Abstract
Background
The degree of effectiveness of condoms in preventing the transmission of herpes simplex virus 2 (HSV-2), is uncertain. We performed a large pooled analysis to address this question.
Methods
We identified prospective studies with individual-level condom use data and laboratory-defined HSV-2 acquisition. Six studies were identified through a review of publications through 2007: three candidate HSV-2 vaccine studies, an HSV-2 drug study, an observational STD incidence study and a behavioral STD intervention study. Study investigators provided us individual-level data to perform a pooled analysis. Effect of condom use was modeled using a continuous percent of sex acts during which a condom was used and, alternatively, using absolute number of unprotected sex acts.
Results
A total of 5384 people who were HSV-2 negative at baseline contributed 2,040,894 follow-up days. 415 persons acquired laboratory-documented HSV-2 during follow-up. Consistent (100%) condom users had a 30% lower risk of HSV-2 acquisition compared to those who never used condoms (hazard ratio: 0.70; 95 percent confidence interval: 0.40, 0.94; p = 0.01). Risk for HSV-2 acquisition increased steadily and significantly with each unprotected sex act (hazard ratio: 1.16; 95 percent confidence interval: 1.08, 1.25); p < 0.001). Condom effectiveness did not vary by gender.
Conclusions
This is the largest analysis using prospective data to assess the effect of condoms in preventing HSV-2 acquisition. Although the magnitude of protection was not as large as has been observed with other STIs, we found that condoms offer moderate protection against HSV-2 acquisition in men and women.
INTRODUCTION
Studies that prospectively measure sexual activity, condom use and STI incidence are necessary to assess preventive effect of condom use on STI acquisition. In the absence of randomized controlled trials, the best evidence available on condom use and STI acquisition comes from prospective observational studies, or intervention trials conducted for other purposes, in which the STI of interest was an endpoint. Compelling evidence from such studies supports that consistent condom use reduces transmission of HIV 1. Additionally, increasingly strong data support condom effectiveness in preventing sexually transmitted infections (STIs) that target urethral or cervical epithelia, such as chlamydia and gonorrhea 2. However, the effectiveness of condoms in preventing the transmission of herpes simplex virus 2 (HSV-2), is less certain 3-5. A 2001 panel convened by the National Institute of Allergy and Infectious Diseases concluded that the available evidence on condom effectiveness was insufficient to establish that condoms were protective against HSV-2 acquisition 6, as the research was derived from studies that used prevalent HSV-2 infection as the outcome and thus were unable to determine the temporal relationship between condom use and HSV-2 acquisition 6,7. Since that time, three studies have been published that show moderate efficacy (~50%) for condom use 8-10. However, in these studies, measures of condom use and definitions of condom effectiveness differed. More precise measurement of condom efficacy, with attention to subgroups in which condom effectiveness may differ, is desirable.
In this study, we sought to increase the precision of the estimates of condom use on HSV-2 acquisition by pooling data from all published studies that prospectively assessed condom use and HSV-2 incidence. We performed an individual-level pooled analysis that combined prospective data from such studies to assess the relationship between condom use and time to HSV-2 acquisition. In addition, we performed additional analyses to assess the relationship between the absolute number of unprotected sex acts per week and HSV-2 acquisition.
MATERIALS AND METHODS
Data Collection
We sought to identify all relevant studies for this analysis by conducting literature searches as well as discussions with other researchers in the field. First we conducted a PubMed search of studies published through October 2004 using the terms “genital herpes AND condom” and “herpes AND condom.” The initial search resulted in 147 articles that were reviewed for inclusion according to three predetermined eligibility criteria: (1) use of prospective cohort study design in which participants were tested with type-specific HSV-2 antibody tests at baseline and follow-up, (2) assessment of both condom use and frequency of sexual activity throughout the study, and (3) laboratory documentation (either culture, PCR, or type-specific serology) of HSV-2 acquisition. From this review, we identified 21 studies as potentially eligible; 17 of these did not meet the inclusion criteria upon detailed review11-27. We found six that met our predetermined criteria (Table 1), including four identified through the literature review process and two identified through asking researchers in the field about their knowledge of studies meeting the eligibility criteria 28,29. These studies included: two candidate HSV-2 vaccine studies, one HSV-2 drug study (placebo arm only), one HSV-2 vaccine study (placebo arm only), one observational STD incidence study and one HIV behavioral intervention study (Table 1)29-32. All studies incorporated safer sex counseling as part of their routine follow-up. Next, we asked the investigators to provide individual-level data from the eligible studies. We were provided data for all participants in four studies 30-32 and for the placebo arm participants in two studies (GSK valacyclovir 28 and SKB vaccine 29). After completing the preliminary analyses, we performed a second literature review of published articles since 2004 and identified 9 additional studies 33-41, none of which met the criteria for inclusion.
Table 1.
