Abstract
Human herpesvirus (HHV)-8, also called Kaposi’s sarcoma-associated herpesvirus, was discovered in 1994 and was rapidly sequenced, revealing several unique and surprising features of its genetic makeup. Among these discoveries was the identification of the first viral homolog of IL-6 and three CC/β-chemokine ligands (viral CCL-1, -2 and -3), not previously found in γ-herpesviruses. Viral IL-6 was immediately recognized as a potential contributor to HHV-8 pathogenesis, specifically endothelial-derived Kaposi’s sarcoma and the B-cell malignancy multicentric Castleman’s disease with which IL-6, a proangiogenic and B-cell growth factor, had previously been implicated. The roles of the viral chemokines were speculated to involve immune evasion; however, like viral IL-6, the viral chemokines have the potential to contribute to pathogenesis through their shared angiogenic activities, known to be important for Kaposi’s sarcoma and HHV-8-associated primary effusion lymphoma, and also via direct prosurvival activities. This article will discuss the molecular properties, activities and functions of viral IL-6 and the viral CCLs, proteins that could provide appropriate targets for antiviral and therapeutic strategies.
Keywords: angiogenesis, HHV-8, human herpesvirus 8, Kaposi’s sarcoma, multicentric Castleman’s disease, pathogenesis, primary effusion lymphoma, signal transduction, vCCL, vIL-6, viral IL-6
Human herpesvirus (HHV)-8 was discovered in 1994 by PCR-based representational difference analysis as a herpesvirus agent associated with Kaposi’s sarcoma (KS) [1]. HHV-8 sequences were subsequently detected in malignant B cells of multicentric Castleman’s disease (MCD) [2]. In both cases, IL-6 had been implicated in disease development; elevated levels of human IL-6 (hIL-6) had been detected in KS lesions and in the circulation of MCD patients, correlating with disease severity, KS cells in culture were reported to be mitogenically responsive to IL-6, and IL-6 was known to promote B-cell proliferation [3–7]. HHV-8 was also identified rapidly to be present in primary effusion lymphomas (PEL), formerly referred to as body cavity-based lymphomas, another B-cell malignancy [8–10]. Thus, when the IL-6 homolog was identified in HHV-8 [11–13] its potential role in virus-associated neoplasia was immediately recognized and work began in several laboratories to characterize the molecular and biological properties of this novel viral cytokine. Like viral IL (vIL)-6, the three chemokines of HHV-8, specified by open reading frames K6 (viral CC/β-chemokine ligand [vCCL]-1/viral macrophage inflammatory protein [vMIP]-1A/MIP-I), K4 (vCCL-2/vMIP-1B/MIP-II) and K4.1 (vCCL-3/viral β-chemokine [BCK]/MIP-III), were identified following partial and complete sequencing of the viral genome [14–16]. It was discovered that each specified angiogenic properties, as recognized by standard chick chorioallantoic and rabbit corneal assays. Therefore, they had the potential to contribute to KS pathogenesis [17,18].
In respect to the roles of vIL-6 and vCCLs in virus biology and their potential contribution to viral pathogenesis, it is important to note that each is expressed during productive replication [11,15,19–21]. Therefore, it is speculated that they promote virus production, either directly via influences on the cell in which they are produced or indirectly by way of paracrine effects on surrounding cells, through their own activities or those of viral cytokine-induced cellular factors. Paracrine effects are likely to include immune evasion functions of the viral chemokines via agonist and inverse-agonist activities on cellular chemokine receptors (see later). However, the viral chemokines, in addition to vIL-6, are also able to exert their effects in an autocrine manner to promote cell survival. The viral chemokines vCCL-1 and -2 enhance endothelial cell survival and virus production under lytic cycle-induced stress [22]. For vIL-6, which, unlike the viral chemokines, can be expressed at low levels during latency (in PEL cells at least), autocrine activity is enhanced by the ability of the viral cytokine to signal intracellularly. Promotion of cell proliferation and survival via this route has been reported [23]. Thus, vIL-6 has the potential to contribute to viral pathogensis in a direct autocrine manner during latency, promoting proliferation and survival, and thereby, viral maintenance. It may also contribute to pathogenesis in a paracrine manner during productive replication, when induced expression of the viral cytokine allows for its abundant secretion from lytically infected cells and influences on the growth, survival or other properties of surrounding cells (latently infected and uninfected). Both intracrine and paracrine activities of vIL-6 are likely to contribute to virus-associated neoplasia in addition to their presumed positive roles in viral latency and productive replication in the context of host infection.
The molecular properties of the viral cytokines and the mechanisms they employ to mediate signal transduction are important to understand in order to elucidate their biological activities in respect of virus replication and viral pathogenesis and to allow the development of therapeutic strategies to inhibit their activities. There are many questions remaining regarding the properties and roles of the viral cytokines, but considerable progress has been made on their characterization. The purpose of this article is to provide an overview of published research in this area and to offer perspectives of the likely biological and pathological roles of the viral cytokines.
Viral IL-6
Viral IL 6 was codiscovered independently by three research groups [11–13]. The viral protein is significantly diverged from its human cellular counterpart (hIL-6), displaying only 25% amino acid identity, but its structure is very similar to hIL-6 and to other cellular IL-6 proteins [24,25]. It utilizes the same signal transducer, gp130, either together with or independently of the nonsignaling α-receptor subunit, gp80, and shares activities similar to hIL-6 and other IL-6 proteins, such as activation of signal transducer and activator of transcription (STAT) and CCAAT/enhancer-binding protein (C/EBP) transcription factors, growth support of IL-6-dependent cells and activation of acute-phase proteins in hepatocytes [11,12,26–29]. The gp80 independence of vIL-6 and its very inefficient secretion from cells are the two major differences between vIL-6 and cellular IL-6 proteins, and these properties have implications for the role of the viral cytokine in virus biology, in addition to its contribution to HHV-8-associated neoplasia. The properties and potential roles of vIL-6 in these processes are categorized and discussed in detail in the following sections.
Receptor binding & signaling by vIL-6
The viral cytokine, like its cellular homologs, possesses a four α-helical bundle structure in which particular surface residues on different parts of the 3D structure are presented to distinct interaction interfaces on each of the two gp130 signal transducer molecules to form a vIL-6-bridged gp130 dimer (vIL-62–gp1302) [24]. In contrast to cellular IL-6 proteins, vIL-6 does not require prior binding to gp80 to allow such interaction and complexing with gp130, but vIL-6 can form equivalent and functional gp80-containing hexameric complexes (vIL-62–g1302–gp802) [27,28,30–32]. The receptor-interaction sites on vIL-6 are, by analogy with cellular counterparts, referred to as site I, II and III. These contact residues in the cytokine-binding homology region (CHR) of gp80, gp130 CHR domains 2 and 3, and gp130 domain 1 (Ig-homology domain), respectively. Detailed information regarding the physical nature of vIL-6-induced tetrameric complexes (vIL-62–gp1302) has been obtained via x-ray crystallographic studies [24]. This work highlighted a vIL-6-unique hydrophobic pocket in site II that was predicted to contribute significantly to interactions with gp130 CHR and hypothesized to provide gp80 independence. However, in subsequent studies, mutagenesis of these residues in vIL-6 did not abolish gp80-independent signaling by vIL-6, nor did their introduction into hIL-6 confer gp80 independence [33]. Moreover, domain (e.g., helix B) and amino acid substitutions involving residues unconnected with the direct interaction with g130 rendered vIL-6 gp80 dependent, implicating the importance of overall cytokine conformation for vIL-6-induced tetrameric signaling [33]. Recently published work has provided further support for this conclusion and determined that substitution of site III interface residues/regions of hIL-6 with those of vIL-6 can confer gp80-independence to the human cytokine [34]. Therefore, it seems that vIL-6 naturally adopts a conformation that is conducive to dimerizing interactions with gp130, whereas cellular IL-6 proteins first require a conformational change mediated via binding to gp80.
