Abstract
Background
To estimate the effect size of tuberculosis preventive therapy (PT) on the public health problem of tuberculosis in contemporary sub-Saharan Africa.
Methods
A compartmental flow model that considers high levels of tuberculosis and human immunodeficiency virus (HIV) infection in contemporary sub-Saharan Africa was used to assess the impact of PT on the prevalence of tuberculosis and tuberculosis-associated mortality.
Results
Model implementation shows that giving PT to 25% of HIV-positive individuals with latent tuberculosis infection (LTBI) leads to a 3.9% reduction in the prevalence of tuberculosis in 10 years and a 5.1% reduction in 20 years. This intervention also prevents a cumulative total of 3.0% of tuberculosis-associated deaths in a decade and 5.5% in two decades. Doubling PT coverage to 50% approximately doubles the effect size, suggesting a linear relationship within the 20-year period. The effect size is slightly sensitive to changes in level of HIV transmission, level of tuberculosis transmission, and level of case detection and treatment cure rates in the population.
Conclusions
Contrary to suggestions by previous authors that PT can significantly reduce the public health problem of tuberculosis in sub-Saharan Africa, this model-based analysis suggests that the impact of PT on tuberculosis in the population is likely to be small.
Keywords: Tuberculosis preventive therapy, Tuberculosis control, Sub-Saharan Africa, HIV-infection, Deterministic model
Introduction
Tuberculosis is a major health problem in many parts of sub-Saharan Africa, where case rates continue to rise [1,2]. Case notification rates have been increasing by approximately 10% per year since 1990 [3], and the current average annual case notification rate is estimated to be approximately 200 cases per 100,000 persons, with some countries reporting rates as high as 600 cases per 100,000 persons [2]. The human immunodeficiency virus (HIV) infection, which dramatically increases the risk of reactivation of latent tuberculosis infection (LTBI) to active disease [4],is highly endemic in this region and is the major cause of increasing tuberculosis rates in sub-Saharan Africa [5]. As a result, HIV-associated tuberculosis is a major cause of morbidity and is the leading cause of adult mortality in this region [1].
LTBI may be treated using one or more of the available anti-tuberculosis drugs as a means of preventing disease in HIV-infected persons. There is ample research evidence demonstrating that tuberculosis preventive therapy (PT) reduces the individual risk of active tuberculosis in HIV-positive persons believed to be latently infected with Mycobacterium tuberculosis [6–8]. In view of these findings, the World Health Organization (WHO), the Joint United Nations Programme on HIV/AIDS (UNAIDS), and the International Union Against Tuberculosis and Lung Disease (IUATLD) recommend PT for tuberculin skin test (TST)-positive, HIV-infected persons who do not have active tuberculosis [9,10]. They emphasize, however, that PT should be promoted as an intervention for individuals living with HIV, rather than as a primary strategy to control the public health burden of tuberculosis [10]. In contrast, several investigators have suggested that PT could significantly reduce the prevalence of tuberculosis in countries with high prevalence of both tuberculosis and HIV infection [11–16]. While there is a general agreement that priority for tuberculosis control in high burden countries should remain the detection and cure of infectious cases, it is not clear if PT makes a significant contribution toward the public health control of tuberculosis in high burden population where most of the cases are due to reactivation of LTBI among HIV-infected persons. Review of literature reveals that the potential impact and the effect size of PT on tuberculosis at a population level have not been adequately assessed in settings with high prevalence of both tuberculosis and HIV infection such as sub-Saharan Africa. We used a compartmental flow model to estimate the probable effect size of PT on tuberculosis in contemporary sub-Saharan Africa. We also examined how the effect size would change over time, and how it might be affected by the different levels of HIV infection and tuberculosis transmission in these settings.
Methods
Description of the model
The compartmental model describes the flow of individuals through the different stages of the natural history of human tuberculosis. It stratifies the population by HIV infection and examines the impact of PT as one of the tuberculosis control interventions in the population. The model is an extended version of that presented by Blower et al. in 1995 [17]. The structure of the model is depicted schematically in Fig. 1.
Fig. 1.
Structure of the model.
The mechanism of the model is such that new individuals are recruited into the population through births that do not have M. tuberculosis infection, and a proportion p1 do not have HIV infection. Because BCG vaccination at birth is widely used in most of the sub-Saharan countries for protection against tuberculosis in childhood [18], it is assumed that a proportion t of the births receives BCG vaccination immediately after birth; whereas the remaining proportion (1 t) do not. Over time (t), the number of HIV-negative individuals without BCG vaccination and without M. tuberculosis infection X0(t) increases due to unvaccinated births (at a constant HIV-negative proportion p1 in the population and a constant proportion of unvaccinated births (1 t) of the birth rate d). They decrease due to the incidence of new infections with M. tuberculosis (at a tuberculosis infection rate k0(t)), due to non-tuberculosis mortality (at a rate l0), and also due to infection with HIV (at a constant HIV infection rate p). Thus, the instantaneous change in the number of HIV-negative individuals without BCG vaccination and without M. tuberculosis infection is therefore represented by the equation:
Similarly, the instantaneous change in the number of HIV-positive individuals without BCG vaccination and without M. tuberculosis infection X1(t) is represented by the equation:
The tuberculosis infection rate k0(t) is determined by the number of infectious cases present in the population and the transmission coefficient b0. It is given by: k0(t)= b0[I0(t) + I1(t)]. In turn, the transmission coefficient b0 is determined by the probability of an infectious individual coming into contact with a susceptible individual and the probability of such a contact resulting in the successful transmission of infection [19].
