Abstract
Purpose
Albumin-bound paclitaxel, ABI-007 (Abraxane ®), has a different toxicity profile than solvent-based paclitaxel, including a lower rate of severe neutropenia. The combination of ABI-007 and carboplatin may have significant activity in a variety of tumor types including non-small and small cell lung cancer, ovarian cancer, and breast cancer. The purpose of this study was to determine the maximum tolerated dose (MTD) of ABI-007, on three different schedules in combination with carboplatin.
Methods
Forty-one patients with solid tumors were enrolled, and received ABI-007 in combination with carboplatin AUC of 6 on day 1. Group A received ABI-007 at doses ranging from 220 to 340 mg/m2 on day 1 every 21 days; group B received ABI-007 at 100 or 125 mg/m2 on days 1, 8, and 15 every 28 days; and group C received ABI-007 125 or 150 mg/m2 on days 1 and 8 every 21 days. Dose-limiting toxicities were assessed after the first cycle. Doses were escalated in cohorts of three to six patients. Fifteen patients participated in a pharmacokinetic study investigating the effects of the sequence of infusion. ABI-007 was infused first followed by carboplatin in cycle 1, and vice versa in cycle 2.
Results
The MTD of ABI-007 in combination with carboplatin was 300, 100, and 125 mg/m2 in groups A, B, and C, respectively. Myelosuppression was the primary dose limiting toxicity. No unexpected or new toxicities were reported. Sequence of infusion did not affect either the pharmacokinetics of ABI-007 or the degree of neutropenia. Responses were seen in melanoma, lung, bladder, esophageal, pancreatic, breast cancer, and cancer of unknown primary.
Conclusions
The recommended dose for phase II studies of ABI-007 in combination with carboplatin (AUC of 6) is 300, 100, 125 mg/m2 for the schedules A, B, and C, respectively. The combination of ABI-007 and carboplatin is well tolerated and active in this heavily pretreated patient population.
Keywords: Dose-limiting toxicity, Maximum tolerated dose, Melanoma, Non-small-cell lung cancer, Small-cell lung cancer, Clinical trial
Introduction
Solvent-based paclitaxel has a wide spectrum of activity and is frequently used as a single agent or in combination with other chemotherapeutic or biologic agents in the treatment of cancers of the breast, ovary, lung, and head and neck. While highly effective, solvent-based paclitaxel has several burdensome side effects, including hypersensitivity reactions, peripheral neuropathy, neutropenia, alopecia, mucositis, arthalgias, myalgias, and mild nausea [1, 2]. Paclitaxel is highly hydrophobic, and commercially available formulations require polyethoxylated Castor oil as a solvent to allow parenteral administration. The polyethoxylated Castor oil solvent may be responsible for the hypersensitivity reactions and the development of neuropathy [3, 4]. ABI-007 (Abraxane®, Abraxis BioScience Inc, Los Angeles, CA) is a solvent-free, albumin-bound paclitaxel that delivers paclitaxel as a suspension of albumin particles in saline. This formulation does not require premedication for hypersensitivity reactions, allows for a shorter infusion time (30 min) compared with solvent-based paclitaxel, and is not associated with hypersensitivity reactions [5].
A phase III clinical trial in patients with metastatic breast cancer compared treatment with solvent-based paclitaxel (175 mg/m2) versus treatment with ABI-007 (260 mg/m2) every 3 weeks [5]. Patients who were treated with ABI-007 had a statistically significant higher objective response rates, and longer time to progression compared with patients who were treated with solvent-based paclitaxel. Patients who were treated with ABI-007 had a statistically significant lower incidence of grade 4 neutropenia (9 vs. 22%, respectively; P < 0.001) and a higher rate of grade 3 sensory neuropathy (10 vs. 2%, respectively; P < 0.001) than patients who were treated with solvent-based paclitaxel.
Carboplatin and solvent-based paclitaxel is a standard therapy for non-small cell lung cancer (NSCLC), ovarian cancer, and metastatic breast cancer [6–8]. There is significant interest in investigating the combination of ABI-007 and carboplatin since the combination may have a lower rate of toxicity and/or greater efficacy than carboplatin and solvent-based paclitaxel in multiple different types of malignancies. ABI-007 and solvent based paclitaxel have significant differences in their standard doses and toxicities. There is also the potential for additive toxicity with the combination of ABI-007 and carboplatin, and safety and pharmacokinetic data of ABI-007 in combination with carboplatin is not available. Therefore, we performed a phase I trial to determine the maximum tolerated dose (MTD) of ABI-007 in combination with carboplatin.