Of significance in consideration of vIL-6 conformational requirements for gp130 interaction, complexing and signaling is the recent elegant work from Dela Cruz and colleagues [35]. These investigators examined the role of N-glycosylation for vIL-6–gp130 interactions and signal transduction. Residues N78 and N89 are glycosylated and while glycosylation is not necessary for the association of vIL-6 with gp130 (glycosidase treatment of the secreted protein does not abrogate ligand–receptor association), N89 glycosylation is required for structural maturation of the protein in order to achieve native conformation. These findings are generally consistent with data demonstrating the interaction between vIL-6 and the endoplasmic reticulum chaperone protein calnexin, involved in protein folding and quality control, and the requirement of the glycosylated asparagine residues of vIL-6 for appropriate protein folding [36]. In contrast to vIL-6, hIL-6, while glycosylated, is not dependent on glycosylation and eukaryotic cell processing for native (active) protein conformation and does not interact with calnexin [36–38]. This explains the 1000-fold reduced specific activity of bacterially produced recombinant vIL-6 compared with eukaryotically produced vIL-6 and recombinant hIL-6 [27,39].
IL-6 signaling via gp130 leads to receptor recruitment and tyrosine phosphorylation-mediated activation of STAT1 and STAT3 in addition to SHP2, which mediates activation of the MAPK signaling pathway [40]. However, the two types of vIL-6-induced gp130 signaling complexes, hexamer (containing gp80) and tetramer (devoid of gp80), have distinguishable signaling profiles and biological activity. Incorporation of gp180 into vIL-6 signaling complexes leads to enhanced signaling for a longer duration, increased STAT1:STAT3 ratios and better promotes the growth of IL-6-responsive cells in culture [31]. This is consistent with the noted stabilization of vIL-6-induced gp130 dimers by gp80 [30,33]. Amplitudes and durations of STAT activation and support of cell growth by vIL-6 are greater than those mediated by hIL-6. However, the molecular and structural basis of gp80-determined and ligand-specific differences in signaling profiles and durations is not clear, and further research needs to be undertaken to understand these phenomena. Subtle conformational differences of complexes may determine the accessibility of gp130 signaling tyrosine residues to activating JAKs, inactivating phosphatases (SHP2), STATs and/or signaling-inhibitory SOCS proteins. The differences in signaling mediated by gp80-containing hexameric and gp80-devoid tetrameric complexes induced by vIL-6 may be significant in virus biology and viral pathogenesis. As discussed later, intracellular activity of vIL-6, mediated from the endoplasmic reticulum, is executed exclusively via tetrameric complexes, whereas both tetrameric and hexameric complexes can form at the cell surface.
General properties & activities of vIL-6
As might be expected from the shared utilization of gp130 for signal transduction by vIL-6 and hIL-6, the two proteins are generally functionally analogous. As mentioned previously, they activate common pathways (STAT and MAPK) and have qualitatively similar biological effects, such as inducing acute-phase gene expression and supporting cell growth [11,26,27,29,39,41]. Interestingly, vIL-6 can induce expression of hIL-6 in certain cell types, suggesting the possibility of signal amplification in certain contexts and/or enabling strong paracrine signaling when most vIL-6 is retained intracellularly [42,43].
However, there are notable differences between vIL-6 and hIL-6 activities, as detected in cell culture assays. Using maximally active concentrations of vIL-6 and hIL-6 in the presence of exogenously added gp80 (required for hIL-6 activity), it has been demonstrated that vIL-6 is better able to support IL-6-dependent Baf-130 cell growth [31]. Furthermore, only vIL-6 is able to stimulate the growth and survival of PEL cells in culture [41,44], although hIL-6 has been reported to be specifically able to support clonal growth of PEL cells in soft agar, as determined by using inhibitory antisense oligonucleotides [45]. The mechanisms underlying these differences have not been determined, but the distinguishable profiles and amplitudes of vIL-6 versus hIL-6 signaling presumably account for the different biological responses observed [31].
Potential roles of vIL-6 in virus biology
In contrast to the extensive research undertaken on receptor interactions of vIL-6, signaling complex formation and resulting signal transduction, little is known regarding the role of the viral cytokine in virus biology. It has been speculated that proproliferative and prosurvival activities of vIL-6 may contribute to the maintenance of latently infected cell pools if vIL-6 is expressed during latency (see later) or from a subset of lytically infected cells within the HHV-8+ population. Both direct and indirect functions of vIL-6 mediated via vIL-6-induced cellular cytokines or other factors could be relevant to such a role during latency. However, the predominant lytic expression of vIL-6 suggests that it functions to promote productive replication, notwithstanding indications that such activity cannot be identified in culture. Experimental utilization of a vIL-6-null virus revealed no effect on reactivated replication in infected BJAB or Vero cells [46]. It remains likely that vIL-6 proreplication functions operate in vivo, via potentially complex interactions with infected and uninfected cells. For example, vIL-6 might enhance, through proinflammatory and/or angiogenic activities, the spread of virus via the recruitment of permissive cells into or infected cells away from sites of active replication. Addressing this aspect of vIL-6 function is, therefore, likely to require the deployment of animal model systems, such as those utilizing vIL-6-encoding rhesus rhadinovirus and appropriate viral mutants [47–49].
Possible contributions of vIL-6 to HHV-8 pathogenesis
Speculation regarding the role of vIL-6 in HHV-8 pathogenesis has focused on paracrine contributions of the viral cytokine, owing to its lytic expression [11,20,21,50,51]. Pathogenesis-relevant activities, including the promotion of cell growth and induction of angiogenesis in support of B-cell and endothelial neoplasia, parallel the hypothesized role of hIL-6 in MCD and KS [3,4,6,7]. Indeed, vIL-6 has been shown to be important for PEL cell growth in culture and vIL-6-inducible VEGF is important for the growth and dissemination of PEL in inoculated mice [41,52–54].