The number of HIV-negative individuals with BCG vaccination but without M. tuberculosis infection V0(t) increases due to new vaccinated births at a constant BCG vaccination coverage proportion m and a constant HIV-negative proportion p1 of the constant birth rate d. They decrease due to the incidence of new infections with M. tuberculosis (at a relative risk ratio r10 of the tuberculosis infection rate k0(t)), due to all-cause mortality in HIV-negative individuals without tuberculosis (at a constant rate l0), and also due to individuals newly infected with HIV (at a constant HIV infection rate p). The risk ratio r10 is the relative risk of tuberculosis infection between vaccinated individuals versus unvaccinated individuals. Therefore, the rate of tuberculosis infection in BCG vaccinated individuals is less by r10 compared to unvaccinated individuals. Thus, the instantaneous change in the number of HIV-negative individuals with BCG vaccination but without M. tuberculosis infection is represented by the equation:
Similarly, the instantaneous change in the number of HIV-positive individuals with BCG vaccination but without M. tuberculosis infection V1(t) is represented by the equation:
Note from the model that a proportion of individuals who get infected p20 are able to contain the infection and thus become latently infected, whereas the remaining proportion (1 p20) are not able to contain the infection and therefore progress to primary active tuberculosis. Further, a proportion p30 of those progressing to primary active tuberculosis will have noninfectious tuberculosis, whereas the remaining proportion (1 p30) will have infectious active tuberculosis.
The number of HIV-negative individuals with LTI L0(t) increases due to HIV-negative individuals without BCG vaccination who get infected with M. tuberculosis and enter the latent phase (at a constant proportion p20 of the infection rate of k0(t)); plus HIV-negative individuals with BCG vaccination who lose protection against M. tuberculosis, get infected, and enter the latent phase (at a constant proportion p20 and risk rate ratio r10 of the infection rate of k0(t)). They also increase due to individuals recovered from active tuberculosis (at a constant case detection rate and treatment cure rate w and q0, respectively). They decrease due to individuals with LTBI who progress to active tuberculosis (at a constant rate r0), due to non-tuberculosis mortality (at a constant rate l0), and also due to newly infected individuals with HIV (at an HIV infection rate p). Note that a proportion of the latently infected HIV-negative individuals who progress to active tuberculosis p40 have noninfectious tuberculosis, and the remaining proportion (1 p40) have infectious tuberculosis. Thus, the instantaneous change in the number of HIV-negative individuals with LTBI is represented by the equation:
The number of HIV-positive individuals with LTBI L1(t) increases due to HIV-positive individuals without BCG vaccination who get infected with M. tuberculosis and enter the latent phase (at a constant proportion p21 of the incidence infection rate of λ0(t)); plus HIV-positive individuals with BCG vaccination who get infected after losing protection provided by BCG and enter the latent phase (at a constant proportion p21 and risk rate ratio r11 of the incidence infection rate of λ1(t)). They also increase due to HIV-negative individuals who get infected with HIV (at a constant infection rate π) and increase due to individuals recovered from active tuberculosis (at a constant case detection rate and treatment cure rate ψ and ρ1, respectively). They decrease due to individuals who progress to active tuberculosis (at a constant case rate σ1), due to non-tuberculosis mortality (at a constant rate μ1), and due to individuals who receive PT (at a constant proportion θ of individuals who have never had active tuberculosis). Thus, the instantaneous change in the number of HIV-positive individuals with LTBI, without PT is represented by the equation:
Because posttreatment PT is currently not widely recommended, only individuals without a history of tuberculosis treatment are offered PT. This is in spite of one randomized controlled trial that demonstrated that posttreatment PT reduces the risk of tuberculosis relapse among HIV-positive individuals [20]. Although not reflected in the diagrammatic representation of the model in Fig. 1, the selective PT is clearly represented in the foregoing equation.
The number of HIV-positive individuals with LTBI receiving PT L2(t) increases due to the number of new HIV-positive individuals with LTBI who start receiving PT. They decrease due to individuals who lose prophylaxis protection and progress to active tuberculosis (at a constant risk ratio r2 of the latent reactivation case rate σ1) and decrease due to all-cause mortality in HIV-positive individuals without tuberculosis (at a constant rate μ1). Thus, the instantaneous change in the number of HIV-positive individuals with LTBI is represented by the equation:
The number of HIV-negative infectious tuberculosis cases I0(t) increases with HIV-negative individuals with LTBI that progress to infectious tuberculosis (at a constant proportion (1 – p40) of the case rate σ0). They also increase due to HIV-negative BCG vaccinated plus HIV-negative individuals without BCG vaccination who get infected with M. tuberculosis and progress to primary infectious tuberculosis (at a constant proportion (1 – p20)(1 – p30) of the infection rate λ0(t)). They decrease due to individuals who recover from active tuberculosis (at a constant case detection and treatment cure rates ψ and ρ0, respectively), due to HIV infection (at an infection rate π), due to non-tuberculosis mortality (at a constant rate μ0), and due to tuberculosis-associated mortality among HIV-negative individuals (at a constant mortality rate φ0). Thus, the instantaneous change in the number of HIV-negative individuals with infectious tuberculosis is represented by the equation:
Similarly, the instantaneous change in the number of HIV-positive individuals with infectious tuberculosis is represented by the equation:
The number of HIV-negative individuals with non-infectious tuberculosis T0(t) increases with HIV-negative individuals with LTBI that develop noninfectious active tuberculosis (at a constant proportion p40 of the case rate σ0). They also increase due to HIV-negative BCG vaccinated plus HIV-negative individuals without BCG vaccination who get infected with M. tuberculosis and progress to primary noninfectious tuberculosis (at a constant proportion (1 – p20)p30 of the infection rate λ0(t)). They decrease due to individuals recovered from active tuberculosis (at constant case detection and treatment cure rates ψ and ρ0, respectively), due to newly infected individuals with HIV (at an infection rate π), due to non-tuberculosis mortality (at a constant rate μ0), and due to tuberculosis-associated mortality (at a constant rate φ0). Thus, the instantaneous change in the number of HIV-negative individuals with noninfectious tuberculosis is represented by the equation:
Similarly, the instantaneous change in the number of HIV-positive individuals with noninfectious tuberculosis is represented by the equation:
Simulations of the above model were run using the ModelMaker software, with the Euler method of numerical integration, and a step length of 1 day.