A phase I trial of cisplatin and solvent-based paclitaxel found the sequence of infusion had a significant impact on the degree of neutropenia [9]. The sequence of cisplatin before solvent-based paclitaxel had more profound neutropenia than the sequence of solvent-based paclitaxel before cisplatin. Pharmacokinetic measurements suggested this difference was related to a 25% lower paclitaxel clearance rates when cisplatin infusion preceded paclitaxel. By contrast, a phase I trial of carboplatin and solvent-based paclitaxel in 55 chemotherapy-naïve NSCLC patients investigated the influence of sequence of infusion of solvent-based paclitaxel and carboplatin, and did not find any sequence-independent pharmacokinetic interaction [10]. There was no significant difference in the area under of the curve of carboplatin between the two sequences of infusions. In a separate phase II trial in 40 patients with gynecologic malignancies the rate of neutropenia was not influenced by the sequence of administration of carboplatin and solvent-based paclitaxel [11]. Given these observations; we investigated whether the sequence of administration of ABI-007 and carboplatin had an effect on the pharmacokinetics of ABI-007 and the degree of neutropenia.
Patients and methods
Patients
Adults with advanced solid tumors, which had progressed on standard therapy or for which there was no standard therapy, were eligible for inclusion in this study. Eligibility criteria included an Eastern Cooperative Oncology Group (ECOG) performance status of zero to two, an absolute neutrophil count (ANC) ≥1.5 × 109 L−1, platelet count ≥100 × 109 L−1, and hemoglobin concentration ≥8.0 g/dL. Adequate renal function, defined as a serum creatinine in the normal range or a Cockcroft-Gault calculated creatinine clearance of ≥60 mL/min, and hepatic function, defined as hepatic transaminases ≤2.5 upper limit of normal and total bilirubin within the normal range, were required. Patients were not eligible if they had received chemotherapy, radiation therapy, or immunotherapy for treatment of malignancy within 3 weeks. No limit was set on the number of previous therapies, and patients with previous exposure to taxanes were eligible for the study. Patients were required to have evaluable disease by RECIST [12] or disease that was evaluable by tumor markers.
The protocol was approved by the Protocol Review Committee of Lineberger Comprehensive Cancer Center at the University of North Carolina at Chapel Hill, and the Institutional Review Board at the University of North Carolina. All patients gave written informed consent before any study-related procedures were done.
Study design
This was a phase I non-randomized single-center trial that evaluated safety of ABI-007 administered with carboplatin. The primary objective was to determine the maximum tolerated dose (MTD) of ABI-007 on three different schedules in combination with carboplatin. The secondary objectives were to evaluate sequence dependent effects on toxicity and pharmacokinetics of ABI-007, and to determine the anti-tumor activity of this combination as assessed by response. The initial study design included three dose levels on two treatment schedules, groups A and B; however, when no dose-limiting toxicities were observed in the first three patients at the third dose level in group A, the trial was amended to include a fourth dose level. The trial was subsequently amended to explore a third schedule of ABI-007 and carboplatin, group C; however, only two treatment arms were open simultaneously. Patients were not randomized between the treatment arms, and patients were enrolled in three patient cohorts alternating between the two open treatment arms. Three patients were enrolled at a dose level on one arm and after completion of enrollment at that dose level, the next three patients would be enrolled at a dose level on the alternate arm that was open at that time. This trial design was used in order to determine the MTD of ABI-007 on multiple schedules within the context of a single trial. The three schedules were evaluated separately and in parallel, and there is no intent to compare the treatment schedules with each other.
Patients in group A received carboplatin on day 1 and ABI-007 on day 1 every 21 days; patients in group B received carboplatin on day 1 and ABI-007 on days 1, 8, and 15 every 28 days; and patients in group C received carboplatin day 1 and ABI-007 on days 1 and 8 every 21 days. For patients enrolled in the pharmacokinetic portion of the trial, in the first cycle of therapy, ABI-007 was infused followed by carboplatin 30 min later, and vice versa during the second cycle.
Study drugs
ABI-007 (Abraxane) was supplied by the manufacturer and carboplatin (Paraplatin) was obtained through commercial sources. The dose of ABI-007 was calculated using the patient’s body surface area (BSA), with the maximum BSA of 2.0. The dose of carboplatin was fixed at an area under the curve (AUC) of six using the Cockcroft-Gault equation to estimate the glomerular filtration rate, and the Calvert equation to calculate the carboplatin dose [13]. Patients received antiemetic therapy with ondansteron and dexamethasone before each treatment with carboplatin and ABI-007 on day 1, and received dexamethasone 4 mg twice daily for 3 days following the day 1 treatment unless contraindicated. On treatment arms B and C patients did not receive antiemetic therapy prior to treatment with single agent ABI-007 on days 8 or 15. No premedications for hypersensitivity were given and the prophylactic use of filgrastim was prohibited during the first cycle. Patients who experienced a dose-limiting toxicity could continue on treatment at a reduced ABI-007 dose level at the discretion of the treating physician.