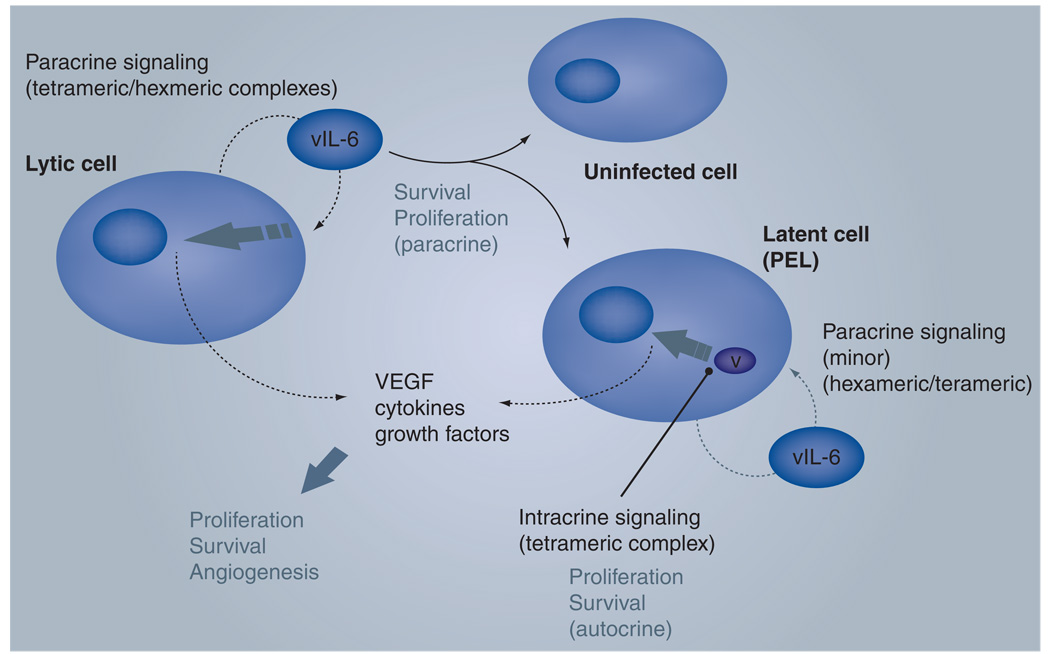
While it is likely that vIL-6 produced and released from lytically infected cells can function in a paracrine fashion to contribute to HHV-8-associated neoplasia, it is important to note that vIL-6 can also be produced during latency, at least in PEL cells. Intracellular, strictly autocrine signaling by latently expressed vIL-6 contributes significantly to PEL cell proliferation and survival via endoplasmic reticulum-localized tetrameric (gp80-devoid) signaling complexes [23]. Thus, the inefficient secretion of vIL-6, noted initially by Meads and Medveczky [43], may be important for restricting vIL-6 activity largely to HHV-8-infected cells during latency, providing them with a growth and survival advantage that may contribute to PEL disease, in addition to the maintenance of latent viral pools in the host. It is also possible that such intracrine vIL-6 activity contributes to the high levels of active STAT3, known to be important for PEL cell survival and implicated in many human cancers [55–58]. Cellular cytokines, such as hIL-6 and VEGF, induced by both intracrine and paracrine signaling by vIL-6 are highly likely to contribute to HHV-8 neoplasia. In the case of latently expressed vIL-6, there would be no restrictions on host gene expression imposed by lytic host shut-off mechanisms [59,60]. Potential mechanisms of vIL-6 involvement in HHV-8 neoplasia are illustrated in Figure 1.
Figure 1. Postulated activities of viral IL-6.
The viral cytokine is expressed predominantly during lytic replication, presumably produced in quantities to allow for an accumulation of significant, biologically active concentrations extracellularly and to be able to mediate paracrine signaling. One consequence is the induction of cellular cytokines from infected and uninfected cells alike; these cytokines can contribute, along with vIL-6, to pathogenesis, for example by promoting cell proliferation and survival in addition to angiogenesis. Both intracrine and cell-surface signaling can occur in lytically infected cells (latter indicated); autocrine signaling by either route may contribute directly to lytic replication. During latency, vIL-6 is expressed at low levels (detected in primary effusion lymphoma cells) and intracrine signal transduction is likely to predominate. This is of demonstrated importance for PELs cell growth; contributions of paracrine signaling in this setting are uncertain but not required. As for paracrine signaling, intracellular activity of vIL-6 would be predicted to induce the expression of pathogenically relevant cellular cytokines.
PEL: Primary effusion lymphoma; vIL: Viral IL.
HHV-8 chemokines
The viral chemokines were discovered as a result of directed and complete sequencing of the HHV-8 genome [11,12,15,16]. The open reading frames were ultimately named K2, K4 and K4.1, encoding vCCL-2, -1 and -3, respectively (previously referred to as vMIP-1B/vMIP-II, vMIP-1A/vMIP-I and vBCK/vMIP-III). While clearly related to cellular CC-chemokines, the viral proteins show limited sequence similarity to their cellular counterparts and direct orthologs are difficult to discern with confidence. vCCL-1 and -2 have the closest amino acid sequence similarity to both CCL-3/ MIP-1α and CCL-4/MIP-1β, while vCCL-3 is related to CCL-2/monocyte chemotactic protein-1 and various other cellular chemokines. Owing to their agonistic binding to Th2-expressed chemokine receptors, it has been proposed that all three chemokines function to antagonize antiviral Th1 (cytotoxic T lymphocyte-mediated) immune responses. In addition, the binding of vCCL-2 as a neutral ligand and cellular chemokine antagonist to a variety of other cellular chemokine receptors has implicated this particular viral chemokine in immune evasion via the blocking of normal chemokine-mediated responses to viral infection. Apart from the likely functions of viral chemokines in immune evasion through these activities, vCCL-1 and -2 also appear to function directly, in an autocrine manner, on the cells in which they are expressed, to prolong cell survival in the face of lytic cycle-induced proapoptotic signals and, therefore, to enhance productive replication of the virus. Prosurvival activity of this sort clearly could contribute in a paracrine manner (as the chemokines are expressed exclusively during lytic replication) to HHV-8 pathogenesis. The properties and activities of the viral chemokines and their postulated roles in viral biology and pathogenesis are discussed in the following sections.