Source of information for initial conditions and parameter estimates of the model
We used the Uganda as the case study sub-Saharan population, a country where a variety of research information on tuberculosis and HIV infection is available. The baseline conditions and most of the parameter estimates of the model used in simulations of the model were obtained from published literature on Uganda. Whenever the appropriate estimates were not available on Uganda, estimates from countries in the Eastern or Southern African region were used. Therefore, baseline values of the population assume a population of 24 million people, with a prevalence of 300 cases tuberculosis/100,000 population [2], and that the prevalence of infection with M. tuberculosis is 40%. The prevalence of HIV infection in the population is taken to be 10% [21,22]. A baseline M. tuberculosis infection rate of 2.76% per year was used and was based on the assumption that the incidence of smear-positive tuberculosis cases is 138 cases/100,000 population [2] and that the relationship between incidence of smear-positive tuberculosis and tuberculosis infection rate as described by Styblo et al. and Sutherland et al. [23,24] applies. The rest of the parameter estimates used in the simulations and their sources are given in Table 1.
Table 1.
Summary of meaning of the parameters of the model
| Parameter | Meaning of parameter | Parameter values used |
|---|---|---|
| δ | Recruitment rate. This represents the introduction of new susceptible individuals into the population, in this case, mostly through births (birth rate) | 4.72% per year [33] |
| μ 0 | All-cause mortality rate among HIV-negative individuals without tuberculosis | 8.1/1000 person-years [34] |
| μ 1 | All-cause mortality rate among HIV-positive individuals without tuberculosis | 129/1000 person-years [34] |
| π | Incidence rate of infection with HIV | 1.65% per year [22] |
| λ0(t) | Baseline annual risk of tuberculosis infection among HIV-negative susceptible individuals | 2.76% per year (derived from estimated incidence of smear-positive cases [35] using the relationship described by Styblo et al. and Sutherland et al. [23,24]) |
| λ1(t) | Baseline annual risk of tuberculosis infection among HIV-positive susceptible individuals (this is assumed to be 2-fold compared to the rate in HIV-negative individuals) | |
| β 0 | Transmission coefficient among HIV-negative individuals | 6.73 × 10−7 per year (derived from number of smear-positive cases and ARTI at baseline) |
| β 1 | Transmission coefficient among HIV-positive individuals | |
| r 10 | BCG vaccine efficacy ratio among HIV-negative individuals | 0.490 [36,37] |
| r 11 | BCG vaccine efficacy ratio among HIV-positive individuals | 0.516 [36,37] |
| σ 0 | Rate of progression from latent tuberculosis infection to active disease among HIV-negative individuals without PT | 74 cases per 100,000 persons/year (derived from estimated annual incidence of active tuberculosis of 200/100,000 population [2]; by assuming that the annual incidence is a pooled case rate between σ0 and σ1) |
| σ 1 | Rate of progression from latent tuberculosis infection to active disease among HIV-positive individuals without PT | 737 cases per 100,000 persons/year (σ1 assumed to be 10 times σ0 [29,38]) |
| r 2 | Tuberculosis preventive therapy efficacy ratio among HIV-positive individuals | 0.40 [27] |
| φ 0 | Tuberculosis-associated mortality rate among HIV – ve tuberculosis patients (derived from the difference between all-cause mortality rate among HIV-negative individuals without tuberculosis [39] and all-cause mortality rate among HIV-negative tuberculosis patients [34] = 65 − 8.1 = 56.9/1000/year) | 56.9/1000/year [34,39] |
| φ 1 | Tuberculosis-associated mortality rate among HIV + ve tuberculosis patients (derived from the difference between all-cause mortality rate among HIV-positive individuals without tuberculosis [39] and all-cause mortality rate among HIV-positive tuberculosis patients [34] = 325 − 129 = 196/1000/year) | 196/1000/year [34,39] |
| ψ | Case detection rate | 60% per year [2] |
| ρ 0 | Tuberculosis treatment cure rate among HIV-negative TB patients | 50% per year [2] |
| ρ 1 | Tuberculosis treatment cure rate among HIV-positive TB patients | 50% per year [2] |
| p 1 | Proportion that HIV-negative individuals among new susceptible individuals introduced into the population | 0.94 |
| p 20 | Of HIV-negative susceptible individuals, the proportion who develop latent infection following initial infection with MTB (remaining proportion get primary tuberculosis) | 0.95 |
| p 21 | Of HIV-positive susceptible individuals, the proportion who develop latent infection following initial infection with MTB (remaining proportion get primary tuberculosis) | 0.80 |
| p 30 | Of all HIV-negative susceptible individuals who progress to primary TB disease, proportion that have noninfectious TB (sputum smear – ve) | 0.43 [35] |
| p 31 | Of all HIV-positive susceptible individuals who progress to primary TB disease, proportion that have noninfectious TB (sputum smear – ve) | 0.5 [35] |
| p 40 | Of all HIV-negative latently infected individuals who progress to active TB disease, proportion that have noninfectious TB (sputum smear – ve) | 0.43 [35] |
| p 41 | Of all HIV-positive latently infected individuals who progress to active TB disease, proportion that have noninfectious TB (sputum smear – ve) | 0.5 [35] |
Results
To estimate the effect size of PT on tuberculosis, simulations were run over a 20-year period, assuming that a predetermined proportion of HIV-positive individuals with LTBI in the population receive PT. Three indicators were used to quantify the effect size of PT on tuberculosis in the population that included: the percentage reduction in the prevalence of tuberculosis due to the PT intervention, the number of tuberculosis-associated deaths prevented, and the percentage of the tuberculosis-associated deaths prevented.
