“Pharmacologic targeted regulation of BMMSCs by aspirin may offer a new approach for estrogen-deficient osteoporosis treatment.”
Osteoporosis, the most prevalent skeletal disorder, is recognized by low bone mineral density (BMD) and structural deterioration of bone tissue, both of which lead to bone fragility fractures [1]. Postmenopausal osteoporosis is the most common and significant form of this disease, whereby the loss of estrogen causes an imbalance in bone metabolism. This imbalance is due to an overactivated osteoclast activity, and a temporal increase in osteoblast activity that is unable to rescue osteoclast-mediated bone resorption [1]. Although many systemic and local regulators are involved in estrogen-deficient osteoporosis, it appears that activated T lymphocytes are the key factor inducing osteoclast overactivation in postmenopausal osteoporosis [2–4]. Investigations focused on understanding the role of osteogenic cells in postmenopausal osteoporosis have been on the rise over the last several years. These studies demonstrate a potential link between cell death and osteoporosis [5,6]. It has been proposed that irregular apoptosis of osteoblasts/osteocytes leads to the imbalanced bone remodeling in osteoporosis [7,8]. To expand the knowledge of this form of osteoporosis to correctly treat the disease, each aspect of the bone resorption and formation must be well understood. Currently, the role of osteoblasts and their progenitor bone marrow mesenchymal stem cells (BMMSCs) in osteoporosis is not well known.
BMMSCs are known as multipotent stem cells and are capable of differentiating into a variety of cell types including osteoblasts, chondrocytes, adipocytes and myoblasts [9–11]. The BMMSC/osteoblast lineage not only participates in de novo bone matrix formation to balance osteoclast-mediated bone resorption during the bone remodeling process, but also plays a critical role in maintaining homeostasis of the bone/marrow system [12]. This homeostasis includes governing the hematopoietic stem cell (HSC) niche [12–15] and modulation of immune cells, such as T and B lymphocytes, dendritic cells (DCs) and natural killer (NK) cells [16–22]. Recently, transplantation of culture-expanded BMMSCs has been successfully used to treat a variety of clinical disorders such as graft-versus-host-disease via inhibiting T-lymphocyte proliferation and activity [23–25] and ameliorating HSC engraftment [26,27]. Since BMMSCs reside in the same marrow compartment with immune cells, it will be interesting to examine whether immune cells affect BMMSCs.
The deficiency of the Fas/Fas ligand system can cause various immune disorders associated with inappropriate T-lymphocyte proliferation, such as organ transplantation graft rejection, systemic lupus erythematosus and lymphoid tumors [28]. Expression of Fas/FasL on the lymphoid/myeloid lineage cells plays an important role in immune homeostasis, T lymphocytes and NK cell-mediated toxicity, as well as Fas-mediated tumor killing [29]. The current study showed that BMMSCs expressing Fas and CD3-activated T lymphocytes were capable of inducing BMMSC apoptosis in a direct cell co-culture system, but not in an indirect cell co-culture system [30]. By contrast, the perforin pathway, one of the major apoptotic mechanisms by T lymphocytes [31], was not involved in CD3-activated T-cell mediated BMMSC apoptosis. Furthermore, it was found that activated T lymphocytes failed to induce apoptosis in Fas-mutated BMMSCs. Therefore, the study suggested that the Fas/FasL pathway is a predominant cell death pathway in T-cell mediated BMMSC apoptosis [32].
In an effort to treat this form of osteoporosis, scientists have begun examining the use of a T-lymphocyte adoptive transfer system. Currently, the most often studied T-lymphocyte adoptive transfer system is used to study inflammatory bowel disease (IBD) [33,34]. Studies have shown that with an application of a widely used T-lymphocyte adoptive transfer system to immune-deficient recipient mice, CD4+CD45RB+/high T lymphocytes account for the development of IBD. By contrast, transfer of the reciprocal CD4+CD45RB−/low population not only failed to induce colitis, but also prevented the symptoms [35]. Interestingly, IBD patients ordinarily express decreased bone mass, an increased risk of developing osteoporosis, and associated fragility fractures and morbidity [36,37]. However, the role of activated T lymphocytes on osteogenic progenitor cells in IBD patients has remained unclear. Adoptive transfer of CD4+CD45RB+/high T lymphocytes into T-lymphocyte deficient mice with ovaryectomy, which lacked the osteoporosis phenotype due to the absence of T lymphocytes [38], demonstrated a typical BMD reduction and trabecular bone resorption in femurs [30]. Also, the impairment of BMMSCs was elucidated by several assays including colony-forming units fibroblastic (CFU-F) number, proliferation capacity and osteogenic capacity in vitro and in vivo [30]. As expected, osteoclast activity was upregulated in these CD4+CD45RB+/high T-lymphocyte transfer mice by in vivo osteoclast assays, including an osteoclast-specific enzyme tartrate resistant acid phosphatase (TRAP) staining and in serum levels of soluble RANKL (sRANKL), a critical osteoclast differentiation factor. Furthermore, this upregulation was also seen in C-terminal telopeptide of type I collagen (CTX), a functional marker for osteoclast resorption. These findings provide direct evidence to support the hypothesis that interplays between T lymphocytes and BMMSCs may be critical for pathogenesis of osteoporosis.
