Abstract
Background
The A2A receptor (A2AR) plays a complex role in inflammation and tissue injury. In this study, we used the mice deficient in both A2AR and apolipoprotein E (A2AR−/−/apoE−/−) to investigate the role of A2AR in mediating the interactions of leukocytes with injured arterial walls and the formation of arterial neointima induced by a guide wire.
Methods and Results
In apoE−/− mice, A2AR deficiency increased the size of arterial neointima in injured carotid arteries by 83%. Arterial neointima formation was also enhanced in bone marrow transplanted chimeric mice that lacked A2AR in their bone marrow-derived cells. Epifluorescence intravital microscopy showed that neutrophil rolling and adherence to the injured arterial area was enhanced by 80% and 110% in A2AR−/−/apoE−/− mice, respectively. This phenomenon occurred even though the protein levels of homing molecules on A2AR-deficient neutrophils were unchanged from those of wild type neutrophils. A2AR-deficient neutrophils exhibited an increase in the phosphorylation of p38 mitogen-activated protein kinase (MAPK), PSGL-1 clustering, and the affinity of b2 integrins. Inhibition of p38 phosphorylation abrogated the increased PSGL-1 clustering and b2 integrin affinity, thereby reversing the increased homing ability of A2AR-deficient leukocytes.
Conclusion
The deficiency of A2AR enhances the homing ability of leukocytes and increases the formation of arterial neointima after injury. A2AR antagonists are being tested for the treatment of neurodegenerative diseases and other chronic diseases. Our results suggest that an evaluation of the effect of A2AR antagonists on arterial restenosis following arterial angioplasty should be conducted.
Introduction
Adenosine receptor A2 (A2AR) is one of the four G-protein coupled receptors for adenosine. It is present on many inflammatory cells, including neutrophils, monocytes, platelets, and all vascular cells 1, 2. A2AR plays different roles in inflammation and tissue injury under different conditions. In many acute inflammatory or injury models of peripheral organs, A2AR acts as an anti-inflammatory molecule. For example, loss of A2AR increases inflammatory responses and causes tissue damage in the liver, lung, and spleen in vivo 3, 4, while the activation of A2AR with agonists reduces inflammation and protects tissues from injury 2. In contrast to the anti-inflammatory effect of A2AR in the acute injury or inflammatory models, the absence or blocking of A2AR appears to offer mice protection from chronic diseases, such as atherosclerosis and liver cirrhosis 5, 6, as well as neurodegenerative diseases 7. Accordingly, many A2AR antagonists are being developed to treat neurological disorders including Parkinson’s disease, and some of them are being evaluated in clinical trials 8.
Arterial restenosis is a serious complication of angioplasty, including percutaneous transluminal coronary intervention 9. In human, VSMCs predominate neointimal hyperplasia. However, it has been demonstrated that in human neointimal hyperplasia, the number of leukocytes in neointima correlates with the severity of restenosis 10, indicating the causal role of infiltrated leukocytes in the formation of arterial restenosis. To study the effect of infiltrated leukocytes on arterial neointima in patients with arterial restenosis, a model of wire-induced neointima formation in the mouse carotid artery has been described and widely used in the field of research 11. The inflammatory response, including the platelet and leukocyte accumulation on the injured arterial areas, as well as the smooth muscle cell migration, is requisite for arterial neointima formation 12–14. Immediately after arterial injury, platelets interact with the injured area via many factors including glycoprotein Ib and glycoprotein IIb/IIIa 15, 16. Upon adherence, platelets become activated and orchestrate the leukocyte recruitment and endothelial regeneration on the injured site 17–19. Studies from our and other groups have demonstrated that the formation of arterial neointima is significantly suppressed following the inhibition of platelet accumulation, leukocyte adhesion, and the improvement of endothelial regeneration on the injured area 20–22.
Many senior patients with neurodegenerative diseases also suffer from atherosclerotic coronary diseases. Therefore, the patients, who might take A2AR antagonists for the treatment of their neurological disease, could possibly need percutaneous transluminal coronary intervention for their coronary artery disease. Given this clinical scenario, it is relevant to study whether the blocking or inactivation of A2AR affects the arterial repair following injury. To our best knowledge, no reports have been published on the effects of the blocking or inactivation of A2AR on the formation of arterial neointima. In this study, we evaluated whether A2AR deficiency affects the injury-induced arterial neointima by using the mice deficient in both A2AR and apolipoprotein E (A2AR−/−/apoE−/−).
Materials and Methods
A2AR−/− mice in a C57BL/6J background 23 were bred with apoE−/− (C57BL/6J background) mice to generate A2AR−/−/apoE−/− mice and their littermate controls. Chimeric mice, with or without A2AR in their bone marrow-derived cells, were produced by bone marrow transplantation 24. The 8-week-old mice were fed a Western diet containing 21% fat, 0.15% cholesterol, and 19.5% casein without sodium cholate for 2 weeks prior to the wire injury of arteries and maintained on the same diet after wire injury until euthanization. Arterial injury was induced by a guide wire 21. Leukocyte interactions with the injured arteries were examined in vivo by intravital epifluorescence microscopy, or ex vivo on a coated surface by a technique described previously 25. All animal experiments and care were approved by the University of Minnesota Animal Care and Use Committee in accordance with AAALAC guidelines. Detailed methods are available inthe supplemental material.