Characteristics of studies included in the pooled analysis
| Study; Year of Publication (reference no.) | Population; Study Years, Location | Number of Participants; Planned Length of Follow-Up | Condom Use Measurement | Objective | Main Finding |
|---|---|---|---|---|---|
| Project RESPECT; 199831 | Individuals ≥14 years of age with heterosexual vaginal intercourse in the previous 3 months. 1993-1996, United States. | 5758; 52 weeks | Percentage of vaginal or anal sex acts | Evaluation of a face-to-face prevention counseling program to reduce HIV and STD acquisition. | 20% reduction in STD acquisition and increase in condom use as result of intervention. |
| Chiron Vaccine Partners; 199930 | Monogamous, HIV(-), HSV-2(-) individuals ≥18 years of age with a HSV-2(+) partner. 1993-1995, United States. | 531; 72 weeks | Percentage of genital sex acts | Evaluation of a candidate subunit glycoprotein vaccine for prevention of HSV-2 acquisition. | Vaccine was found not to prevent HSV-2 acquisition. |
| Chiron Vaccine STD Clinic; 199930 | HIV(-), HSV-2(-) STD clinic attendees ≥18 years of age with STD diagnosis or ≥4 partners in previous year. 1993-1995, United States. | 1862; 72 weeks | Percentage of genital sex acts | Evaluation of a candidate subunit glycoprotein vaccine for prevention of HSV-2 acquisition. | Vaccine was found not to prevent HSV-2 acquisition. |
| Adolescent STD Incidence; 200132 | Individuals ≤21 years of age. 1994-1997, Unites States. | 536; 24 weeks | Never; Sometimes; About Half; Most Times; or Every Time | Assessment of sexual behaviors and STD incidence of homeless adolescents. | Incidence of HSV-2 and C. trachomatis was relatively high in females. Inconsistent condom use was the primary factor associated with increased risk. |
| SKB Vaccine Trial; 200229 | HSV-1 & -2 (-), HIV(-), individuals 19-45 years of age with a partner with a history of genital herpes. 1995-1997; N. America, Europe, Australia. | 847; 19 months | Never (0%); Sometimes (<50%); Usually (>50%); Always (100%) | Evaluation of a candidate subunit gD glycoprotein vaccine for prevention of HSV-2 acquisition. | Vaccine efficacy only in women. |
| GSK Valacyclovir; 200428 | Heterosexual, monogamous, immunocompetent individuals ≥18 years of age with a HSV-2(+) partner. 1998-2001, N. America, Europe, Latin America, Australia. | 741; 56 weeks | Never (0%); Sometimes (1-90%); or Nearly Always (91-100%) | Evaluation of the effect of suppressive valacyclovir use in HSV-2 (+) individuals in reducing HSV-2 acquisition in susceptible partners | HSV-2 acquisition was significantly reduced in susceptible participants. |
Statistical Methods
Information on participant characteristics, sexual behaviors, and HSV-2 acquisition was standardized across each dataset. The frequency of genital or anal sex acts and the proportion of condom use for sex acts were available in each study. For studies that reported categories of condom use (such as “never”, “sometimes”, or “always”), the corresponding ranges of percent condom use for each category were obtained from the study questionnaire. The numeric value at the midpoint of each response category's range was used in analyses that included condom use as a continuous variable. We included only participants who were known to be HSV-2-negative at baseline (based on laboratory determination). We also excluded data from participants who, during the follow up period, reported no sex, had no laboratory tests done, or who were not interviewed about condom use. At each assessment time, HSV-2 status was compared with sexual risk behavior and other potential risk factors that were available across studies (age, race, sexual practices, prior STI history, etc.) over the preceding time period. Race data were obtained from the original studies and categorized by using indicator variables for “African American” and “other” with “white” used as the reference group. This variable was included because of the higher incidence of HSV-2 in African Americans42. When values for potential risk factors were missing, data from previous visits up to 6 months before the time of the missing data were carried forward. Date of HSV-2 acquisition was set at the midpoint between the date of the most recent negative test and the date of the first positive test. Annualized incidence of HSV-2 acquisition was calculated by study and gender. Continuous measures taken repeatedly on individuals were summarized by first averaging over all timepoints and then by computing study-specific and overall means or medians of these values.
A univariate Cox regression model stratified by study was generated for each potential risk factor and tested using a likelihood ratio test at a significance level of 0.05. Stratified Cox models were used throughout the analysis to allow for differing baseline hazards between studies, possibly due to any effects of study-specific interventions, which may not satisfy the proportional hazards assumption. The proportional hazards assumption was examined using plots of scaled Schoenfeld's residuals for univariate and multivariate models and no violations were found. Graphical analysis was also used to assess parameterization of continuous variables and to select cutpoints or categories. Frequency of genital or anal sex acts per week was included in the models as a categorical variable (0-1, 2, 3-5, 6-10, >10). Age was grouped into tertiles (≤S23, 24-31,31+) and included as a grouped linear variable. Gender, prior STI history (ever, never), baseline HSV-1 status, sexual orientation during study (heterosexual, other), and monogamy (one partner) during study were included as binary variables. The effect of having one partner versus multiple partners in a measurement period on time until HSV-2 acquisition was hypothesized to differ based on whether the subject participated in a study that recruited monogamous couples. Therefore, an interaction was tested between number of partners and participation in a couples’ study as some persons reported having multiple partners despite their participation in a couples’ study.
The association between condom use and the risk of HSV-2 acquisition was initially evaluated with a Cox regression model stratified by study and controlling for categorical frequency of sex acts by week. Condom use was included in the model as a continuous variable (percent of sex acts during which a condom was used). Coefficients were multiplied by 25 to ascertain the aggregate effect of condoms for every 25 percent increase in use. We examined whether the effect of condom use differed by gender using an interaction term for condom use by gender.
Adjusted models for the effect of condom use on the risk of HSV-2 acquisition were generated with the Hosmer and Lemeshow stepwise method 43. An adjusted model was generated for the effect of percent condom use, stratified on study and adjusted for frequency of sex acts per week and other significant covariates by this method. Two-way interaction terms for condom use by gender and by study were also evaluated in this model.
