Abstract
Purpose
Conventional medicine has had little to offer patients with inoperable pancreatic adenocarcinoma; thus, many patients seek alternative treatments. The National Cancer Institute, in 1998, sponsored a randomized, phase III, controlled trial of proteolytic enzyme therapy versus chemotherapy. Because most eligible patients refused random assignment, the trial was changed in 2001 to a controlled, observational study.
Methods
All patients were seen by one of the investigators at Columbia University, and patients who received enzyme therapy were seen by the participating alternative practitioner. Of 55 patients who had inoperable pancreatic cancer, 23 elected gemcitabine-based chemotherapy, and 32 elected enzyme treatment, which included pancreatic enzymes, nutritional supplements, detoxification, and an organic diet. Primary and secondary outcomes were overall survival and quality of life, respectively.
Results
At enrollment, the treatment groups had no statistically significant differences in patient characteristics, pathology, quality of life, or clinically meaningful laboratory values. Kaplan-Meier analysis found a 9.7-month difference in median survival between the chemotherapy group (median survival, 14 months) and enzyme treatment groups (median survival, 4.3 months) and found an adjusted-mortality hazard ratio of the enzyme group compared with the chemotherapy group of 6.96 (P < .001). At 1 year, 56% of chemotherapy-group patients were alive, and 16% of enzyme-therapy patients were alive. The quality of life ratings were better in the chemotherapy group than in the enzyme-treated group (P < .01).
Conclusion
Among patients who have pancreatic cancer, those who chose gemcitabine-based chemotherapy survived more than three times as long (14.0 v 4.3 months) and had better quality of life than those who chose proteolytic enzyme treatment.
INTRODUCTION
Pancreatic cancer is one of the deadliest of all malignancies; its rate of mortality and its incidence are nearly equal.1 Only patients with localized disease or those with unusual histology obtain long-term survival benefits from therapy. In 1998, the median survival of patients with inoperable disease was approximately 6 months.
The Scottish embryologist John Beard first proposed pancreatic proteolytic enzyme treatment in 1906 and soon after published a monograph, entitled The Enzyme Therapy of Cancer.2 In 1981, Nicholas Gonzalez began to evaluate the use of proteolytic enzyme therapy. Twelve years later, in 1993, he was invited to present a series of cases at the National Cancer Institute (NCI), which led him to undertake a case series of alternative medical therapy that included proteolytic enzymes, diet, nutritional supplements, and detoxification procedures. Among 11 patients with inoperable, biopsy-proven, stages II to IV pancreatic adenocarcinoma, he reported 81% survival at 1 year and 45% at 2 years.3 Four of the 11 patients survived for 3 years.
At nearly the same time, Burris et al4 reported a trial in 126 patients who were randomly assigned to receive either difluorodeoxycytidine (ie, gemcitabine) chemotherapy or fluorouracil. The median survival was only 5.7 months for gemcitabine and was 4.4 months for fluorouracil, and this supported improvement in patient-reported outcomes that led to approval of the drug by the US Food and Drug Administration.4 Several subsequent studies have shown similar results among patients with advanced disease.5–7 As a result of these bleak survival reports and the promise of proteolytic enzyme therapy, the NCI, in November 1998, funded a randomized, controlled, phase III trial (No. NCT00003851). The primary objective of the study was to compare the overall survival of patients with inoperable pancreatic adenocarcinoma treated with standard gemcitabine-based chemotherapy (ie, control arm) and an experimental proteolytic enzyme regimen with adjunctive dietary and nutritional support plus detoxification procedures (ie, experimental arm).
METHODS
Study Design
On November 13, 1998, the institutional review board for research with human participants of the Columbia University Medical Center approved the phase III trial protocol, but only three patients agreed to enter the trial during the next 14 months if they were given enzyme therapy with ancillary nutritional support. The protocol was amended on July 5, 2001, to a controlled, observational design that compared patients with inoperable pancreatic adenocarcinoma treated with either a control arm of gemcitabine-based chemotherapy or an experimental arm of proteolytic enzyme treatment. The primary end point was overall survival, defined as time from enrollment to confirmed date of death, and the secondary end point was quality of life. The design called for the analyses to be monitored by an independent data safety monitoring committee. The institutional review board approved the study protocol and several amendment changes during the subsequent 5 years.
Recruitment
This study was widely publicized through the NCI and its Web site. All patients who were interested were reviewed by the study chair or by one of the coinvestigators. All laboratory, radiologic, and pathologic findings were reviewed at Columbia University Medical Center.