Results
A2AR deficiency leads to the formation of large neointima in the injured arteries of apoE−/− mice
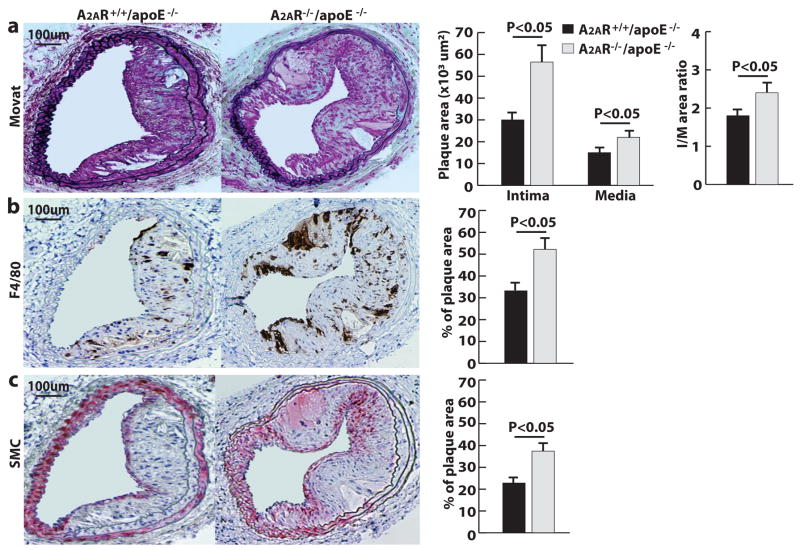
To determine the role of A2AR in the formation of arterial neointima, the carotid arteries of A2AR−/−/apoE−/− mice and the littermate A2AR+/+/apoE−/− mice were injured with a guide wire. Four weeks after the injury, their arteries were excised and processed for analysis. The neointima lesions were 83% larger in A2AR−/−/apoE−/− mice than those in A2AR+/+/apoE−/− mice (Fig. 1a). Additionally, the numbers of macrophages and α-actin-positive smooth muscle cells in the neointima of A2AR−/−/apoE−/− mice were 57% and 68% greater than the numbers in A2AR+/+/apoE−/− mice, respectively (Fig. 1b and 1c). A2AR−/−/apoE−/− mice had slightly higher body weights and blood cholesterol levels than those of A2AR+/+/apoE−/−mice after 6 weeks on a Western diet (Supplementary table I and II); however, these differences were not statistically significant.
Figure 1. A2ARdeficiency increases injury-induced arterial neointima formation.
a, Movat staining and size quantification of neointima (I), media (M) and ratio of intima to media (I/M). b, Anti-F4/80 staining of infiltrated macrophages. c, Anti-α-actin staining of smooth muscle cells (SMCs). Data from 12 sections of 12 mice.
A2AR deficiency in bone marrow–derived cells increases injury-induced arterial neointima formation
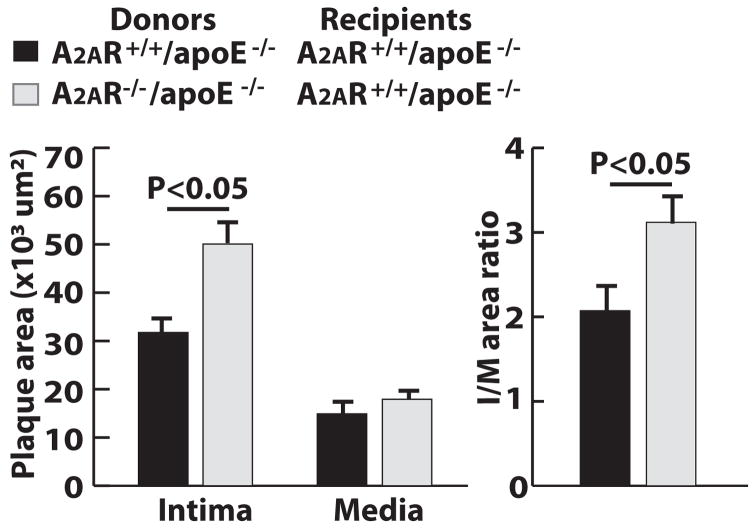
We next determined whether, and to what extent, the presence of A2AR in bone marrow–derived cells (BMDCs) influences the formation of arterial neointima. We used bone marrow transplantation to generate the apoE−/− chimeric mice that lacked A2AR in their BMDCs and the apoE−/− chimeric control mice that retained A2AR in their BMDCs. Their carotid arteries were injured as described above. At the time of euthanization, both groups of mice were identical in blood cholesterol level, peripheral leukocyte count, and body weight (data not shown). However, the neointima lesions in chimeric mice lacking A2AR in their BMDCs were 60% larger than the lesions in chimeric control mice (Fig. 2 and supplementary Fig. Ia). The arterial neointima was stained heavily for the marker of macrophages but not that of vascular smooth muscle cells (supplementary Figs. Ib and Ic). Additionally, we performed wire injury in chimeric mice that were A2AR−/−/apoE−/− mice receiving bone marrow from A2AR+/+/apoE−/− mice. The size of their arterial neointima was similar to that in the A2AR+/+/apoE−/− control mice, which were A2AR+/+/apoE−/− mice receiving bone marrow from A2AR+/+/apoE−/− mice (data not shown). This result suggests that the enhanced neointima formation observed in A2AR−/−/apoE−/− mice was mainly due to the deficiency of A2AR in BMDCs
Figure 2. A2AR deficiency in bone marrow derived cells increases injury-induced arterial neointima formation.
Size quantification of neointima (I), media (M) and ratio of intima to media (I/M). Data from 12 sections of 12 mice.