The frequency of unprotected sex acts was determined by multiplying the frequency of total sex acts per week by the percentage condom use for each time interval and subtracting this from the total sex acts per week. Frequency of unprotected sex acts per week was included in a secondary model as a categorical variable with categories for values zero, one, two, three, and four or more. The effect of increasing unprotected sex acts on risk of HSV-2 acquisition was estimated using a Cox regression model stratified on study. An adjusted model for the effect of unprotected sex acts included interaction terms for frequency of unprotected sex acts by study and by gender. All reported p values are two-sided. All statistical analyses were performed with STATA 8.0 (StataCorp, College Station, TX).
RESULTS
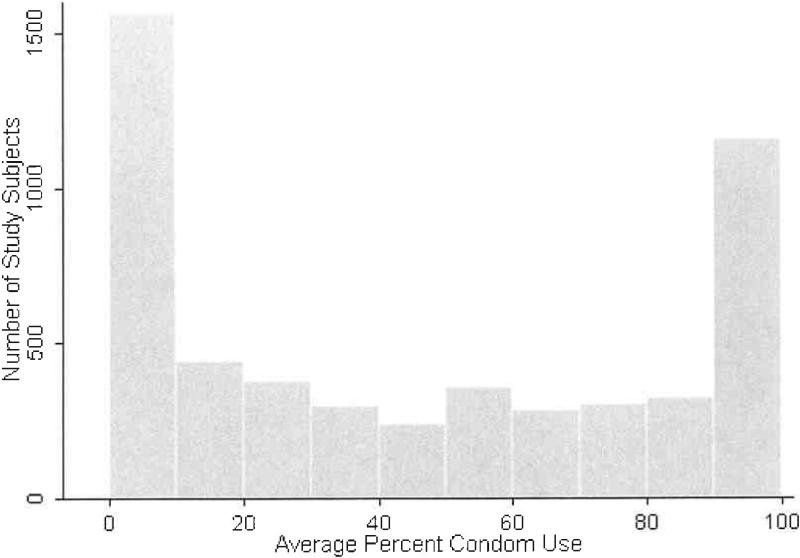
The six studies yielded a total of 5384 subjects whose baseline HSV-2 tests were negative and who were included in this pooled analysis. Four-hundred seventy five subjects were excluded because they reported no sex, or had no laboratory test or condom use interview during the follow up period (40 from the GSK valacyclovir study, 9 from the SKB vaccine study, 10 from the Chiron Partners study, 118 from the Chiron STD Clinic study, 38 from the Project RESPECT, and 260 from the Adolescent STD Incidence study). Overall, subjects had a mean age of 29, 66.2 percent were male, 60.4 percent were white, 94.1 percent were heterosexual, and most reported no prior STIs (Table 2). Sixty percent of the subjects were HSV-1 seropositive at study entry. The 5384 subjects contributed 2,040,894 follow-up days with a median follow withup of 374 days (range: 4, 987 days). Overall, 415 persons acquired laboratory-documented HSV-2 during follow-up. The overall incidence was 7.4 per 100 person years (95 percent confidence interval: 6.7, 8.2), but varied among studies (Table 3). Incidence was consistently higher for women than for men though the differences between genders varied greatly (Table 3). The study-specific median frequency of sex acts averaged 1.4 per week (range: 0.6, 1.9). The median number of partners reported during study was 1 (interquartile range: 1, 1.74) in the pooled data set and matched the study-specific median values except for the Adolescent STD Incidence Study (median: 2) and the Chiron vaccine STD Clinic study (median: 1.4). The median percent condom use during follow-up was lower in the studies that recruited discordant couples (7, 14 and 18 in Chiron vaccine Partners, SKB, and GSK valacyclovir, respectively) compared with other studies (from 46 to 53). In the pooled data, the median percent condom use was 39, and the subject averages over study follow-up had a u-shaped distribution (Figure 1) 44. Variables univariately associated with HSV-2 acquisition included female gender, younger age, non-white race, and history of STIs (Table 4).
Table 2.
Characteristics of subjects included in the pooled analysis, by study and in the pooled dataset
| Chiron Vaccine Partners | Chiron Vaccine STD Clinic | GSK Valacyclovir | Adolescent STD Incidence | Project RESPECT | SKB Vaccine Trial | Pooled Dataset | |
|---|---|---|---|---|---|---|---|
| Total N | 521 | 1744 | 701 | 277 | 1728 | 413 | 5384 |
| Continuous Variables | |||||||
| Duration of Follow-up, median days (range) | 561 (18-706) | 553 (17-819) | 242 (14-337) | 182 (46-255) | 365 (4-542) | 546 (14-987) | 374 (4-987) |
| Age, mean years(range)* | 36.0 (18-62) | 29.0 (17-64) | 35.9 (18-76) | 18.1 (13-22) | 25.9 (14-60) | 32.1 (18-46) | 29.2 (13-76) |
| Categorical variables, n(percent) | |||||||
| Gender: | |||||||
| Male | 255 (49.0) | 1296 (74.3) | 470 (67.1) | 167 (60.3) | 1095 (63.4) | 283 (68.5) | 3566 (66.2) |
| Female | 266 (51.1) | 448 (25.7) | 231 (33.0) | 110 (39.7) | 633 (36.6) | 130 (31.5) | 1818 (33.8) |
| Race:† | |||||||
| White | 483 (92.7) | 1093 (62.7) | 636 (90.7) | 213 (78.0) | 431 (24.9) | 395 (95.6) | 3251 (60.4) |
| Black | 14 (2.7) | 536 (30.7) | 18 (2.6) | 6 (2.2) | 893 (51.7) | 6 (1.5) | 1473 (27.4) |
| Other | 24 (4.6) | 115 (66) | 47 (6.7) | 54 (19.8) | 404 (23.4) | 12 (2.9) | 656 (12.2) |
| Sexual Orientation:‡ | |||||||
| Heterosexual | 507 (97.5) | 1523 (87.3) | 701 (100) | 204 (73.7) | 1728 (100) | 401 (97.3) | 5064 (94.1) |
| Other | 13 (2.6) | 221 (12.7) | 0 | 73 (26.3) | 0 | 11 (2.7) | 318 (5.9) |
| Baseline HSV1 status:§ | |||||||
| HSV1 Positive | 309 (59.3) | 1111 (63.7) | 487 (69.5) | 135 (49.8) | 1183 (68.5) | 0 | 3225 (60.0) |
| Past STI Diagnosis:# | 16 (3.1) | 704 (40.4) | 155 (22.1) | 95 (34.3) | 963 (56.0) | NA | NA |
Missing values:
8 for Age
for Race
1 for Sexual Orientation
8 for Baseline HSV1 Status
9 for Any Past STI Diagnosis.