Receptor recognition
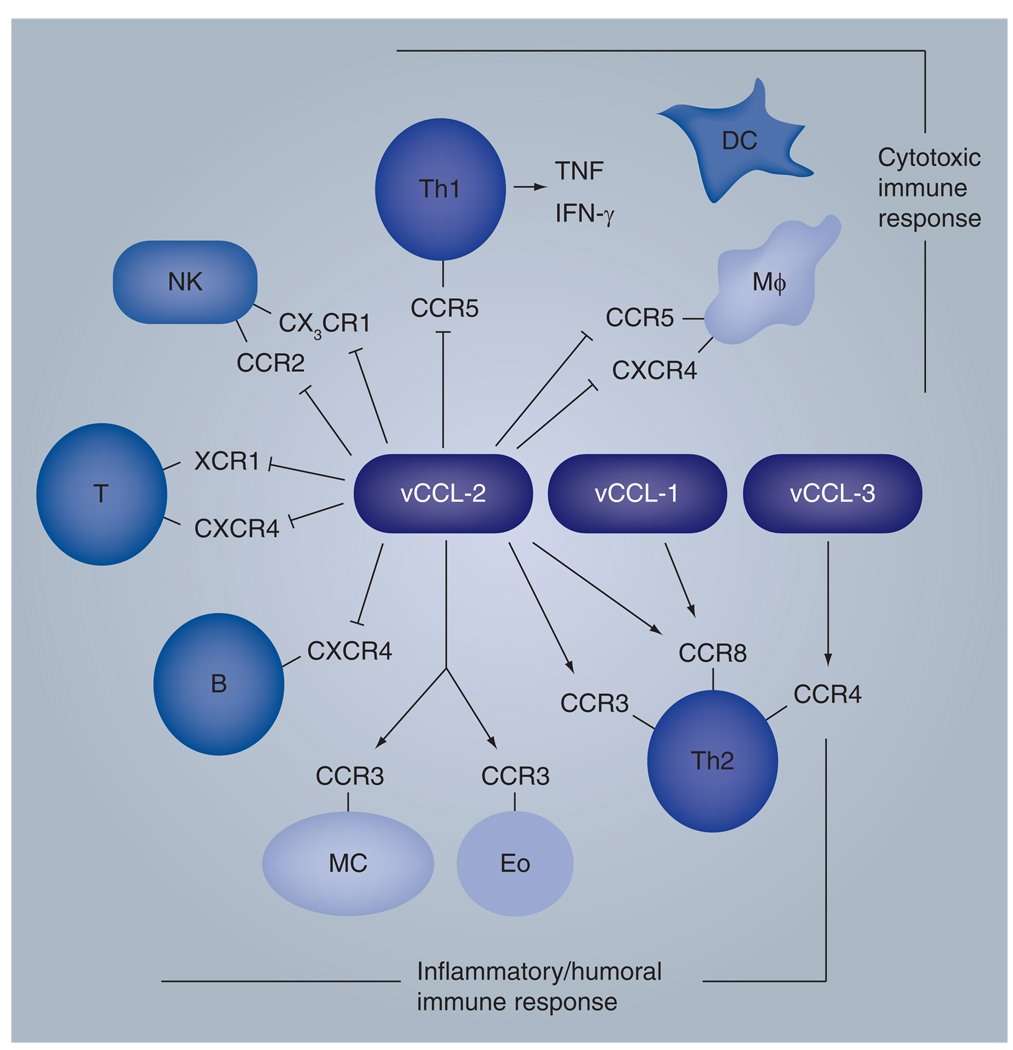
Once the HHV-8 chemokines were identified, researchers focused on identifying the targeted receptors. vCCL-1 and -2 were both shown to target CC chemokine receptor (CCR)8 to mediate signaling, with vCCL-2 also reported to bind to and activate CCR3, and vCCL-3 identified as an agonist for CCR4 [17,18,61–63]. vCCL-2 also targets the HHV-8 chemokine receptor, viral G-protein-coupled receptor, to inhibit its constitutive (ligand-independent) activity [64]. More recently, vCCL-2 and-3 agonistic targeting of XCR1 has been reported, in addition to productive interactions of vCCL-1 and -2 with CCR5 [65–67]. The findings for vCCL-2 are discrepant with previous reports of neutral (nonsignaling) binding of XCR1 and CCR5 by vCCL-2 and functionally antagonistic interactions of vCCL-2 with CCR5 and XCR1 agonists [68–70]; the reasons for these discrepancies are uncertain. vCCL-2 has been reported to bind to a variety of other receptors as a neutral ligand, therefore having the ability to block the actions of cellular chemokines recognizing these same receptors; the receptors include CCR1, CCR2, CCR10, CXCR4 and CX3CR1 [68,69,71,72]. As discussed later, the receptor binding patterns exhibited by the chemokines, as agonists or antagonists, together with their lytic expression kinetics, suggests that they may play an important role in immune evasion (Figure 2).
Figure 2. Properties of human herpesvirus 8 chemokines.
The viral chemokines are produced during lytic replication and are presumed to contribute positively to virus replication both directly via autocrine activities and indirectly via modulation of the immune system. Autocrine functions appear to include prosurvival signaling to enhance human herpesvirus 8 replication, as determined in cultured endothelial cells (see text for details). Immune modulation is hypothesized based on agonistic (signaling) and antagonistic (competitive) interactions with specific cellular chemokine receptors, as indicated. In general, chemokine agonistic interactions mediate Th2 polarization to inhibit antiviral Th1 responses, while neutral binding of several receptors by vCCL-2 prevents immune activities mediated by cellular chemokines recognizing these receptors. The receptors include CCR2, CXCR4, XCR1 and CX3CR1 expressed on NK, T and B cells. Recently, vCCL-1 and -2 have been reported to be CCR5 agonists, in contrast to previous findings of vCCL-2 antagonist activity on CCR5 (indicated in figure), and monocyte recruitment is reported to be mediated by vCCL-1 and -2 [67]. In addition, vCCL-3 has been reported to activate XCR1 (not indicated in figure) [68]. Monocyte recruitment by vCCL-1 and -2 may enhance virus dissemination in the host, as monocytes are known to be infected by human herpesvirus 8 in vivo.
CCR: CC-chemokine receptor; CXCR: CXC-chemokine receptor; DC: Dendritic cell; Eo: Eosinophil; Mφ: Macrophage; MC: Monocytic cell; NK: Natural killer; vCCL: Viral CC/β-chemokine ligand; XCR: XC-chemokine receptor.
Potential roles of the viral chemokines in virus biology
All three of the viral chemokines are agonists for receptors that are expressed on Th2 cells and, therefore, activate the humoral arm of the immune response rather than Th1 responses that are involved in mediating a cytolytic attack of infected cells by the host. In consideration of such functions, it is worth noting that vCCL-2 has been demonstrated, under flow culture conditions, to mediate firm arrest on activated endothelial cells of Th2 cells (CCR3+/8+), via agonistic activities, while blocking CCL5-mediated firm arrest and transmigration of Th1 cells (CCR1+/5+) through antagonistic, neutral receptor binding [73]. Furthermore, in vivo experiments using animal models have demonstrated effective blocking of immune cytotoxicity by vCCL-2 and inhibition of CCL-5-mediated recruitment of CCR1+/5+ Th1 cells [70,71]. In addition, KS tissues expressing vCCL-2 have been shown to display Th2 polarization, as evidenced by a preponderance of CCR3+ (Th2) over CCR5+/CXCR3+ (Th1) leukocytes [73]. Clearly, the ability of vCCL-2 to act as a neutral ligand and antagonist and target several chemokine receptors expressed on natural killer cells, Th1 and other T cells indicates that the viral chemokine may act generally to inhibit T-cell-mediated immunity. Such activities of vCCL-2 together with Th2 polarization by vCCL-1, -2 and -3 would be envisioned to enhance productive replication of the virus and help prevent viral clearance during de novo infection of the host (Figure 2).
It is likely that viral chemokines have other functions in virus biology. It has been reported, for example, that vCCL-1 and -2 are able to promote chemotaxis and recruitment of monocytic cells (THP-1) in vitro [67]. As monocytes are infected in vivo by HHV-8, these findings indicate that viral chemokines may promote the recruitment of virus-permissive cells into sites of ongoing productive replication and thereby facilitate viral amplification and dissemination. Recruitment of permissive cells and spread of virus via infected cells may also be promoted by virtue of the proangiogenic properties of all three chemokines [17,18]. In this model, increased vascular permeability at sites of viral chemokine production (during lytic replication) would enhance cellular migration into sites of productive replication and exit and dissemination of newly infected cells from these sites. A distinct mechanism by which viral chemokines may contribute to virus biology is through direct autocrine influence on the cells in which they are expressed, during the virus lytic replication cycle. This hypothesis is based on the recognition that vCCL-1 and -2 function to protect endothelial cells from stress-induced apoptosis, such as that which occurs in lytically infected cells, and are required for optimal HHV-8 replication in endothelial cells in culture [22]. Such survival signaling and proreplication activity is mediated via CCR8, upon which viral chemokine signaling in endothelial cells is dependent. CCR8 or vCCL-1/-2 depletion was found to lead to reduced viral titers in endothelial cell-based reactivation experiments [22].