A2AR deficiency increases leukocyte interactions with the injured arteries
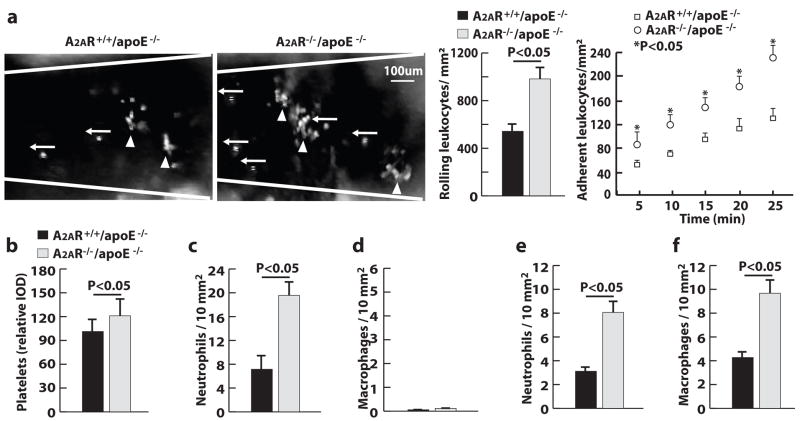
Using epifluorescence intravital microscopy, we examined the interactions of leukocytes with injured mouse carotid arteries in vivo. Immediately after the arterial injury, circulating leukocytes (fluorescently labeled with rhodamine 6G) were able to roll onto, and adhere to, the injured arterial vessel wall. Within 30 minutes after arterial injury, the number of leukocytes rolling on and adhering to the arterial wall were 1.8 and 1.7 fold greater, respectively, in A2AR−/−/apoE−/− mice than in A2AR+/+/apoE−/− mice (Fig. 3a).
Figure 3. A2AR deficiency increases leukocyte interactions with injured arteries.
a, Images and quantification of leukocyte rolling (←) and adhesion (▲) in injured carotid arteries. b to f, Quantification of carotid arteries stained for (b) platelets 1 hour after wire injury (WI), (c) neutrophils 1 hour after WI, (d) macrophages 1 hour after WI, (e) neutrophils 7 days after WI and (f) macrophages 7 days after WI (n=5).
To determine which types of cells were adhering to the injured arteries, we immunostained arterial cross-sections with markers specific for platelets, neutrophils, and monocytes. At 1 hour after the arterial injury, platelets and leukocytes covered the denuded luminal surface (Fig. 3b and supplementary Fig. IIa). Many more leukocytes bound to the injured area of carotid arteries in A2AR−/−/apoE−/− mice than in A2AR+/+/apoE−/− mice. Nearly all of the adherent leukocytes were neutrophils (Fig. 3c and supplementary Fig. IIb); the presence of monocytes (macrophages) was rare (Fig. 3d and supplementary Fig. IIc). At 7 days after wire injury, the adhesion and infiltration of both neutrophils and macrophages were observed in the injured carotid arteries, and more cells were observed in the injured vessel walls of A2AR−/−/apoE−/− mice than in A2AR+/+/apoE−/− mice (Fig. 3e, 3f and supplementary Fig. IId, IIe).
A2AR deficiency increases the interactions of leukocytes with the P-selectin expressing surface
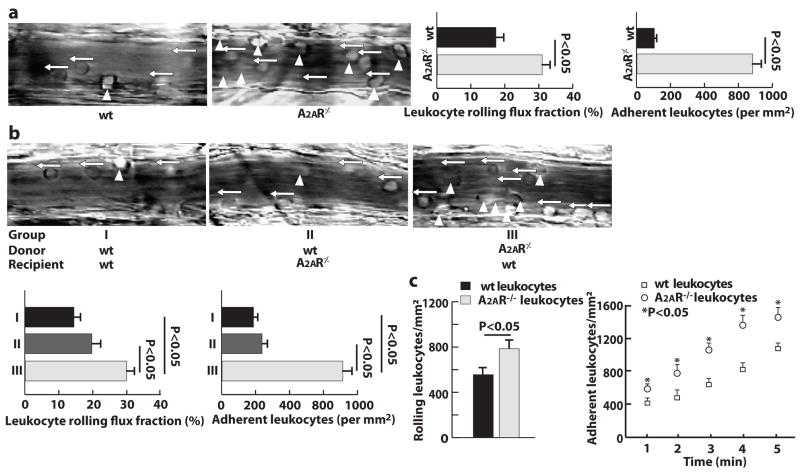
The homing ability of wild type (wt) and A2AR-deficient neutrophils was further studied by intravital microscopy in an in vivo trauma model of mouse cremaster muscle. Mild trauma caused by the exteriorization of the cremaster muscle is an inflammatory stimulus. This stimulus induces the presentation of P-selectin and fibrinogen on the endothelium of postcapillary venules, and it mediates both P-selectin-dependent neutrophil rolling and b2 integrin-dependent neutrophil adhesion 26. To evaluate the role of A2AR in leukocyte-endothelial interactions, we measured the neutrophil rolling flux fraction, defined as the number of rolling neutrophils divided by the total numberof neutrophils passing through the same vessel. Compared to wt mice, A2AR-deficient mice exhibited a 2-fold increase in the neutrophil rolling flux fraction (Fig. 4a). Additionally, many more neutrophils adhered to the endothelium of postcapillary venules in A2AR−/− mice than in wt mice.
Figure 4. A2AR deficiency increases neutrophil homing ability.
a and b, Images and quantification of neutrophil rolling (←) and adhesion (▲) on the endothelium of postcapillary venules of mouse cremaster muscle (n=6). c and d, Neutrophil rolling and adhesion on activated platelets through micro-flow chambers (n=6).
To further determine which cellular A2AR is responsible for these increased neutrophil-endothelial interactions in A2AR−/− mice, we performed an intravital microscopy study in chimeric mice with (control) or without A2AR in their BMDCs. Compared to control mice, chimeric mice with A2AR-deficient BMDCs had significantly increased neutrophil rolling and adhesion, and chimeric mice with A2AR-deficient vessel walls had a similar level of neutrophil rolling and adhesion (Fig. 4b).
