Abstract
Purpose
Ewing sarcoma family tumors (ESFTs) exhibit chromosomal translocations that lead to the creation of chimeric fusion oncogenes. Combinatorial diversity among chromosomal breakpoints produces varying fusions. The type 1 EWS-FLI1 transcript is created as a result of fusion between exons 7 of EWS and 6 of FLI1, and retrospective studies have reported that type 1 tumors are associated with an improved outcome. We have re-examined this association in a prospective cohort of patients with ESFT treated according to current Children's Oncology Group (COG) treatment protocols.
Methods
Frozen tumor tissue was prospectively obtained from patients diagnosed with ESFT, and reverse transcriptase polymerase chain reaction (RT-PCR) was used to determine translocation status. Analysis was confined to patients with localized tumors who were diagnosed after 1994 and treated according to COG protocols. Translocation status was correlated with disease characteristics, event-free survival (EFS), and overall survival (OS).
Results
RT-PCR identified chimeric fusion oncogenes in 119 of 132 ESFTs. Eighty-nine percent of identified transcripts were EWS-FLI1, and of these, 58.8% were type 1. Five-year EFS and OS rates for patients with type 1 and non–type 1 fusions diagnosed between 2001 and 2005 were equivalent (type 1: EFS, 63% ± 7%; OS, 83% ± 6%; non–type 1: EFS, 71% ± 9%; OS, 79% ± 8%).
Conclusion
Current intensive treatment protocols for localized ESFT have erased the clinical disadvantage that was formerly observed in patients with non–type 1 EWS-FLI1 fusions.
INTRODUCTION
The Ewing sarcoma family of tumors (ESFT) are highly malignant neoplasms of bone and soft tissue that affect approximately one per 2.9 million people younger than 20 years of age.1 Despite multimodality therapy, overall survival (OS) is approximately 70% for patients with localized ESFT and only 20% to 30% for patients with metastatic disease.2,3 Various clinicopathologic variables have been investigated as prognostic indicators, with hopes that clinicians may tailor therapy for tumors predicted to have favorable versus refractory response. Evidence of gross metastatic disease at the time of diagnosis is the worst prognostic factor, in particular for patients with extrapulmonary metastases.2 Adverse outcome has also been associated with older age at presentation (age ≥ 14 years2,4 or ≥ 18 years5), larger tumor volume,4,6,7 poor response to induction therapy,4 axial tumor location,2,4 elevated lactate dehydrogenase,8 secondary cytogenetic abnormalities,9 deletion of p16,10 and mutation of p53.11,12 However, despite these associations, no pathologic or laboratory criteria exist in current clinical use that allow prediction of response to therapy.
Cytogenetically, ESFTs are identified by reciprocal chromosomal translocations. In nearly all cases, these translocations result in fusion of the amino-terminal domain of EWSR1 (EWS) or, in rare cases, the related TLS/FUS gene to the DNA-binding domain of an ETS gene family member.13 In 90% to 95% of cases, the EWS fusion partner is the FLI1 gene, resulting in creation of an EWS-FLI1 fusion oncoprotein.13 Other ETS family members that pair with EWSR1 include the ERG gene in up to 10% of cases and, in the remainder of cases, the ETV1, E1AF, and FEV genes.14 Combinatorial diversity among the involved chromosomal breakpoints produces even more heterogeneity at the molecular level. Specifically, breakpoints in EWS can occur between introns 7 to 9, and breakpoints in FLI1 can occur between introns 3 to 9.15 Most often, exon 7 of EWS is fused to exon 6 (60%) or exon 5 (20%) of FLI1, creating type 1 and type 2 EWS-FLI1 fusions, respectively. Other EWS-FLI1 fusions occur less frequently, and to date, at least 18 variations of the fusion transcript have been described.14
In two independent retrospective studies published in 1996 and 1998, EWS-FLI1 fusion type was found to be associated with significant differences in clinical outcome in patients with localized ESFT.14,16 Specifically, these studies revealed that patients with nonmetastatic disease whose tumors harbored the most common fusion, type 1 EWS-FLI1, had a better event-free survival (EFS) and OS than patients with non–type 1 translocations. These studies together suggested that EWS-FLI1 fusion type might be useful as a means to stratify patients into different risk groups at the time of diagnosis.