Eligibility Criteria
The enrollment criteria were carefully defined. To be eligible, study patients had to be older than age 18 years, had to have an estimated life expectancy of greater than 2 months, had to have an Eastern Cooperative Oncology Group (ECOG) performance status of less than 3, and had to have a histologically confirmed adenocarcinoma of the pancreas that was inoperable because of advanced primary tumor or metastases (ie, stages II to IV). No prior treatment was allowed, except surgery with noncurative intent, and patients had to begin therapy within 8 weeks of diagnosis.
Patients who had undergone a prior Whipple procedure, who smoked during the previous year, who had daily alcohol consumption during the past year, who had prior illicit drug addiction, or who had allergy or intolerance to pork were ineligible. Pregnant or lactating women were excluded. Enrollment restrictions for the study also recognized the rigor of proteolytic enzyme therapy; patients and their families had to be willing to undertake and be able to administer the treatment at home, and to be able to eat solid food.
Proteolytic Enzyme Treatment
The enzyme treatment included orally ingested proteolytic enzymes, nutritional supplements, detoxification, and an organic diet (unaltered from the pilot study).3 Patients received three pancreatic enzyme and two magnesium citrate capsules with each meal. The patients also took specified numbers of capsules with magnesium citrate and Papaya Plus every 4 hours on an empty stomach. The dose for patients with stage II disease was 69 enzyme capsules, and the dose for patients with stages III or IV was 81 capsules per day. After day 16, patients had a 5-day rest period and then resumed treatment on day 22. Treatment could be adjusted by the physician and could be increased for cancer progression. A diet that required at least 70% of the food to be raw or minimally cooked was required. All food was organic. Prescribed detoxification procedures included coffee enemas twice each day; skin brushing and cleansing; salt and soda baths; and a liver flush, clean sweep, and purging.
Chemotherapy
Gemcitabine-based treatments were given on schedules outlined by the treating oncologist. Nineteen of the patients received capecitabine (Xeloda, Roche, Nutley, NJ) and docetaxel (Taxotere, sanofi-aventis, Bridgewater, NJ) with gemcitabine every 21 days, as developed by one of the coinvestigators.8,9 One of the initial patients received gemcitabine alone; one patient received gemcitabine plus taxotere; and two patients received gemcitabine plus cisplatin and taxotere. Patients were not monitored for dose and frequency of treatments, both of which were left to the discretion of the treating physicians.
Modification of Study Design
Because of poor accrual in 2001, the study was changed to a nonrandomized, controlled trial. Patients who wished to enroll were given a detailed entrance interview; the patients were allowed to select which treatment arm they preferred. Initial intention-to-treat procedure was followed for data analysis throughout the study.
Quality-of-life evaluation by the Functional Assessment of Cancer Therapy questionnaire for pancreatic cancer (FACT-PA) was given to patients at the time of enrollment and every 3 months for the first year of participation. Pain and analgesic use was collected in a similar fashion. Patients in the experimental arm received proteolytic treatment under the care of a practitioner familiar with the regime; those in the chemotherapy arm received treatment by whichever oncologist they selected. All patients were observed in addition by their own physicians and could receive treatments unrelated to the study from them. No attempt was made by the study team to influence any treatment decisions.
Statistical Analyses
A patient's propensity score is a measure of the likelihood that a patient will receive a treatment that is not randomly assigned on the basis of his or her covariate information, and it is used to control for differences in measured selection factors for observational studies.10 To obtain the propensity scores, we developed a logistic regression model in which the treatment group was the dependent variable and the predictors of treatment were covariates (Table 1). A log-rank statistic stratified by the three propensity score strata was used to compare the treatment effects. Cox proportional hazard models then were used to compare the relative risks of death as a result of the initial decision, and analysis was adjusted for potential confounders, such as age at diagnosis, stage, histology, performance status, and weight (Table 2).11
Table 1.
Logistic Regression Model for Propensity Score
| Parameter | Estimate | P |
|---|---|---|
| Intercept | −1.90 | .35 |
| Age | 0.016 | .62 |
| Sex | ||
| Male | 0.00 | |
| Female | −0.90 | .16 |
| ECOG performance status | ||
| 0 | 0.00 | |
| 1 | 1.09 | .12 |
| 2 | 1.07 | .36 |
| Stage | ||
| II to III | 0.00 | |
| IV | 0.44 | .46 |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Table 2.