While studies have investigated the role of T lymphocytes and osteoporosis, treatment measures have been researched to determine an appropriate pathway for these patients. Aspirin is a hugely popular and widely used NSAID. This drug is also known to prevent heart attacks by daily low dose intervention. The effect of aspirin is shown in multiple biological pathways, such as inhibiting cyclooxygenase 2 (COX2) and cyclooxygenase 1 (COX1), and prostaglandin E2 activities. According to epidemiological studies, the regular use of aspirin or NSAIDs may have a moderate beneficial effect on BMD in postmenopausal women [39]; however, there appears no clinical significance regarding the protective effect on the subsequent risk of fractures [40]. Therefore, more detailed studies are necessary to examine whether aspirin is able to offer therapeutic effects to patients suffering from osteoporosis and, more importantly, to elucidate the mechanism by which aspirin may affect bone integrity.
“According to epidemiological studies, the regular use of aspirin or NSAIDs may have a moderate beneficial effect on BMD in postmenopausal women.”
Women lose bone at a high rate during the initial years following menopause. Therefore, ovary-removed (ovariectomized [OVX]) mice are a suitable model to study osteoporosis. The estrogen-deficient mice show typical osteoporosis hallmarks such as reduction of BMD, reduced trabecular bone mass associated with overactivated osteoclast function (excess bone resorption) [41] and activation of T lymphocytes linked to osteoblast/osteocyte cell death [38]. Interestingly, estrogen-deficient OVX mice showed significant BMMSC damages including an increase in CFU-F number and cell proliferation, and a decrease in osteogenic capacity in vitro and in vivo [30]. When aspirin (0.6 mg/ml) was continuously given to OVX mice, their femurs showed a higher level of BMD than those control OXV, following 4 weeks of treatment (Figure 1) [30]. Aspirin was also shown to rescue impaired BMMSC function, such as recovering CFU-F number and osteogenic capacities. In addition, aspirin lessened osteoclast activity in OVX mice, as seen by decreased TRAP-positive cells and serum levels of sRANKL and CTX. When cultured BMMSCs were treated with aspirin, they showed improved anti-apoptotic capacity (Figure 2) and elevated mineralized tissue formation in vitro and in vivo [30]. Interestingly, aspirin was able to upregulate telomerase activity in BMMSCs in vitro [30], as seen in other cell types [42]. It was known that acquired telomerase activity in BMMSCs enhanced osteogenesis in vitro and in vivo via the Runx2 pathway [43]. Therefore, upregulation of telomerase activity in BMMSCs may contribute to aspirin-mediated improvement of osteogenesis. Aspirin-elevated telomerase levels in BMMSCs was much lower than that in cancer cells, implying a safe use of aspirin to improve BMMSC functions.
Figure 1. Representative horizontal μCT images of femurs.
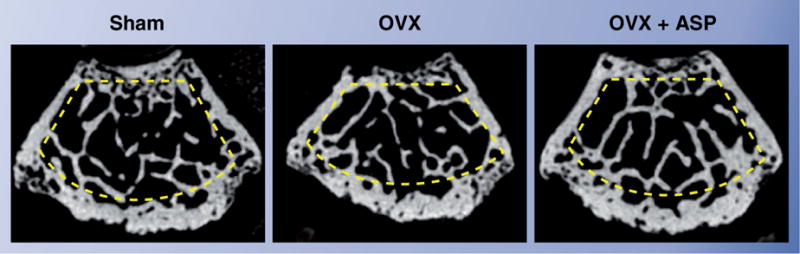
In OVX mice (B), the femur showed the decrement of trabecular bone mass (yellow circled area) when compared with the nonsurgery group (A). Aspirin treatment (OVX + ASP; (C)) improved the bone mass in OVX mice.
ASP: Aspirin; OVX: Ovariectomized.