Potential contributions of vCCLs to pathogenesis
With regard to possible contributions of the viral chemokines to pathogenesis, it is of major importance that they are each able to promote angiogenesis [17,18]. Presumably this property is mediated via the induction of cellular angiogenic cytokines, such as VEGF, which has been shown to be induced by vCCL-1 in PEL cells [74]. VEGF, while likely to be relevent in KS, is also a probable contributor to PEL disease, having been demonstrated to promote growth and dissemination of PEL cells in inoculated mice [53]. vCCL-induced cytokines have the potential to influence pathogenesis via their effects on cells surrounding those undergoing lytic replication, for example by promoting cell proliferation, survival and inflammatory responses. However, as mentioned previously, vCCL-1 and -2, at least, have the ability to mediate survival signaling directly, and it is conceivable that this activity, demonstrated in endothelial cells [22], could contribute to KS. Unlike other anti-apoptotic proteins of HHV-8 [75,76], the viral chemokines can mediate their prosurvival activities via paracrine mechanisms, exerting their influence on latently infected and other cells neighboring the lytically infected cells from which the viral chemokines are secreted. In this way, their production in a minority of cells undergoing lytic replication can support pathogenesis mediated primarily by latently infected cells (the majority) in the population. This provides a means of latent–lytic cooperation in HHV-8-associated neoplasia, a concept that is widely accepted for KS, but which also may apply to PEL and MCD, in which VEGF, IL-6 and other activities may be relevant [5,7,52,53]. Another contributory factor in KS again involves vCCL-1 and -2 via CCR8-mediated signaling, namely the noted role of CCR8 in inducing vascular smooth muscle reorganization and activation of angiogenic metalloproteinase-2 [77]. Thus, the viral chemokines may promote HHV-8 neoplasia via their angiogenic activities, their induction of various cytokines that influence cell morphology and growth, and direct prosurvival activities.
Future perspective
The IL-6 and chemokine homologs encoded by HHV-8 provided the first examples of a viral IL-6 homolog and γ-herpesvirus-specified chemokines. This, together with the associated proangiogenic activities and consequent implications for potential roles in HHV-8-associated neoplasia, led to great interest in these viral proteins. While it is difficult to determine with precision the actual influences of these proteins in virus-associated pathogenesis, their properties, as outlined in this article, provide support for the hypothesis that they are involved. Not only do they promote angiogenesis, a key feature of KS and a likely contributor to PEL and MCD, but vIL-6 also promotes B-cell growth and vIL-6, vCCL-1 and -2 mediate prosurvival signaling in B lymphocytes and endothelial cells, which is likely to be of significance in HHV-8 neoplasia affecting these cell types. While paracrine signaling is key for contributions of the viral cytokines to HHV-8 neoplasia, an important and newly identified property of vIL-6 is that it can be expressed as a bone fide latency protein (at low but biologically active levels) and that intracellular signaling by the viral cytokine is important for PEL cell growth, promoting both proliferation and survival. Thus, future therapeutic strategies targeting vIL-6 could be useful for attacking latently infected cells, as well as productively infected cells, but will require methods (e.g., RNAi-based methodologies) to inhibit vIL-6 functions intracellularly.
Ideas regarding the roles of the vIL-6 and the viral chemokines in virus biology have essentially the same experimental basis as do hypotheses regarding the roles of the viral cytokines in virus-associated neoplasia. Thus, proangiogenic and prosurvival properties of the cytokines, identified in culture and in some in vivo experimental systems, point to functions that promote virus productive replication (all viral cytokines) or maintain latent viral pools (vIL-6). The proangiogenic activities are predicted to enhance the recruitment of HHV-8-permissive cells and the spread of virus infection; the prosurvival functions to increase virus yield per cell, and proproliferative and prosurvival activity of vIL-6 expressed during latency (in PEL cells at least) to help maintain latent virus load in the host. The finding that vCCL-1 and -2 act in an autocrine manner to promote endothelial cell survival and virus production during lytic replication is a novel and unexpected finding, demonstrating that the viral chemokines can act entirely independently of immune regulatory mechanisms to impact virus biology. It is possible that vIL-6 also functions in this manner, but supporting evidence is so far lacking. The likely importance of cell-surface autocrine and paracrine signaling by the viral cytokines in promotion of lytic replication suggests that the development of strategies that extracellularly target and inactivate the viral cytokines may be particularly effective in blocking virus production and, thereby, inhibiting likely contributions of lytic replication to HHV-8-associated disease, in particular KS.
Future research on the biological consequences of viral chemokine signaling in cell types infected with HHV-8 (B cells, monocytes and endothelial cells), in addition to the influence of vIL-6 on virus lytic gene expression and virus productive replication, are warranted. These avenues are likely to yield novel information regarding the functions of these cytokines in virus biology and to provide new avenues to antiviral therapy.
Human herpesvirus 8 & pathogenesis
Human herpesvirus (HHV)-8 is associated with Kaposi’s sarcoma, an endothelial tumor, and two B-cell malignancies: primary effusion lymphoma (PEL) and multicentric Castleman’s disease.
Angiogenic and proinflammatory cytokines contribute to Kaposi’s sarcoma development and may also play roles in PEL and multicentric Castleman’s disease.
HHV-8 cytokines
HHV-8 cytokines comprise viral IL-6 (vIL-6) and three CC-chemokines (vCCL-1, -2 and -3). All four are angiogenic.
All HHV-8 cytokines are expressed during lytic (productive) replication; vIL-6 can be expressed at low, functional levels during latency in PEL.
Viral cytokines have the potential to contribute to HHV-8 pathogenesis via paracrine mechanisms; vIL-6 may also do so by autocrine signaling during latency.
Viral IL-6
vIL-6 signals via the gp130 signal transducer and does not require the gp80 subunit of the IL-6 receptor.
vIL-6 can signal intracellularly from the endoplasmic reticulum and this is important for PEL cell growth.
Their role in HHV-8 replication is unknown.
Viral chemokines
Chemokine receptor targeting (as agonists) suggests roles in immune evasion via the suppression of Th1 responses.
vCCL-2 is a broadly acting antagonist of cellular chemokines and is likely to mediate immune evasion via this route.
vCCL-1 and -2 support endothelial cell survival, contributing to HHV-8 replication efficiency and, potentially, to pathogeneis.
Acknowledgements
The author would like to thank his laboratory colleagues G Sandford, D Chen, Y Bong Choi and K Karen for their support.
Footnotes
Financial & competing interests disclosure
Research undertaken in J Nicholas’ laboratory is supported by the National Cancer Institute, USA, under grants CA76445, CA119887, CA136356 and CA13239. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. ▪ First identification of human herpesvirus (HHV)-8 sequences and recognition of a new human γ-herpesvirus.