Dose escalation
Three patients were treated at the initial dose level for each treatment group, and if no cycle-1 dose-limiting toxicities were observed, three additional patients were treated at the next dose level. If one of three initial patients experienced a dose-limiting toxicity at any given dose level, three additional patients were treated at that same dose. If a dose-limiting toxicity occurred in at least two patients at any dose level, dose escalation was halted, and the next three patients enrolled onto that treatment group were treated at the next lower dose level. The MTD and the recommended treatment dose for each schedule were defined as the highest dose level at which fewer than two of six patients experienced a dose-limiting toxicity in cycle 1. Toxicity was assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3.0.
Dose-limiting toxicities were assessed after the first cycle, and were defined as grade ≥ 3 nonhematologic toxicity, grade 4 anemia or thrombocytopenia, or grade 4 neutropenia lasting ≥7 days or associated with fever. Patients were required to have all treatment-related toxicity from the previous cycle resolve to baseline or NCI CTCAE grade ≤ 1 before initiating the second cycle of therapy. A >2-week treatment delay of initiating the second cycle of chemotherapy was considered a dose-limiting toxicity. For patients receiving weekly treatment of ABI-007, treatment was omitted for patients with ANC <1.5 × 109 L−1 or a platelet count <100 × 109 L−1. Patients in group B who had myelosuppression that required omission of two of the three weekly treatments, and patients in group C who had myelosuppression that required omission of one of the two weekly treatments were considered to have had a dose-limiting toxicity.
Pharmacokinetic analysis
Whole blood was obtained from an indwelling venous catheter in the arm contralateral to the arm into which the drug was infused and samples were drawn at 11 prespecified time points (baseline, end of ABI-007 infusion, 1, 2, 3, 4, 5, 6, 20–28, 40–48, and 70–78 h after ABI-007 infusion). Approximately 5 mL of whole blood was drawn in sodium EDTA-containing tubes and an additional 7 mL of whole blood was drawn into heparinized tubes for each time point; the heparinized sample was centrifuged at 1,000×g for 10 min at 4°C and aliquots of plasma were collected and stored with the whole blood at −80°C until analysis. Paclitaxel was extracted from 10 µL whole blood and plasma samples using protein precipitation with methanol containing docetaxel as the internal standard (5:1 methanol:sample). Paclitaxel concentration was quantitated using high-performance liquid chromatography, and detection was performed by triple quad mass-spectrometry using electrospray atmospheric pressure ionization. (SCIEX API 4000, Applied Biosystems Concord, Ontario, Canada). The lower limit of quantitation was 10 nM, and the range for the standard curve was 10–30,000 nM. Quality controls were back calculated to within 15% of nominal.
Individual paclitaxel whole blood concentrations using WinNonlin 4.0 (Pharsight Corp., Mountain View, CA) were used to estimate maximum plasma concentration (Cmax), area under the concentration–time cure through infinity (AUC∞), area under the concentration time curve through the last measurable time point (AUCt); terminal half-life [t1/2], whole blood clearance (Cl), and apparent steady-state volume of distribution (Vss). The AUC was calculated using the Log-linear trapezoidal method. Vss was estimated from the product of mean residence time and Cl.
Statistical analysis
All pharmacokinetic data were log-transformed before analysis and pharmacokinetic parameters are reported as geometric mean and coefficient of variation. For AUC, data are reported as a ratio of log-transformed data and 90% confidence interval. A 2-tailed, paired students’ t test was used to test for significant differences between cycles. The correlation between AUC and ANC nadir was compared using Pearson’s correlation coefficient. Significance was set at P < 0.05.
Results
Patients
Forty-seven patients were enrolled on the trial, and 41 patients were treated. Most patients had either lung cancer or melanoma, were heavily pretreated, and most patients were white (Table 1). The dose levels of ABI-007, number of patients, and number of cycles received for each dose level are included in the toxicity tables (Tables 2, 3).
Table 1.
Patient characteristics at baseline
| Characteristic | |
| Total no. patients | 41 |
| Age (years) | |
| Median (range) | 58 (32–75) |
| ECOG Performance Status | |
| 0 | 16 (39%) |
| 1 | 22 (54%) |
| 2 | 3 (7%) |
| Sex | |
| Men | 21 (51%) |
| Women | 20 (49%) |
| Race | |
| White | 32 (78%) |
| Black | 8 (20%) |
| Latino | 1 (2%) |
| Median number previous therapies | 3 |
| Malignancy | |
| Non-small-cell lung | 12 (29%) |
| Melanoma | 10 (24%) |
| Small-cell lung | 3 (7%) |
| Breasta | 3 (7%) |
| Soft tissue sarcoma | 3 (7%) |
| Unknown primary | 2 (5%) |
| Gastric | 2 (5%) |
| Otherb | 6 (10%) |
ECOG, Eastern Cooperative Oncology Group
One patient with breast cancer had phyllodes tumor
One each: bladder, colorectal, esophageal, pancreatic, penile, prostate
Table 2.