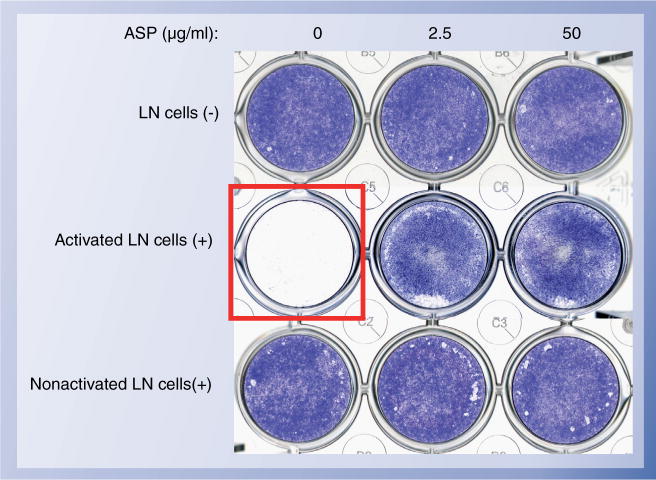
Figure 2. Representative images of co-culture with BMMSCs and LN cells.
BMMSCs were seeded on the culture wells, followed by the co-culture with [LN+] or without [LN−] LN cells in the presence or absence of ASP at indicated concentrations. LN cells were activated by plate-bounded anti-CD3 antibody (1 μg/ml) for three days or not before the co-culture. Three days after the co-culture, the wells were washed well and stained with toluidine blue (red box). Activated LN cells induced BMMSC death as shown BMMSC-non-staining well, but ASP treatment rescues the BMMSC death under the co-culture with activated LN cells. Nonactivated LN cells were not capable of the BMMSC death stimulated with or without ASP.
ASP: Aspirin; BMMSC: Bone marrow mesenchymal stem cell; LN: Lymph node.
Here, we provided experimental evidence that activated T lymphocytes are responsible for the BMMSC apoptosis through the Fas/FasL pathway, resulting in an accelerated osteoporosis phenotype in OVX mice. Moreover, aspirin appears to prevent osteoporosis by inhibiting BMMSC apoptosis and osteoclast-mediated bone resorption. Therefore, pharmacologic targeted regulation of BMMSCs by aspirin may offer a new approach for estrogen-deficient osteoporosis treatment. However, more detailed studies on the mechanism of aspirin-mediated anti-osteoporosis and proper dosing is critical to elucidate the role of aspirin in osteoporosis treatment.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Songtao Shi, Associate Professor, Center for Craniofacial Molecular Biology, University of Southern California School of Dentistry, 2250 Alcazar Street, CSA 103, Los Angeles, CA 90033, USA, Tel.: +1 323 442 3038; Fax: +1 323 442 2981; songtaos@usc.edu.
Takayoshi Yamaza, Center for Craniofacial Molecular Biology, University of Southern California School of Dentistry, 2250 Alcazar Street, CSA 103, Los Angeles, CA 90033, USA.
Kentaro Akiyama, Center for Craniofacial Molecular Biology, University of Southern California School of Dentistry, 2250 Alcazar Street, CSA 103, Los Angeles, CA 90033, USA.
Bibliography
- 1.Raisz LG. Pathogenesis of osteoporosis: concepts, conflict, and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh MC, Kim N, Kadono Y, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- 3.Weitzmann MN, Pacifici R. The role of T lymphocytes in bone metabolism. Immunol Rev. 2005;208:154–168. doi: 10.1111/j.0105-2896.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 4.Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev. 2005;208:207–227. doi: 10.1111/j.0105-2896.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 5.Hock JM, Krishnan V, Onyia JE, et al. Osteoblast apoptosis and bone turnover. J Bone Miner Res. 2001;16:975–984. doi: 10.1359/jbmr.2001.16.6.975. [DOI] [PubMed] [Google Scholar]
- 6.Manolagas SC. Cell number versus cell vigor: what really matters to a regenerating skeleton? Endocrinology. 1999;140:4377–4381. doi: 10.1210/endo.140.10.7129. [DOI] [PubMed] [Google Scholar]
- 7.Xing L, Boyce BF. Regulation of apoptosis in osteoclasts and osteoblastic cells. Biochem Biophys Res Commun. 2005;328:709–720. doi: 10.1016/j.bbrc.2004.11.072. [DOI] [PubMed] [Google Scholar]
- 8.Jilka RL, Weinstein RS, Parfitt AM, Manolagas SC. Quantifying osteoblast and osteocyte apoptosis: challenge and rewards. J Bone Miner Res. 2007;22:1492–1501. doi: 10.1359/jbmr.070518. [DOI] [PubMed] [Google Scholar]
- 9.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 11.Prockop DJ. Marrow stromal cells as stem cells for non-hematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka Y, Nakayamada S, Okada Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:325–328. doi: 10.2174/1568010054022015. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 14.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 15.Arai F, Hirano A, Ohmura M, et al. Tie2/angiopoietin-1 signalling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 17.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 18.Uccelli A, Pistoia V, Moretta L. Mesenchymal stem cells: A new strategy for immunosuppression? Trends Immunol. 2007;28:219–226. doi: 10.1016/j.it.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 20.Rasmusson I, Le Blanc K, Sundberg B, Ringdén O. Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand J Immunol. 2007;65:336–343. doi: 10.1111/j.1365-3083.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 21.Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 22.Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymalstemcell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 24.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Armstrong MA, Li G. Mesenchymal stem cells in immunoregulation. Immunol Cell Biol. 2006;84:413–421. doi: 10.1111/j.1440-1711.2006.01458.x. [DOI] [PubMed] [Google Scholar]
