Abstract
Oncolytic herpes simplex virus (oHSV) mutants are under development as anticancer therapeutics. One such vector, rRp450, is ICP6-deleted and expresses a prodrug enzyme for cyclophosphamide (CPA) (rat CYP2B1). Little is known about rRp450’s toxicity profile, especially in combination with CPA. We tested rRp450/CPA for antitumor efficacy in an aggressive human xenograft sarcoma model, measured virus production in primary cells, and tested rRp450/CPA for safety in immunocompetent mice. CPA enhanced the antitumor efficacy of rRp450. Relative to wild-type HSV-1, rRp450 replication was attenuated ~10,000-fold in human primary hepatocytes, differentiated primary foreskin keratinocytes, and primary Schwann cells. In vivo, intravenous and intracranial (IC) rRp450 injection at the strength of 108 plaque-forming units (pfu) alone or followed 24 hours later by intraperitoneal (IP) CPA was well tolerated and had no significant effect clinically on blood counts or chemistries. By contrast, intravenous KOS was found to be uniformly neurotoxic at 105 and fatal at 106 pfu, and IC virus was fatal in most mice at 104 pfu. Low levels of virus DNA were detected in some organs following intravenous and IC virus injection, but were not significantly altered by CPA. HSV replication was not detected in reactivation studies of isolated organs. Our findings suggest rRp450/CPA is safe and warrants further study as a potential combination in anticancer therapeutics.
INTRODUCTION
The use of multiagent chemotherapy combined with surgery and radiation has significantly advanced the treatment of many solid tumors, particularly sarcomas, occurring in childhood. Most sarcomas in adults, however, are resistant to chemotherapy and radiation.1 Even with chemosensitive cancers in children such as neuroblastoma, less than half the patients with metastatic disease survive up to 5 years from the time of diagnosis when undergoing high dose intensity therapies,2 and <30% of patients with metastatic sarcomas of most histological types are curable.3 Therefore, new treatment options are desperately needed for these diseases.
Oncolytic herpes simplex virus (oHSV) mutants are being actively pursued as novel therapeutic agents in preclinical4-7 and early phase clinical studies.8-11 One strategy to enhance efficacy is the use of viruses “armed” to express prodrug-activating enzymes to provide a bystander effect. rRp450 is a HSV-1 KOS-derived mutant deleted for the UL39 gene that encodes ICP6, the large subunit of ribonucleotide reductase, causing it to be permissive for replication selectively in rapidly dividing cells. In rRp450, the UL39 gene is replaced by the rat CYP2B1 gene, encoding a cytochrome P450 enzyme able to activate oxazophosphorines such as cyclophosphamide (CPA).7 In preclinical studies, rRp450 kills tumor cells by direct lysis and potentiates the antitumor effect of CPA.12,13 In turn, CPA enhances viral replication in vivo by counteracting the host response against the virus.14,15
Clinical safety studies of oHSVs have been limited to viruses attenuated by deletion of both copies of the so-called “neurovirulence” gene, RL1, encoding ICP34.5 (refs. 5,9,10,16) or multimutated viruses such as G207 (ref. 17) and NV1020 (ref. 18). The antitumor effect of G207 is significantly diminished compared with a wild-type virus or viruses containing single gene mutations.5,6,19 The increased lytic potency of single gene mutants raises the question of increased toxicity. Extensive toxicity studies of viruses dependent solely on deletion of ICP6 to confer tumor-selective replication have not been conducted, so it remains unclear whether viruses such as rRp450 are also clinically safe. Furthermore, little safety data exist regarding the combination of the virus with the prodrug. The potential for latent infection and the risk of virus re-activation for ICP6 mutants have been studied only using corneal and intracranial (IC) inoculation models20,21; the safety of the virus when directly entering the bloodstream as might occur during intratumoral (IT) injection or with systemic therapy is unknown. Finally, the influence of an immunomodulating drug such as CPA on the incidence of latency has not been examined.
We have found oHSV to be efficacious in preclinical models of sarcomas,6,19,22-24 and seek to develop clinical trials of IT injection as a novel method of “local control” for unresectable solid tumors. Here, we demonstrate that systemic CPA enhances the efficacy of IT rRp450 in a human sarcoma xenograft model. To address issues of safety, we first conducted in vitro studies to evaluate virus attenuation. We then sought to determine the in vivo clinical safety of rRp450 administered alone or followed by systemic CPA in the brain, as it would be the site most likely to be susceptible to HSV-1 infection, and in the blood to account for the worst-case scenario of systemic leakage with IT injection. Furthermore, we evaluated the potential of rRp450 to establish latency and re-activate following intravenous (IV) or IC injection alone or in combination with CPA. Our findings suggest rRp450 is >10,000-fold attenuated relative to wild-type HSV-1, and that local and systemic administration of rRp450 alone and when followed by a dose of CPA is well tolerated.
RESULTS
CPA enhances the antitumor efficacy of rRp450 in an aggressive sarcoma xenograft model
To confirm the antitumor activity of rRp450 and test for enhancement with CPA, athymic nude mice bearing subcutaneous human alveolar rhabdomyosarcoma Rh30 xenograft tumors (200–500 mm3) were treated two times with IT rRp450 alone or followed by intraperitoneal (IP) CPA. CPA was administered 24 hours after the virus to allow time for virus-mediated expression of the prodrug enzyme, CYP2B1. The rRp450 virus alone significantly prolonged animal survival relative to control animals injected with phosphate-buffered saline (PBS; Figure 1, log-rank P < 0.001). Although CPA showed only a minimal antitumor effect by itself (P = 0.03), addition of CPA to the virus further extended survival time (P = 0.0001 rRp450/CPA versus CPA, P = 0.003 rRp450/CPA versus rRp450), consistent with the previously described potentiating effects of rRp450/CPA.12,14 The antitumor effect of IT rRp450 was confirmed in a second human xenograft model of alveolar rhabdomyosarcoma (Rh18) and a human xenograft model of neuroblastoma (CHLA-20), where we observed significant tumor shrinkage in 13/13 injected tumors, 8 of which were initially complete responses (Supplementary Figure S1).
Figure 1. Antitumor effect of rRp450/cyclophosphamide (CPA).