Cox Proportional Hazard Mortality Rate Ratios for Treatment and Other Risk Factors
| Variable | Hazard Ratio | 95% CL | P |
|---|---|---|---|
| Group | |||
| Chemotherapy | 1.00 | ||
| Enzyme | 6.96 | 3.06 to 15.81 | < .0001 |
| Age | |||
| < 59 years | 1.00 | ||
| > 59 years | 1.80 | 1.00 to 3.25 | .05 |
| Stage | |||
| II to III | 1.00 | ||
| IV | 2.21 | 1.19 to 4.10 | .01 |
| ECOG performance status | |||
| 0 | 1.00 | ||
| 1 | 4.44 | 1.86 to 10.62 | .0008 |
| 2 | 4.66 | 1.28 to 16.94 | .02 |
Abbreviations: CL, confidence limit; ECOG, Eastern Cooperative Oncology Group.
We used the FACT-PA questionnaire12,13 to measure quality of life at baseline and at 3-month intervals for the next year. We assigned study participants a zero score for each measurement scheduled after the date of their death. Pain was assessed on a pain scale of 0 to 100, and analgesic use per milligram of morphine equivalency was averaged over 24 hours.14 SAS version 9.0 (SAS Institute, Cary, NC) was used in all statistical analyses.
Stopping Rule
On February 17, 2005, because of the number of events in the enzyme arm, the data safety monitoring committee established the following stopping rule: At the 10th death among patients in the chemotherapy arm, if the two-sided, adjusted, log-rank test rejected the null hypothesis at a type I error rate of .001, the study was to be closed to accrual.
RESULTS
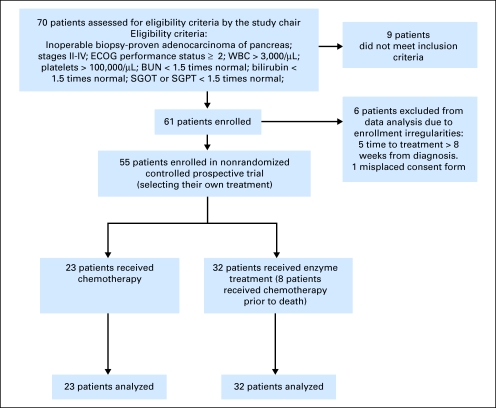
Of 70 patients with pancreatic cancer who expressed interest in the study, 15 patients were excluded (Fig 1); nine did not meet the trial inclusion criteria; and one additional patient signed a consent form that was misplaced because of a change in staffing. Five additional patients had a delay in enrollment of greater than 8 weeks from the date of diagnosis and, thus, were ineligible. Several of these subjects were originally treated as part of the trial due to extenuating circumstances at the time of evaluation resulting in excessive elapsed time between diagnosis and therapy. Subjects often traveled great distances to be considered for this trial, and on several occasions, obtaining data at long distance or the requirement for subject travel (eg, travel to New York around the time of September 11, 2001) resulted in excessive delay. Data from these subjects are not included in the data reported in this article, in order that the analysis apply to subjects that strictly meet the inclusion criteria.
Fig 1.
tudy design of controlled, nonrandomized trial. ECOG, Eastern Cooperative Oncology Group; BUN, blood urea nitrogen; SGOT, aspartate aminotransferase; SGPT, alanine aminotransferase.
On October 17, 2005, the stopping rule criterion was met, and the study was closed to accrual. Fifty-five patients, 23 on the control arm and 32 on the experimental arm, enrolled on the study and were available for analysis.
Table 3 presents the demographic and clinical characteristics of the participating patients by treatment group. The patients in both the control and experimental arms were carefully enrolled according to identical entry criteria. There were no statistically significant differences at the time of enrollment in age, sex, weight, ECOG performance status, stage of disease, pathology, quality of life, or CA19-9. Bilirubin and albumen were significantly higher in the chemotherapy group, but all values were clinically within normal limits and met eligibility criteria.
Table 3.