In the present study, we evaluated the prognostic significance of EWS-FLI1 fusion type in a prospectively acquired group of localized ESFT samples obtained from patients treated on Children's Oncology Group (COG) clinical trials after 1994. Importantly, in contrast to earlier retrospective studies, fusion type was not found to be a significant prognostic variable in this recently treated cohort of patients. These data suggest that current treatment protocols, which routinely add ifosfamide and etoposide (IE) to the standard three-drug backbone (vincristine, doxorubicin, and cyclophosphamide [VDC]), have dramatically improved outcomes for tumors with non–type 1 fusions and have eliminated the previously reported prognostic advantage of type 1 fusion–positive tumors.
METHODS
Sample Accrual
ESFT biopsy specimens were obtained from the COG Biorepository in Columbus, OH (Cooperative Human Tissue Network) and the Childrens Hospital Los Angeles (CHLA) tumor bank. Prospectively acquired diagnostic samples from patients treated on clinical trials INT-0154 (Children's Cancer Group trial 7942 and Pediatric Oncology Group trial 9354) and AEWS0031 were assessed. These were the two most recent COG clinical protocols for patients diagnosed with localized disease. Study INT-0154 ran from 1995 to 1998, and AEWS0031 ran from 2001 to 2005. INT-0154 evaluated whether dose intensification of VDC/IE over 30 weeks would improve outcome compared with the standard 48-week regimen.17 AEWS0031 was designed to determine whether delivery of the VDC/IE regimen in every-2-week rather than every-3-week cycles would improve outcome.18 Inclusion criteria for the current molecular study included confirmation of localized disease, registration or treatment on either of the two COG trials, and availability of frozen tissue in either the COG or CHLA tumor bank.
Diagnosis of ESFT was reaffirmed by central pathologic review for COG specimens or by local pathologists for CHLA specimens. An estimate of percent viable tumor cells was made for all samples using hematoxylin and eosin–stained sections of the frozen tissue blocks. Tumors were assigned an anonymous identifier, and specimens and correlative data were obtained in compliance with the Health Insurance Portability and Accountability Act. Review and approval by the Committee for Clinical Investigations at CHLA was obtained in accordance with an assurance filed with and approved by the Department of Health and Human Services. Informed consent for use of tumor samples for research purposes was obtained from each patient or the patient's guardian.
Translocation Type Determination
Total RNA was isolated from fresh-frozen tumor sections using RNAeasy or miRNAeasy kits (Qiagen, Valencia, CA) according to the manufacturer's instructions. Quantitative reverse transcriptase polymerase chain reaction (RT-PCR) was performed on first-strand cDNA using SYBR green incorporation and the following primer pairs: ESBP1(EWS) forward (CGACTAGTTATGATCAGAGCAGT) and ESBP2 (FLI1) reverse (CCGTTGCTCTGT ATTCTTACTGA) or EWS3tqm forward (GTCAACCTCAATCTAGCACAGGG) and ERG3tqm reverse (CTGTCCGACAGGAGCTCCAG). These primer pairs will detect the most common fusions (ie, those that occur between EWS and exons 5 to 7 of FLI1 or exons 4 to 8 of ERG, respectively).15 RNA quality of each sample was determined by concurrent amplification of β-actin. Samples in which β-actin failed to amplify within 30 cycles were considered inadequate. In samples with amplifiable β-actin but no detectable EWS-ETS fusion using the aforementioned primers, RT-PCR was repeated using alternate primer pairs that will detect less common fusions between EWS and exons 8 and 9 of FLI1.19,20 All amplified RT-PCR products were run on agarose gels, and bands were isolated and sequenced.
Statistical Methods
Correlations between translocation type and patient or disease characteristics were analyzed via logistic regression, Pearson's χ2 test, or Fisher's exact test when the number of samples was limited. EFS was defined as the minimum interval from the date of diagnosis to the date of tumor recurrence, progression, death, or last follow-up. OS was defined as the interval from the date of diagnosis to the date of death or last follow-up. Two patients had a second malignancy before disease relapse/progression and were censored at the time of the occurrence of the second malignancy for both EFS and OS analyses. Estimates of EFS and OS rates were based on the product-limit (Kaplan-Meier) estimate with Greenwood SEs.21 The association of EFS and OS with translocation type was tested using the log-rank test, either univariately or with stratification based on treatment era (1996 to 2000 v 2001 to 2005).