All episodes of treatment-related hematologic toxicities
| Treatment cohort (mg/m2) |
No. patients |
No. cycles |
Neutropenia | Anemia | Thrombocytopenia | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | ||||||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |||
| Group A | ||||||||||||||
| 220 | 3 | 10 | 1 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 2 | 1 | 1 | 0 |
| 260 | 3 | 11 | 0 | 1 | 0 | 0 | 2 | 3 | 0 | 0 | 2 | 1 | 1 | 1 |
| 300 | 6 | 18 | 3 | 0 | 2 | 0 | 5 | 2 | 0 | 0 | 3 | 1 | 0 | 0 |
| 340 | 5 | 15 | 2 | 1 | 1 | 0 | 5 | 3 | 1 | 0 | 3 | 3 | 1 | 1 |
| Group B | ||||||||||||||
| 100 | 7 | 24 | 3 | 2 | 4 | 3 | 7 | 4 | 1 | 0 | 6 | 3 | 2 | 0 |
| 125 | 6 | 23 | 4 | 3 | 2 | 0 | 6 | 4 | 0 | 0 | 4 | 2 | 2 | 1 |
| Group C | ||||||||||||||
| 125 | 7 | 23 | 6 | 6 | 3 | 3 | 7 | 4 | 1 | 0 | 5 | 3 | 3 | 2 |
| 150 | 4 | 11 | 1 | 2 | 2 | 1 | 4 | 2 | 1 | 0 | 3 | 3 | 2 | 1 |
Numbers represent episodes of toxicity observed during all cycles
Table 3.
All episodes of treatment-related nonhematologic toxicities
| Treatment cohort (mg/m2) |
No. patients |
No. cycles |
Myalgia/ arthralgia |
Neuropathy | Nausea/ vomiting |
Fatigue | Alopecia | Infection | Febrile neutropenia |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | ||||||||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |||
| Group A | ||||||||||||||||||||||||||||
| 220 | 3 | 10 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 260 | 3 | 11 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 300 | 6 | 18 | 2 | 0 | 0 | 0 | 3 | 2 | 2 | 0 | 3 | 0 | 0 | 0 | 3 | 2 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 340 | 5 | 15 | 0 | 0 | 1 | 0 | 5 | 2 | 0 | 0 | 2 | 3 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Group B | ||||||||||||||||||||||||||||
| 100 | 7 | 24 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 3 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 125 | 6 | 23 | 0 | 1 | 0 | 0 | 4 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 5 | 0 | 0 | 3 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Group C | ||||||||||||||||||||||||||||
| 125 | 7 | 23 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 150 | 4 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
Numbers represent episodes of toxicity observed during all cycles
Determination of the maximum tolerated dose
In group A, no dose-limiting toxicities were observed at the first three dose levels (220, 260, or 300 mg/m2). At 340 mg/m2, one of the first three patients developed a dose-limiting toxicity (grade 3 myalgias/arthralgias) so the cohort was expanded. The first two patients of the expanded cohort were enrolled simultaneously, and experienced a dose-limiting toxicity (grade 3 nausea/vomiting and hyperglycemia (n = 1); and grade 3 nonneutropenic infection and nausea/vomiting (n = 1). In the interim, the third patient, who had not experienced significant toxicity prior to the expansion, experienced a 2-week treatment delay in initiating the second cycle of chemotherapy related to thrombocytopenia (platelet count 75–100 × 109 L−1). Three additional patients were enrolled at the previous dose level, 300 mg/m2, and none experienced a dose-limiting toxicity.
In group B, no dose-limiting toxicities were observed in the first three patients at 100 mg/m2. At the next dose level, 125 mg/m2, one of the first three patients experienced a dose-limiting toxicity (grade 3 dehydration and grade 4 thrombocytopenia). Three additional patients were enrolled, and one patient had a dose-limiting toxicity (grade 3 thrombocytopenia requiring the omission of day-8 and day-15 treatment). An additional three patients were enrolled at the previous dose level, 100 mg/m2. One patient received treatment on day 8 with grade 3 thrombocytopenia in violation of the treatment protocol and was considered nonevaluable for determination of the MTD. Three additional patients did not experience a dose-limiting toxicity; however, two of the six evaluable patients had omission of the day-15 treatment related to myelosuppression [grade 3 neutropenia (n = 1); and grade 3 neutropenia/thrombocytopenia (n = 1)].