Demographic and Clinical Characteristics of Patients in the Chemotherapy and Enzyme-Treatment Groups
| Variable | Treatment |
P | |
|---|---|---|---|
| Chemotherapy (n = 23) | Enzyme Treatment (n = 32) | ||
| Sex | .22 | ||
| Male | |||
| No. | 16 | 17 | |
| % | 70 | 53.1 | |
| Female | |||
| No. | 7 | 15 | |
| % | 30 | 46.9 | |
| Stage | .54 | ||
| II to III | |||
| No. | 9 | 10 | |
| % | 39.1 | 31.3 | |
| IV | |||
| No. | 14 | 22 | |
| % | 60.9 | 68.7 | |
| ECOG performance status | |||
| 0 | |||
| No. | 4 | 11 | |
| % | 17 | 34.4 | |
| 1 | |||
| No. | 17 | 19 | |
| % | 74 | 59.4 | |
| 2 | |||
| No. | 2 | 2 | |
| % | 9 | 6.2 | |
| Age, years | .98 | ||
| Mean | 58.44 | 58.47 | |
| SD | 9.38 | 9.23 | |
| Weight, kg | .93 | ||
| Mean | 70.88 | 71.22 | |
| SD | 14.37 | 12.05 | |
| Total bilirubin, mg/dL | .01 | ||
| Mean | 1.04 | 0.63 | |
| SD | 0.64 | 0.35 | |
| Alkaline phosphatase, U/L | .86 | ||
| Mean | 126.09 | 121.31 | |
| SD | 82.20 | 105.59 | |
| WBC, 109/L | .18 | ||
| Mean | 7.78 | 6.81 | |
| SD | 2.73 | 2.45 | |
| Hematocrit, % | .92 | ||
| Mean | 37.08 | 37.32 | |
| SD | 7.03 | 9.39 | |
| Platelet, 109/L | .53 | ||
| Mean | 245.91 | 230.28 | |
| SD | 110.86 | 73.71 | |
| BUN, mg/dL | .35 | ||
| Mean | 14.78 | 13.44 | |
| SD | 4.48 | 6.11 | |
| Creatinine, mg/dL | .24 | ||
| Mean | 1.21 | 0.84 | |
| SD | 1.73 | 0.22 | |
| Albumin, g/dl | .04 | ||
| Mean | 4.27 | 4.00 | |
| SD | 0.52 | 0.47 | |
| AST, U/L | .80 | ||
| Mean | 25.09 | 26 | |
| SD | 15.16 | 10.43 | |
| ALT, U/L | .41 | ||
| Mean | 38.26 | 31.43 | |
| SD | 35.24 | 18.60 | |
| CA19-9, U/mL | .9219 | ||
| Mean | 9,117 | 9,700 | |
| SD | 15,945 | 20,347 | |
| Total QOL score | .42 | ||
| Mean | 107.31 | 111.69 | |
| SD | 18.42 | 15.45 | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; SD, standard deviation; BUN, blood urea nitrogen; QOL, quality of life.
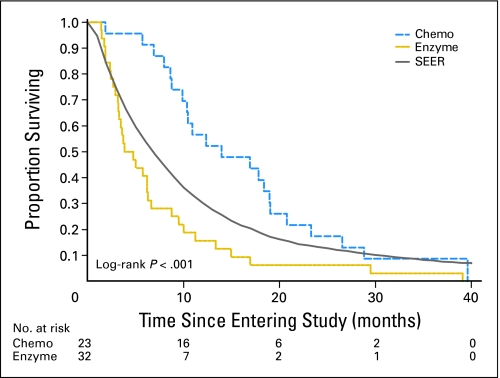
The primary end point was overall survival. As the Kaplan-Meier curves demonstrate (Fig 2), there was a 9.7-month median survival advantage for patients on chemotherapy treatment (median survival, 14 months) compared with those on enzyme treatment (median survival, 4.3 months; P < .001). The accompanying SEER (Surveillance, Epidemiology, and End Results) survival curve in Figure 2 has the same distribution of local and regional disease as the study population.
Fig 2.
Kaplan-Meier curves for the enzyme and chemotherapy (chemo) groups and for patients with stages II to IV pancreatic cancer in SEER (Surveillance, Epidemiology, and End Results).
Twelve months after enrollment, 56% of chemotherapy-group patients were alive; 16% of the enzyme-group patients were alive. The longest survivors were one chemotherapy-group patient who died at 39.5 months and one chemotherapy-group patient who was censored at 37.5 months (ie, the closing date of the data analysis) and, at the time of manuscript submission, was still alive at 40 months.
Both propensity score–adjusted and multivariate Cox proportional hazards models were used to estimate the relative risk for mortality. The log-rank test for analyzing the group effect on survival times adjusted by propensity scores was less than .001. Both methods gave similar results (Table 1).