RESULTS
Population Characteristics
RNA from 132 patients with localized ESFT was analyzed by RT-PCR. Patient characteristics are listed in Table 1. The median age of all patients was 13 years (range, 1 to 25 years), and 54.5% were male. Tumors arising from bone and soft tissue tumors represented 72.7% and 27.3% of the study population, respectively. The majority of tumors included in this analysis arose outside the pelvis (n = 112). Importantly, comparison of the clinical characteristics of 100 patients from the AEWS0031 study who were included in this analysis with the 468 patients in AEWS0031 whose translocation status was not available for this study revealed no significant differences in age, sex, incidence of bone versus soft tissue origin, pelvic disease, or outcome (unpublished data). Thus, the patients whose tumors were analyzed for this study are representative of the larger population of patients with localized ESFT diagnosed and treated on COG clinical trials in the last 10 years.
Table 1.
Demographics and Clinical and Molecular Characteristics of Patients With ESFT
| Characteristic | All Patients (N = 132) |
Fusion (No. of patients) |
|||||
|---|---|---|---|---|---|---|---|
| No. | % | EWS-FLI1 Type 1 (n = 70) | EWS-FLI1 Type 2 (n = 19) | EWS-FLI1 Other (n = 17) | EWS-ERG (n = 13) | No Fusion Detected (n = 13) | |
| Positive patients, % | 58.8 | 16.0 | 14.3 | 10.9 | |||
| Age at diagnosis, years | |||||||
| Median | 13 | 13 | 14 | 11 | 12 | 11 | |
| Range | 1-25 | 2-25 | 1-17 | 3-19 | 1-19 | 1-19 | |
| Sex | |||||||
| Male | 72 | 54.5 | 36 | 12 | 9 | 7 | 8 |
| Female | 60 | 45.5 | 34 | 7 | 8 | 6 | 5 |
| Region of tumor | |||||||
| Pelvic | 20 | 15.2 | 12 | 2 | 1 | 2 | 3 |
| Nonpelvic | 112 | 84.8 | 58 | 17 | 16 | 11 | 10 |
| Systemic therapy | |||||||
| AEWS0031 | 100 | 75.8 | 56 | 17 | 10 | 8 | 9 |
| INT-0154 | 32 | 24.2 | 14 | 2 | 7 | 5 | 4 |
| Treatment era | |||||||
| 1996-2000 | 31 | 23.1 | 14 | 0 | 7 | 5 | 5 |
| 2001-2005 | 103 | 76.9 | 56 | 19 | 10 | 8 | 10 |
| Site of origin | |||||||
| Bone | 96 | 72.7 | 50 | 12 | 15 | 10 | 9 |
| Soft tissue | 36 | 27.3 | 20 | 7 | 2 | 3 | 4 |
| Follow-up, years | |||||||
| Median | 4.2 | 4.3 | 3.8 | 4.0 | 5.0 | 4.1 | |
| Range | 0.45-10.9 | 1.2-10.9 | 1.6-5.4 | 0.45-9.2 | 1.4-10.3 | 3.2-8.9 | |
Abbreviation: ESFT, Ewing sarcoma family of tumors.