In group C, one of the first three patients experienced a dose-limiting toxicity (grade 4 thrombocytopenia), and four additional patients were enrolled, none of whom experienced a dose-limiting toxicity. At the next dose level, one of the first three patients, and the fourth patient experienced a dose-limiting toxicity [grade 3 infection (n = 1); grade 3 febrile neutropenia/grade 4 thrombocytopenia (n = 1)].
The primary dose-limiting toxicity was myelosuppression, and the MTD of ABI-007 in combination with full dose carboplatin (AUC = 6) was 300, 100, and 125 mg/m2 for groups A, B, and C, respectively.
Toxicities
Hematologic toxicity
All the hematologic toxicity is summarized in Table 2. Fourteen patients experienced grade 3 or 4 neutropenia, and three patients experienced grade 3 febrile neutropenia. Twelve patients developed grade 3 or 4 thrombocytopenia, and three patients required platelet transfusion for an absolute platelet count <10 × 109 L−1 (1 patient) or <25 × 109 L−1 with mucocutaneous bleeding (2 patients). Five patients developed grade 3 anemia, 13 patients required transfusion of packed red blood cells, and 14 patients required erythropoietin growth factor support.
Nonhematologic toxicity
All the non-hematologic toxicities consisted of mylagias, nausea, vomiting, fatigue and alopecia, and toxicities for all cycles are summarized in Table 3. Five patients developed grade 2 and three patients developed grade 3 sensory neuropathy. Most of these patients had resolution of the neuropathy or return to baseline or grade ≤ 1 with discontinuation of the drug; however, one patient had persistent grade 3 neuropathy for 6 months. Six of the eight patients who developed ≥2 grade neuropathy had previous treatment with a drug known to cause sensory neuropathy (i.e., oxaliplatin, carboplatin, interferon, docetaxel, or solvent-based paclitaxel).
Responses
Thirty-seven patients were evaluable for response according to RECIST after two cycles of therapy. The best response for the patient is reported, and patients who experienced stable disease or response did not undergo radiological confirmation of response. Responses were noted in 12 patients (melanoma, n = 3; NSCLC, n = 3; and one each for small cell lung cancer (SCLC), breast, pancreatic, bladder, esophageal, and cancer of unknown primary). Twelve additional patients had stable disease (NSCLC, n = 6; melanoma, n = 3, SCLC, n = 2, breast, n = 1). One additional patient with adenocarcinoma of unknown primary did not have evaluable disease by RECIST, but had an elevated of CA-125 that had correlated with disease progression and response in the past. At baseline, this patient’s CA-125 value was 503 U/mL, and declined to normal (15.2 U/mL) after three cycles, and remained within normal limits for nine months. A patient with prostate cancer had radiographically stable disease; however, his prostate-specific antigen concentration (PSA) increased from a baseline of 1,810–2,760 ng/mL after two cycles, and was classified as progressive disease.
Pharmacokinetic results
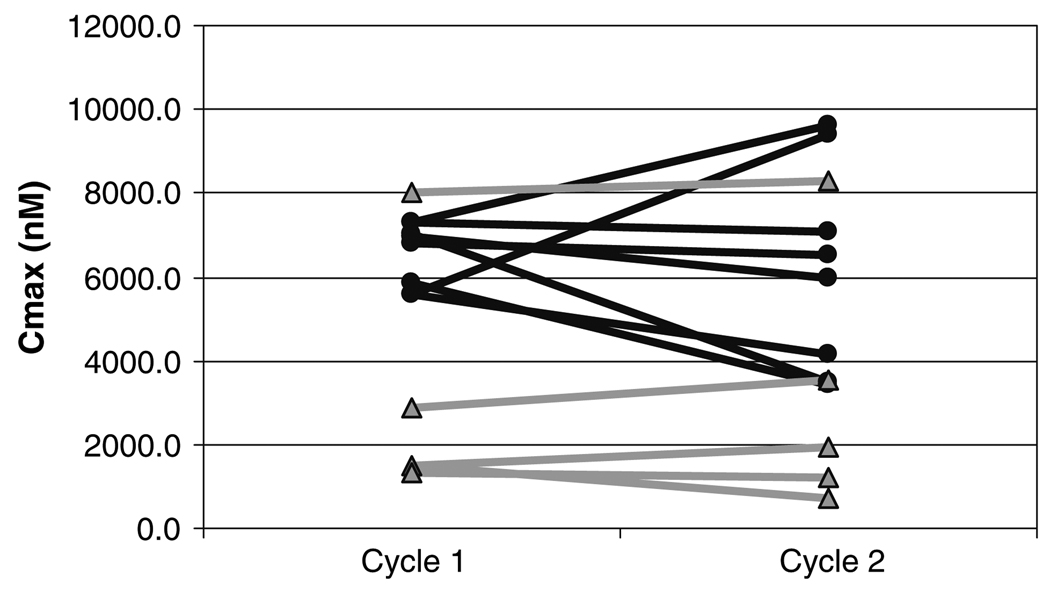
Fifteen patients completed the first two cycles of chemotherapy, and 14 were evaluable for analysis. The pharmacokinetic parameters are shown in Table 4. The point estimate of the log transformed AUC ratio between cycle 1 and cycle 2 was 1.01 with a coefficient variance of 1.604. The AUC of ABI-007 was not significantly different between cycle 1 and cycle 2 (P = 0.20) (Fig. 1). No difference was seen between the ANC nadir between cycle 1 and cycle 2 (2.2 × 109 L−1 vs. 1.9 × 109 L−1, respectively; R2 = 0.17, P = 0.33).