- 2.Soulier J, Grollet L, Oksenhendler E, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 3.Burger R, Wendler J, Antoni K, Helm G, Kalden JR, Gramatzki M. Interleukin-6 production in B-cell neoplasias and Castleman’s disease: evidence for an additional paracrine loop. Ann. Hematol. 1994;69:25–31. doi: 10.1007/BF01757344. [DOI] [PubMed] [Google Scholar]
- 4.Hsu SM, Waldron JA, Xie SS, Barlogie B. Expression of interleukin-6 in Castleman’s disease. Hum. Pathol. 1993;24:833–839. doi: 10.1016/0046-8177(93)90132-z. [DOI] [PubMed] [Google Scholar]
- 5.Ishiyama T, Nakamura S, Akimoto Y, et al. Immunodeficiency and IL-6 production by peripheral blood monocytes in multicentric Castleman’s disease. Br. J. Haematol. 1994;86:483–489. doi: 10.1111/j.1365-2141.1994.tb04777.x. [DOI] [PubMed] [Google Scholar]
- 6.Miles SA, Rezai AR, Salazar-Gonzalez JF, et al. AIDS Kaposi sarcoma-derived cells produce and respond to interleukin 6. Proc. Natl Acad. Sci. USA. 1990;87:4068–4072. doi: 10.1073/pnas.87.11.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshizaki K, Matsuda T, Nishimoto N, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman’s disease. Blood. 1989;74:1360–1367. [PubMed] [Google Scholar]
- 8.Arvanitakis L, Mesri EA, Nador RG, et al. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein–Barr virus. Blood. 1996;88:2648–2654. [PubMed] [Google Scholar]
- 9.Carbone A, Gloghini A, Vaccher E, et al. Kaposi’s sarcoma-associated herpesvirus DNA sequences in AIDS-related and AIDS-unrelated lymphomatous effusions. Br. J. Haematol. 1996;94:533–543. doi: 10.1046/j.1365-2141.1996.d01-1826.x. [DOI] [PubMed] [Google Scholar]
- 10.Gaidano G, Cechova K, Chang Y, Moore PS, Knowles DM, Dalla-Favera R. Establishment of AIDS-related lymphoma cell lines from lymphomatous effusions. Leukemia. 1996;10:1237–1240. [PubMed] [Google Scholar]
- 11.Nicholas J, Ruvolo VR, Burns WH, et al. Kaposi’s sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat. Med. 1997;3:287–292. doi: 10.1038/nm0397-287. [DOI] [PubMed] [Google Scholar]
- 12.Moore PS, Boshoff C, Weiss RA, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274:1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 13.Neipel F, Albrecht JC, Ensser A, et al. Human herpesvirus 8 encodes a homolog of interleukin-6. J. Virol. 1997;71:839–842. doi: 10.1128/jvi.71.1.839-842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neipel F, Albrecht JC, Fleckenstein B. Cell-homologous genes in the Kaposi’s sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J. Virol. 1997;71:4187–4192. doi: 10.1128/jvi.71.6.4187-4192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicholas J, Ruvolo V, Zong J, et al. A single 13-kilobase divergent locus in the Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genome contains nine open reading frames that are homologous to or related to cellular proteins. J. Virol. 1997;71:1963–1974. doi: 10.1128/jvi.71.3.1963-1974.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russo JJ, Bohenzky RA, Chien MC, et al. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc. Natl Acad. Sci. USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boshoff C, Endo Y, Collins PD, et al. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278:290–294. doi: 10.1126/science.278.5336.290. ▪ First report of angiogenic activities of HHV-8 chemokines, shown for viral CC-chemokine ligand (vCCL)-1 (viral macrophage inflammatory protein [vMIP]-I) and vCCL-2 (vMIP-II).
- 18.Stine JT, Wood C, Hill M, et al. KSHV-encoded CC chemokine vMIP-III is a CCR4 agonist, stimulates angiogenesis, and selectively chemoattracts TH2 cells. Blood. 2000;95:1151–1157. [PubMed] [Google Scholar]
- 19.Krishnan HH, Naranatt PP, Smith MS, Zeng L, Bloomer C, Chandran B. Concurrent expression of latent and a limited number of lytic genes with immune modulation and anti-apoptotic function by Kaposi’s sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J. Virol. 2004;78:3601–3620. doi: 10.1128/JVI.78.7.3601-3620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenner RG, Alba MM, Boshoff C, Kellam P. Kaposi’s sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J. Virol. 2001;75:891–902. doi: 10.1128/JVI.75.2.891-902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulose-Murphy M, Ha NK, Xiang C, et al. Transcription program of human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus) J. Virol. 2001;75:4843–4853. doi: 10.1128/JVI.75.10.4843-4853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choi YB, Nicholas J. Autocrine and paracrine promotion of cell survival and virus replication by human herpesvirus 8 chemokines. J. Virol. 2008;82:6501–6513. doi: 10.1128/JVI.02396-07. ▪ Evidence of direct (autocrine) proreplication functions of endogenously produced viral chemokines (vCCL-1 and -2), independent of hypothesized influences on the immune system. The authors also report anti-apoptotic activities of the viral chemokines.
- 23. Chen D, Sandford G, Nicholas J. Intracellular signaling mechanisms and activities of human herpesvirus 8 interleukin-6. J. Virol. 2009;83:722–733. doi: 10.1128/JVI.01517-08. ▪ Identifies viral IL(vIL)-6 expression and function via intracellular signaling in latently infected primary effusion lymphoma cells.
- 24. Chow D, He X, Snow AL, Rose-John S, Garcia KC. Structure of an extracellular gp130 cytokine receptor signaling complex. Science. 2001;291:2150–2155. doi: 10.1126/science.1058308. ▪ First crystal structure of a ligand–gp130 complex, the vIL-62–gp1302 tetramer.
- 25. Boulanger MJ, Chow DC, Brevnova EE, Garcia KC. Hexameric structure and assembly of the interleukin-6/IL-6 α-receptor/gp130 complex. Science. 2003;300:2101–2104. doi: 10.1126/science.1083901. ▪ First crystal structure of a hexameric gp130 complex, human IL-62–gp1302–gp802.
- 26.Hideshima T, Chauhan D, Teoh G, et al. Characterization of signaling cascades triggered by human interleukin-6 versus Kaposi’s sarcoma-associated herpes virus-encoded viral interleukin 6. Clin. Cancer Res. 2000;6:1180–1189. [PubMed] [Google Scholar]
- 27.Wan X, Wang H, Nicholas J. Human herpesvirus 8 interleukin-6 (vIL-6) signals through gp130 but has structural and receptor-binding properties distinct from those of human IL-6. J. Virol. 1999;73:8268–8278. doi: 10.1128/jvi.73.10.8268-8278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Molden J, Chang Y, You Y, Moore PS, Goldsmith MA. A Kaposi’s sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J. Biol. Chem. 1997;272:19625–19631. doi: 10.1074/jbc.272.31.19625. ▪ Demonstrates that vIL-6, in contrast to cellular IL-6 proteins, can signal via gp130 in the absence of the α-receptor subunit, gp80.
- 29.Osborne J, Moore PS, Chang Y. KSHV-encoded viral IL-6 activates multiple human IL-6 signaling pathways. Hum. Immunol. 1999;60:921–927. doi: 10.1016/s0198-8859(99)00083-x. [DOI] [PubMed] [Google Scholar]
- 30.Boulanger MJ, Chow DC, Brevnova E, et al. Molecular mechanisms for viral mimicry of a human cytokine: activation of gp130 by HHV-8 interleukin-6. J. Mol. Biol. 2004;335:641–654. doi: 10.1016/j.jmb.2003.10.070. [DOI] [PubMed] [Google Scholar]
- 31. Hu F, Nicholas J. Signal transduction by human herpesvirus 8 viral interleukin-6 (vIL-6) is modulated by the nonsignaling gp80 subunit of the IL-6 receptor complex and is distinct from signaling induced by human IL-6. J. Virol. 2006;80:10874–10878. doi: 10.1128/JVI.00767-06. ▪ Data are presented revealing the influence of gp80 on vIL-6 signal transduction and demonstrating signaling differences between vIL-6 and human IL-6.