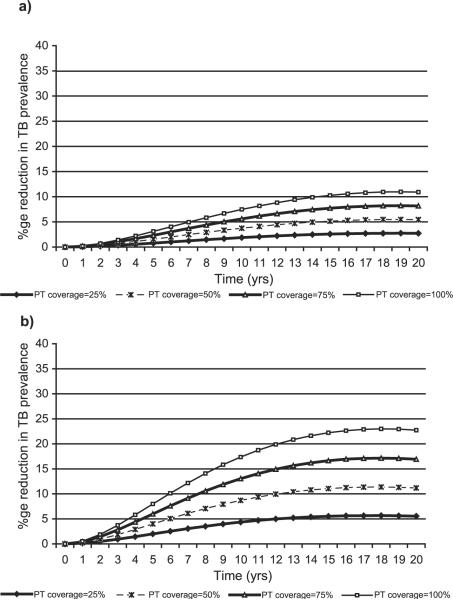
Fig. 2 shows trends in the percentage reduction in the prevalence of tuberculosis in the population over a 20-year period, when PT coverage to HIV-positive individuals with LTBI is maintained at θ = 25%, 50%, 75%, and 100%. The reduction in tuberculosis is relative to no PT intervention in the population. We note that for each of the four levels of PT coverage, the effect of PT builds up almost linearly and attains its maximum effect by the 16th year. A maximum reduction in the prevalence of tuberculosis of approximately 5% is attained if PT coverage is maintained at 25%, a maximum reduction of approximately 10% is attained if PT coverage is maintained at 50%, a maximum reduction of approximately 16% is attained if PT coverage is maintained at 75%, and a maximum reduction of approximately 21% is attained if it were possible to maintain PT coverage at 100%. For the Ugandan population with approximately 24 million people, a cumulative total of approximately 12,906 tuberculosis cases would be prevented out of a total of 481,871 new tuberculosis cases that would occur over a 10-year period if 25% of HIV-positive persons with LTBI received PT; and a cumulative total of approximately 52,398 tuberculosis cases would be prevented out of a total of 1,253,589 new tuberculosis cases that would occur over a 20-year period if there were no PT intervention.
Fig. 2.
Percentage reduction in the prevalence of tuberculosis when PT is provided to HIV-positive individuals with LTBI, assuming population case detection rate = 60%, case cure rate = 50%, and all other parameters at status quo.
Table 2 gives the cumulative number of tuberculosis-associated deaths that would occur in a population of 24 million people and the corresponding percentage of the deaths prevented at each level of PT coverage. Without PT intervention (θ = 0%), a cumulative total of 41,506 tuberculosis-associated deaths would have occurred by the 5th year. With a 25% level of PT coverage, a cumulative total of 477 tuberculosis-associated deaths would have been prevented within the first 5 years of initiation of the PT intervention, and this is equivalent to 1.1% of the tuberculosis-associated deaths that would have occurred without the intervention. By the 20th year, 5.5% of tuberculosis-associated deaths would have been prevented if PT coverage was maintained at 25%. Further, although not shown in the table, at each time point more than 80% of the tuberculosis-associated deaths would be among HIV-positive individuals.
Table 2.
Cumulative number of tuberculosis cases prevented in a population of 24 million persons
| Time from baseline (years) | Level of PT coverage |
||||
|---|---|---|---|---|---|
| 0% |
25% |
50% |
75% |
100% |
|
| No. of TB deaths | TB deaths prevented (%) | TB deaths prevented (%) | TB deaths prevented (%) | TB deaths prevented (%) | |
| 5 | 41,506 | 477 (1.1) | 954 (2.3) | 1431 (3.4) | 1906 (4.6) |
| 10 | 86,835 | 2600 (3.0) | 5191 (6.0) | 7774 (9.0) | 10,348 (11.9) |
| 15 | 143,138 | 6438 (4.5) | 12,846 (9.0) | 19,223 (13.4) | 25,569 (17.9) |
| 20 | 214,525 | 11,743 (5.5) | 23,433 (10.9) | 35,069 (16.3) | 46,651 (21.7) |
Sensitivity of the effect size of pt due to selected factors
We investigated the possibility that the level of HIV infection transmission in the population might affect the effect size of PT. Variation of the baseline prevalence of HIV infection in the population in the range 5–30% showed negligible sensitivity in the impact of PT on tuberculosis in the population. At 25% PT coverage, the maximum sensitivity is seen at 10 years with a change in the impact of PT of 1%, while at 50% PT coverage, the maximum sensitivity is seen at 10 years with a change in the impact of PT of 2% (Table 3). Further, sensitivity of the impact of PT due to changes in the incidence of HIV infection in the population was assessed by varying the value of the parameter π in the range 0.5–2.0% per year. At 25% PT coverage, the maximum reduction in prevalence of TB of 2.9%, while at 50% PT coverage, the maximum reduction is 5.8% (Fig. 3a). Fig. 3b assumes an HIV incidence rate of 2.0%, and we note that the impact of PT is higher—a maximum reduction in prevalence of TB of 5.6% at 25% PT coverage, and a maximum reduction in prevalence of TB of 11.2% at 50% PT coverage.