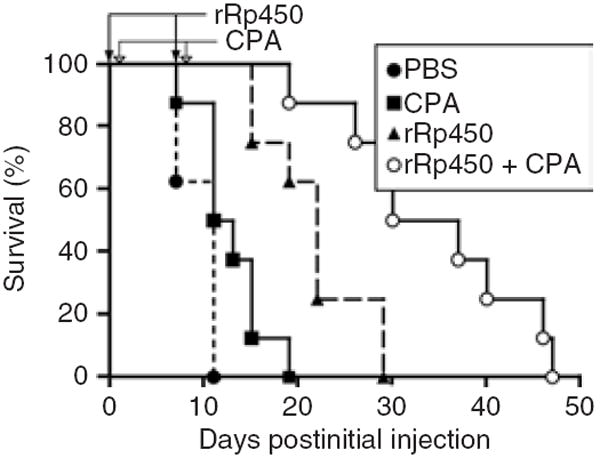
Mice bearing Rh30 xenografts were given direct intratumoral injections of phosphate-buffered saline (PBS) (control), CPA alone, rRp450 alone, or rRp450 followed by CPA. The timings of virus (days 0 and 7) and CPA (days 1 and 8) administration are shown by the closed and open arrows, respectively.
rRp450 is attenuated in primary human cells
To assess the attenuation of rRp450 replication, we measured the ability of normal human hepatocytes, primary human foreskin keratinocytes (HFKs), and normal human Schwann cells (NHSCs) to support rRp450 production relative to its wild-type parent virus, KOS, or the multimutated virus NV1020 (Figure 2). In normal hepatocytes, the amount of infectious virus declined over time, suggesting a nonproductive infection (Figure 2a). By contrast, a robust increase in virus production of the parent virus (KOS) was observed; at the time point of 72 hours after infection there was a 4-log difference (10,000-fold) in titers between rRp450 and KOS. On account of concerns about the possible neurological side effects with HSV-based vectors, we also tested replication of rRp450 in NHSCs. In addition to rRp450 and wild-type KOS, an oHSV currently under clinical trial, NV1020, was also included. In NHSCs, virus production of rRp450 was attenuated relative to KOS and NV1020 (Figure 2b). In 48 hours, KOS titers increased by 3 logs, NV1020 increased 2 logs, and rRp450 increased ~1 log.
Figure 2. Herpes simplex virus production of oncolytic mutants in normal human cells.
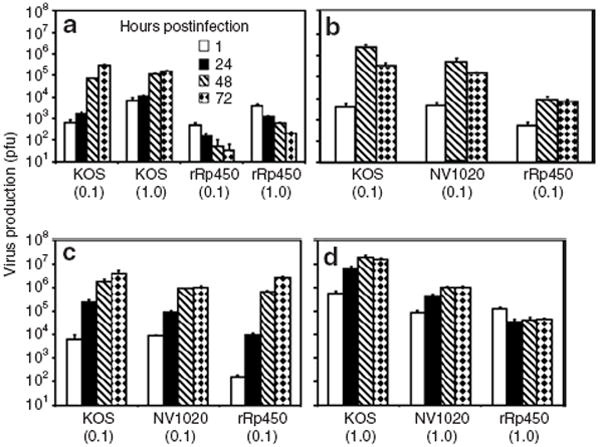
Using different multiplicities of infection (plaque-forming units/cell) as shown in parentheses, cell lysates were titered at times indicated following inoculation of cultures of (a) hepatocytes, (b) Schwann cells, (c) proliferating keratinocytes, and (d) differentiated keratinocytes. Bars indicate SDs of triplicate points. Similar data were obtained on a repeat experiment. SD is shown.
In dividing primary HFKs, all three viruses showed 3–4 logs increases in virus production over 48–72 hours (Figure 2c). By contrast, the replication of rRp450 was severely attenuated in quiescent, differentiated cultures of HFKs (Figure 2d). Virus production of both wild-type KOS and the NV1020 mutant under these conditions persisted, increasing by 1–2 logs. These data suggest that rRp450 is more attenuated than the wild-type virus or NV1020.
Mice administered rRp450/CPA survive long term
To assess the effects on survival of administering rRp450 alone or in combination with CPA, and to capture any toxicity due to an antiviral immune response, we injected rRp450, rRp450/CPA, and wild-type KOS (positive control) into immunocompetent FVB/N mice. Although the animals tolerated a single IV dose of KOS at 104 plaque-forming units (pfu) without any signs of illness, a single IV injection of KOS at 105 pfu caused a reversible neurotoxicity; all mice were observed to have an abnormal gait for 2–3 days with apparent hind-limb paralysis. A dose of 106 pfu was uniformly lethal within 2–3 days (Figure 3a). These results defined the level at which FVB/N mice are susceptible to human HSV-1 infection. By contrast, following a single IV injection of 108 pfu rRp450, all mice survived >100 days and 95% (19/20) survived >300 days (the cause of the isolated death is unknown; log-rank versus KOS P < 0.0001) (Figure 3a). A relatively high dose of IP CPA (200 mg/kg) without the virus showed some toxicity as only 70% of the mice (7/10) survived beyond 50 days (Figure 3a). Intravenous administration of rRp450 24 hours before administration of the high dose IP CPA (200 mg/kg) did not significantly increase toxicity (P > 0.5). Necropsy of the mice given both the virus and CPA that died on days 20 and 22 showed evidence of bacterial pneumonia, likely related to CPA-induced immunosuppression. There were no lesions noted in the heart, kidney, liver, pancreas, femur, cerebrum, or cerebellum. CPA-induced toxicity was reduced at a lower dose of CPA (50 mg/kg); nevertheless, this dose was sufficient to cause marrow suppression with a nadir at 6 days (Supplementary Figure S2). Pretreatment of animals with IV administration of rRp450 prior to that of CPA at 50 mg/kg did not increase toxicity as ≥85% of mice survived >100 days in both groups (Figure 3b, P > 0.3).
Figure 3. Effect of rRp450/cyclophosphamide (CPA) on long-term survival.