Table 3.
HSV-2 incidence and 95 percent confidence intervals per 100 person years, by study and gender
| Chiron Vaccine Partners | Chiron Vaccine STD Clinic | GSK Valacyclovir | Adolescent STD Incidence | Project RESPECT | SKB Vaccine Trial | Overall | ||
|---|---|---|---|---|---|---|---|---|
| Women | Incidence | 8.7 | 5.4 | 11.5 | 21.8 | 15.5 | 15.0 | 10.8 |
| 95 percent confidence interval | 6.1, 12.5 | 3.8, 7.7 | 7.1, 18.8 | 12.1, 39.4 | 12.6, 19.2 | 9.9, 22.8 | 9.4, 12.5 | |
| Men | Incidence | 1.9 | 5.1 | 3.2 | 12.3 | 9.5 | 3.0 | 5.8 |
| 95 percent confidence interval | 0.9, 3.9 | 4.1, 6.3 | 1.7, 6.2 | 6.4, 23.6 | 7.8, 11.6 | 1.7, 5.4 | 5.1, 6.6 | |
| Total | Incidence | 5.1 | 5.1 | 6.0 | 16.2 | 11.7 | 6.6 | 7.4 |
| 95 percent confidence interval | 3.7, 7.1 | 4.3, 6.2 | 4.1, 8.9 | 10.4, 25.0 | 10.1, 13.5 | 4.8, 9.3 | 6.7, 8.2 |
Figure 1.
Distribution of average percent condom use during study follow-up
Table 4.
Univariate associations with HSV-2 acquisition in the pooled analysis*
| Covariate | Univariate Hazard Ratio | 95 percent confidence interval | P value | |
|---|---|---|---|---|
| Frequency of sexual activity per week: | 0.08 | |||
| 0 to1 | 1.0 | |||
| 2 | 0.92 | 0.70, 1.20 | ||
| 3 to 5 | 1.07 | 0.83, 1.36 | ||
| 6 to 10 | 1.32 | 0.89, 1.95 | ||
| > 10 | 2.21 | 1.08, 4.49 | ||
| Race: | <0.001 | |||
| White | 1.0 | |||
| African American | 2.46 | 1.90, 3.21 | ||
| Other | 1.48 | 1.07, 2.09 | ||
| Women | 1.79 | 1.45, 2.17 | <0.001 | |
| HSV-1 positive at baseline | 1.21 | 0.97, 1.50 | 0.09 | |
| Had an STI prior to study† | 1.73 | 1.39, 2.16 | <0.001 | |
| Homosexual or bisexual MSM | 1.08 | 0.70, 1.68 | 0.73 | |
| Study: | <0.001 | |||
| Chiron Partners | 1.0 | |||
| Chiron STD Clinics | 0.98 | 0.68, 1.42 | ||
| GSK | 0.87 | 0.52, 1.45 | ||
| Project RESPECT | 1.88 | 1.31, 2.69 | ||
| Homeless Adolescent | 2.06 | 1.18, 3.58 | ||
| SKB Vaccine Trial | 1.20 | 0.75, 1.93 | ||
| Multiple partners vs. 1 partner | 1.02 | 0.81, 1.29 | 0.88 | |
| Age‡ | 0.83 | 0.75, 0.92 | 0.001 | |
| Condom Use (per every 25 percent increase)§ | 0.95 | 0.88, 1.00 | 0.09 | |
All estimates except for “Study” are from a univariate model stratified by study
Does not include SKB data
For increasing categories of age (≤23, 24-31, 31+)
Adjusted for weekly frequency of sex acts
The association between a 25 percent increase in condom use and HSV-2 acquisition, adjusted for frequency of sex acts and stratified by study, indicated a weak protective effect that approached statistical significance (HR: 0.95; 95 percent confidence interval: 0.88, 1.00; P = 0.09). This effect did not significantly differ by gender (P = 0.22 for interaction).
In a multivariate model (Table 5), a 25 percent increase in condom use significantly decreased the risk of HSV-2 acquisition (HR: 0.93; 95 percent confidence interval: 0.85, 0.99; P = 0.01). Similarly, the aggregate hazard ratio for 100% condom use compared to 0% use was 0.70 (95 percent confidence interval: 0.40, 0.94; P = 0.01). No evidence of heterogeneity was found by study (P = 0.24) or gender (P = 0.22) in the adjusted model. In separate analyses for each study, increasing condom use was found to decrease the adjusted risk of HSV-2 acquisition; however, these estimates were only statistically significant for one study (Figure 2). Baseline HSV-1 status, prior STI history, sexual orientation during study, and monogamy during study did not significantly predict HSV-2 acquisition during model selection and were not included in the final multivariate model.
Table 5.