- 32.Li H, Nicholas J. Identification of amino acid residues of gp130 signal transducer and gp80 α receptor subunit that are involved in ligand binding and signaling by human herpesvirus 8-encoded interleukin-6. J. Virol. 2002;76:5627–5636. doi: 10.1128/JVI.76.11.5627-5636.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen D, Nicholas J. Structural requirements for gp80 independence of human herpesvirus 8 interleukin-6 (vIL-6) and evidence for gp80 stabilization of gp130 signaling complexes induced by vIL-6. J. Virol. 2006;80:9811–9821. doi: 10.1128/JVI.00872-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adam N, Rabe B, Suthaus J, Grotzinger J, Rose-John S, Scheller J. Unraveling viral interleukin-6 binding to gp130 and activation of STAT-signaling pathways independently of the interleukin-6 receptor. J. Virol. 2009;83:5117–5126. doi: 10.1128/JVI.01601-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dela Cruz CS, Viswanathan SR, El-Guindy AS, Shedd D, Miller G. Complex N-linked glycans on Asn-89 of Kaposi sarcoma herpes virus-encoded interleukin-6 mediate optimal function by affecting cytokine protein conformation. J. Biol. Chem. 2009;284:29269–29282. doi: 10.1074/jbc.M109.039115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen D, Choi YB, Sandford G, Nicholas J. Determinants of secretion and intracellular localization of human herpesvirus 8 interleukin-6. J. Virol. 2009;83(13):6874–6882. doi: 10.1128/JVI.02625-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parekh RB, Dwek RA, Rademacher TW, Opdenakker G, Van Damme J. Glycosylation of interleukin-6 purified from normal human blood mononuclear cells. Eur. J. Biochem. 1992;203:135–141. doi: 10.1111/j.1432-1033.1992.tb19838.x. [DOI] [PubMed] [Google Scholar]
- 38.Santhanam U, Ghrayeb J, Sehgal PB, May LT. Post-translational modifications of human interleukin-6. Arch. Biochem. Biophys. 1989;274:161–170. doi: 10.1016/0003-9861(89)90427-x. [DOI] [PubMed] [Google Scholar]
- 39.Burger R, Neipel F, Fleckenstein B, et al. Human herpesvirus type 8 interleukin-6 homologue is functionally active on human myeloma cells. Blood. 1998;91:1858–1863. [PubMed] [Google Scholar]
- 40.Hirano T, Nakajima K, Hibi M. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev. 1997;8:241–252. doi: 10.1016/s1359-6101(98)80005-1. [DOI] [PubMed] [Google Scholar]
- 41. Jones KD, Aoki Y, Chang Y, Moore PS, Yarchoan R, Tosato G. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi’s sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood. 1999;94:2871–2879. ▪ First report of the influence of vIL-6 on primary effusion lymphoma cell growth.
- 42.Mori Y, Nishimoto N, Ohno M, et al. Human herpesvirus 8-encoded interleukin-6 homologue (viral IL-6) induces endogenous human IL-6 secretion. J. Med. Virol. 2000;61:332–335. doi: 10.1002/1096-9071(200007)61:3<332::aid-jmv8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 43. Meads MB, Medveczky PG. Kaposi’s sarcoma-associated herpesvirus-encoded viral interleukin-6 is secreted and modified differently than human interleukin-6: evidence for a unique autocrine signaling mechanism. J. Biol. Chem. 2004;279:51793–51803. doi: 10.1074/jbc.M407382200. ▪ Documents the slow secretion kinetics of vIL-6 and demonstrates that vIL-6 can activate gp130 intracellularly. Data presented here also characterize glycosylation of vIL-6 and indicate that its signal sequence remains uncleaved.
- 44.Chatterjee M, Osborne J, Bestetti G, Chang Y, Moore PS. Viral IL-6-induced cell proliferation and immune evasion of interferon activity. Science. 2002;298:1432–1435. doi: 10.1126/science.1074883. [DOI] [PubMed] [Google Scholar]
- 45.Asou H, Said JW, Yang R, et al. Mechanisms of growth control of Kaposi’s sarcoma-associated herpes virus-associated primary effusion lymphoma cells. Blood. 1998;91:2475–2481. [PubMed] [Google Scholar]
- 46. Chen L, Lagunoff M. The KSHV viral interleukin-6 is not essential for latency or lytic replication in BJAB cells. Virology. 2007;359:425–435. doi: 10.1016/j.virol.2006.09.044. ▪ Only paper to date to investigate the role of vIL-6 in virus biology. An engineered, vIL-6-null virus was used in cell culture assays; no influence of vIL-6 deletion on establishment of latency or lytic gene expression during reactivation was identified in BJAB nor on virus production in Vero cells.
- 47.Dittmer DP, Gonzalez CM, Vahrson W, DeWire SM, Hines-Boykin R, Damania B. Whole-genome transcription profiling of rhesus monkey rhadinovirus. J. Virol. 2005;79:8637–8650. doi: 10.1128/JVI.79.13.8637-8650.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Connor CM, Kedes DH. Rhesus monkey rhadinovirus: a model for the study of KSHV. Curr. Top. Microbiol. Immunol. 2007;312:43–69. doi: 10.1007/978-3-540-34344-8_2. [DOI] [PubMed] [Google Scholar]
- 49.Orzechowska BU, Powers MF, Sprague J, et al. Rhesus macaque rhadinovirus-associated non-Hodgkin lymphoma: animal model for KSHV-associated malignancies. Blood. 2008;112:4227–4234. doi: 10.1182/blood-2008-04-151498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu M, Suen J, Frias C, et al. Dissection of the Kaposi’s sarcoma-associated herpesvirus gene expression program by using the viral DNA replication inhibitor cidofovir. J. Virol. 2004;78:13637–13652. doi: 10.1128/JVI.78.24.13637-13652.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun R, Lin SF, Staskus K, et al. Kinetics of Kaposi’s sarcoma-associated herpesvirus gene expression. J. Virol. 1999;73:2232–2242. doi: 10.1128/jvi.73.3.2232-2242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aoki Y, Jaffe ES, Chang Y, et al. Angiogenesis and hematopoiesis induced by Kaposi’s sarcoma-associated herpesvirus-encoded interleukin-6. Blood. 1999;93:4034–4043. ▪ Reports VEGF-inducing, angiogenic and tumorigenic activities of vIL-6.
- 53. Aoki Y, Tosato G. Role of vascular endothelial growth factor/vascular permeability factor in the pathogenesis of Kaposi’s sarcoma-associated herpesvirus-infected primary effusion lymphomas. Blood. 1999;94:4247–4254. ▪ Demonstrates that VEGF is an important factor for primary effusion lymphoma cell growth and dissemination in a xenograft (murine) model.