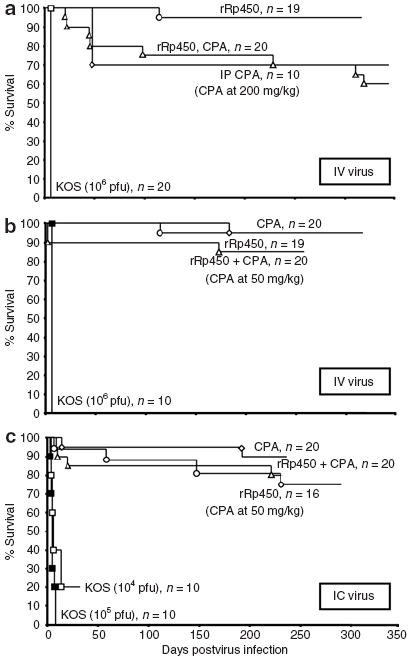
Groups of mice were administered rRp450 at 108 plaque-forming units (pfu) or lower doses of wild-type KOS as indicated. Viruses were given (a) intravenous (IV) alone or followed 24 hours later by 200 mg/kg intraperitoneal (IP) CPA, (b) IV alone or followed 24 hours later by 50 mg/kg IP CPA, and (c) IC alone or followed 24 hours later by 50 mg/kg IP CPA.
We also tested IC virus injections. IC injection of wild-type 104 pfu of KOS caused death in 7 days in four of five male mice and in 12 days in four of five female mice, suggesting that the IC lethal dose in 50% of KOS is <104 pfu in FVB/N mice. With the administration of 108 pfu rRp450 IC injection alone, >90% of mice survived with only 1 of 16 mice dying before day 55 (Figure 3c). The addition of IP CPA (50 mg/kg) 24 hours after administering IC rRp450 did not significantly alter the effect on survival seen with IP CPA alone, as 90% (18/20) and 80% (16/20) of the mice survived >200 days, respectively (Figure 3c). Consistent with the low incidence of global toxicity, mice given IV rRp450, IC rRp450, or IC rRp450/CPA showed no differences in their weights measured 20–30 days after the injections compared with age-matched controls (Supplementary Figure S3).
Normal organ function following systemic rRp450
To address the concern of possible virus replication in dividing normal cells such as hematopoietic progenitors, we measured complete blood counts in mice at early (day 3) and late (day 28) time points following administration of IV and IT rRp450 alone or when followed 24 hours later by CPA. With the IV virus, we observed an increase in the total white blood count and absolute lymphocyte count at both time points (Figure 4a and Supplementary Figure S4), likely the result of an antiviral immune response. The increase in neutrophils seen in tumor-bearing animals was unaffected by virus (Supplementary Figure S5). We found only minor, statistically significant but clinically insignificant changes in electrolytes (Supplementary Figure S6) and liver function tests (Supplementary Figure S7).
Figure 4. Effect of systemic rRp450 on bone marrow function.
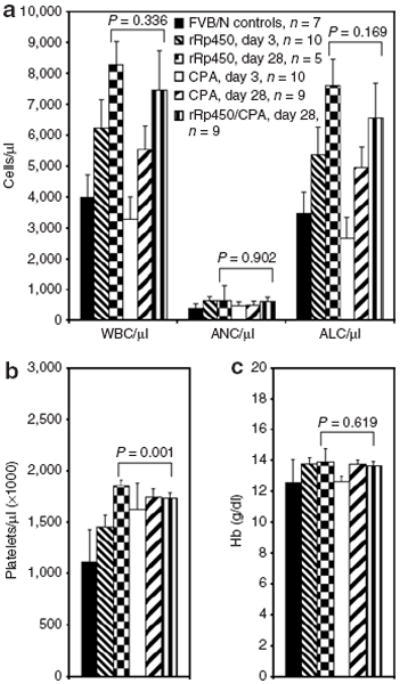
Animals were given 108 plaque-forming units (pfu) rRp450 intravenous alone or followed 24 hours later by cyclophosphamide (CPA) (50 mg/kg), and blood was collected at early (day 3) and late (day 28) time-points for (a) total white blood cell count (WBC) and differential including absolute neutrophil count (ANC) and absolute lymphocyte count (ALC), (b) platelet count, and (c) hemoglobin (Hb). Numbers of mice in each group are shown, as are SD within each group. SD is shown.
Given the increase in lymphocytes and because a host immune reaction to IT virus injection could contribute to toxicity, we sought to characterize the cellular immune response to IT oHSV in immunocompetent animals bearing syngeneic sarcomas. We analyzed tumors at the time points of 2 and 6 days following virus injection. Despite the neutrophilia induced in the peripheral blood, there was only a low-level immune infiltrate of scattered neutrophils and a few identifiable lymphocytes in uninjected tumors. The infiltrate was essentially unchanged following virus injection alone or with CPA treatment at either time point (data not shown). These data suggest there was not a significant inflammatory reaction to the virus within the tumor, which was also not significantly altered by a single dose of postvirus CPA.
rRp450 exhibits an altered pattern of latent viral DNA
To determine the tissue distribution of latent virus infection with rRp450, genomic DNA was isolated from tissues of mice >100 days following IV and IC virus administration. In IV-treated mice, viral DNA was detectable by quantitative PCR in a subset of dorsal root ganglia (DRGs), trigeminal ganglia (TGs), spleens, and in one of eight bone marrow (BM) samples (Figure 5a), with no virus detected in any of the eight brains. Taking into account the total amount of DNA isolated on a per organ basis, the highest viral DNA load was in the spleen (~500,000 copies) with only <1% of that level in each of the other organs (~2,000–5,000 copies each; note that “whole organ” for DRGs represents pooled 5–20 single DRGs that were harvested from each animal and BM represents the total isolated from two femurs). In IV-treated mice followed 24 hours later by CPA (50 mg/kg) administration, for organs harvested >300 days later, viral DNA was found in even fewer mice and at somewhat lower levels (Figure 5b), again without being detected in the brain. In comparison, mice treated with 1,000-fold less wild-type KOS virus (105 pfu) showed detectable HSV DNA in the majority of DRGs and TGs, but in only one of seven spleens. In contrast to rRp450, wild-type virus DNA was found in all but one brain (Figure 5c).