Molecular Phenotypes
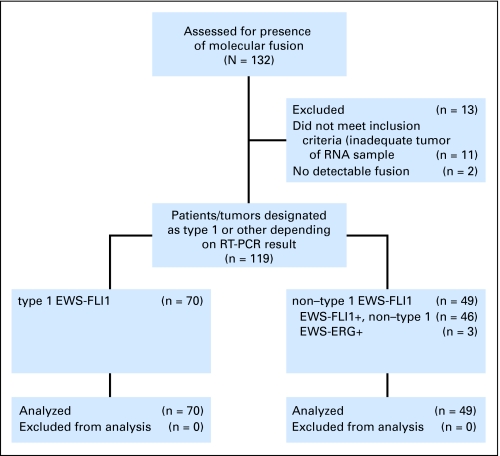
A fusion gene was identified by RT-PCR in 119 (90.2%) of 132 samples (Fig 1). Sequencing of the amplified fusion products revealed 106 EWS-FLI1 fusions (89.1%) and 13 EWS-ERG fusions (10.9%; Table 1). Among EWS-FLI1 fusions, 70 (66%) were type 1 (exons 7 and 6), 19 (18%) were type 2 (exons 7 and 5), and 17 (16%) had other variants including exons 10 and 5 (n = 6), exons 10 and 6 (n = 6), exons 7 and 7 (n = 3), and exons 10 and 7 (n = 2). In 13 samples, no fusion was detected. However, in three of these samples, the percentage of viable tumor on pathology review was less than 10%. Of the remaining 10 fusion-negative samples, seven required 30 or more RT-PCR cycles (range, 30 to 37 cycles) to amplify the control gene β-actin, indicating that the quality of the RNA sample was inadequate to reliably amplify a fusion gene even if one were present. In one additional fusion-negative sample, β-actin was detected at cycle 27, so although this sample met our criteria for RNA adequacy (< 30 cycles), low-level expression of a fusion transcript may have been missed in this RT-PCR assay. Therefore, it is highly probable that 11 of 13 samples with no detectable fusion were falsely negative as a result of inadequate tumor cell content (n = 3) and/or inadequate RNA quality (n = 8). In two samples, no fusion was detected with either EWS-FLI1 or EWS-ERG primer pairs despite amplification of β-actin at 16 and 17 cycles. Although it is possible that these two samples represent true fusion-negative ESFT, it is more likely that they harbored rare variant translocations involving other fusion partners (eg, TLS-ERG, EWS-ETV1) that would not have been detected with the EWS-FLI1– and EWS-ERG–specific primer sets used in this study.
Fig 1.
Translocation types of Ewing sarcoma family of tumor patients as determined by reverse transcriptase polymerase chain reaction (RT-PCR).
Non–Type 1 Fusion Type Is No Longer Associated With Adverse Outcome
Previous retrospective studies found that the type 1 EWS-FLI1 fusion was associated with improved EFS and OS in patients with newly diagnosed localized disease.14,16 To determine whether this observation was also true in our more recently diagnosed and treated prospective cohort of patients with ESFT, we first asked whether specific translocations occurred in conjunction with specific clinical or pathologic features. As shown in Table 2, no significant association was observed between age and translocation type, either in the proportion of tumors with EWS-FLI1 type 1 fusion (P = .41) or in the proportion of tumors with EWS-ERG fusion (P = .62). Similarly, no significant association was found between translocation type and location of tumor or tissue of origin (bone v soft tissue; Table 2). These results are consistent with earlier data that showed no differences in clinical presentation between the different molecular phenotypes.22
Table 2.
Association of EWS-FLI1 Translocation Type With Other Predictors of Outcome
| Factor | Translocation Type (No. of patients) |
EWS-FLI1 Type I |
EWS-ERG |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
EWS-FLI1 |
EWS-ERG | |||||||||
| Type 1 | Type 2 | Other | No. of Patients/Total | % | P | No. of Patients/Total | % | P | ||
| Age, years | ||||||||||
| < 14 | 46 | 9 | 10 | 10 | 46/75 | 61 | .41* | 10/75 | 13 | .62* |
| ≥ 14 | 24 | 10 | 7 | 3 | 24/44 | 55 | 3/44 | 7 | ||
| < 18 | 66 | 19 | 15 | 11 | 66/111 | 59 | 11/111 | 10 | ||
| ≥ 18 | 4 | 0 | 2 | 2 | 4/8 | 50 | 2/8 | 25 | ||
| Location | ||||||||||
| Pelvic | 12 | 2 | 1 | 2 | 12/17 | 71 | .43† | 2/17 | 12 | .99† |
| Nonpelvic | 58 | 17 | 16 | 11 | 58/102 | 57 | 11/102 | 11 | ||
| Site of origin | ||||||||||
| Bone | 50 | 12 | 15 | 10 | 50/87 | 57 | .68† | 10/87 | 11 | .99† |
| Soft tissue | 20 | 7 | 2 | 3 | 20/32 | 63 | 3/32 | 9 | ||
P value from logistic regression analysis with age as a continuous variable.