Adverse events appeared similar in both groups and were difficult to distinguish from the morbidity of progressive pancreatic cancer. One patient in the chemotherapy group died as a result of a pulmonary embolus.
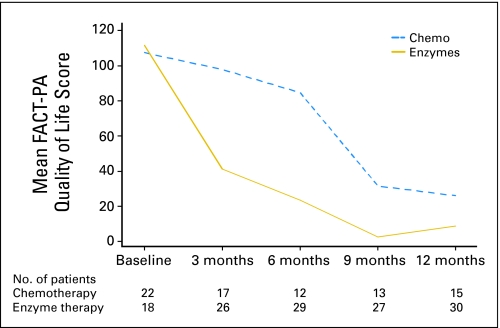
Patients in the two groups responded similarly to the questionnaires on quality of life before initiation of therapy, but the overall FACT-PA scores during 12 months decreased more in the enzyme group than in the gemcitabine group (Fig 3). Twenty-four percent of total measurements were missing. Quality of life scores of both groups were significantly different (P < .01). During the first 6 months of the study, pain scores increased in the enzyme group, but they decreased in the chemotherapy group (P < .05); however, few patients reported on use of analgesics. (Table 2).
Fig 3.
Mean Functional Assessment of Cancer questionnaire for pancreatic cancer (FACT-PA; ie, quality of life) scores of the chemotherapy (chemo) group and the enzyme-therapy group in the first year of the study.
DISCUSSION
Most patients with cancer use some form of complementary medicine,15–17 such as vitamins, nutritional supplements, diet, exercise, or prayer, in addition to cancer therapy. Many studies have been published on the merits and dangers of these additional remedies.18–20 Little is known, however, about either the prevalence or the benefits and harms of alternative medicine used in place of conventional, evidence-based treatments.
Many patients believe that alternative medicine offers better quality of life than standard cancer therapies.21,22 In this study, patients in the enzyme-treatment and chemotherapy groups had similar scores in quality of life at enrollment, but the enzyme group fared much worse than the gemcitabine group in the subsequent year. In part, this was due to missing data related to active disease and early deaths among the enzyme-group patients (Figs 2 and 3). Pain levels appeared to diverge during the initial 6 months of the study and were more severe among the enzyme group.
This report may be among the first controlled, clinical studies to compare allopathic treatment to an alternative medicine program for a survival end point.20 Other studies have investigated mixtures of herbal or vitamin therapies or compounds isolated from natural substances for symptom relief.23
Despite the intent of all the investigators involved to complete the study as an initially planned (ie, a randomized, controlled trial), physicians and patients were strongly committed to their own beliefs. Unless physicians convey clinical equipoise and sincere uncertainty about which treatment is better, patients rarely submit to random assignment, especially in the setting of a lethal disease.24 However, the contemporaneous enrollment of participants who met the eligibility criteria into two well-balanced groups increased the study design to the second highest level of evidence-based quality, as defined by the US Preventive Services Task Force.25,26
Although randomized, phase III trials are considered the gold standard in evidence-based medicine,27,28 many treatments have been accepted as beneficial on the basis of phase II trials. These include penicillin for the treatment of pneumococcal pneumonia,29,30 methotrexate for the treatment of acute lymphocytic leukemia,31 and—more recently—imatinib for the treatment of chronic myelogenous leukemia.32
This study avoided many of the weaknesses of observational studies, because the two groups were similar in demographic and clinical characteristics; propensity scores were used; the end point of overall survival was clearly defined; and patients in both groups were encouraged to use comparable additional medical care. Each group was allowed to cross over to other therapies, including chemotherapy, and the patients were observed for intent to treat until death. These criteria have been stressed by Hartz et al.24,33,34
The chemotherapy chosen for the control arm was the best reported when this study was initiated in 1998.4 Since that time, additional agents have been added, such as capecitabine and docetaxel, the latter of which is being studied currently in a phase II trial at Columbia University Medical Center.35,36 Other regimens have been explored, but no published reports of controlled randomized trials to date have indicated that the newer treatments improve overall survival more than a few months.5,37–39 Erlotinib (Tarceva, OSI Pharmaceuticals, Melville, NY), a biologic response modifier, combined with gemcitabine and compared with gemcitabine alone has been shown to increase the median overall survival by 0.5 months in a phase III trial.40
The difference in survival between patients who chose gemcitabine-based chemotherapy and those who chose enzyme treatment was statistically significant. The median survival of 4.3 months among patients who chose enzyme therapy was less than that observed in the most recently published SEER database (with survival through 2002), among patients with similarly staged disease (Fig 2).41
The median survival in the enzyme-treatment group also was shorter than that of the gemcitabine (ie, control) arm in the recently presented ECOG trial that compared two schedules of gemcitabine with the combination of gemcitabine and oxaliplatin,42 and median survival was shorter than that of the gemcitabine arm in several other reported studies.38–40,43
The findings in this study suggest that controlled studies of alternative medicine regimens are feasible44 and that gemcitabine-based chemotherapy appears superior to pancreatic enzyme therapy at these doses and schedules. The unexpectedly long survival observed in the gemcitabine group also may have been due to the selection criteria and changes in supportive care (eg, better use of surgical procedures, antibiotics, pain medication, and noninvasive placement of biliary stents).45 The addition of biologic response modifiers may additionally improve the results we observed.