Figure 5. Long-term detection of herpes simplex virus (HSV) DNA in mouse organs following systemic virus.
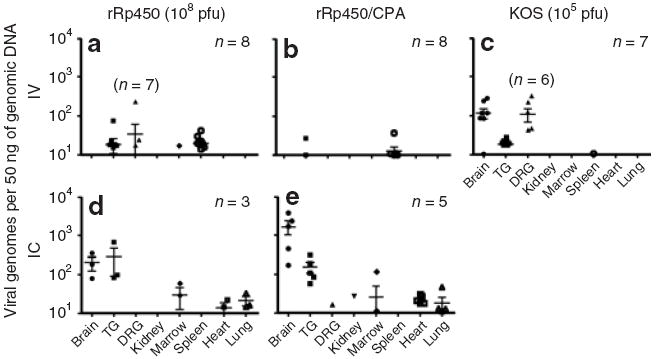
108 Plaque-forming units (pfu) rRp450 or 105 pfu KOS were given as indicated by (a–c) intravenous (IV) or (d,e) IC either as (a,c,d) a single injection alone or (b,e) followed 24 hours later by 50 mg/kg intraperitoneal cyclophosphamide (CPA). Quantitative PCR for HSV-1 genomes was performed on organs harvested >100 days following virus injections. Numbers in each group are indicated, with exceptions for a given organ shown in parentheses. Each data point represents a single organ and the bars indicate the average and SD of the positive samples for each group. DRG, dorsal root ganglion; TG, trigeminal ganglia.
Not unexpectedly, at a period >100 days (in mice given IC rRp450) latent viral DNA was detected in all brains and TGs (Figure 5d). Somewhat surprisingly, HSV DNA was also detected in the BM, heart, and lungs. On a per organ basis, the highest viral DNA load was found in the brain (~1,000,000 copies) followed by the lungs (~200,000 copies), the heart (~40,000 copies), the TGs (~25,000 copies), and the BM (~2,000 copies). No viral DNA was detected in the spleen following IC delivery. We also evaluated the presence of HSV DNA in tissues of mice given IC rRp450 followed 24 hours later by IP CPA (Figure 5e). Viral DNA was found in most brains, TGs, hearts, and lungs, and rarely in kidneys, DRGs, and BMs. On a per organ basis, the highest viral DNA load was in the brain (~5,000,000 copies) followed by the lungs (~100,000 copies), the heart (~65,000 copies), the kidney (~15,000 copies), the TGs (~6,000 copies), the BM (~2,000 copies), and the DRGs (~300 copies). The difference between the amount of viral DNA in the brains of IC rRp450 and IC rRp450/CPA-treated mice (approximately fivefold) showed a trend toward but did not reach statistical significance (P = 0.07), suggesting that CPA may increase the risk of latency when there is IC administration of the virus. No other tissues approached a statistically significant difference in their viral load as a result of these two treatments.
Virus reactivation was not observed following IV rRp450
We sought to determine whether rRp450 could be reactivated from tissues found to be positive for HSV DNA. Organs were harvested from mice that were infected with IV rRp450 at a strength of 108 pfu or KOS at 104 pfu 30 days after infection. As a positive control, we used TGs isolated from mice inoculated via corneal scratch with wild-type HSV-1 17syn+. Reactivation was only detected in the positive control, 17syn+ latently infected TGs (Figure 6). One KOS-treated mouse did show an obvious cytopathic effect and HSV-1 staining in the kidney (Supplementary Figure S8). To test the possibility that the oncolytic virus excited latency but failed to produce an infectious virus, each of the tissues was examined for lytic viral protein expression by immunohistochemistry. Lytic viral protein was not detected in tissues explanted from rRp450-infected mice (data not shown).
Figure 6. Reactivation of latent herpes simplex virus.
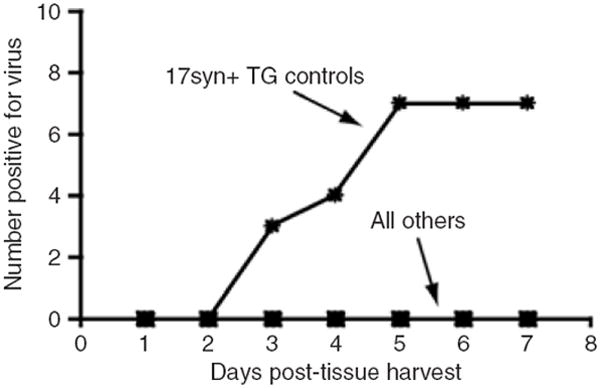
Organs were harvested from mice >30 days after being given 108 rRp450 intravenous and explanted into cultures. Infectious virus production was detected in 7/8 trigeminal ganglia (TGs) harvested from four animals inoculated into the cornea with a wild-type virus (positive control), but not in organs from animals given systemic rRp450 and KOS (“all others” include TG, dorsal root ganglion, spleen, kidney, liver, adrenal).
DISCUSSION
The therapeutic efficacy of multimutated HSV-1 mutants as single agents in early phase clinical trials has been promising yet limited.8,25 The use of singly mutated viruses, especially those expressing a prodrug enzyme in combination with the prodrug, offers the possibility of increased efficacy, yet the toxicity of these viruses has not been well studied. After demonstrating that coadministration of CPA enhanced the antitumor efficacy of rRp450 in a xenograft sarcoma model, we established a preclinical safety profile for rRp450 alone as well as in combination with CPA.
Our data showed attenuation of virus replication in normal cells and support a previous report about attenuation in hepatocytes,12 which is quite different from the high replication of rRp450 observed in human cancer cell lines.12,13,26,27 Furthermore, the replication and virus production of rRp450 were reduced in normal cells compared with the virus mutant NV1020, which has been shown to be safe for use in humans by intrahepatic artery administration.18 Although the effect of CPA on virus replication in normal cells was not tested, CPA does not affect replication of rRp450 in tumor cells.12 A further safety feature is the retention of thymidine kinase expression by rRp450, conferring sensitivity to antiviral drugs.