To compare the binding affinity of wt and A2AR-deficient neutrophils, we employed an ex vivo micro-flow chamber27, 25 whose surface was coated with thrombin-activated wt platelets. Whole blood from A2AR+/+ or A2AR−/− mice was then perfused through the chamber. Immediately following the perfusion, the numbers of rolling and adherent neutrophils were 60% and 50% higher, respectively, in the blood of A2AR−/− mice than that of A2AR+/+ mice. After 5 minutes of perfusion, these numbers further increased to 80% and 90%, respectively (Fig. 4c). In addition, A2AR -deficient neutrophils rolled much more slowly than wt neutrophils did (velocity data not shown).
Mechanisms for the increased homing ability of A2AR-deficient neutrophils
To determine the influence of A2AR deficiency in neutrophil homing ability, we first measured the expression of homing molecules on wt and A2AR-deficient neutrophils by flow cytometry. The levels of PSGL-1, L-selectin, LFA-1, CD11b, and CXCR2 were identical between both types of neutrophils (supplementary Fig. IIIa).
PSGL-1 is the key molecule responsible for P-selectin-mediated neutrophil rolling on the injured arteries or postcapillary venules 26. To explore the mechanisms responsible for the increased rolling of A2AR-deficient neutrophils, we first used a flow cytometry assay to examine the binding of neutrophil PSGL-1 to P-selectin. In accordance with previously measured levels of PSGL-1 expression (above), the levels of P-selectin binding to wt and A2AR-deficient neutrophils were identical (supplementary Fig. IIIb). Thus, under static conditions, PSGL-1 has the same level of P-selectin binding in A2AR-deficient and wt neutrophils.
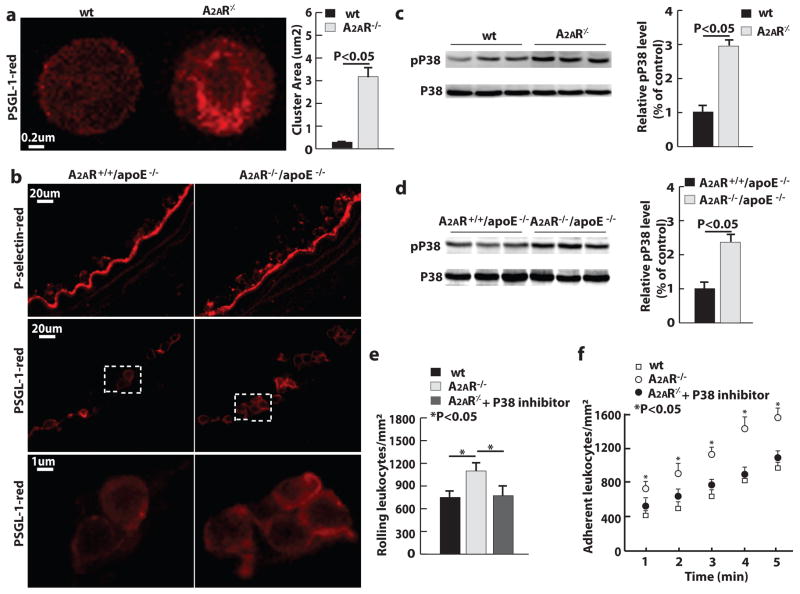
Using confocal microscopy, we investigated the distribution of PSGL-1 following the immunofluorescent labeling of neutrophils with PSGL-1 antibody. PSGL-1 was much more clustered on the surface of A2AR-deficient neutrophils than that of wt neutrophils. This result was observed on neutrophils isolated from blood and on those adhering to injured arteries (Fig. 5a and 5b). Also, we explored several intracellular signaling mechanisms – i.e., the activity of phosphoinositide 3-kinase (PI3K)/Akt and mitogen-activated protein kinases (MAPKs) – that might be responsible for the increased homing ability of A2AR-deficient neutrophils. In a western blot assay of neutrophils isolated from mouse spleen, the level of phosphorylated p38 MAPK in A2AR-deficient neutrophils was 2.5 times higher than that in wt neutrophils (Fig. 5c). This phenomenon was also observed in injured arteries (Fig. 5d). In contrast, the levels of phosphorylated Akt and other MAPKs were not significantly different between wt and A2AR-deficient neutrophils (supplementary Fig. IV).
Figure 5. A2AR deficiency increases neutrophil p38 activity, and PSGL-1 clustering.
a, PSGL-1 distribution on neutrophil membranes. b, P-selectin and PSGL-1 on injured arteries. c and d, Western blot of P38 and phosphorylated p38 (pP38) of (c) spleen neutrophils and (d) carotid arteries 1 hour after injury (n = 3). e and f, Leukocyte rolling and adhesion through (e) P-selectin coated and (f) P-selectin and ICAM-1 coated micro-flow chambers (n=3).
Consistent with the PSGL-1 distribution under flow conditions, A2AR-deficient neutrophils exhibited greater PSGL-1 binding than wt neutrophils did. This result was demonstrated by an ex vivo micro-flow chamber experiment 27, 25, in which mouse whole blood was perfused through a P-selectin coated surface. After 5 minutes of perfusion, the whole blood from A2AR−/− mice presented an average of 1200 ± 80 rolling neutrophils per mm,2 whereas the whole blood from wt mice presented 800 ± 70 rolling neutrophils per mm2 (Fig. 5e). In a similar setup, 5-minute perfusion of mouse whole blood through the flow chamber coated with P-selectin and ICAM-1 led to a 2-fold higher number of A2AR-deficient neutrophils adhering to the surface than the number of adhered wt neutrophils (Fig. 5f), indicating an increase in the affinity of b2 integrins on A2AR-deficient neutrophils.