Table 3.
Sensitivity range of impact of PT on percentage reduction in prevalence of tuberculosis
| Parameter range tested | Time from baseline |
|||
|---|---|---|---|---|
| 5 years | 10 years | 15 years | 20 years | |
| Sensitivity range of percentage reduction in prevalence of TB, if PT coverage is 25% | ||||
| HIV infection prevalence at baseline 5–30% | 1.9–1.4 | 4.2–3.2 | 5.3–4.7 | 5.1–5.5 |
| TB transmission coefficient, β = 4.88–9.75 × 10−7 | 1.9–1.5 | 4.5–2.9 | 6.2–3.5 | 7.0–3.6 |
| Proportion of smear – ve TB among HIV-positive, p31 = p41 = 0.5–0.7 | 1.7–1.6 | 3.9–3.6 | 5.1–4.8 | 5.1–5.2 |
| Efficacy of PT, r2 = 0.24–0.65 | 2.2–1.0 | 5.0–2.3 | 6.4–2.9 | 6.6–3.0 |
| Sensitivity range of percentage reduction in prevalence of TB, if PT coverage is 50% | ||||
| HIV infection prevalence at baseline 5–30% | 3.8–2.8 | 8.4–6.4 | 10.5–9.4 | 10.2–11.1 |
| TB transmission coefficient, β = 4.88–9.75 × 10−7 | 3.9–2.9 | 9.0–5.8 | 12.3–7.0 | 13.9–7.2 |
| Proportion of smear – ve TB among HIV-positive, p31 = p41 = 0.5–0.7 | 3.5–3.2 | 7.8–7.3 | 10.1–9.6 | 10.3–10.3 |
| Efficacy of PT, r2 = 0.24–0.65 | 4.4–1.0 | 9.9–4.5 | 12.9–5.9 | 13.2–6.0 |
Fig. 3.
(a) Percentage reduction in the prevalence of tuberculosis when PT is provided to HIV-positive individuals with LTBI, assuming HIV infection incidence rate of 0.5% per year, and all other parameters at status quo. (b) Percentage reduction in the prevalence of tuberculosis when PT is provided to HIV-positive individuals with LTBI, assuming HIV infection incidence rate of 2.0% per year, and all other parameters at status quo.
We also investigated the level of case detection and treatment cure rates in the population on the effect size of PT. Fig. 4 shows trends in the percentage reduction in the prevalence of tuberculosis in the population due to PT intervention when the case detection and treatment cure rates are as recommended by the WHO of 70% and 85%, respectively. This figure is in contrast to Fig. 2, where we assumed that the case detection and treatment cure rates in the population are 60% and 50%, respectively. We note from Fig. 2, which assumes a population with case detection and treatment cure rates in the population of 60% and 50%, respectively, the percentage reduction in tuberculosis with 25% PT coverage is 3.9% after 10 years. This is in comparison to a reduction of 5.7% after 10 years in Fig. 4, which assumes case detection and treatment cure rates in the population of 70% and 85%, respectively. The effect size of PT is better in Fig. 4.
Fig. 4.
Percentage reduction in the prevalence of tuberculosis when PT is provided to HIV-positive individuals with LTBI, assuming population case detection rate = 70%, case cure rate = 85%, and all other parameters at status quo.
Because the tuberculosis transmission coefficient β is a population characteristic that might vary across sub-Saharan Africa, we performed sensitivity analysis to assess how the impact of PT would change in settings with different values of β in the range 4.88–9.75 × 10−7 per year. These are values that would correspond to baseline tuberculosis infection rates of 2.0–4.0% per year in the Ugandan population. We note from Table 3 that when the transmission of tuberculosis is high, as indexed by the transmission coefficient, the effect size of PT is smaller than when the transmission is low. For example, when the tuberculosis transmission coefficient in the population β = 4.88 × 10−7 per year, providing PT at 25% level of coverage would lead to a reduction in the prevalence of tuberculosis of 4.5% by the 10th year, compared to a reduction of 2.9% when the tuberculosis transmission coefficient in the population is β = 9.75 × 10−7 per year.
Smear-negative tuberculosis has been observed to occur more often among HIV-positive than among HIV-negative individuals [25,26]. However, there has been no reliable study to provide incidence rate estimates for smear-negative tuberculosis among HIV-positive individuals. Because we were uncertain about the true proportions of smear-negative tuberculosis among HIV + ve individuals, therefore, sensitivity analyses simulations were performed with values of p31 and p41 ranging from 0.5 to 0.7. Results of these sensitivity simulations are also presented in Table 3. We note that the change in the effect size of PT is negligible over the 0.5–0.7 sensitivity range for these two parameters.
Finally, as expected, the effect size of PT is higher when efficacy of the prophylactic drug is stronger, as indexed by the risk ratio r2 (Table 3).