Table 4.
Paclitaxel whole blood pharmacokinetic parameters for cycle 1 and cycle 2
| Dose (mg/m2) | N | Cmax (ng/mL) | AUCinf (ng h/mL) | T1/2 (12) | CL (L/h) | CL (L/h/m2) | Vd (L/m2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geo mean |
CV% | Geo mean |
CV% | Geo mean |
CV% | Geo mean |
CV% | Geo mean |
CV% | Geo mean |
CV% | ||
| Cycle 1: ABI-007→Carboplatin | |||||||||||||
| 100 | 2 | 1275.23 | 0.21 | 1756.03 | 9.18 | 10.43 | 30.61 | 111.01 | 10.92 | 50.24 | 1.09 | 461.63 | 31.61 |
| 125 | 4 | 2467.79 | 38.84 | 5086.65 | 19.15 | 13.98 | 35.72 | 44.64 | 21.31 | 24.57 | 19.19 | 397.49 | 3.75 |
| 220 | 2 | 4894.96 | 1.40 | 5630.09 | 0.57 | 13.03 | 26.05 | 74.14 | 3.88 | 35.67 | 5.16 | 325.18 | 14.74 |
| 260 | 3 | 6058.19 | 0.95 | 9312.31 | 13.57 | 10.94 | 10.71 | 48.27 | 15.47 | 27.92 | 13.57 | 243.37 | 5.19 |
| 300 | 3 | 5558.61 | 6.14 | 12163.00 | 10.93 | 8.37 | 8.16 | 45.16 | 15.25 | 24.27 | 9.59 | 164.96 | 6.64 |
| Cycle 2: carboplatin→ABI-007 | |||||||||||||
| 100 | 3 | 1155.70 | 25.90 | 1970.26 | 13.01 | 14.62 | 2.65 | 100.66 | 12.91 | 45.57 | 10.71 | 628.39 | 8.73 |
| 125 | 3 | 2820.76 | 51.39 | 5637.54 | 25.52 | 19.90 | 39.68 | 39.25 | 26.47 | 22.22 | 25.62 | 399.76 | 12.44 |
| 220 | 2 | 3205.81 | 5.96 | 4793.92 | 1.01 | 14.11 | 17.63 | 88.27 | 1.40 | 41.89 | 6.83 | 523.68 | 1.11 |
| 260 | 3 | 5006.85 | 24.60 | 7659.23 | 21.45 | 11.31 | 34.71 | 57.97 | 22.51 | 33.95 | 21.46 | 277.32 | 2.84 |
| 300 | 3 | 6462.87 | 8.73 | 13191.32 | 10.60 | 11.52 | 24.06 | 41.62 | 15.84 | 22.38 | 9.26 | 217.80 | 23.35 |
Fig. 1.
Comparison of AUC of ABI-007 between cycle 1 and cycle 2 (filled circle q 3 weekly patients, filled triangle weekly patients, P = 0.20 for all patients)
Discussion
This phase I trial was designed to evaluate the feasibility of administering ABI-007 in combination in carboplatin using three different ABI-007 treatment schedules. In group A, we were able to combine ABI-007 at 300 mg/m2 every 21 days with standard-dose carboplatin. This ABI-007 dose was the MTD reported in the single-agent, phase I trial and the dose used in a previously reported single agent phase II trial [14, 15] This dose is higher than that achieved with solvent-based paclitaxel, which is typically combined at a dose of 175–225 mg/m2 with carboplatin AUC = 6 [6, 16, 17].