P value from Fisher's exact test.
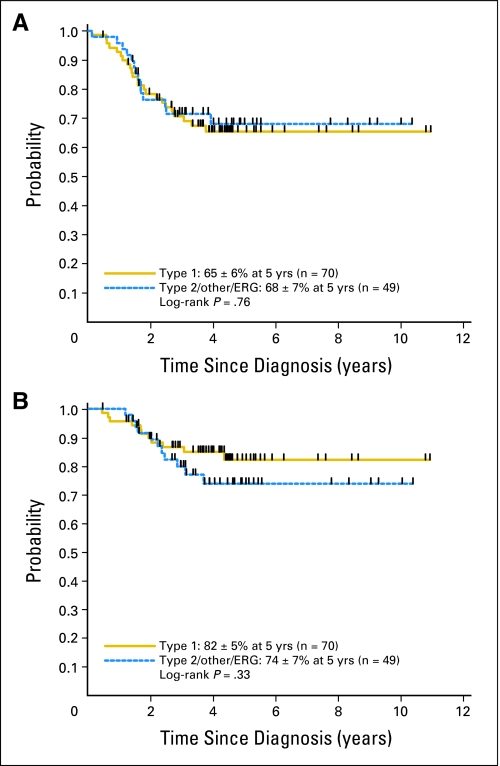
Next, we evaluated whether fusion type was associated with outcome. Interestingly, survival analysis revealed no significant difference in outcome between patients with type 1 EWS-FLI1 and non–type 1 fusions (Figs 2A and 2B). EFS and OS rates for 70 patients with a type 1 fusion were 65% ± 6% and 82% ± 5%, respectively, at 5 years. For the 49 patients with an alternate fusion, 5-year EFS and OS rates were 68% ± 7% and 74% ± 7%, respectively. Limiting the analysis to include only patients with an EWS-FLI1 fusion also failed to identify a significant prognostic advantage of the type 1 fusion compared with other EWS-FLI1 fusions (type 1: EFS, 65% ± 6%; OS, 82% ± 5% at 5 years; other EWS-FLI1: EFS, 63% ± 9%; OS, 71% ± 8% at 5 years; P = .85 for EFS; P = .25 for OS). Outcomes of patients with type 1 EWS-FLI1 versus type 2 EWS-FLI1, the two most common fusion types, were also statistically equivalent (type 1: EFS, 65% ± 6%; OS, 82% ± 5% at 5 years; type 2: EFS, 67% ± 13%; OS, 78% ± 11% at 5 years; P = .56 for EFS; P = .98 for OS).
Fig 2.
(A) Event-free survival and (B) overall survival for patients with Ewing sarcoma family tumors with type 1 EWS-FLI1 fusions and non–type 1 fusions.
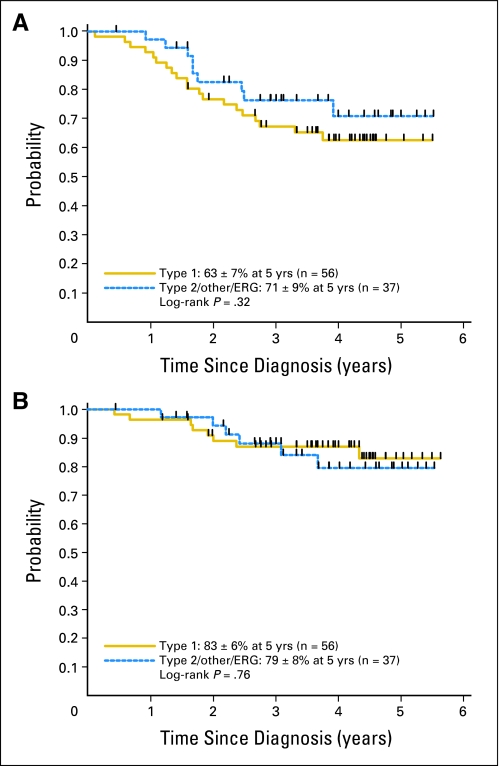
We hypothesized that the absence of observed difference in outcome in our study might be a result of treatment advances that had occurred since the time of the retrospective studies. To further address this possibility, we compared outcomes of patients diagnosed between 2001 and 2005 with outcomes previously reported in a multi-institutional study of patients diagnosed in the 1980s and 1990s.14 For the 93 patients with identified translocations who were treated after the year 2000, 5-year EFS rates were 63% ± 7% and 71% ± 9% (P = .32) and OS rates were 83% ± 6% and 79% ± 8% (P = .76) for type 1 (n = 56) and non–type 1 (n = 37) fusions, respectively (Figs 3A and 3B). In contrast, the 5-year OS rate was approximately 70% for type 1 patients and only 20% for non–type 1 patients in the historical patient cohort.14 Therefore, using historical cohorts as controls, a weighted improvement in outcome has been achieved in non–type 1 patients over the last decade.