Multivariate model of risk of HSV-2 acquisition, including condom effect and stratified on study
| Covariate | Adjusted Hazard Ratio | 95 percent confidence interval | P value | |
|---|---|---|---|---|
| Age* | 0.86 | 0.75, 0.99 | 0.04 | |
| Women | 2.00 | 1.64, 2.50 | <0.001 | |
| Race: | <0.001 | |||
| White | 1.0 | |||
| African American | 2.60 | 1.99, 3.42 | ||
| Other | 1.29 | 0.90, 1.83 | ||
| Sex Acts per Week: | 0.07 | |||
| 0 to 1 | 1.0 | |||
| 2 | 0.96 | 0.73, 1.27 | ||
| 3 to 5 | 1.12 | 0.87, 1.45 | ||
| 6 to 10 | 1.44 | 0.97, 2.15 | ||
| >10 | 2.57 | 1.25, 5.27 | ||
| Condom Use (per every 25 percent increase) | 0.93 | 0.85, 0.99 | 0.01 | |
For increasing categories of age (≤23, 24-31, 31+)
Figure 2. Study-specific hazard ratios (and 95 percent confidence intervals) for the effect of (a) a 25 percent increase in condom use and the effect of (b) increasing unprotected sex acts on HSV-2 acquisition.
Size of dark squares are proportional to the inverse variance of the estimate and centered on the hazard ratio. Horizontal lines indicate the 95% confidence interval for effect on time until HSV-2 acquisition. Diamonds are centered on the pooled hazard ratio estimate (dashed line) and width indicates the 95% confidence interval.
Secondary analysis of the effect of each additional unprotected sex act
In a univariate model stratified by study, the risk of HSV-2 acquisition increased significantly with increasing unprotected sex acts per week (0, 1, 2, 3,4 or more) (HR: 1.10; 95 percent confidence interval 1.02, 1.19; P=0.01). After adjustment for age, race, and gender, the estimate showed an increased risk of HSV-2 acquisition with increasing numbers of unprotected sex acts per week (HR: 1.16; 95 percent confidence interval: 1.08, 1.25; P<0.001) (Table 6). We observed no evidence for significant variation of this effect by gender (p = 0.41)). Overall estimates of the impact of the number of unprotected sex acts were also relatively consistent between study subgroups, except smaller effect was observed in the Project RESPECT and SKB vaccine subgroups (Figure 2b). However, an interaction between study and frequency of unprotected sex acts was not significant (P = 0.41).
Table 6.
Multivariate model of risk of HSV-2 acquisition, including unprotected sex act effect and stratified by study
| Covariate | Adjusted Hazard Ratio | 95 percent confidence interval | P value | |
|---|---|---|---|---|
| Age* | 0.87 | 0.75, 1.00 | 0.05 | |
| Female | 2.00 | 1.64, 2.50 | <0.001 | |
| Race: | <0.001 | |||
| White | 1.0 | |||
| African American | 2.62 | 2.00, 3.43 | ||
| Other | 1.31 | 0.92, 1.86 | ||
| Unprotected Sex Acts per Week† | 1.16 | 1.08, 1.25 | <0.001 | |
For increasing categories of age (≤23, 24-31, 31+)
For increasing categories of frequency of unprotected sex acts (0, 1, 2, 3, 4+)
DISCUSSION
In our pooled analysis of data from all studies to date that have prospectively assessed condom use and HSV-2 incidence, we found that condom use moderately, albeit significantly, protected against HSV-2 acquisition. Persons who always used condoms had a 30 percent decreased risk of acquiring HSV-2 compared to persons who reported no condom use. Risk of HSV-2 acquisition decreased by seven percent for every additional 25 percent of the time that condoms were used during anal or vaginal sex. Risk of HSV-2 acquisition also rose steadily and significantly with increasing frequency of unprotected sex acts, and our findings were consistent throughout multiple analysis strategies. Our method of pooled analysis circumvented the obstacles and expense of recruiting and following a large cohort of individuals and the use of individual-level data in the pooled analysis allowed for uniform coding of the relevant variables and assessment of relationships that may not have been explored as part of the original results 45. Since we did not find strong evidence of heterogeneity between studies for the effectiveness of condoms, we believe that this was a valid approach.
In some cases our pooled estimates of condom effects on HSV-2 acquisition varied from earlier published reports on those studies. For example, previously published analyses of the Project RESPECT study data found subjects who used condoms less than 50 percent of the time with occasional partners had twice the risk of acquiring HSV-2 than those with 100 percent condom use or no occasional partners (Hazard ratio: 2.0; 95 percent confidence interval: 1.2,3.3) 8. We found a lower estimate in this analysis that is likely due to the inclusion of condom use between self-reported monogamous partners; level of condom use with main partners was not associated with reduced HSV-2 acquisition in the previous analysis. Also, in an analysis of the Chiron vaccine Partners study, Wald, et al. 10 reported an adjusted hazard ratio of 0.085 for women (95 percent confidence interval: 0.01,0.67) using condoms more than 25 percent of the time; yet this protection was not observed in men. We did not find any significant differences in condom effectiveness between men and women, despite a higher incidence of HSV-2 acquisition in women (Table 3). This suggests that lack of effect in the earlier publication may have been related to few cases of HSV-2 acquisition in men and the large sample size in the pooled analysis allowed a more robust estimate. .