Pancreatic cancer is the fourth major cause of cancer death. Together with other studies, these findings suggest that recent advances in conventional treatment have improved survival and offer longer survival and better quality of life to patients with pancreatic cancer. These observations should spark additional clinical research in this relatively neglected disease.
Supplementary Material
Acknowledgment
We thank Judith Jacobson, DPH, for her helpful advice and editorial comments.
Footnotes
See accompanying editorial on page 1979
Supported in part by National Cancer Institute Grant No. P-3P30CA 13696-2653 from the National Center of Complementary and Alternative Medicine and by Grant No. RSGT-01-024-04-CPHPS from the American Cancer Society (V.R.G.).
The National Institutes of Health, which was the funding agency, had oversight of the design and monitored the conduct of the study; however, the National Institutes of Health had no direct involvement in patient management; data collection; analysis and interpretation of the data; or preparation, review, or approval of the manuscript. W.-Y.T. performed all statistical analyses.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00003851.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: John A. Chabot, Wei-Yann Tsai, Robert L. Fine, Chunxia Chen, Carolyn K. Kumah, Karen A. Antman, Victor R. Grann
Financial support: John A. Chabot, Karen A. Antman, Victor R. Grann
Administrative support: John A. Chabot, Wei-Yann Tsai, Robert L. Fine, Chunxia Chen, Carolyn K. Kumah, Karen A. Antman, Victor R. Grann
Provision of study materials or patients: John A. Chabot, Wei-Yann Tsai, Robert L. Fine, Carolyn K. Kumah, Karen A. Antman, Victor R. Grann
Collection and assembly of data: John A. Chabot, Wei-Yann Tsai, Robert L. Fine, Chunxia Chen, Karen A. Antman, Victor R. Grann
Data analysis and interpretation: John A. Chabot, Wei-Yann Tsai, Robert L. Fine, Chunxia Chen, Carolyn K. Kumah, Karen A. Antman, Victor R. Grann
Manuscript writing: John A. Chabot, Wei-Yann Tsai, Robert L. Fine, Chunxia Chen, Carolyn K. Kumah, Karen A. Antman, Victor R. Grann
Final approval of manuscript: John A. Chabot, Wei-Yann Tsai, Robert L. Fine, Chunxia Chen, Carolyn K. Kumah, Karen A. Antman, Victor R. Grann
REFERENCES
- 1.Ries L, Malbert D, Krapcho M, et al. Bethesda, MD: National Cancer Institute; Surveillance, Epidemiology, and End Results (SEER) Cancer Statistics Review, 1975-2005 (of November 2007 SEER data submission) http://seer.cancer.gov/csr/1975_2005/ [Google Scholar]
- 2.Gonzalez NJ. Enzyme Therapy and Cancer. http://www.dr-gonzalez.com/history_of_treatment.htm.
