw cycles, after that the levels started to decline. We saw no change in cholesterol level during storage without thawing after 1 and 2 years; hence we believe the change was due primarily to the freeze-thaw cycles alone. For triglycerides, we saw a significant decrease (6%) after 12 months without thawing. This suggests that the decrease we observed after 50 freeze-thaw cycles mainly was a result of the freeze-thaw process but also that storage time may influence concentration. Other investigators have explored the impact of plasma cholesterol and triglycerides over time [7–10]. Using 10 samples at each time point, Comstock and colleagues showed a small change for cholesterol concentration of −0.4 % per freeze-thaw cycles during three cycles in plasma stored at −70 °C [12]; we would be unable to detect such a small change in concentration. Other studies including our own have shown stability during 3–10 cycles in human plasma stored at −80 °C and for 10 cycles in baboon serum stored at −20 °C [10, 13]. For triglycerides it has been reported stability for 3 cycles in plasma stored at −80 °C [10].
Our results showed that free fatty acid levels increased with the number of freeze-thaw cycles. Already after 10 cycles we saw an 11% increase and after 20 cycles it was 31%. At the same time as the free fatty acids increased, we also saw, as already discussed, that cholesterol and triglycerides decreased; this may explain the increase in free fatty acids concentration. The 12 and 24 month samples showed that the increase in free fatty acids was not associated with storage time but the freeze-thaw cycles alone. Hirsch and colleagues reported overall free fatty acids in plasma were stable when stored at −20 °C for 12 months. Additionally, they indicated that some individual fatty acids in plasma changed over the course of one year, but that this was not due to lipid peroxidation [14]. Taken together these analyses suggest that free fatty acids should not be analysed after repeated freeze-thaw cycles.
The AST level was stable within the first 10–15 freeze-thaw cycles. Earlier the enzyme was shown to be relatively stable through six cycles in serum stored at −70 °C to −76 °C [15], and the same study found only small changes during long-term storage over 10 years. The changes observed were statistically significant, but the authors suggest that the changes were not clinically relevant. We will continue to analyze AST and the other components with yearly intervals to be able to say more about the behaviour after long-term storage.
In our study the vitamin E concentration was relatively stable during the first 20–30 cycles. Gunter et al has found decreasing concentrations (−13% and −22%) after five freeze-thaw cycles in two serum pools stored at −20 °C for 7 to 13 months, suggesting that our colder storage temperature may minimize degradation [16]. Further work by the NHANES laboratory and others have indicated that vitamin E is stable for up to 5 years at −70 °C [17,18]
Our study represents just one aspect of a quality control program for stored specimens. For this quality control study, we chose to use one plasma pool with an average concentration, thus we are limited in our ability to evaluate what would happen at the different analyte levels. We chose to use a balanced design that had the same number of replicates at each time period. In retrospect, the statistical analysis would have been strengthened by having more replicates at t1. While we selected a wide range of analytes, clearly, they do not represent the full spectrum of analytes which may be considered in future investigations.
The laboratory accuracy of the analyses was very good. The coefficients of analytical variations were 1.0% for sodium, 3.1% for AST, 3.4% for triglycerides, 4.0% for cholesterol, 4.8% for Vitamin E and and 5.0% for free fatty acids. Because the analyses were run over a two-year period, we have to consider that small changes in the laboratories over this period could contribute to the observed changes after multiple freeze-thaw cycles. However, given that we did not observe any differences with samples stored for 12 and 24 months, it is unlikely that laboratory variability explained our results. Because we had such good analytical capabilities, small changes in concentration may have been statistically significant and yet may not represent meaningful changes in concentration compared to the population variability.
In design of biobanks or in the use of stored plasma, these kinds of analyses can be beneficial in the evaluation of sample integrity. We showed that most components can go through up to 10 or more freeze-thaw cycles. While the 96-well plate format is an inexpensive way to store plasma, we do not recommend this for all analytes. As a result of this study, it is clear that in the The Norwegian Mother and Child Cohort Study we will have to re-aliquot at least some of our plasma samples aliquots stored in 96-well plate format to a single tube format to avoid more than 10–20 freeze-thaw cycles.
Acknowledgments
This work was supported in part by the intramural research program of the National Institutes of Health (National Institute of Environmental Health Sciences). We thank Trine Skjerden, Vibeke Nordanger, Jeanette Aarem and Rune Kjølstad for their help with the freezing and thawing of the specimens.