We also determined that rRp450 is safe for injection by multiple routes in FVB/N mice. We chose this mouse model because of the availability of genetically susceptible mutations for cancer, including a rhabdomyosarcoma model used in our studies.28 Our toxicity data using wild-type HSV-1 suggest it a suitable model. Nakamura et al. assessed the safety of the ICP6 mutant hrR3 and found one of four mice died when receiving 108 pfu, when administered intrasplenically,29 though the significance in toxicity observed via this route is unclear. Certainly, the administration of IV or IC rRp450, even when combined with systemic CPA (50 mg/kg), produced no adverse signs and symptoms in our studies. By contrast, IV wild-type virus was uniformly neurotoxic at 105 pfu (resulting in a transient abnormal gait) and fatal at 106 pfu. When the administration was IC, wild-type virus was 80 and 100% fatal at 104 pfu and 105 pfu, respectively, indicating that the lethal dose in 50% of animals is <10,000 pfu. These data suggest rRp450 is attenuated relative to wild type by at least 10,000-fold in vivo.
In this study, we did not analyze the long-term effects of rRp450/CPA in the mouse brain. There have been reports in the past that some, but not all mouse strains, can develop a multifocal demyelination process upon peripheral inoculation with wild-type HSV-1 and chronic CPA immunosuppression.30 Although this is a valid concern, we do note several lines of evidence suggesting this is unlikely: (i) we have not employed a wild-type HSV-1, (ii) we have only employed a short-term (one dose) treatment of CPA, and (iii) the occurrence of demyelination occurs only in a minor subset of mouse strains. We do not suspect demyelination to be an issue as no brain lesions were seen in two mice given intravenous virus and high-dose CPA that died of pneumonia on days 20 and 22, and we did not observe any neurological impairment in mice that were followed long term.
To determine the extent of viral latency, quantitative PCR was performed on tissues harvested from mice that had been administered >100 days earlier with 108 pfu IV or IC rRp450 alone or in combination with IP CPA. The detection of virus DNA in several organs at late time points following IV administration of rRp450 suggests there is a risk of latency, with the DRG, TG, and spleen being the most likely sites. Interestingly, although most mice given wild-type HSV-1 showed latent DNA in the brain, we were unable to detect rRp450 DNA in the brain after IV administration. We postulate that access to the brain following IV virus requires viral replication, with the infection ascending from peripheral nerve ganglia to central nervous system ganglia, a process that appears to be significantly attenuated with rRp450. Our study did not address whether the DNA we detected represents intact genomes, or may in fact be genomic fragments, particularly in the spleen where virus fragments may have been scavenged by macrophages.
A single dose of CPA following the IV rRp450 did not alter the virus DNA biodistribution or quantity, though there was a trend toward an increase in the brain following IC administration of the virus. These results are consistent with those of Yamada et al.20 who demonstrated genomic DNA of ICP6 mutants persisting in the brain >3 months after IC injection. Interestingly, we detected latent virus following IC administration of rRp450 not only in the brain, but also in the TG, BM, heart, and lungs. The discovery of the virus in TG following IC injection may have resulted from the virus being taken up by trigeminal nerve sensory innervations of the dura mater that refluxed during viral injection. For the distant sites, we presume there was blood-borne dissemination. In fact, more latent virus was detected in the BM, heart, and lungs (though not spleen) than was found following IV virus administration. The explanation for this finding is unclear, but it suggests these sites should be monitored in clinical trials where oHSV mutants are administered to IC sites.
Despite finding a sporadic distribution of viral DNA in various organs following IV administration, we were unable to detect any reactivation of the virus. Our results are similar to the findings of Jacobson et al.21 who showed that a HSV-1 ICP6 deletion mutant could not be reactivated from ganglia tissue by cocultivation. In addition, we were unable to detect reactivated virus in the TG, DRG, spleen, kidney, liver, or adrenals of mice infected with IV rRp450. Further, the absence of lytic viral proteins in these tissues indicates that even if latent genomes were present, exit from latency did not occur. However, Yamada et al.20 were able to reactivate the mutant by superinfection with wild-type HSV. We did not test reactivation following wild-type superinfection, but the clinical relevance of any reactivation of latent rRp450 in the context of a wild-type infection is likely to be minimal. Similarly, we did not test the effect of CPA or its metabolites on reactivation of rRp450; however, patients who previously received rRp450 would be at much higher risk for reactivation of the highly prevalent, latent wild-type virus than of rRp450.
Our data suggest the combination of rRp450 plus CPA is more efficacious than rRp450 alone in the treatment of sarcoma xenografts. In addition to inducing a bystander effect, the addition of CPA to rRp450 has also shown to increase virus uptake and IT replication by abrogating innate and adaptive immune responses to the virus.14,15 Importantly, the combination of rRp450 and CPA was found to be as safe as CPA alone by all routes tested using a single dose. We did not test repeated dosing of the virus or CPA in this study, which would likely be required to support a multidose clinical trial. Also, the effect that differences in CPA metabolism between individuals31 might have on rRp450/CPA efficacy is unknown. Despite the presence of viral DNA long term, our findings indicate there is little risk of reactivation of the latent virus following intravenous virus administration. Based on these and other preclinical studies, we expect oncolytic viral therapy will be one additional treatment modality available in the future for oncologists. The challenge over the next decade will be to determine which viruses work best for which cancers, at what doses, schedules, and routes of administration, and in what combinations with other treatments. Our results suggest that further development of rRp450/CPA as a combination cancer therapeutic application is warranted.