- 54.Zhang YJ, Bonaparte RS, Patel D, Stein DA, Iversen PL. Blockade of viral interleukin-6 expression of Kaposi’s sarcoma-associated herpesvirus. Mol. Cancer Ther. 2008;7:712–720. doi: 10.1158/1535-7163.MCT-07-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–1542. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- 56.Benekli M, Baer MR, Baumann H, Wetzler M. Signal transducer and activator of transcription proteins in leukemias. Blood. 2003;101:2940–2954. doi: 10.1182/blood-2002-04-1204. [DOI] [PubMed] [Google Scholar]
- 57.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 58.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur. J. Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 59.Glaunsinger B, Ganem D. Highly selective escape from KSHV-mediated host mRNA shutoff and its implications for viral pathogenesis. J. Exp. Med. 2004;(200):391–398. doi: 10.1084/jem.20031881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Glaunsinger B, Ganem D. Lytic KSHV infection inhibits host gene expression by accelerating global mRNA turnover. Mol. Cell. 2004;13:713–723. doi: 10.1016/s1097-2765(04)00091-7. ▪ Demonstrates that host protein expression is inhibited during HHV-8 lytic replication, emphasizing the relevance of this phenomenon for models of viral pathogenesis via lytic protein-induced cellular cytokines.
- 61.Dairaghi DJ, Fan RA, McMaster BE, Hanley MR, Schall TJ. HHV8-encoded vMIP-I selectively engages chemokine receptor CCR8. Agonist and antagonist profiles of viral chemokines. J. Biol. Chem. 1999;274:21569–21574. doi: 10.1074/jbc.274.31.21569. [DOI] [PubMed] [Google Scholar]
- 62.Endres MJ, Garlisi CG, Xiao H, Shan L, Hedrick JA. The Kaposi’s sarcoma-related herpesvirus (KSHV)-encoded chemokine vMIP-I is a specific agonist for the CC chemokine receptor (CCR)8. J. Exp. Med. 1999;189:1993–1998. doi: 10.1084/jem.189.12.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sozzani S, Luini W, Bianchi G, et al. The viral chemokine macrophage inflammatory protein-II is a selective Th2 chemoattractant. Blood. 1998;92:4036–4039. ▪ Demonstrates Th2 selectivity of an HHV-8 chemokine, suggesting that vCCL-2 (vMIP-II), and potentially other HHV-8 viral chemokines, can contribute to immune evasion via polarization away from antiviral Th1 responses.
- 64.Geras-Raaka E, Varma A, Clark-Lewis I, Gershengorn MC. Kaposi’s sarcoma-associated herpesvirus (KSHV) chemokine vMIP-II and human SDF-1 α inhibit signaling by KSHV G protein-coupled receptor. Biochem. Biophys. Res. Commun. 1998;253:725–727. doi: 10.1006/bbrc.1998.9557. [DOI] [PubMed] [Google Scholar]
- 65.Luttichau HR. The herpesvirus 8 encoded chemokines vCCL2 (vMIP-II) and vCCL3 (vMIP-III) target the human but not the murine lymphotactin receptor. Virol. J. 2008;5:50. doi: 10.1186/1743-422X-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luttichau HR, Johnsen AH, Jurlander J, Rosenkilde MM, Schwartz TW. Kaposi sarcoma-associated herpes virus targets the lymphotactin receptor with both a broad spectrum antagonist vCCL2 and a highly selective and potent agonist vCCL3. J. Biol. Chem. 2007;282:17794–17805. doi: 10.1074/jbc.M702001200. [DOI] [PubMed] [Google Scholar]
- 67.Nakano K, Isegawa Y, Zou P, Tadagaki K, Inagi R, Yamanishi K. Kaposi’s sarcoma-associated herpesvirus (KSHV)-encoded vMIP-I and vMIP-II induce signal transduction and chemotaxis in monocytic cells. Arch. Virol. 2003;148:871–890. doi: 10.1007/s00705-002-0971-7. [DOI] [PubMed] [Google Scholar]
- 68.Shan L, Qiao X, Oldham E, et al. Identification of viral macrophage inflammatory protein (vMIP)-II as a ligand for GPR5/XCR1. Biochem. Biophys. Res. Commun. 2000;268:938–941. doi: 10.1006/bbrc.2000.2235. [DOI] [PubMed] [Google Scholar]
- 69. Kledal TN, Rosenkilde MM, Coulin F, et al. A broad-spectrum chemokine antagonist encoded by Kaposi’s sarcoma-associated herpesvirus. Science. 1997;277:1656–1659. doi: 10.1126/science.277.5332.1656. ▪ First report of broad antagonistic activity of vCCL-2 via neutral ligand binding to a variety of chemokine receptors.
- 70.Rubant S, Ludwig RJ, Pfeffer J, et al. Eukaryotic expression of the broad-spectrum chemokine receptor antagonist vMIP-II and its effects on T-cell function in vitro and in vivo. Exp. Dermatol. 2006;15:634–642. doi: 10.1111/j.1600-0625.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 71.Chen S, Bacon KB, Li L, et al. In vivo inhibition of CC and CX3C chemokine-induced leukocyte infiltration and attenuation of glomerulonephritis in Wistar-Kyoto (WKY) rats by vMIP-II. J. Exp. Med. 1998;188:193–198. doi: 10.1084/jem.188.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luttichau HR, Lewis IC, Gerstoft J, Schwartz TW. The herpesvirus 8-encoded chemokine vMIP-II, but not the poxvirus-encoded chemokine MC148, inhibits the CCR10 receptor. Eur. J. Immunol. 2001;31:1217–1220. doi: 10.1002/1521-4141(200104)31:4<1217::aid-immu1217>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 73.Weber KS, Grone HJ, Rocken M, et al. Selective recruitment of Th2-type cells and evasion from a cytotoxic immune response mediated by viral macrophage inhibitory protein-II. Eur. J. Immunol. 2001;31:2458–2466. doi: 10.1002/1521-4141(200108)31:8<2458::aid-immu2458>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 74.Liu C, Okruzhnov Y, Li H, Nicholas J. Human herpesvirus 8 (HHV-8)-encoded cytokines induce expression of and autocrine signaling by vascular endothelial growth factor (VEGF) in HHV-8-infected primary-effusion lymphoma cell lines and mediate VEGF-independent anti-apoptotic effects. J. Virol. 2001;75:10933–10940. doi: 10.1128/JVI.75.22.10933-10940.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feng P, Scott C, Lee SH, Cho NH, Jung JU. Manipulation of apoptosis by herpes viruses (Kaposi’s sarcoma pathogenesis) Prog. Mol. Subcell. Biol. 2004;36:191–205. doi: 10.1007/978-3-540-74264-7_10. [DOI] [PubMed] [Google Scholar]
- 76.Tschopp J, Thome M, Hofmann K, Meinl E. The fight of viruses against apoptosis. Curr. Opin. Genet. Dev. 1998;8:82–87. doi: 10.1016/s0959-437x(98)80066-x. [DOI] [PubMed] [Google Scholar]
- 77.Haque NS, Fallon JT, Pan JJ, Taubman MB, Harpel PC. Chemokine receptor-8 (CCR8) mediates human vascular smooth muscle cell chemotaxis and metalloproteinase-2 secretion. Blood. 2004;103:1296–1304. doi: 10.1182/blood-2002-05-1480. [DOI] [PubMed] [Google Scholar]