- 26.Koç ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 27.Noort WA, Kruisselbrink AB, in’t Anker PS, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34 cells in NOD/SCID mice. Exp Hematol. 2002;30:870–878. doi: 10.1016/s0301-472x(02)00820-2. [DOI] [PubMed] [Google Scholar]
- 28.Scholz M, Cinatl J. Fas/FasL interaction: a novel immune therapy approach with immobilized biologicals. Med Res Rev. 2005;25:331–342. doi: 10.1002/med.20025. [DOI] [PubMed] [Google Scholar]
- 29.Brunner T, Wasem C, Torgler R, et al. Fas (CD95/Apo-1) ligand regulation in T-cell homeostasis, cell-mediated cytotoxicity and immune pathology. Semin Immunol. 2003;15:167–176. doi: 10.1016/s1044-5323(03)00035-6. [DOI] [PubMed] [Google Scholar]
- 30.Yamaza T, Miura Y, Bi Y, et al. Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS ONE. 2008;3(7):e2615. doi: 10.1371/journal.pone.0002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kataoka T, Shinohara N, Takayama H, et al. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol. 1996;156:3678–3686. [PubMed] [Google Scholar]
- 32.Kogianni G, Mann V, Ebetino F, et al. Fas/CD95 is associated with glucocorticoid-induced osteocyte apoptosis. Life Sci. 2004;75:2879–2895. doi: 10.1016/j.lfs.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 33.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD41 T cells induce or protect from chronic intestinal inflammation in CB-17 SCID mice. Int Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 34.Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD41 T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice Disease development is prevented by cotransfer of purified CD41 T cells. J Exp Med. 1993;178:237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Read S, Malmstrom V, Powrie F. Cytotoxic T-lymphocyte associated antigen 4 plays an essential role in the function of CD251CD41 regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sylvester FA. IBD and skeletal health: children are not small adults! Inflamm Bowel Dis. 2005;11:1020–1023. doi: 10.1097/01.mib.0000188341.96726.15. [DOI] [PubMed] [Google Scholar]
- 37.Rothfuss KS, Stange EF, Herrlinger KR. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J Gastroenterol. 2006;12:4819–4831. doi: 10.3748/wjg.v12.i30.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roggia C, Gao Y, Cenci S, et al. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci USA. 2001;98:13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carbone LD, Tylavsky FA, Cauley JA, et al. Association between bone mineral density and the use of nonsteroidal anti-inflammatory drugs and aspirin: impact of cyclooxygenase selectivity. J Bone Miner Res. 2003;18:1795–1802. doi: 10.1359/jbmr.2003.18.10.1795. [DOI] [PubMed] [Google Scholar]
- 40.Bauer DC, Orwoll ES, Fox KM, et al. Aspirin and NSAID use in older women: effect on bone mineral density and fracture risk: study of osteoporotic fractures research group. J Bone Miner Res. 1996;11:29–35. doi: 10.1002/jbmr.5650110106. [DOI] [PubMed] [Google Scholar]
- 41.Miura M, Chen X-D, Allen MR, et al. A crucial role of Caspase-3 in osteogenic differentiation of bone marrow stromal stem cells. J Clin Invest. 2004;114:1704–1713. doi: 10.1172/JCI20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bode-Boger SM, Martens-Lobenhoffer J, Tager M, Schroder H, Scalera F. Aspirin reduces endothelial cell senescence. Biochem Biophys Res Commun. 2005;334:1226–1232. doi: 10.1016/j.bbrc.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 43.Shi S, Gronthos S, Chen S, Counter CM, Robey PG, Wang CY. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002;20:587–591. doi: 10.1038/nbt0602-587. [DOI] [PubMed] [Google Scholar]