MATERIALS AND METHODS
Cells and viruses
Rh30 cells (American Type Culture Collection, Manassas, VA) were grown in Dulbecco’s modified Eagle’s medium media with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin. Rabbit skin cells were grown as previously described.22 Normal HFKs were obtained from normal newborns following circumcision through an institutional review board-approved protocol and were a kind gift from Susa Wells (Cincinnati Children’s Research Foundation, Cincinnati, OH). HFKs were grown in EpiLife Media (Cascade Biologics) supplemented with human growth supplement keratinocyte according to the manufacturer’s instructions. Quiescent HFKs were obtained by the addition of 10% FBS and 1 mmol/l CaCl2 (ref. 32). Primary hepatocytes were purchased from Cambrex (East Rutherford, NJ). NHSCs were obtained from adult trauma victims through an institutional review board-approved protocol and provided kindly by Nancy Ratner (Cincinnati Children’s Research Foundation, Cincinnati, OH). NHSCs were grown as previously described.24 FVB/N mouse rhabdomyosarcoma cell line 0845848 (termed MR848 in this article), derived from a p53+/− mouse transgenic for hepatocyte growth factor/scatter factor, was a kind gift from Glenn Merlino (National Cancer Institute, Bethesda, MD). MR848 cells were grown in Dulbecco’s modified Eagle’s medium containing l-glutamine, penicillin/streptomycin, insulin (20 ng/ml), epidermal growth factor (30 ng/ml), and 10% FBS. NV1020 was kindly provided by MediGene (San Diego, CA). Briefly, NV1020 (derivative of R7020, strain F) is deleted for the internal repeats, with insertion of HSV-2 glycoprotein sequences, and is haplotype for ICP34.5 (ref. 33); the stock concentration was 4.4 × 108 pfu/ml. 17syn+ is a wild-type virus that has been previously described.34 rRp450 contains the rat CYP2B1 gene in place of UL39 and has been previously described.26 The concentrated stock for rRp450 was prepared at the University of Pittsburgh under good laboratory practice conditions at 8.5 × 1010 pfu/ml. Viral plaque assays were performed on rabbit skin cells by standard methods as previously described.24
Viral replication assays
Cells were plated in 12-well dishes at 1 × 105 to 5 × 105 per well, adhered at 37 °C for 2 hours, and infected using 104 (multiplicity of infection of 0.1) or 105 (multiplicity of infection of 1) virus in a total volume of 0.1 ml. Plates were gently shaken every 10 minutes for 1 hour. Inoculum was removed after 1 hour and replaced with 0.5 ml fresh media. At times indicated, cells were scraped, freeze-thawed two times, diluted, and titered by plaque assay.
Animal studies
Animal studies were approved by the Cincinnati Children’s Hospital Institutional Animal Care and Use Committee. For efficacy studies, 5.0 × 106 human rhabdomyosarcoma Rh30 cells in 100 μl PBS were injected subcutaneously with 33% Matrigel (Becton Dickinson, Bedford, MA) in 5–6-week-old female Balb/c athymic nude mice (Harlan Sprague Dawley, Indianapolis, IN). When tumors reached 200–500 mm3, animals were treated either with IT rRp450 (107 pfu, days 0 and 7) alone or IT rRp450 followed 24 hours later by IP CPA (50 mg/kg, days 1 and 8). Control mice received IT PBS (days 0 and 7) or IP CPA (50 mg/kg, days 1 and 8). For survival studies, 5–6-week-old male and female FVB/N mice (Harlan Sprague Dawley, Indianapolis, IN) received IV 108 pfu rRp450, IV 108 pfu rRp450 followed 24 hours later by IP CPA (200 mg/ml), IV 108 pfu rRp450 followed 24 hours later by IP CPA (50 mg/ml), IC 108 pfu rRp450, or IC 108 pfu rRp450 followed 24 hours later by CPA (50 mg/ml). Animals were followed >100 days. Control mice received IV 105 pfu or IC 104–5 pfu wild-type KOS. To determine the cellular immune components in a tumor treated with rRp450, MR848 subcutaneous tumors were generated by injecting 1 × 106 cells in 100 μl PBS with 33% Matrigel in 5–6-week-old female FVB/N mice. When tumors reached 125–200 mm3, animals received either IT HSV rRp450 (1 × 107 pfu) or PBS as a single unfractionated dose. Ten days after IT treatment, tumors were harvested and analyzed by flow cytometry for cellular infiltrate.
Blood analysis
Blood was obtained from FVB/N mice at the indicated times by cardiac puncture during killing. Blood samples were analyzed by a metabolic panel using a SYNCHRON LX (Beckman-Coulter, Brea, CA) or assessed for blood cell counts using a ADVIA 120 (Siemens, Malvern, PA). The blood samples were processed by TriHealth Bethesda Laboratory Services (Cincinnati, OH).
Immunohistochemistry
Excised xenografts were fixed in 10% formalin and routinely processed and cut in five 5-μm sections. For T-cell detection, sections were blocked with 2% donkey/goat immunoglobulin, stained with rabbit anti-CD3 (1:200; Calbiochem, Gibbstown), using citrate antigen retrieval, and Vector ABC detection. For polymorphonouclear leukocyte detection, sections were stained with Leder stain (Poly Scientific, Bay Shore, NY).
Quantitative PCR
At >100 days after IV injection of rRp450, tissues were harvested. To increase sensitivity of detection, one-quarter of large organs were selected for analysis as well as two TGs (pooled), 5–20 DRGs (pooled), marrow from two femurs (pooled), and an ~0.5-cm section of colon. These tissues were homogenized in 25 mmol/l EDTA–0.2% sodium dodecyl sulfate containing 1 mm zirconia beads (BioSpec Products, Bartlesville, OK) using a MiniBeadbeater (BioSpec Products, Bartlesville, OK) for 5 minutes. The homogenate was incubated with 25 μg/ml proteinase K. DNA was isolated from the homogenate by standard phenol–chloroform extraction, resuspended in Tris–EDTA buffer (10 mmol/l Tris–HCl, 1 mmol/l EDTA, pH 7.5) and quantified using the PicoGreen double-stranded DNA quantification kit according to manufacturer’s instructions (Invitrogen, Carlsbad, CA). Real-time PCR was performed using the ABI 7500 system (Applied Biosystems, Foster City, CA). For standards, purified good manufacturing practice-produced rRp450 viral DNA was serially diluted and 105–101 viral genome copy equivalents were mixed with 50 ng of uninfected mouse DNA. After adding 12.5 μl 2× SYBR green PCR mix (Qiagen, Valencia, CA) and 10 pmol of each thymidine kinase primer (forward primer 5′-TCCGCCTGGAGCAGAAAATG-3′, reverse primer 5′-AACACCCGCCAGTAAGTCATC-3′), each dilution was brought to 25 ml with PCR-grade water. DNA extracted from infected mice (50 ng) was brought to 25 ml with PCR-grade water after adding 12.5 μl 2× SYBR green PCR mix and 10 pmol of each thymidine kinase primer. As a control for DNA integrity and quantity, samples were also analyzed using 10 pmol of a primer set for mouse intestinal fatty acid binding protein, Fabpi, a house keeping gene as described.35 These data were used to normalize for minor variations in DNA quantities. Standards were used in every reaction plate analyzed, and all samples were run in triplicate. PCR included one cycle of 50 °C for 2 minutes, 95 °C for 10 minutes, 40 cycles of 94 °C for 15 seconds, 55 °C for 30 seconds, 72 °C for 30 seconds, and 65 °C for 35 seconds with fluorescence detection during the 65 °C extension. A standard dissociation stage followed to determine the melting temperature of each amplification product. The standard curve was used to calculate copy numbers and was linear down to 10 copies per reaction; therefore, experimental values <10 copies were considered below the limit of detection and scored as negative.