Discussion
Whereas there is ample research evidence showing that PT reduces the individual risk of active tuberculosis in HIV-positive persons with LTBI [6–8], the impact of this intervention toward the public health problem of tuberculosis in contemporary sub-Saharan African populations has hitherto not been adequately analyzed. Our model-based analysis that took into consideration the high prevalence of tuberculosis and HIV infection in this region provides estimates of the likely effect size of PT on tuberculosis case rates in these populations. Our findings show that the effect size of PT on tuberculosis case rates in these populations is likely to be small. With 25% PT coverage, a maximum reduction in prevalence of tuberculosis would be only 5%, and at 50% PT coverage, a maximum reduction of only 10%. Moreover, these effect sizes are attained over a 16-year period of sustained implementation of PT. Our estimates are much smaller than those obtained by Heymann who used a 10-stage Markov model to predict the efficacy of PT on tuberculosis in sub-Saharan Africa. In his model, Heymann [16] found that providing PT to 50% of individuals with LTBI would result in a 98% decline of active tuberculosis in the population within 10 years. Whereas these contrasting results might be attributed in part to differences in the models used, it is also noted that Heymann assumed that the efficacy of PT in reducing the individual risk of tuberculosis is 70%, which is much higher than that established by recent meta-analyses results. Our simulations used meta-analysis results by Bucher et al. [27] who showed that PT using Isoniazid, given to HIV-positive individuals with a positive tuberculin skin test, reduces the individual risk of active tuberculosis by approximately 60% or a risk ratio of 0.40.
We have presented our results in relative terms, that is, relative to a situation without the PT intervention. It is therefore not clear if the absolute number of tuberculosis cases prevented by the small effect size of PT found in our analysis would translate into a cost-effective intervention, especially when applied in large populations. If PT is to be adopted in the sub-Saharan African region as an intervention for the public health control of tuberculosis, more detailed studies are needed to assess its cost-effectiveness in these settings. In most of the affected sub-Saharan African countries, the decision against initiating PT on a large scale has mostly been based on the relative cost-effectiveness of tuberculosis interventions, which are strongly in favor of case curative therapy [11,16]. Bell et al. [14] have conducted a model-based cost-effectiveness analysis of PT using the Ugandan population and showed that PT is cost-effective if added to the current case-finding and treatment strategy; their analysis also assumed that PT reduces the individual risk of tuberculosis in HIV-positive persons with LTBI 75%, which is much higher than the current meta-analyses estimates of 60% [27] and 68% [28]. However, two findings from our simulations are consistent with findings by Bell et al., that is, PT is more effective when tuberculosis transmission has been substantially controlled. The effect size of PT was bigger when the case detection and treatment cure rates were higher compared to a situation when the case detection and treatment cure rates are low. The effect size of PT was also bigger when the transmission of tuberculosis in the population was low (as indicated by the tuberculosis transmission coefficient) than when the transmission of tuberculosis in the population was high. The implications of these two findings suggest that a PT intervention program is likely to be more effective in reducing the prevalence tuberculosis if it is accompanied with a tuberculosis control program with good case detection and treatment cure rates. High levels of case detection and cure rates reduce the probability of susceptible individuals coming into contact with an infectious individual, thus lowering the value of β in the population—a situation under which our simulations indicate that PT will be more effective. This is likely to be achieved under a good DOTS program as advocated for by the WHO [29].
In contemplating the implementation of PT as an intervention for the public health control of tuberculosis in the sub-Saharan African setting, it is also important to consider the feasibility of successfully implementing such a program. Feasibility issues of concern include the ability to identify HIV-infected persons, ability to avail voluntary counseling and testing centers for HIV infection, ability to exclude active tuberculosis in HIV-positive persons, tuberculin skin testing to identify reactors who are more likely to benefit from PT, supervision of PT treatment, and monitoring of adverse drug reactions [30]. These concerns present operational challenges that are especially enormous in the resource-constrained countries of sub-Saharan Africa, where the public health infrastructure may not be adequate to support successful implementation of the PT intervention. Such challenges might constitute the biggest cost toward initiating such an intervention.
An important intervention not considered in our model-based analysis is the effect of highly active anti-retroviral therapy (HAART) on the incidence of active tuberculosis. We were not able to include this intervention in our analysis because risk ratio estimates of the effect of HAART on the incidence of active tuberculosis are not yet available. HAART significantly enhances host immunity in HIV-infected persons, thereby reducing the risk of opportunistic infections in these individuals, including reactivation of LTBI [31]. Since most of the increase in tuberculosis cases in sub-Saharan Africa is attributed to reactivation of LTBI in HIV-positive persons, as access to HAART continues to improve in the coming years, it should significantly contribute toward the reduction of HIV-infection-associated tuberculosis morbidity and mortality. The biggest challenge for most of sub-Saharan African countries, however, will be the creation of a good infrastructure to ensure that HAART can be administered in a well-structured, safe, and secure way; a prerequisite for successful benefit from use of HAART [32]. As part of this infrastructure, more people will be motivated to take tests for HIV infection, which will not only identify individuals eligible for initiating HAART, but will in turn also provide an opportunity for identification of individuals eligible for PT.
We conclude that the potential impact of PT on tuberculosis case rates in contemporary sub-Saharan Africa is likely to be small. However, studies based on current estimates of the efficacy of PT in reducing the individual risk of tuberculosis in HIV-positive persons with latent tuberculosis infection that also consider the implementation challenges of PT are needed to evaluate if the intervention is cost-effective. Further, population-based epidemiological pilot studies aimed at estimating the impact of PT on tuberculosis case rates in the sub-Saharan African setting might be useful in further resolving questions about the impact of PT on tuberculosis case rates in populations with high prevalence of tuberculosis and HIV infection. Finally, the model developed for this analysis can be used to assess a series of other parameters that may have practical usefulness in the public health control of tuberculosis in sub-Saharan Africa.