The limitations of our study include the availability of only those covariates for the adjusted analyses that were collected in a consistent way across every study. For example, we were unable to adjust for some known risk factors for HSV-2 acquisition, such as the number of new sexual partners and the HSV status of each partner, which may have led to uncontrolled confounding 46. Warner et al. have described unmeasured confounding in a similar cohort analysis of condom effectiveness as differences between consistent and inconsistent condom users related to unmeasured factors which led to an underestimate of the magnitude of the protective effect of condoms on STI acquisition 47. Condom use may have been inaccurately reported 48 due to social desirability bias or incorrect usage49, or affected by the recent HSV-2 acquisition. Condom use and HSV-2 acquisition were ascertained after various follow-up intervals, and it is possible that a primary HSV-2 episode in some cases could lead to increased condom use within the same measurement interval. These misclassifications attenuate the observed estimate toward the null and are present in other studies of condom effectiveness. Additionally, we identified studies to include in this analysis through a literature search, which may have led to publication bias. We believe, but cannot be certain, that our solicitations among other investigators identified most relevant studies.
This analysis adds to the growing number of condom analyses that use an absolute number of unprotected sex acts for exposure as opposed to the more traditional measure of percent condom use 47,50-52. Analyses of these two outcomes gave roughly the same conclusion.. The unprotected sex act models may be more appropriate as they do not require the impact of percent condom use to be consistent across varying numbers of sex acts, emphasizing that one's risk of acquiring HSV-2 is specific to each unprotected sex act.
The 30 percent reduction in HSV-2 acquisition observed in this pooled analysis was less than the reported 87 percent reduction associated with condom use on HIV acquisition 3. This difference likely reflects different transmission mechanisms. While HIV is transmitted via contact with bodily fluids, HSV-2 is primarily transmitted through direct skin-to-skin or skin-to-mucosa contact. Therefore, some HSV-2 transmission can occur despite condom use when viral shedding is present in areas not covered by the condom. Nonetheless, based on findings of this large analysis using all available prospective data, condom use should continue to be recommended to both men and women for reducing risk of HSV-2 acquisition. Although the magnitude of the protective effect was not as large as has been observed with other STIs, a 30 percent reduction in HSV-2 incidence can have a substantial benefit for individuals as well as a public health impact at the population level.
Acknowledgments
We wish to acknowledge the investigators of the original studies that generously shared data with us: Dr. Lawrence Corey and the GlaxoSmithKine Valacyclovir Transmission Study Team, the Project RESPECT Study Group, the Chiron HSV Vaccine Study Group, and the SKB HSV Vaccine Study Group, and Dr. John Noell. Funding for this project was provided by National Institutes of Health / National Institute of Allergy and Infectious Diseases (P01 AI-030731; K24 AI-107113). Dr. Anna Wald had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Davis KR, Weller SC. The effectiveness of condoms in reducing heterosexual transmission of HIV. Fam Plann Perspect. 1999 Nov-Dec;31(6):272–279. [PubMed] [Google Scholar]
- 2.Fact Sheet for Public Health Personnel: Male Latex Condoms and Sexually Transmitted Diseases: Centers for Disease Control and Prevention. 2003 [Google Scholar]
- 3.Holmes KK, Levine R, Weaver M. Effectiveness of condoms in preventing sexually transmitted infections. Bull World Health Organ. 2004 Jun;82(6):454–461. [PMC free article] [PubMed] [Google Scholar]
- 4.Casper C, Wald A. Condom use and the prevention of genital herpes acquisition. Herpes. 2002 Apr;9(1):10–14. [PubMed] [Google Scholar]
- 5.Corey L. Increasing prevalence of HSV-2 points to need for more effective prevention strategies. Herpes. 2002;9:3. [PubMed] [Google Scholar]
- 6.Workshop Summary: Scientific Evidence on Condom Effectiveness for Sexually Transmitted Disease Prevention. Vol. 2001. Herndon, Virginia: Jul 20, 2001. [Google Scholar]
- 7.Langenberg A. Interrupting herpes simplex virus type 2 transmission: the role of condoms and microbicides. Herpes. 2004 Aug;11(Suppl 3):147A–154A. [PubMed] [Google Scholar]
- 8.Gottlieb SL, Douglas JM, Jr., Foster M, et al. Incidence of herpes simplex virus type 2 infection in 5 sexually transmitted disease (STD) clinics and the effect of HIV/STD risk-reduction counseling. J Infect Dis. 2004 Sep 15;190(6):1059–1067. doi: 10.1086/423323. [DOI] [PubMed] [Google Scholar]
- 9.Wald A, Langenberg AG, Krantz E, et al. The relationship between condom use and herpes simplex virus acquisition. Ann Intern Med. 2005 Nov 15;143(10):707–713. doi: 10.7326/0003-4819-143-10-200511150-00007. [DOI] [PubMed] [Google Scholar]
- 10.Wald A, Langenberg AG, Link K, et al. Effect of condoms on reducing the transmission of herpes simplex virus type 2 from men to women. Jama. 2001 Jun 27;285(24):3100–3106. doi: 10.1001/jama.285.24.3100. [DOI] [PubMed] [Google Scholar]