Virus reactivation
Mice were given IV dose of either 108 pfu rRp450 or 104 pfu KOS. After 60 days, various tissues including TGs, DRGs, kidney, liver, spleen, and adrenal were removed to evaluate the ability of potentially latent rRp450 and KOS viral genomes to reactivate. Eight TGs isolated from four animals inoculated into the cornea with 2 × 105 pfu wild-type 17syn+ served as positive controls. Tissues were aseptically removed from three mice from each group and cultured in minimal essential medium + 10% FBS at 37 °C and 5% CO2 for 7 days. Larger tissues including liver, spleen, and kidney were minced prior to plating. The entire spleen and kidney and approximately one-third of the liver from each animal were tested. Media from each tissue culture well was tested daily for infectious virus by plating 200 μl onto rabbit skin cell monlayers. At the end of 7 days, all tissues were homogenized in media (minimal essential medium + 5% FBS), centrifuged to remove cellular debris, and supernatants were placed on newly confluent RSC monolayers and observed for viral plaque formation. Lytic viral gene expression in tissues was examined by immunohistochemistry as detailed previously.36
Statistical analysis
Statistical analyses including Student’s t-test, Fisher exact test, and log-rank were performed using GraphPad Prism v. 5.0 (GraphPad Software, San Diego, CA).
Supplementary Material
Acknowledgments
We thank MediGene for providing G207 and NV1020, Susa Wells (Cincinnati Children’s Hospital Medical Center) for providing keratinocytes, Nancy Ratner (Cincinnati Children’s Hospital Medical Center) for NHSCs, Glenn Merlino (National Cancer Institute) for mouse rhabdomyosarcoma cells, David Krisky (University of Pittsburgh) for good laboratory practice production of rRp450, and Yoshinaga Saeki (The Ohio State University) for technical assistance. This work was supported by http://www.TeeOffAgainstCancer.org, the Katie Linz Foundation, the National Institutes of Health (NIH) grant R01-CA114004 to T.P.C. and NIH grant P01-CA069246 to E.A.C.
References
- 1.Maki RG. Role of chemotherapy in patients with soft tissue sarcomas. Expert Rev Anticancer Ther. 2004;4:229–236. doi: 10.1586/14737140.4.2.229. [DOI] [PubMed] [Google Scholar]
- 2.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 3.Wunder JS, Nielsen TO, Maki RG, O’Sullivan B, Alman BA. Opportunities for improving the therapeutic ratio for patients with sarcoma. Lancet Oncol. 2007;8:513–524. doi: 10.1016/S1470-2045(07)70169-9. [DOI] [PubMed] [Google Scholar]
- 4.Martuza RL. Conditionally replicating herpes vectors for cancer therapy. J Clin Invest. 2000;105:841–846. doi: 10.1172/JCI9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett JJ, Delman KA, Burt BM, Mariotti A, Malhotra S, Zager J, et al. Comparison of safety, delivery, and efficacy of two oncolytic herpes viruses (G207 and NV1020) for peritoneal cancer. Cancer Gene Ther. 2002;9:935–945. doi: 10.1038/sj.cgt.7700510. [DOI] [PubMed] [Google Scholar]
- 6.Bharatan NS, Currier MA, Cripe TP. Differential susceptibility of pediatric sarcoma cells to oncolysis by conditionally replication-competent herpes simplex viruses. J Pediatr Hematol Oncol. 2001;24:447–453. doi: 10.1097/00043426-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Chase M, Chung RY, Chiocca EA. An oncolytic viral mutant that delivers the CYP2B1 transgene and augments cyclophosphamide chemotherapy. Nat Biotechnol. 1998;16:444–448. doi: 10.1038/nbt0598-444. [DOI] [PubMed] [Google Scholar]
- 8.Varghese S, Rabkin SD. Oncolytic herpes simplex virus vectors for cancer virotherapy. Cancer Gene Ther. 2002;9:967–978. doi: 10.1038/sj.cgt.7700537. [DOI] [PubMed] [Google Scholar]
- 9.Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 10.Harrow S, Papanastassiou V, Harland J, Mabbs R, Petty R, Fraser M, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11:1648–1658. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- 11.MacKie RM, Stewart B, Brown SM. Intralesional injection of herpes simplex virus 1716 in metastatic melanoma. Lancet. 2001;357:525–526. doi: 10.1016/S0140-6736(00)04048-4. [DOI] [PubMed] [Google Scholar]
- 12.Pawlik TM, Nakamura H, Mullen JT, Kasuya H, Yoon SS, Chandrasekhar S, et al. Prodrug bioactivation and oncolysis of diffuse liver metastases by a herpes simplex virus 1 mutant that expresses the CYP2B1 transgene. Cancer. 2002;95:1171–1181. doi: 10.1002/cncr.10776. [DOI] [PubMed] [Google Scholar]
- 13.Pawlik TM, Nakamura H, Yoon SS, Mullen JT, Chandrasekhar S, Chiocca EA, et al. Oncolysis of diffuse hepatocellular carcinoma by intravascular administration of a replication-competent, genetically engineered herpesvirus. Cancer Res. 2000;60:2790–2795. [PubMed] [Google Scholar]
- 14.Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci USA. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5:881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 16.Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 17.Varghese S, Newsome JT, Rabkin SD, McGeagh K, Mahoney D, Nielsen P, et al. Preclinical safety evaluation of G207, a replication-competent herpes simplex virus type 1, inoculated intraprostatically in mice and nonhuman primates. Hum Gene Ther. 2001;12:999–1010. doi: 10.1089/104303401750195944. [DOI] [PubMed] [Google Scholar]