Fig 3.
(A) Event-free survival and (B) overall survival for patients with Ewing sarcoma family tumors with type 1 EWS-FLI1 fusions and non–type 1 fusions treated between 2001 and 2005.
DISCUSSION
This study shows that the outcome for patients diagnosed with localized ESFT after 1995 was not significantly impacted by the tumor's molecular phenotype. This result is in contrast to retrospective studies from the 1980s and early 1990s, in which the most common type 1 EWS-FLI1 fusion was associated with significantly improved EFS and OS in patients with localized disease.14,16 This raises the possibility that either the retrospective nature of the earlier studies in some way biased the patient and tumor characteristics or that changes in clinical protocols over the last decade have erased what was formerly a true prognostic advantage of the type 1 fusion. We propose that advances in therapy, in particular the routine inclusion of IE into ESFT protocols, have erased the prognostic disadvantage of non–type 1 fusions.
In a cohort of patients treated before April 1997, de Alava et al14 found that patients with localized ESFT and non–type 1 EWS-FLI1 fusions had a 5-year OS rate of only 20%, corresponding to a relative failure rate (RFR) of approximately 4.5 compared with type 1 patients. With the 70 type 1 patients and 49 non–type 1 patients available for survival analysis in the current study and accounting for censoring, there would be at least 95% power to detect an RFR of 4.5 between these two groups and at least 80% power to detect an RFR of 3.0, based on a two-sided log-rank test with 5% type I error and assuming 5-year survival rate in type 1 patients of 70% to 80%. Similar numbers apply to the comparison of EFS. Hence, there was sufficient power in this analysis to detect even a smaller difference than was observed in the previous study. Thus, there has been a statistically and clinically significant improvement in the outcome of patients with non–type 1 tumors over the last decade. By contrast, improvement in the outcome of patients with type 1 transcripts has been more modest. Localized patients with type 1 tumors in the study by de Alava et al14 had an OS rate approaching 70%, whereas the same group treated between 2001 and 2005 in our study had an OS rate of 76%. This observation suggests that advances in treatment and supportive therapy in the intervening period have had their most profound effect on improving the survival of patients with non–type 1 tumors.
The reason for this marked and selective improvement in outcome for non–type 1 tumors is unknown, but our findings suggest that routine use of five-drug, intensified treatment protocols has likely overcome differences in the biology of these tumors that formerly portended a worse outcome. Interestingly, de Alava et al23 reported that the proliferative index of ESFT with non–type 1 fusions is higher than type 1 fusions and that this is associated with differential activation of IGF1-R signaling. We have performed a preliminary comparison of the molecular profiles of ESFT samples expressing type 1 and non–type 1 EWS-FLI1 and have also identified relative downregulation of cell cycle pathways in type 1 tumors (unpublished data). In addition, these molecular profiling studies suggest that host immune response pathways might be relatively upregulated in type 1 tumors. Further studies are now required to determine whether these differences could be exploited to develop novel, pathway-targeted therapies in the future that may further improve outcomes for patients with ESFT.
In summary, we have found that the most common type 1 EWS-FLI1 translocation is no longer associated with a more favorable clinical outcome in patients with newly diagnosed localized ESFT. Instead, our data show that modern treatment protocols have resulted in a marked improvement in the survival of patients with non–type 1 EWS-FLI1 fusion types and that this has eliminated the previously reported prognostic advantage of type 1 tumors. Eradication of translocation type as a prognostic variable in ESFT can be viewed as a tangible marker of success resulting from therapeutic advances of the last 20 years.