To determine whether the enhanced p38 MAPK activity is responsible for the increased homing ability of A2AR-deficient neutrophils, we treated A2AR−/− mice with the p38 inhibitor SB203580 (1mg/kg/day) intraperitoneally for 3 days before the perfusion. The treatment of SB203580 reduced the number of rolling cells on both the P-selectin coated surface and the surface coated with P-selectin and ICAM-1 to the levels in wt mice (Figs. 5e and 5f).
Discussion
Our results demonstrate that leukocyte A2AR is critical for the protection of atherosclerotic mice from the injury-induced arterial neointima formation. A2AR deficiency in neutrophils increases p38 activation, resulting in an increase in the clustering of PSGL-1 and the affinity of b2 integrins. Ultimately, A2AR deficiency enhances neutrophil recruitment to the injured arteries and augments the formation of the injury-induced arterial neointima.
Adenosine is known to inhibit a variety of neutrophil functions, including the production of TNF-a and superoxide anions 28. Several studies have demonstrated that adenosine or adenosine receptor agonists inhibit the neutrophil adhesion to endothelial cells and fibrinogen by suppressing the upregulation of neutrophil b2 integrins, as stimulated by N-formyl-methionyl-leucyl-phenylalanine (fMLP) 29–31. When neutrophils are treated with an inhibitor of adenosine kinase (a major intracellular adenosine removal enzyme), adenosine levels increase, and the adhesion of activated neutrophils to cultured endothelial cells decreases through the alteration of L-selectin and the neutrophil cytoskeleton 32. Recently, adenosine or A2AR agonists have been shown to induce heterologous desensitization of chemokine receptors and suppress the expression of very late antigen-4 (VLA-4) on stimulated human neutrophils 33, 34. Notably, these previous studies revealed the pharmacological effects of adenosine or adenosine receptors on leukocytes. Also, the neutrophils used in the studies were activated in vitro by fMLP, TNF-a or other stimuli. The present study shows a physiological role of A 2AR in the regulation of neutrophil homing ability. Under physiological conditions, A2AR deficiency did not affect the expression of adhesion molecules on neutrophils. Rather, A2AR deficiency altered the cell membrane distribution of PSGL-1and the affinity of b2 integrins, thereafter inhibiting neutrophil recruitment to the injured arteries in vivo.
We explored several possible intracellular signaling mechanisms for the increased homing ability of A2AR-deficient neutrophils, including the activity of PI3K/Akt and MAPKs. We found that A2AR regulates the neutrophil homing ability through MAPK p38. Our finding that A2AR-deficient neutrophils exhibited increased p38 MAPK activation could result from a change in the signaling associated with intracellular cAMP 1. A2AR occupancy elevates the intracellular cAMP, thereafter inhibiting the activation of p38 via the cAMP response element–binding protein–induced dynein light chain 35, 36. Leukocyte p38 is usually activated by outside-in signals, and the activated p38 is able to signal many pathways related to different cellular functions 35, 36. In the early phase of arterial injury, the binding of neutrophil PSGL-1 with platelet P-selectin predominates neutrophilic interactions with the vessel wall. Therefore, we focused on the role of p38 activation on the regulation of PSGL-1 binding function. A2AR-deficient neutrophils exhibit increased PSGL-1 clustering. Consequently, these cells have elevated levels of P-selectin binding. In our ex vivo micro-flow chamber assay, the inhibition of p38 activation significantly reversed the increased neutrophil rolling and adhesion, demonstrating that p38 is a key modulator in regulating A2AR-mediated leukocyte homing. The enhanced PSGL-1 clustering can be regulated directly by p38 or indirectly through p38 altered cytoskeletal protein organization 37, 38.
Increased neutrophil recruitment to the injured arteries contributes to neointima formation through many mechanisms 39, 40, 25. Additionally, monocyte recruitment and activation could also play a role in the increased neointima formation following the arterial injury in A2AR−/−/apoE−/− mice. Similar to neutrophils, A2AR-deficient monocytes express adhesion molecules at the same levels seen in wt cells 5. However, A2AR-deficient monocytes showed increased adhesion on atherosclerotic endothelium in an ex vivo mouse carotid artery perfusion model (data not shown). Furthermore, NF-kB was highly activated in A2AR-deficient macrophages in atherosclerotic lesions 5. In a short-term arterial neointima formation model, these factors might contribute to the aggravation of arterial neointima formation. However, in a long-term spontaneous atherosclerosis model, A2AR-deficiency results in a decrease in atherosclerosis 5. This discrepancy may be due to the fact that many resolution aspects of inflammation take actions to reduce the size of lesions during a long-term chronic process of spontaneous lesion formation. These resolution aspects include macrophage apoptosis, macrophage emigration from lesions, and the reduction of the vasa vasorum density of atherosclerotic arteries 41, 42, 43, 44. We have previously shown that A2AR-deficiency increases the apoptosis of macrophages 5. In addition, CD8 T cells target the neovascularization of atherosclerotic arteries to reduce atherosclerosis 45. Dr. Sitkovsky’s group has reported that A2AR-deficient T cells are able to suppress or even regress the tumor growth 46, which may be achieved through the elimination of neovascularization by A2AR-deficient CD8 T cells (personal communication). Therefore, the reduction of atherosclerosis in A2AR−/−/apoE−/− mice may also be due to the suppression of neovascularization by A2AR-deficient CD8 T cells.