- 18.Kemeny N, Brown K, Covey A, Kim T, Bhargava A, Brody L, et al. Phase I, open-label, dose-escalating study of a genetically engineered herpes simplex virus, NV1020, in subjects with metastatic colorectal carcinoma to the liver. Hum Gene Ther. 2006;17:1214–1224. doi: 10.1089/hum.2006.17.1214. [DOI] [PubMed] [Google Scholar]
- 19.Mahller YY, Vaikunth SS, Currier MA, Miller SJ, Ripberger MC, Hsu YH, et al. Oncolytic HSV and erlotinib inhibit tumor growth and angiogenesis in a novel malignant peripheral nerve sheath tumor xenograft model. Mol Ther. 2007;15:279–286. doi: 10.1038/sj.mt.6300038. [DOI] [PubMed] [Google Scholar]
- 20.Yamada Y, Kimura H, Morishima T, Daikoku T, Maeno K, Nishiyama Y. The pathogenicity of ribonucleotide reductase-null mutants of herpes simplex virus type 1 in mice. J Infect Dis. 1991;164:1091–1097. doi: 10.1093/infdis/164.6.1091. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson JG, Leib DA, Goldstein DJ, Bogard CL, Schaffer PA, Weller SK, et al. A herpes simplex virus ribonucleotide reductase deletion mutant is defective for productive acute and reactivatable latent infections of mice and for replication in mouse cells. Virology. 1989;173:276–283. doi: 10.1016/0042-6822(89)90244-4. [DOI] [PubMed] [Google Scholar]
- 22.Currier MA, Adams LC, Mahller YY, Cripe TP. Widespread intratumoral virus distribution with fractionated injection enables local control of large human rhabdomyosarcoma xenografts by oncolytic herpes simplex viruses. Cancer Gene Ther. 2005;12:407–416. doi: 10.1038/sj.cgt.7700799. [DOI] [PubMed] [Google Scholar]
- 23.Parikh NS, Currier MA, Mahller YY, Adams LC, Di Pasquale B, Collins MH, et al. Oncolytic herpes simplex virus mutants are more efficacious than wild-type adenovirus type 5 for the treatment of high-risk neuroblastomas in preclinical models. Pediatr Blood Cancer. 2005;44:469–478. doi: 10.1002/pbc.20268. [DOI] [PubMed] [Google Scholar]
- 24.Mahller YY, Rangwala F, Ratner N, Cripe TP. Malignant peripheral nerve sheath tumors with high and low Ras-GTP are permissive for oncolytic herpes simplex virus mutants. Pediatr Blood Cancer. 2006;46:745–754. doi: 10.1002/pbc.20565. [DOI] [PubMed] [Google Scholar]
- 25.Aghi M, Martuza RL. Oncolytic viral therapies—the clinical experience. Oncogene. 2005;24:7802–7816. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 26.Aghi M, Chou TC, Suling K, Breakefield XO, Chiocca EA. Multimodal cancer treatment mediated by a replicating oncolytic virus that delivers the oxazaphosphorine/rat cytochrome P450 2B1 and ganciclovir/herpes simplex virus thymidine kinase gene therapies. Cancer Res. 1999;59:3861–3865. [PubMed] [Google Scholar]
- 27.Ichikawa T, Petros WP, Ludeman SM, Fangmeier J, Hochberg FH, Colvin OM, et al. Intraneoplastic polymer-based delivery of cyclophosphamide for intratumoral bioconversion by a replicating oncolytic viral vector. Cancer Res. 2001;61:864–868. [PubMed] [Google Scholar]
- 28.Sharp R, Recio JA, Jhappan C, Otsuka T, Liu S, Yu W, et al. Synergism between INK4a/ARF inactivation and aberrant HGF/SF signaling in rhabdomyosarcomagenesis. Nat Med. 2002;8:1276–1280. doi: 10.1038/nm787. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura H, Kasuya H, Mullen JT, Yoon SS, Pawlik TM, Chandrasekhar S, et al. Regulation of herpes simplex virus γ-1 34.5 expression and oncolysis of diffuse liver metastases by Myb34.5. J Clin Invest. 2002;109:871–882. doi: 10.1172/JCI10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kastruokoff LF, Lau AS, Leung GY, Thomas EE. Contrasting effects of immunosuppression on herpes simplex virus type I (HSV I) induced central nervous system (CNS) demyelination in mice. J Neurol Sci. 1993;117:148–158. doi: 10.1016/0022-510X(93)90167-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yule SM, Boddy AV, Cole M, Price L, Wyllie R, Tasso MJ, et al. Cyclophosphamide pharmacokinetics in children. Br J Clin Pharmacol. 1996;41:13–19. doi: 10.1111/j.1365-2125.1996.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 32.Boyce ST, Ham RG. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 1983;81:33s–40s. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- 33.Meignier B, Longnecker R, Roizman B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020: construction and evaluation in rodents. J Infect Dis. 1988;158:602–614. doi: 10.1093/infdis/158.3.602. [DOI] [PubMed] [Google Scholar]
- 34.Bolovan CA, Sawtell NM, Thompson RL. ICP34.5 mutants of herpes simplex virus type 1 strain 17syn+ are attenuated for neurovirulence in mice and for replication in confluent primary mouse embryo cell cultures. J Virol. 1994;68:48–55. doi: 10.1128/jvi.68.1.48-55.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stratman JL, Barnes WM, Simon TC. Universal PCR genotyping assay that achieves single copy sensitivity with any primer pair. Transgenic Res. 2003;12:521–522. doi: 10.1023/a:1024225408961. [DOI] [PubMed] [Google Scholar]
- 36.Sawtell NM. Quantitative analysis of herpes simplex virus reactivation in vivo demonstrates that reactivation in the nervous system is not inhibited at early times postinoculation. J Virol. 2003;77:4127–4138. doi: 10.1128/JVI.77.7.4127-4138.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