Acknowledgment
We thank the Children's Oncology Group Biopathology Center (Columbus, OH) for their exceptional service and members of the Lawlor Lab for helpful discussion.
Footnotes
Supported by National Institutes of Health (NIH) Strategic Partnering to Evaluate Cancer Signatures Grant No. 1U01CA11475-04, NIH Grant No. T32 CA09659, National Cancer Institute Grant No. CA98543-07, Children's Oncology Group Statistics and Data Center Grant No. SDC CA98413, and the My Brother Joey and T.J. Martell Foundations.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00006734.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: John A. van Doorninck, Michele R. Wing, Timothy J. Triche, Elizabeth R. Lawlor
Financial support: Timothy J. Triche, Elizabeth R. Lawlor
Administrative support: Michele R. Wing, Timothy J. Triche, Elizabeth R. Lawlor
Provision of study materials or patients: Stephen L. Lessnick, Timothy J. Triche, Richard B. Womer, Elizabeth R. Lawlor
Collection and assembly of data: John A. van Doorninck, Betty Schaub, Hiroyuki Shimada, Michele R. Wing, Mark D. Krailo, Timothy J. Triche, Elizabeth R. Lawlor
Data analysis and interpretation: John A. van Doorninck, Lingyun Ji, Hiroyuki Shimada, Michele R. Wing, Mark D. Krailo, Stephen L. Lessnick, Timothy J. Triche, Richard Sposto, Elizabeth R. Lawlor
Manuscript writing: John A. van Doorninck, Mark D. Krailo, Neyssa Marina, Timothy J. Triche, Richard Sposto, Richard B. Womer, Elizabeth R. Lawlor
Final approval of manuscript: Michele R. Wing, Mark D. Krailo, Stephen L. Lessnick, Neyssa Marina, Timothy J. Triche, Richard Sposto, Richard B. Womer, Elizabeth R. Lawlor
REFERENCES
- 1.Bernstein M, Kovar H, Paulussen M, et al. Ewing's sarcoma family of tumors: Ewing sarcoma of bone and soft tissue and the peripheral primitive neuroectodermal tumors. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. ed 5. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. pp. 1002–1032. [Google Scholar]
- 2.Rodríguez-Galindo C, Liu T, Krasin MJ, et al. Analysis of prognostic factors in Ewing sarcoma family of tumors: Review of St. Jude Children's Research Hospital studies. Cancer. 2007;110:375–384. doi: 10.1002/cncr.22821. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Galindo C, Spunt SL, Pappo AS. Treatment of Ewing sarcoma family of tumors: Current status and outlook for the future. Med Pediatr Oncol. 2003;40:276–287. doi: 10.1002/mpo.10240. [DOI] [PubMed] [Google Scholar]
- 4.Jenkin RD, Al-Fawaz I, Al-Shabanah M, et al. Localised Ewing sarcoma/PNET of bone: Prognostic factors and international data comparison. Med Pediatr Oncol. 2002;39:586–593. doi: 10.1002/mpo.10212. [DOI] [PubMed] [Google Scholar]
- 5.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 6.Ahrens S, Hoffmann C, Jabar S, et al. Evaluation of prognostic factors in a tumor volume-adapted treatment strategy for localized Ewing sarcoma of bone: The CESS 86 experience—Cooperative Ewing Sarcoma Study. Med Pediatr Oncol. 1999;32:186–195. doi: 10.1002/(sici)1096-911x(199903)32:3<186::aid-mpo5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Paulussen M, Ahrens S, Dunst J, et al. Localized Ewing tumor of bone: Final results of the cooperative Ewing's Sarcoma Study CESS 86. J Clin Oncol. 2001;19:1818–1829. doi: 10.1200/JCO.2001.19.6.1818. [DOI] [PubMed] [Google Scholar]
- 8.Bacci G, Ferrari S, Longhi A, et al. Prognostic significance of serum LDH in Ewing's sarcoma of bone. Oncol Rep. 1999;6:807–811. doi: 10.3892/or.6.4.807. [DOI] [PubMed] [Google Scholar]