The MTD seen with single-agent ABI-007 on the treatment schedule evaluated in group B (ABI-007 on days 1,8, and 15 every 28 days) was 100 mg/m2 in the heavily pretreated patients and 150 mg/m2 in the less heavily pretreated patients [18]. The MTD of ABI-007 in combination with carboplatin in of group B corresponds to the MTD in the heavily pretreated population of the single agent ABI-007 on the same schedule. We did not prospectively stratify patients based on their previous therapy; however, five of the seven patients at the 100 mg/m2 dose level, and the two patients who experienced dose-limiting toxicities at the 125 mg/m2 dose would be considered as heavily pretreated using the criteria in the single-agent phase I study [18].
Two heavily pretreated patients at the 100 mg/m2 required omission of the day-15 treatment because of myelosuppression [grade 3 neutropenia (n = 1); and grade 3 neutropenia/thrombocytopenia (n = 1)]. Treatment in a chemotherapy naïve patient population may result in a lower rate of myelosuppresion and improve the delivery of the day 15 chemotherapy. Preliminary data from two phase II trials of carboplatin (AUC = 6) and ABI-007 100 mg/m2 on the same treatment schedule have demonstrated an acceptable toxicity profile [19, 20]. ABI-007 has not been investigated as a single agent on the treatment schedule of days 1, 8 every 21 days as used in group C. The schedule of carboplatin every 3 weeks may be advantageous in diseases that respond to carboplatin-based therapy. This schedule is being further investigated as part of an ongoing phase II trial in NSCLC.
The most frequent toxicities seen in this study were myelosuppression, sensory neuropathy, myalgias/athralgias, nausea, vomiting, and alopecia, which are the toxicities typically seen with carboplatin and solvent-based paclitaxel [21]. The incidence sensory neuropathy is higher with ABI-007 than with solvent-based paclitaxel [5]. The incidence of neuropathy is known to increase significantly after four cycles of carboplatin and solvent-based paclitaxel and the incidence of sensory neuropathy with carboplatin and ABI-007 may be cumulative as well [21]. Given the small size of this study, the differences in ABI-007 doses and number of cycles, and prior therapy patients received, it is difficult to determine if there was a relationship between the dose of ABI-007 and the development of neuropathy in this study.
The optimal method of dosing of palliative chemotherapy in patients with a significantly elevated BSA is controversial. The practice of using a maximum BSA is a debatable; however, frequently used method to address this clinical situation. This trial used a maximum BSA of 2.0, and this practice resulted in a ≥10% change in the dose of ABI-007 in five patients. In reviewing the toxicity data and the dose escalation process in this trial the use of a maximum BSA of 2.0 did not influence the determination of the MTD.
The infusion sequence did not affect the pharmacokinetics of ABI-007, and the ANC nadir. This finding is suggests that alteration of the sequence of infusion of ABI-007 and carboplatin may not result in a clinically relevant outcome such as seen with cisplatin and solvent-based paclitaxel [9]. Our results are consistent with previous data that has shown that sequence of infusion of carboplatin and solvent-based paclitaxel did not impact the pharmacokinetics of solvent-based paclitaxel or the degree of neutropenia [10, 11].
In conclusion, in this heavily pretreated population of refractory cancer patients, the combination of ABI-007 and carboplatin was well tolerated with an acceptable toxicity profile on all three schedules.
Acknowledgments
The authors thank Paul E Jones, Tammy Allred, Susan Natoli and Henry Bell for their help with the study, and the patients whose participation made this trial possible. Financially supported by the Grant for General Clinical Research Center (Grant # RR00046) and Abraxis Bioscience.
Footnotes
Previously presented as an abstract and poster presentation at the San Antonio Breast Cancer Symposium, San Antonio, TX, December 8–11, 2005.
Contributor Information
Thomas E. Stinchcombe, Department of Hematology/Oncology, Developmental Therapeutics Group Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC, USA
Mark A. Socinski, Department of Hematology/Oncology, Developmental Therapeutics Group Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC, USA
Christine M. Walko, School of Pharmacy, University of North Carolina, Chapel Hill, NC, USA
Bert H. O’Neil, Department of Hematology/Oncology, Developmental Therapeutics Group Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC, USA
Frances A. Collichio, Department of Hematology/Oncology, Developmental Therapeutics Group Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC, USA
Anastasia Ivanova, Department of Biostatics, Lineberger Comprehensive Cancer Center, Chapel Hill, NC, USA.
Hua Mu, Abraxis BioScience, Los Angeles, CA, USA.
Michael J. Hawkins, Abraxis BioScience, Los Angeles, CA, USA
Richard M. Goldberg, Department of Hematology/Oncology, Developmental Therapeutics Group Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC, USA
Celeste Lindley, School of Pharmacy, University of North Carolina, Chapel Hill, NC, USA.
E Claire Dees, Department of Hematology/Oncology, Developmental Therapeutics Group Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC, USA.