Acknowledgments
We acknowledge support by a training grant (TW-00011) from the Fogarty International Center at the National Institutes of Health through the AIDS International Training and Research Program at Case Western Reserve University.
References
- [1].Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement: global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999 Aug;282(7):677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- [2].World Health Organization . WHO Report 2003. Geneva, Switzerland: Global tuberculosis control: surveillance, planning, financing. WHO/CDS/TB/2003.316. [Google Scholar]
- [3].Dye C. Tuberculosis 2000–2010: control, but not elimination. Int J Tuberc Lung Dis. 2000 Dec;4(12 Suppl 2):S146–52. [PubMed] [Google Scholar]
- [4].Harries AD. Tuberculosis and human immunodeficiency virus infection in developing countries. Lancet. 1990 Feb 17;335(8686):387–90. doi: 10.1016/0140-6736(90)90216-r. [DOI] [PubMed] [Google Scholar]
- [5].Raviglione MC, Snider DE, Kochi A. Global epidemiology of tuberculosis: morbidity and mortality of a worldwide epidemic. JAMA. 1995 Jan;273(3):220–6. [PubMed] [Google Scholar]
- [6].Hawken MP, Meme HK, Elliott LC, Chakaya JM, Morris JS, Githui WA, et al. Isoniazid preventive therapy for tuberculosis in HIV-1-infected adults: results of a randomized controlled trial. AIDS. 1997 Jun;11(7):875–82. doi: 10.1097/00002030-199707000-00006. [DOI] [PubMed] [Google Scholar]
- [7].Pape JW, Jean SS, Ho JL, Hafner A, Johnson WD. Effect of Isoniazid prophylaxis on incidence of active tuberculosis and progression of HIV infection. Lancet. 1993 Jul;342(8866):268–72. doi: 10.1016/0140-6736(93)91817-6. [DOI] [PubMed] [Google Scholar]
- [8].Whalen CC, Johnson JL, Okwera A, Hom DL, Huebner R, Mugyenyi P, et al. A trial of three regimens to prevent tuberculosis in Ugandan adults infected with the human immunodeficiency virus. Uganda–Case Western Reserve University Research Collaboration. New Engl J Med. 1997 Sep;337(12):801–8. doi: 10.1056/NEJM199709183371201. [DOI] [PubMed] [Google Scholar]
- [9].WHO. IUATLD Tuberculosis preventive therapy in HIV infected individuals: a joint statement of the WHO Tuberculosis Programme and the Global Programme on AIDS, and the International Union Against Tuberculosis and Lund Disease (IUATLD) Wkly Epidemiol Rec. 1993 Dec;68(49):361–4. [PubMed] [Google Scholar]
- [10].WHO. UNAIDS Policy statement on preventive therapy against tuberculosis in persons living with HIV. WHO/TB/98.255 or UNAIDS/98.34. 1998 [Google Scholar]
- [11].Borgdorff MW, Floyd K, Broekmans JF. Interventions to reduce tuberculosis mortality and transmission in low- and middle-income countries. Bull World Health Organ. 2002;80(3):217–27. [PMC free article] [PubMed] [Google Scholar]
- [12].Faussett GP, Ayles H. The impact of HIV on tuberculosis control—Towards concerted action. Lepr Rev. 2002 Dec;73(4):376–85. [PubMed] [Google Scholar]
- [13].Blower SM, Daley CL. Problems and solutions for the Stop TB partnership. Lancet Infect Dis. 2002 Jun;2(6):374–6. doi: 10.1016/s1473-3099(02)00292-x. [DOI] [PubMed] [Google Scholar]
- [14].Bell JC, Rose DN, Sacks HS. Tuberculosis preventive therapy for HIV-infected people in sub-Saharan Africa is cost-effective. AIDS. 1999 Aug 20;13(12):1549–56. doi: 10.1097/00002030-199908200-00016. [DOI] [PubMed] [Google Scholar]
- [15].Murray CJ, Salomon JA. Modeling the impact of global tuberculosis control strategies. Proc Natl Acad Sci U S A. 1998 Nov;95(23):13881–6. doi: 10.1073/pnas.95.23.13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Heymann SJ. Modeling the efficacy of prophylactic and curative therapies for preventing the spread of tuberculosis in Africa. Trans R Soc Trop Med Hyg. 1993 Jul;87(4):406–11. doi: 10.1016/0035-9203(93)90014-h. [DOI] [PubMed] [Google Scholar]
- [17].Blower SM, McLean AR, Parco TC, Small PM, Hopewell PC, Sancez MA, et al. The intrinsic transmission dynamics of tuberculosis epidemics. Nat Med. 1995 Aug;1(8):815–21. doi: 10.1038/nm0895-815. [DOI] [PubMed] [Google Scholar]
- [18].World Health Organization Expanded programme on immunization (EPI). Immunization schedules in the WHO African region, 1995. Wkly Epidemiol Rec. 1996 Mar 22;71(12):90–4. [PubMed] [Google Scholar]
- [19].Anderson R, May R. Infectious diseases of humans: dynamics and control. Oxford Univ. Press; Oxford: 1991. Chaps. 1 and 2. [Google Scholar]
- [20].Fitzgerald DW, Desvarieux M, Severe P, Joseph P, Johnson WD, Jr, Pape JW. Effect of post-treatment isoniazid on prevention of recurrent tuberculosis in HIV-1-infected individuals: a randomized trial. Lancet. 2000 Oct 28;356(9240):1470–4. doi: 10.1016/S0140-6736(00)02870-1. [DOI] [PubMed] [Google Scholar]
- [21].Uganda Ministry of Health Highlights of sector performance for financial year 2000 – 01. 2002 Jan; Brochure or website: www.health.go.ug.