- 9.Hattinger CM, Potschger U, Tarkkanen M, et al. Prognostic impact of chromosomal aberrations in Ewing tumours. Br J Cancer. 2002;86:1763–1769. doi: 10.1038/sj.bjc.6600332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei G, Antonescu CR, de Alava E, et al. Prognostic impact of INK4A deletion in Ewing sarcoma. Cancer. 2000;89:793–799. doi: 10.1002/1097-0142(20000815)89:4<793::aid-cncr11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.Abudu A, Mangham DC, Reynolds GM, et al. Overexpression of p53 protein in primary Ewing's sarcoma of bone: Relationship to tumour stage, response and prognosis. Br J Cancer. 1999;79:1185–1189. doi: 10.1038/sj.bjc.6690190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Alava E, Antonescu CR, Panizo A, et al. Prognostic impact of P53 status in Ewing sarcoma. Cancer. 2000;89:783–792. [PubMed] [Google Scholar]
- 13.Lessnick SL, Dei Tos AP, Sorensen PH, et al. Small round cell sarcomas. Semin Oncol. 2009;36:338–346. doi: 10.1053/j.seminoncol.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 14.de Alava E, Kawai A, Healey JH, et al. EWS-FLI1 fusion transcript structure is an independent determinant of prognosis in Ewing's sarcoma. J Clin Oncol. 1998;16:1248–1255. doi: 10.1200/JCO.1998.16.4.1248. [DOI] [PubMed] [Google Scholar]
- 15.Zucman J, Melot T, Desmaze C, et al. Combinatorial generation of variable fusion proteins in the Ewing family of tumours. EMBO J. 1993;12:4481–4487. doi: 10.1002/j.1460-2075.1993.tb06137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zoubek A, Dockhorn-Dworniczak B, Delattre O, et al. Does expression of different EWS chimeric transcripts define clinically distinct risk groups of Ewing tumor patients? J Clin Oncol. 1996;14:1245–1251. doi: 10.1200/JCO.1996.14.4.1245. [DOI] [PubMed] [Google Scholar]
- 17.Granowetter L, Womer R, Devidas M, et al. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: A Children's Oncology Group study. J Clin Oncol. 2009;27:2536–2541. doi: 10.1200/JCO.2008.19.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Womer R, Krailo M, Dickman P, et al. Randomized comparison of every-two-week vs. every-three-week chemotherapy in Ewing family tumors (ESFT) J Clin Oncol. 2008;26(suppl):554s. abstr 10504. [Google Scholar]
- 19.Ishii N, Hiraga H, Sawamura Y, et al. Alternative EWS-FLI1 fusion gene and MIC2 expression in peripheral and central primitive neuroectodermal tumors. Neuropathology. 2001;21:40–44. doi: 10.1046/j.1440-1789.2001.00367.x. [DOI] [PubMed] [Google Scholar]
- 20.Krams M, Peters J, Boeckel F, et al. In situ reverse-transcriptase polymerase chain reaction demonstration of the EWS/FLI-1 fusion transcript in Ewing's sarcomas and peripheral primitive neuroectodermal tumors. Virchows Arch. 2000;437:234–240. doi: 10.1007/s004280000252. [DOI] [PubMed] [Google Scholar]
- 21.Cox DR, Oakes D. Analysis of Survival Data. Boca Raton, FL: CRC Press; 1984. [Google Scholar]
- 22.Ginsberg JP, de Alava E, Ladanyi M, et al. EWS-FLI1 and EWS-ERG gene fusions are associated with similar clinical phenotypes in Ewing's sarcoma. J Clin Oncol. 1999;17:1809–1814. doi: 10.1200/JCO.1999.17.6.1809. [DOI] [PubMed] [Google Scholar]
- 23.de Alava E, Panizo A, Antonescu CR, et al. Association of EWS-FLI1 type 1 fusion with lower proliferative rate in Ewing's sarcoma. Am J Pathol. 2000;156:849–855. doi: 10.1016/S0002-9440(10)64953-X. [DOI] [PMC free article] [PubMed] [Google Scholar]