References
- 1.Socinski MA. Single-agent paclitaxel in the treatment of advanced non-small cell lung cancer. Oncologist. 1999;4(5):408–416. [PubMed] [Google Scholar]
- 2.Crown J, O’Leary M. The taxanes: an update. Lancet. 2000;355(9210):1176–1178. doi: 10.1016/S0140-6736(00)02074-2. [DOI] [PubMed] [Google Scholar]
- 3.Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590–1598. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 4.Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. Assessment of neurotoxicity following repeated cremophor/ethanol injections in rats. Neurotox Res. 2001;3(3):301–306. doi: 10.1007/BF03033269. [DOI] [PubMed] [Google Scholar]
- 5.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 6.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 7.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 8.Burris H, 3rd, Yardley D, Jones S, Houston G, Broome C, Thompson D, et al. Phase II trial of trastuzumab followed by weekly paclitaxel/carboplatin as first-line treatment for patients with metastatic breast cancer. J Clin Oncol. 2004;22(9):1621–1629. doi: 10.1200/JCO.2004.08.065. [DOI] [PubMed] [Google Scholar]
- 9.Rowinsky EK, Gilbert MR, McGuire WP, Noe DA, Grochow LB, Forastiere AA, et al. Sequences of taxol and cisplatin: a phase I and pharmacologic study. J Clin Oncol. 1991;9(9):1692–1703. doi: 10.1200/JCO.1991.9.9.1692. [DOI] [PubMed] [Google Scholar]
- 10.Huizing MT, Giaccone G, van Warmerdam LJ, Rosing H, Bakker PJ, Vermorken JB, et al. Pharmacokinetics of paclitaxel and carboplatin in a dose-escalating and dose-sequencing study in patients with non-small-cell lung cancer The European cancer centre. J Clin Oncol. 1997;15(1):317–329. doi: 10.1200/JCO.1997.15.1.317. [DOI] [PubMed] [Google Scholar]
- 11.Markman M, Elson P, Kulp B, Peterson G, Zanotti K, Webster K, et al. Carboplatin plus paclitaxel combination chemotherapy: impact of sequence of drug administration on treatment-induced neutropenia. Gynecol Oncol. 2003;91(1):118–122. doi: 10.1016/s0090-8258(03)00517-1. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, National cancer institute of the United States, National cancer institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Calvert AH, Newell DR, Gumbrell LA, O’Reilly S, Burnell M, Boxall FE, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7(11):1748–1756. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim NK, Samuels B, Page R, Doval D, Patel KM, Rao SC, et al. Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J Clin Oncol. 2005;23(25):6019–6026. doi: 10.1200/JCO.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8(5):1038–1044. [PubMed] [Google Scholar]
- 16.Kosmidis P, Mylonakis N, Skarlos D, Samantas E, Dimopoulos M, Papadimitriou C, et al. Paclitaxel (175 mg/m2) plus carboplatin (6 AUC) versus paclitaxel (225 mg/m2) plus carboplatin (6 AUC) in advanced non-small-cell lung cancer (NSCLC): a multicenter randomized trial. Hellenic cooperative oncology group (HeCOG) Ann Oncol. 2000;11(7):799–805. doi: 10.1023/a:1008389402580. [DOI] [PubMed] [Google Scholar]
- 17.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 18.Nyman DW, Campbell KJ, Hersh E, Long K, Richardson K, Trieu V, et al. Phase I and pharmacokinetics trial of ABI-007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. J Clin Oncol. 2005;23(31):7785–7793. doi: 10.1200/JCO.2004.00.6148. [DOI] [PubMed] [Google Scholar]
- 19.Allerton JP, Hagenstad CT, Webb RT, Smith GB, Birch R, Goggins TF, et al. A phase II evaluation of the combination of paclitaxel protein-bound and carboplatin in the first line treatment of advanced non-small cell lung cancer (NSCLC) J Clin Oncol (ASCO Annual Meetings Proceedings) 2006;24(18S):7127. (abstract) [Google Scholar]
- 20.Hawkins MJ, Georgy M, Makhson A, Cheporov S, Sergey O, Yablonsky P, et al. Dose escalation study of nab-paclitaxel followed by carboplatin a first line therapy in advanced non-small cell lung cancer (NSCLC) J Clin Oncol (ASCO Annual Meetings Proceedings) 2006;24(18S):7132–(abstract). [Google Scholar]
- 21.Socinski MA, Schell MJ, Peterman A, Bakri K, Yates S, Gitten R, et al. Phase III trial comparing a defined duration of therapy versus continuous therapy followed by second-line therapy in advanced-stage IIIB/IV non-small-cell lung cancer. J Clin Oncol. 2002;20(5):1335–1343. doi: 10.1200/JCO.2002.20.5.1335. [DOI] [PubMed] [Google Scholar]