- 3.Gonzalez NJ, Isaaacs LL. Evaluation of pancreatic proteolytic enzyme treatment of adenocarcinoma of the pancreas, with nutrition and detoxification support. Nutr Cancer. 1999;33:117–124. doi: 10.1207/S15327914NC330201. [DOI] [PubMed] [Google Scholar]
- 4.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 5.Burris HA., 3rd Recent updates on the role of chemotherapy in pancreatic cancer. Semin Oncol. 2005;32(suppl 6):S1–S3. doi: 10.1053/j.seminoncol.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 6.El-Rayes BF, Philip PA. A review of systemic therapy for advanced pancreatic cancer. Clin Adv Hematol Oncol. 2003;1:430–434. [PubMed] [Google Scholar]
- 7.Tempero M, Plunkett W, Ruiz Van Haperen V, et al. Randomized phase II comparison of dose-intense gemcitabine: Thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21:3402–3408. doi: 10.1200/JCO.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 8.Fine R, Fogelman D, Sherman JW, et al. The GTX regimen: A biochemically synergistic combination for advanced pancreatic cancer (PC) J Clin Oncol. 2003;22(suppl):281s. abstr 1129. [Google Scholar]
- 9.Fogelman DR, Chen J, Chabot JA, et al. The evolution of adjuvant and neoadjuvant chemotherapy and radiation for advanced pancreatic cancer: From 5-fluorouracil to GTX. Surg Oncol Clin N Am. 2004;13:711–735. doi: 10.1016/j.soc.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaum PR, Rubin DB. The bias due to incomplete matching. Biometrics. 1985;41:103–116. [PubMed] [Google Scholar]
- 12.Cella D, Hahn EA, Dineen K. Meaningful change in cancer-specific quality of life scores: Differences between improvement and worsening. Qual Life Res. 2002;11:207–221. doi: 10.1023/a:1015276414526. [DOI] [PubMed] [Google Scholar]
- 13.Wong GY, Schroeder DR, Carns PE, et al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: A randomized controlled trial. JAMA. 2004;291:1092–1099. doi: 10.1001/jama.291.9.1092. [DOI] [PubMed] [Google Scholar]
- 14.Kelsen DP, Portenoy RK, Thaler HT, et al. Pain and depression in patients with newly diagnosed pancreas cancer. J Clin Oncol. 1995;13:748–755. doi: 10.1200/JCO.1995.13.3.748. [DOI] [PubMed] [Google Scholar]
- 15.Nahleh Z, Tabbara IA. Complementary and alternative medicine in breast cancer patients. Palliat Support Care. 2003;1:267–273. doi: 10.1017/s1478951503030256. [DOI] [PubMed] [Google Scholar]
- 16.Yates JS, Mustian KM, Morrow GR, et al. Prevalence of complementary and alternative medicine use in cancer patients during treatment. Support Care Cancer. 2005;13:806–811. doi: 10.1007/s00520-004-0770-7. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson JS, Workman SB, Kronenberg F. Research on complementary/alternative medicine for patients with breast cancer: A review of the biomedical literature. J Clin Oncol. 2000;18:668–683. doi: 10.1200/JCO.2000.18.3.668. [DOI] [PubMed] [Google Scholar]
- 18.Small EJ, Frohlich MW, Bok R, et al. Prospective trial of the herbal supplement PC-SPES in patients with progressive prostate cancer. J Clin Oncol. 2000;18:3595–3603. doi: 10.1200/JCO.2000.18.21.3595. [DOI] [PubMed] [Google Scholar]
- 19.Sovak M, Seligson AL, Konas M, et al. Herbal composition PC-SPES for management of prostate cancer: Identification of active principles. J Natl Cancer Inst. 2002;94:1275–1281. doi: 10.1093/jnci/94.17.1275. [DOI] [PubMed] [Google Scholar]
- 20.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers: The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 21.Evans M, Shaw A, Thompson EA, et al. Decisions to use complementary and alternative medicine (CAM) by male cancer patients: Information-seeking roles and types of evidence used. BMC Complement Altern Med. 2007;7:25. doi: 10.1186/1472-6882-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu L, Lao LX, Ge A, et al. Chinese herbal medicine for cancer pain. Integr Cancer Ther. 2007;6:208–234. doi: 10.1177/1534735407305705. [DOI] [PubMed] [Google Scholar]
- 23.Antman K, Benson MC, Chabot J, et al. Complementary and alternative medicine: The role of the cancer center. J Clin Oncol. 2001;19:55S–60S. [PubMed] [Google Scholar]