- [22].Mbulaiteye SM, Mahe C, Whitworth JAG, Ruberantwari A, Nakiyingi JS, Ojwiya A, et al. Declining HIV-1 incidence and associated prevalence over 10 years in a rural population in south-west Uganda: a cohort study. Lancet. 2002 Jul;360:41–6. doi: 10.1016/s0140-6736(02)09331-5. [DOI] [PubMed] [Google Scholar]
- [23].Styblo K, Meijer J, Sutherland I. Tuberculosis surveillance unit, report no 1: the transmission of tubercle bacilli: its trend in a human population. Bull Int Union Tuberc. 1969 Aug;42:1–104. [PubMed] [Google Scholar]
- [24].Sutherland I, Svandova E, Radhakrishma S. Alternative models for development of tuberculosis disease following infection with tubercle bacilli. Bull Int Union Tuberc. 1976;51(1):171–9. [PubMed] [Google Scholar]
- [25].Elliott AM, Namaambo K, Allen BW, Luo N, Hayes RJ, Pobee JOM, et al. Negative sputum smear results in HIV-positive patients with pulmonary tuberculosis in Lusaka, Zambia. Tuber Lung Dis. 1993 Jun;74(3):191–4. doi: 10.1016/0962-8479(93)90010-U. [DOI] [PubMed] [Google Scholar]
- [26].Palmieri F, Girardi E, Pellicelli AM, Rianda A, Bordi E, Busi RE, et al. Pulmonary tuberculosis in HIV-infected patients presenting with normal chest radiograph and negative sputum smear. Infection. 2002;30(2):68–74. doi: 10.1007/s15010-002-2062-9. [DOI] [PubMed] [Google Scholar]
- [27].Bucher HC, Griffith LE, Guyatt GH, Sudre P, Naef M, Sendi P, et al. Isoniazid prophylaxis for tuberculosis in HIV infection: a meta-analysis of randomized controlled trials. AIDS. 1999 Mar;13(4):501–7. doi: 10.1097/00002030-199903110-00009. [DOI] [PubMed] [Google Scholar]
- [28].Wilkinson D, Squire SB, Garner P. Effect of preventive treatment for tuberculosis in adults infected with HIV: systematic review of rando mized placebo controlled trials. BMJ. 1998 Sep;317(7159):625–9. doi: 10.1136/bmj.317.7159.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].World Health Organization . WHO tuberculosis programme: framework for effective tuberculosis control. WHO; Geneva: 1994. WHO/TB/94.179. [Google Scholar]
- [30].Hawken MP, Muhindi DW. Tuberculosis preventive therapy in HIV-infected persons: feasibility issues in developing countries. Int J Tuberc Lung Dis. 1999 Aug;3(8):646–50. [PubMed] [Google Scholar]
- [31].Holmes CB, Losina E, Walensky RP, Yazdanpanah Y, Freedberg KA. Review of human immunodeficiency virus type 1-related opportunistic infections in sub-Saharan Africa. Clin Infect Dis. 2003 Mar 1;36(5):652–62. doi: 10.1086/367655. [DOI] [PubMed] [Google Scholar]
- [32].Harries AD, Hargreaves NJ, Chimzizi R, Salaniponi FM. Highly active antiretroviral therapy and tuberculosis control in Africa: synergies and potential. Bull World Health Organ. 2002;80(6):464–9. [PMC free article] [PubMed] [Google Scholar]
- [33].Uganda Bureau of Statistics (UBOS) ORC Macro . Uganda demographic and health survey 2000 – 2001. UBOS and ORC Macro; Calverton, MD, USA: 2001. [Google Scholar]
- [34].Nunn AJ, Mulder DW, Kamali A, Ruberantwari A, Kengeya-Kayondo JF, Whitworth J. Mortality associated with HIV-1 infection over five years in a rural Ugandan population: cohort study. BMJ. 1997 Sept;315(7111):767–71. doi: 10.1136/bmj.315.7111.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Uganda Ministry of Health Tuberculosis surveillance report. Qt Bull Integr Dis Surv. 2001 Mar;1:19–21. [Google Scholar]
- [36].Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta analysis of the published literature. JAMA. 1994 Mar 2;271(9):698–702. [PubMed] [Google Scholar]
- [37].Arbelaez MP, Nelson KE, Munoz A. BCG vaccine effectiveness in preventing tuberculosis and its interaction with human immunodeficiency virus infection. Int J Epidemiol. 2000 Dec;29(6):1085–91. doi: 10.1093/ije/29.6.1085. [DOI] [PubMed] [Google Scholar]
- [38].Raviglione MC, Harries AD, Msiska R, Wilkinson D, Nunn P. Tuberculosis and HIV: current status in Africa. AIDS. 1997;11(Suppl B):S115–23. [PubMed] [Google Scholar]
- [39].Elliott AM, Halwiindi B, Hayes RJ, Luo N, Mwinga AG, Tembo G, et al. The impact of human immunodeficiency virus on mortality of patients treated for tuberculosis in a cohort study in Zambia. Trans R Soc Trop Med Hyg. 1995 Jan– Feb;89(1):78–82. doi: 10.1016/0035-9203(95)90668-1. [DOI] [PubMed] [Google Scholar]