Increased neointima formation in A2AR−/− mice is not attributed to the A2AR deficiency in endothelial cells. In a mouse carotid artery wire injury model, the regeneration of endothelial cells on the injured area occurred at a few days after wire injury. These regenerated endothelial cells were inflamed and expressing a variety of adhesion molecules, which is the key for leukocyte recruitment to the injured arteries and the formation of arterial neointima 47. In a similar mouse carotid artery injury model, endogenous extracellular adenosine, generated through CD73, protected the regenerated endothelial cells from inflammatory activation 48. Also, in a mouse carotid artery ligation model, an A2AR agonist inhibited adhesion molecule expression and consequent neointima formation in injured arteries 49. Thus, it has been speculated that adenosine reduces endothelial activation through A2ARs 1. In this study, we found that A2AR deficiency in endothelial cells does not affect the inflammatory response of endothelial cells that are regenerated on the injured area. This finding is supported by our immunostaining and western blot results from an in vivo carotid artery injury model and our flow cytometry results of an in vitro cell culture system to determine the role of A2AR deficiency in endothelial inflammatory responses (Supplementary Figs. Va to Vc). Thus, different from its pharmacological effect as reported in other studies 50, 49, endothelial A2AR does not play a physiological role in determining the inflammatory status of endothelial cells in the arterial neointima model.
Augmented arterial neointima formation in A2AR−/− mice may not be associated with theA2AR deficiency in platelets. Immediately after wire injury, platelets accumulated on the injured area. These platelets were activated and presenting P-selectin and other integrins to serve as a platform for leukocyte recruitment to the injured arterial vessel wall 17. In a dog coronary hypoperfusion model, it has been demonstrated that platelet A2AR is critically involved in the effect of adenosine on the inhibition of platelet-leukocyte interactions and platelet aggregation 51. In humans, upregulated A2AR on platelets, as a result of chronic caffeine intake, is beneficial for the prevention of platelet aggregation 52, 53. Different from above studies, we did not find increased platelet activation mediated by thrombin in A2AR- deficient platelets (Supplementary Fig. VIa). In response to thrombin stimulation, A2AR-deficient mouse platelets presented P-selectin and exhibited platelet-leukocyte interactions at the same levels as seen in wt mouse platelets (Supplementary Fig. VIb). It is likely that the role of A2AR in platelet activation is strain dependent. Otherwise, adenosine and adenosine receptors are not critically involved in platelet activation either in response to thrombin or in the milieu of arterial injury.
Taken together, the deficiency of A2AR enhances leukocyte recruitment to the injured arteries and aggravates the formation of injury-induced arterial neointima. A2AR antagonists are being tested in clinical trials for the treatment of neurodegenerative diseases and other chronic conditions. The results from this study indicate that the evaluation on the effect of A2AR antagonists on arterial restenosis following arterial angioplasty should be considered.
Supplementary Material
Acknowledgments
The authors thank Dr. Anne Marie Weber-Main for her critical review and editing of manuscript drafts.
Sources and Funding
This work was supported by AHA 0430151N, NIH HL78679 and HL080569 to Y. Huo.
Footnotes
Disclosures
None.
Reference List
- 1.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Lappas CM, Sullivan GW, Linden J. Adenosine A2A agonists in development for the treatment of inflammation. Expert Opin Investig Drugs. 2005;14:797–806. doi: 10.1517/13543784.14.7.797. [DOI] [PubMed] [Google Scholar]
- 3.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 4.Lukashev D, Ohta A, Apasov S, Chen JF, Sitkovsky M. Cutting edge: Physiologic attenuation of proinflammatory transcription by the Gs protein-coupled A2A adenosine receptor in vivo. J Immunol. 2004;173:21–24. doi: 10.4049/jimmunol.173.1.21. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Zhang W, Zhu C, Bucher C, Blazar BR, Zhang C, Chen JF, Linden J, Wu C, Huo Y. Inactivation of the adenosine A2A receptor protects apolipoprotein E-deficient mice from atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1046–1052. doi: 10.1161/ATVBAHA.109.188839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan ES, Montesinos MC, Fernandez P, Desai A, Delano DL, Yee H, Reiss AB, Pillinger MH, Chen JF, Schwarzschild MA, Friedman SL, Cronstein BN. Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br J Pharmacol. 2006;148:1144–1155. doi: 10.1038/sj.bjp.0706812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen JF, Sonsalla PK, Pedata F, Melani A, Domenici MR, Popoli P, Geiger J, Lopes LV, de MA. Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and “fine tuning” modulation. Prog Neurobiol. 2007;83:310–331. doi: 10.1016/j.pneurobio.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 2006;29:647–654. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Weintraub WS. The pathophysiology and burden of restenosis. Am J Cardiol. 2007;100:3K–9K. doi: 10.1016/j.amjcard.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Moreno PR, Bernardi VH, Lopez-Cuellar J, Newell JB, McMellon C, Gold HK, Palacios IF, Fuster V, Fallon JT. Macrophage infiltration predicts restenosis after coronary intervention in patients with unstable angina. Circulation. 1996;94:3098–3102. doi: 10.1161/01.cir.94.12.3098. [DOI] [PubMed] [Google Scholar]