- 11.HIV spread in four sub-Saharan African cities. AIDS Anal Afr. 2000 Jan;10(4):9–10. [PubMed] [Google Scholar]
- 12.Abraham CD, Conde-Glez CJ, Cruz-Valdez A, Sanchez-Zamorano L, Hernandez-Marquez C, Lazcano-Ponce E. Sexual and demographic risk factors for herpes simplex virus type 2 according to schooling level among Mexican youths. Sex Transm Dis. 2003 Jul;30(7):549–555. doi: 10.1097/00007435-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Bryson Y, Dillon M, Bernstein DI, Radolf J, Zakowski P, Garratty E. Risk of acquisition of genital herpes simplex virus type 2 in sex partners of persons with genital herpes: a prospective couple study. J Infect Dis. 1993 Apr;167(4):942–946. doi: 10.1093/infdis/167.4.942. [DOI] [PubMed] [Google Scholar]
- 14.Butler T, Donovan B, Taylor J, et al. Herpes simplex virus type 2 in prisoners, New South Wales, Australia. Int J STD AIDS. 2000 Nov;11(11):743–747. doi: 10.1258/0956462001915174. [DOI] [PubMed] [Google Scholar]
- 15.Chen YM, Yu PS, Lin CC, Jen I. Surveys ofHIV-1, HTLV-I, and other sexually transmitted diseases in female sex workers in Taipei City, Taiwan, from 1993 to 1996. J Acquir Immune Defic Syndr Hum Retrovirol. 1998 Jul 1;18(3):299–303. doi: 10.1097/00042560-199807010-00016. [DOI] [PubMed] [Google Scholar]
- 16.Dobbins JG, Mastro TD, Nopkesorn T, et al. Herpes in the time of AIDS: a comparison of the epidemiology of HIV-1 and HSV-2 in young men in northern Thailand. Sex Transm Dis. 1999 Feb;26(2):67–74. doi: 10.1097/00007435-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Jones DL, Irwin KL, Inciardi J, et al. The high-risk sexual practices of crack-smoking sex workers recruited from the streets of three American cities. The Multicenter Crack Cocaine and HIV Infection Study Team. Sex Transm Dis. 1998 Apr;25(4):187–193. doi: 10.1097/00007435-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Lowhagen GB, Tunback P, Andersson K, Johannisson G. Recurrent genital herpes in a population attending a clinic for sexually transmitted diseases. Acta Derm Venereol. 2001 Jan-Feb;81(1):35–37. doi: 10.1080/000155501750208164. [DOI] [PubMed] [Google Scholar]
- 19.Mertz GJ, Benedetti J, Ashley R, Selke SA, Corey L. Risk factors for the sexual transmission of genital herpes. Ann Intern Med. 1992 Feb 1;116(3):197–202. doi: 10.7326/0003-4819-116-3-197. [DOI] [PubMed] [Google Scholar]
- 20.Mihret W, Rinke de Wit TF, Petros B, et al. Herpes simplex virus type 2 seropositivity among urban adults in Africa: results from two cross-sectional surveys in Addis Ababa, Ethiopia. Sex Transm Dis. 2002 Mar;29(3):175–181. doi: 10.1097/00007435-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Munoz-Perez MA, Rodriguez-Pichardo A, Camacho Martinez F. Sexually transmitted diseases in 1161 HIV-positive patients: a 38-month prospective study in southern Spain. J Eur Acad Dermatol Venereol. 1998 Nov;11(3):221–226. [PubMed] [Google Scholar]
- 22.Shlay JC, McClung MW, Patnaik JL, Douglas JM., Jr Comparison of Sexually Transmitted Disease Prevalence by Reported Condom Use: Errors Among Consistent Condom Users Seen at an Urban Sexually Transmitted Disease Clinic. Sex Transm Dis. 2004;31(9):526–532. doi: 10.1097/01.olq.0000137897.17919.d1. [DOI] [PubMed] [Google Scholar]
- 23.Smith JS, Herrero R, Munoz N, et al. Prevalence and risk factors for herpes simplex virus type 2 infection among middle-age women in Brazil and the Philippines. Sex Transm Dis. 2001 Apr;28(4):187–194. doi: 10.1097/00007435-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Stroffolini T, Corona R, Giglio A, et al. Risk factors for hepatitis B virus infection among homosexual men attending a sexually transmitted diseases clinic in Italy. New Microbiol. 1997 Oct;20(4):333–338. [PubMed] [Google Scholar]
- 25.Sucato G, Celum C, Dithmer D, Ashley R, Wald A. Demographic rather than behavioral risk factors predict herpes simplex virus type 2 infection in sexually active adolescents. Pediatr Infect Dis J. 2001 Apr;20(4):422–426. doi: 10.1097/00006454-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Tideman RL, Pitts MK, Fairley CK. Effects of a change from an appointment service to a walk-in triage service at a sexual health centre. Int J STD AIDS. 2003 Dec;14(12):793–795. doi: 10.1258/095646203322556093. [DOI] [PubMed] [Google Scholar]
- 27.Ward H, Day S, Weber J. Risky business: health and safety in the sex industry over a 9 year period. Sex Transm Infect. 1999 Oct;75(5):340–343. doi: 10.1136/sti.75.5.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corey L, Wald A, Patel R, et al. Once-daily val acyclovir to reduce the risk of transmission of genital herpes. N Engl J Med. 2004 Jan 1;350(1):11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 29.Stanberry LR, Spruance SL, Cunningham AL, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002 Nov 21;347(21):1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 30.Corey L, Langenberg AG, Ashley R, et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. Jama. 1999 Jul 28;282(4):331–340. doi: 10.1001/jama.282.4.331. [DOI] [PubMed] [Google Scholar]
- 31.Kamb ML, Fishbein M, Douglas JM, Jr., et al. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: a randomized controlled trial. Project RESPECT Study Group. Jama. 1998 Oct 7;280(13):1161–1167. doi: 10.1001/jama.280.13.1161. [DOI] [PubMed] [Google Scholar]