Abbreviations
- AST
Aspartate aminotransferase
- MoBa
The Norwegian Mother and Child Cohort Study
Reference List
- 1.Holland NT, Pfleger L, Berger E, Ho A, Bastaki M. Molecular epidemiology biomarkers--sample collection and processing considerations. Toxicol Appl Pharmacol. 2005;206:261–268. doi: 10.1016/j.taap.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 2.Winn DM, Reichman ME, Gunter E. Epidemiologic issues in the design and use of biologic specimen banks. Epidemiol Rev. 1990;12:56–70. doi: 10.1093/oxfordjournals.epirev.a036062. [DOI] [PubMed] [Google Scholar]
- 3.International Society for Biological and Environmental Repositories (ISBER) 2008 Best Practices for Repositories: Collection, Storage, Retrieval and Distribution of Biological Materials for Research. Cell Preservation Technology. 2008;6 (1):3–58. [Google Scholar]
- 4.Ronningen KS, Paltiel L, Meltzer HM, Nordhagen R, Lie KK, Hovengen R, Haugen M, Nystad W, Magnus P, Hoppin JA. The biobank of the Norwegian Mother and Child Cohort Study: a resource for the next 100 years. Eur J Epidemiol. 2006;21:619–625. doi: 10.1007/s10654-006-9041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magnus P, Irgens LM, Haug K, Nystad W, Skjærven R, Stoltenberg C the MoBa Study Group. Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35:1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 6.Longnecker MP, Zhou H, Klebanoff MA, Brock JW. An unexpected distribution of sodium concentration in serum specimens stored for more than 30 years. Ann Epidemiol. 2003;13:178–181. doi: 10.1016/s1047-2797(02)00415-5. [DOI] [PubMed] [Google Scholar]
- 7.Shih WJ, Bachorik PS, Haga JA, Myers GL, Stein EA. Estimating the long-term effects of storage at −70 degrees C on cholesterol, triglyceride, and HDL-cholesterol measurements in stored sera. Clin Chem. 2000;46:351–364. [PubMed] [Google Scholar]
- 8.Bachorik PS, Walker R, Brownell KD, Stunkard AJ, Kwiterovich PO. Determination of high density lipoprotein-cholesterol in stored human plasma. J Lipid Res. 1980;21:608–616. [PubMed] [Google Scholar]
- 9.Devanapalli B, Bermingham MA, Mahajan D. Effect of long-term storage at −80 degrees C on the various lipid parameters in stored plasma samples. Clin Chim Acta. 2002;322:179–181. doi: 10.1016/s0009-8981(02)00137-7. [DOI] [PubMed] [Google Scholar]
- 10.Kronenberg F, Lobentanz EM, Konig P, Utermann G, Dieplinger H. Effect of sample storage on the measurement of lipoprotein[a], apolipoproteins B and A-IV, total and high density lipoprotein cholesterol and triglycerides. J Lipid Res. 1994;35:1318–1328. [PubMed] [Google Scholar]
- 11.Grubbs F. Procedures for Detecting Outlying Observations in Samples. Technometrics. 1969;11:1–21. [Google Scholar]
- 12.Comstock GW, Burke AE, Norkus EP, Gordon GB, Hoffman SC, Helzlsouer KJ. Effects of repeated freeze-thaw cycles on concentrations of cholesterol, micronutrients, and hormones in human plasma and serum. Clin Chem. 2001;47:139–142. [PubMed] [Google Scholar]
- 13.Jennings C, Weaver DM, Kruski AW. Effects of freeze-thawing on determinations of cholesterol and high-density lipoproteins in baboon sera. Clin Chem. 1979;25:490. [PubMed] [Google Scholar]
- 14.Hirsch EZ, Slivka S, Gibbons AP. Stability of fatty acids on hyperlipoproteinemic plasma during long-term storage. Clinical Chemistry. 1976;22:445–448. [PubMed] [Google Scholar]
- 15.DiMagno EP, Corle D, O’Brien JF, Masnyk IJ, Go VL, Aamodt R. Effect of long-term freezer storage, thawing, and refreezing on selected constituents of serum. Mayo Clin Proc. 1989;64:1226–1234. doi: 10.1016/s0025-6196(12)61285-3. [DOI] [PubMed] [Google Scholar]
- 16.Gunter EW, Driskell WJ, Yeager PR. Stability of vitamin E in long-term stored serum. Clin Chim Acta. 1988;175:329–335. doi: 10.1016/0009-8981(88)90110-6. [DOI] [PubMed] [Google Scholar]
- 17.NHANES Laoratory Manual. www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l45_c_met_vitAE_carotenoids.pdf.
- 18.Craft NE, Brown ED, Smith JC. Effects of Storage and handling conditions on concentrations of individual carotenoids, retinol, and tocopherol in plasma. Clin Chem. 1988;34:44–48. [PubMed] [Google Scholar]