- 11.Lindner V, Fingerle J, Reidy MA. Mouse model of arterial injury. Circ Res. 1993;73:792–796. doi: 10.1161/01.res.73.5.792. [DOI] [PubMed] [Google Scholar]
- 12.Ross R. Atherosclerosis--an inflammatory disease [see comments] N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 13.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 14.Glass CK, Witztum JL. Atherosclerosis. the road ahead Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 15.Andrews RK, Shen Y, Gardiner EE, Dong JF, Lopez JA, Berndt MC. The glycoprotein Ib-IX-V complex in platelet adhesion and signaling. Thromb Haemost. 1999;82:357–364. [PubMed] [Google Scholar]
- 16.Phillips DR, Charo IF, Scarborough RM. GPIIb–IIIa - The responsive integrin. Cell. 1991;65:359–362. doi: 10.1016/0092-8674(91)90451-4. [DOI] [PubMed] [Google Scholar]
- 17.Manka D, Forlow SB, Sanders JM, Hurwitz D, Bennett DK, Green SA, Ley K, Sarembock IJ. Critical Role of Platelet P-Selectin in the Response to Arterial Injury in Apolipoprotein-E-Deficient Mice. Arterioscler Thromb Vasc Biol. 2004;24:1124–1129. doi: 10.1161/01.ATV.0000127619.04687.f4. [DOI] [PubMed] [Google Scholar]
- 18.Simon DI, Chen Z, Xu H, Li CQ, Dong J, McIntire LV, Ballantyne CM, Zhang L, Furman MI, Berndt MC, Lopez JA. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18) J Exp Med. 2000;192:193–204. doi: 10.1084/jem.192.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber C. Platelets and chemokines in atherosclerosis: partners in crime. Circ Res. 2005;96:612–616. doi: 10.1161/01.RES.0000160077.17427.57. [DOI] [PubMed] [Google Scholar]
- 20.Manka D, Collins RG, Ley K, Beaudet AL, Sarembock IJ. Absence of p-selectin, but not intercellular adhesion molecule-1, attenuates neointimal growth after arterial injury in apolipoprotein e-deficient mice. Circulation. 2001;103:1000–1005. doi: 10.1161/01.cir.103.7.1000. [DOI] [PubMed] [Google Scholar]
- 21.An G, Wang H, Tang R, Yago T, McDaniel JM, McGee S, Huo Y, Xia L. P-selectin glycoprotein ligand-1 is highly expressed on Ly-6Chi monocytes and a major determinant for Ly-6Chi monocyte recruitment to sites of atherosclerosis in mice. Circulation. 2008;117:3227–3237. doi: 10.1161/CIRCULATIONAHA.108.771048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips JW, Barringhaus KG, Sanders JM, Hesselbacher SE, Czarnik AC, Manka D, Vestweber D, Ley K, Sarembock IJ. Single injection of P-selectin or P-selectin glycoprotein ligand-1 monoclonal antibody blocks neointima formation after arterial injury in apolipoprotein E-deficient mice. Circulation. 2003;107:2244–2249. doi: 10.1161/01.CIR.0000065604.56839.18. [DOI] [PubMed] [Google Scholar]
- 23.Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huo Y, Zhao L, Hyman MC, Shashkin P, Harry BL, Burcin T, Forlow SB, Stark MA, Smith DF, Clarke S, Srinivasan S, Hedrick CC, Pratico D, Witztum JL, Nadler JL, Funk CD, Ley K. Critical role of macrophage 12/15-lipoxygenase for atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;110:2024–2031. doi: 10.1161/01.CIR.0000143628.37680.F6. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Zhang W, Tang R, Hebbel RP, Kowalska MA, Zhang C, Marth JD, Fukuda M, Zhu C, Huo Y. Core2 1-6-N-Glucosaminyltransferase-I Deficiency Protects Injured Arteries From Neointima Formation in ApoE-Deficient Mice. Arterioscler Thromb Vasc Biol. 2009;29:1053–1059. doi: 10.1161/ATVBAHA.109.187716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 27.Smith ML, Sperandio M, Galkina EV, Ley K. Autoperfused mouse flow chamber reveals synergistic neutrophil accumulation through P-selectin and E-selectin. J Leukoc Biol. 2004;76:985–993. doi: 10.1189/jlb.1003483. [DOI] [PubMed] [Google Scholar]
- 28.Thiel M, Chouker A. Acting via A2 receptors, adenosine inhibits the production of tumor necrosis factor-alpha of endotoxin-stimulated human polymorphonuclear leukocytes. J Lab Clin Med. 1995;126:275–282. [PubMed] [Google Scholar]
- 29.Cronstein BN, Levin RI, Philips M, Hirschhorn R, Abramson SB, Weissmann G. Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J Immunol. 1992;148:2201–2206. [PubMed] [Google Scholar]
- 30.Wollner A, Wollner S, Smith JB. Acting via A2 receptors, adenosine inhibits the upregulation of Mac-1 (Cd11b/CD18) expression on FMLP-stimulated neutrophils. Am J Respir Cell Mol Biol. 1993;9:179–185. doi: 10.1165/ajrcmb/9.2.179. [DOI] [PubMed] [Google Scholar]
- 31.Thiel M, Chambers JD, Chouker A, Fischer S, Zourelidis C, Bardenheuer HJ, Arfors KE, Peter K. Effect of adenosine on the expression of b2 integrins and L-selectin of human polymorphonuclear leukocytes in vitro. J Leukocyte Biol. 1996;59:671–682. doi: 10.1002/jlb.59.5.671. [DOI] [PubMed] [Google Scholar]
- 32.Firestein GS, Bullough DA, Erion MD, Jimenez R, Ramirez-Weinhouse M, Barankiewicz J, Smith CW, Gruber HE, Mullane KM. Inhibition of neutrophil adhesion by adenosine and an adenosine kinase inhibitor: The role of selectins. J Immunol. 1995;154:326–334. [PubMed] [Google Scholar]