- 32.Noell J, Rohde P, Ochs L, et al. Incidence and prevalence of chlamydia, herpes, and viral hepatitis in a homeless adolescent population. Sex Transm Dis. 2001 Jan;28(1):4–10. doi: 10.1097/00007435-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Ao TT, Sam NE, Masenga EJ, Seage GR, 3rd, Kapiga SH. Human immunodeficiency virus type 1 among bar and hotel workers in northern Tanzania: the role of alcohol, sexual behavior, and herpes simplex virus type 2. Sex Transm Dis. 2006 Mar;33(3):163–169. doi: 10.1097/01.olq.0000187204.57006.b3. [DOI] [PubMed] [Google Scholar]
- 34.Fox J, Taylor GP, Day S, Parry J, Ward H. How safe is safer sex? High levels of HSV-1 and HSV-2 in female sex workers in London. Epidemiol Infect. 2006 Oct;134(5):1114–1119. doi: 10.1017/S0950268806006133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lama JR, Lucchetti A, Suarez L, et al. Association of herpes simplex virus type 2 infection and syphilis with human immunodeficiency virus infection among men who have sex with men in Peru. J Infect Dis. 2006 Nov 15;194(10):1459–1466. doi: 10.1086/508548. [DOI] [PubMed] [Google Scholar]
- 36.Mehta SD, Moses S, Agot K, et al. Herpes simplex virus type 2 infection among young uncircumcised men in Kisumu, Kenya. Sex Transm Infect. 2008 Feb;84(1):42–48. doi: 10.1136/sti.2007.025718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moss NJ, Harper CC, Ahrens K, et al. Predictors of incident herpes simplex virus type 2 infections in young women at risk for unintended pregnancy in San Francisco. BMC Infect Dis. 2007;7:113. doi: 10.1186/1471-2334-7-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Msuya SE, Mbizvo EM, Stray-Pedersen B, et al. Decline in HIV prevalence among women of childbearing age in Moshi urban, Tanzania. Int J STD AIDS. 2007 Oct;18(10):680–687. doi: 10.1258/095646207782193858. [DOI] [PubMed] [Google Scholar]
- 39.Nagot N, Ouedraogo A, Defer MC, Vallo R, Mayaud P, Van de Perre P. Association between bacterial vaginosis and Herpes simplex virus type-2 infection: implications for HIV acquisition studies. Sex Transm Infect. 2007 Aug;83(5):365–368. doi: 10.1136/sti.2007.024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soto RJ, Ghee AE, Nunez CA, et al. Sentinel surveillance of sexually transmitted infections/HIV and risk behaviors in vulnerable populations in 5 Central American countries. J Acquir Immune Defic Syndr. 2007 Sep 1;46(1):101–111. [PubMed] [Google Scholar]
- 41.Theng TS, Sen PR, Tan HH, Wong ML, Chan KW. Seroprevalence of HSV-1 and 2 among sex workers attending a sexually transmitted infection clinic in Singapore. Int J STD AIDS. 2006 Jun;17(6):395–399. doi: 10.1258/095646206777323364. [DOI] [PubMed] [Google Scholar]
- 42.Wald A. Herpes simplex virus type 2 transmission: risk factors and virus shedding. Herpes. 2004 Aug;11(Suppl 3):130A–137A. [PubMed] [Google Scholar]
- 43.Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. 1st Ed. Wiley-Interscience; New York: 1999. [Google Scholar]
- 44.Crosby RA, Yarber WL, Sanders SA, Graham CA. Condom use as a dependent variable: a brief commentary about classification of inconsistent users. AIDS Behav. 2004 Mar;8(1):99–103. doi: 10.1023/b:aibe.0000017529.20932.73. [DOI] [PubMed] [Google Scholar]
- 45.Petitti D. Meta-Analysis, Decision Analysis, and Cost-Effectiveness Analysis: Methods for Quantitative Synthesis in Medicine. 2nd Ed Oxford University Press; New York: 2000. [Google Scholar]
- 46.Peterman TA, Lin LS, Newman DR, et al. Does measured behavior reflect STD risk? An analysis of data from a randomized controlled behavioral intervention study. Project RESPECT Study Group. Sex Transm Dis. 2000 Sep;27(8):446–451. doi: 10.1097/00007435-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Warner L, Macaluso M, Austin HD, et al. Application ofthe case-crossover design to reduce unmeasured confounding in studies of condom effectiveness. Am J Epidemiol. 2005 Apr 15;161(8):765–773. doi: 10.1093/aje/kwi094. [DOI] [PubMed] [Google Scholar]
- 48.Rose E, Diclemente RJ, Wingood GM, et al. The Validity of Teens’ and Young Adults’ Self-reported Condom Use. Arch Pediatr Adolesc Med. 2009 Jan;163(1):61–64. doi: 10.1001/archpediatrics.2008.509. [DOI] [PubMed] [Google Scholar]
- 49.Warner L, Clay-Warner J, Boles J, Williamson J. Assessing condom use practices. Implications for evaluating method and user effectiveness. Sex Transm Dis. 1998 Jul;25(6):273–277. doi: 10.1097/00007435-199807000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Crosby R, DiClemente RJ, Holtgrave DR, Wingood GM. Design, measurement, and analytical considerations for testing hypotheses relative to condom effectiveness against non-viral STIs. Sex Transm Infect. 2002 Aug;78(4):228–231. doi: 10.1136/sti.78.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warner L, Newman DR, Austin HD, et al. Condom effectiveness for reducing transmission of gonorrhea and chlamydia: the importance of assessing partner infection status. Am J Epidemiol. 2004 Feb 1;159(3):242–251. doi: 10.1093/aje/kwh044. [DOI] [PubMed] [Google Scholar]
- 52.Crosby RA. Condom use as a dependent variable: measurement issues relevant to HIV prevention programs. AIDS Educ Prev. 1998 Dec;10(6):548–557. [PubMed] [Google Scholar]