- 24.Soares HP, Kumar A, Daniels S, et al. Evaluation of new treatments in radiation oncology: Are they better than standard treatments? JAMA. 2005;293:970–978. doi: 10.1001/jama.293.8.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preventive Services Task Force. ed 2. Baltimore, MD: Williams and Wilkins; 1996. Guide to Clinical Preventive Services: Report of the US Preventive Services Task Force. [Google Scholar]
- 27.Grann A, Grann VR. The case for randomized trials in cancer treatment: New is not always better. JAMA. 2005;293:1001–1003. doi: 10.1001/jama.293.8.1001. [DOI] [PubMed] [Google Scholar]
- 28.Pocock SJ, Elbourne DR. Randomized trials or observational tribulations? N Engl J Med. 2000;342:1907–1909. doi: 10.1056/NEJM200006223422511. [DOI] [PubMed] [Google Scholar]
- 29.Abraham EP, Chain CM, Fletcher HW. Further observations on penicillin. Lancet. 1941;2:177–188. [Google Scholar]
- 30.Ligon BL. Penicillin: Its discovery and early development. Semin Pediatr Infect Dis. 2004;15:52–57. doi: 10.1053/j.spid.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Sacks MS, Bradford GT, Schoenbach EB. The response of acute leukemia to the administration of the folic acid antagonists, aminopterin and a-methopterin: Report of 14 cases. Ann Intern Med. 1950;32:80–115. doi: 10.7326/0003-4819-32-1-80. [DOI] [PubMed] [Google Scholar]
- 32.Cohen MH, Williams G, Johnson JR, et al. Approval summary for imatinib mesylate capsules in the treatment of chronic myelogenous leukemia. Clin Cancer Res. 2002;8:935–942. [PubMed] [Google Scholar]
- 33.Hartz A, Benson K, Glaser J, et al. Assessing observational studies of spinal fusion and chemonucleolysis. Spine. 2003;28:2268–2275. doi: 10.1097/01.BRS.0000085093.68773.EC. [DOI] [PubMed] [Google Scholar]
- 34.Hartz A, Bentler S, Charlton M, et al. Assessing observational studies of medical treatments. Emerg Themes Epidemiol. 2005;2:8. doi: 10.1186/1742-7622-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fine RL, Fogelman DR, Schreibman SM, et al. The gemcitabine, docetaxel, and capecitabine (GTX) regimen for metastatic pancreatic cancer: A retrospective analysis. Cancer Chemother Pharmacol. 2008;61:167–175. doi: 10.1007/s00280-007-0473-0. [DOI] [PubMed] [Google Scholar]
- 36.Fine RL, Sherman WH, Moorer M, et al. Prospective phase II trial of GTX chemotherapy for metastatic pancreatic cancer. J Clin Oncol. 2009;27(suppl):232s. abstr 4623. [Google Scholar]
- 37.Ko AH, Tempero MA. Treatment of metastatic pancreatic cancer. J Natl Compr Canc Netw. 2005;3:627–636. doi: 10.6004/jnccn.2005.0036. [DOI] [PubMed] [Google Scholar]
- 38.Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: Results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 39.Cunningham D, Chau I, Stocken D, et al. Phase III randomised comparison of gemictabine (GEM) versus gemcitabine plus capecitabine (GEM-CAP) in patients with advanced pancreatic cancer. Eur J Cancer. 2005;4(suppl):1–14. [Google Scholar]
- 40.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 41.Ries L, Eisner M, Kosary C, et al. Bethesda, MD: 2005. Surveillance, Epidemiology, and End Results (SEER) Cancer Statistics Review, 1975-2002 (of November 2004 SEER data submission) http://seer.cancer.gov/csr/1975_2002/ [Google Scholar]
- 42.Poplin E, Levy D, Berlin J, et al. Phase III trial of gemcitabine (30-minute infusion), versus gemcitabine (fixed-dose-rate infusion (FSR) versus gemcitabine + oxaliplatin (GEMOX) in patients with advanced pancreatic cancer. 42nd Annual Meeting of the American Society of Clinical Oncology; June 2-6, 2006; Atlanta, GA. abstr E6201. [Google Scholar]
- 43.Nieto J, Grossbard ML, Kozuch P. Metastatic pancreatic cancer 2008: Is the glass less empty? Oncologist. 2008;13:562–576. doi: 10.1634/theoncologist.2007-0181. [DOI] [PubMed] [Google Scholar]
- 44.Dagenais S, Tricco AC, Bian ZX, et al. Critical appraisal of clinical studies in Chinese herbal medicine. Zhong Xi Yi Jie He Xue Bao. 2006;4:455–466. doi: 10.3736/jcim20060504. [DOI] [PubMed] [Google Scholar]
- 45.Allendorf J, Ippagunta N, Emond J. Management of liver metastases: New horizons for biologically based therapy. J Surg Res. 2004;117:144–153. doi: 10.1016/j.jss.2003.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.