- 33.Zhang N, Yang D, Dong H, Chen Q, Dimitrova DI, Rogers TJ, Sitkovsky M, Oppenheim JJ. Adenosine A2a receptors induce heterologous desensitization of chemokine receptors. Blood. 2006;108:38–44. doi: 10.1182/blood-2005-06-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan GW, Lee DD, Ross WG, DiVietro JA, Lappas CM, Lawrence MB, Linden J. Activation of A2A adenosine receptors inhibits expression of alpha 4/beta 1 integrin (very late antigen-4) on stimulated human neutrophils. J Leukoc Biol. 2004;75:127–134. doi: 10.1189/jlb.0603300. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Bui TN, Xiang J, Lin A. Cyclic AMP inhibits p38 activation via CREB-induced dynein light chain. Mol Cell Biol. 2006;26:1223–1234. doi: 10.1128/MCB.26.4.1223-1234.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 37.Green CE, Pearson DN, Camphausen RT, Staunton DE, Simon SI. Shear-dependent capping of L-selectin and P-selectin glycoprotein ligand 1 by E-selectin signals activation of high-avidity beta2-integrin on neutrophils. J Immunol. 2004;172:7780–7790. doi: 10.4049/jimmunol.172.12.7780. [DOI] [PubMed] [Google Scholar]
- 38.Ley K. Integration of inflammatory signals by rolling neutrophils. Immunol Rev. 2002;186:8–18. doi: 10.1034/j.1600-065x.2002.18602.x. [DOI] [PubMed] [Google Scholar]
- 39.Shimazawa M, Watanabe S, Kondo K, Hara H, Nakashima M, Umemura K. Neutrophil accumulation promotes intimal hyperplasia after photochemically induced arterial injury in mice. Eur J Pharmacol. 2005;520:156–163. doi: 10.1016/j.ejphar.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 40.Welt FG, Edelman ER, Simon DI, Rogers C. Neutrophil, not macrophage, infiltration precedes neointimal thickening in balloon-injured arteries. Arterioscler Thromb Vasc Biol. 2000;8(20):2553–2558. doi: 10.1161/01.atv.20.12.2553. [DOI] [PubMed] [Google Scholar]
- 41.Arai S, Shelton JM, Chen M, Bradley MN, Castrillo A, Bookout AL, Mak PA, Edwards PA, Mangelsdorf DJ, Tontonoz P, Miyazaki T. A role for the apoptosis inhibitory factor AIM/Spalpha/Api6 in atherosclerosis development. Cell Metab. 2005;1:201–213. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Liu B, Wang P, Dong X, Fernandez-Hernando C, Li Z, Hla T, Li Z, Claffey K, Smith JD, Wu D. Phospholipase C beta3 deficiency leads to macrophage hypersensitivity to apoptotic induction and reduction of atherosclerosis in mice. J Clin Invest. 2008;118:195–204. doi: 10.1172/JCI33139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herrmann J, Lerman LO, Mukhopadhyay D, Napoli C, Lerman A. Angiogenesis in atherogenesis. Arterioscler Thromb Vasc Biol. 2006;26:1948–1957. doi: 10.1161/01.ATV.0000233387.90257.9b. [DOI] [PubMed] [Google Scholar]
- 45.Hauer AD, Habets KL, van Wanrooij EJ, de VP, Krueger J, Reisfeld RA, Van Berkel TJ, Kuiper J. Vaccination against TIE2 reduces atherosclerosis. Atherosclerosis. 2009;204:365–371. doi: 10.1016/j.atherosclerosis.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 46.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, Chen JF, Jackson EK, Apasov S, Abrams S, Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li G, Sanders JM, Phan ET, Ley K, Sarembock IJ. Arterial macrophages and regenerating endothelial cells express P-selectin in atherosclerosis-prone apolipoprotein E-deficient mice. Am J Pathol. 2005;167:1511–1518. doi: 10.1016/S0002-9440(10)61237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zernecke A, Bidzhekov K, Ozuyaman B, Fraemohs L, Liehn EA, Luscher-Firzlaff JM, Luscher B, Schrader J, Weber C. CD73/ecto-5′-nucleotidase protects against vascular inflammation and neointima formation. Circulation. 2006;113:2120–2127. doi: 10.1161/CIRCULATIONAHA.105.595249. [DOI] [PubMed] [Google Scholar]
- 49.McPherson JA, Barringhaus KG, Bishop GG, Sanders JM, Rieger JM, Hesselbacher SE, Gimple LW, Powers ER, Macdonald T, Sullivan G, Linden J, Sarembock IJ. Adenosine A(2A) receptor stimulation reduces inflammation and neointimal growth in a murine carotid ligation model. Arterioscler Thromb Vasc Biol. 2001;21:791–796. doi: 10.1161/01.atv.21.5.791. [DOI] [PubMed] [Google Scholar]
- 50.Sands WA, Martin AF, Strong EW, Palmer TM. Specific inhibition of nuclear factor-kappaB-dependent inflammatory responses by cell type-specific mechanisms upon A2A adenosine receptor gene transfer. Mol Pharmacol. 2004;66:1147–1159. doi: 10.1124/mol.104.001107. [DOI] [PubMed] [Google Scholar]
- 51.Minamino T, Kitakaze M, Asanuma H, Tomiyama Y, Shiraga M, Sato H, Ueda Y, Funaya H, Kuzuya T, Matsuzawa K, Hori M. Endogenous adenosine inhibits p-selectin-dependent formation of coronary thromboemboli during hypoperfusion in dogs. J Clin Invest. 1998;101:1643–1653. doi: 10.1172/JCI635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Varani K, Portaluppi F, Gessi S, Merighi S, Ongini E, Belardinelli L, Borea PA. Dose and time effects of caffeine intake on human platelet adenosine A(2A) receptors: functional and biochemical aspects. Circulation. 2000;102:285–289. doi: 10.1161/01.cir.102.3.285. [DOI] [PubMed] [Google Scholar]
- 53.Varani K, Portaluppi F, Merighi S, Ongini E, Belardinelli L, Borea PA. Caffeine alters A2A adenosine receptors and their function in human platelets. Circulation. 1999;99:2499–2502. doi: 10.1161/01.cir.99.19.2499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.