Abstract
Purpose
We previously demonstrated that thalidomide appears to add to the activity of docetaxel in metastatic castration-resistant prostate cancer (CRPC). Phase II studies combining docetaxel with bevacizumab have had substantial antitumor activity. We hypothesized that the combination of docetaxel plus these antiangiogenic drugs with different targets would have substantial clinical activity. To explore safety and efficacy, this was tested in mice and in human patients.
Patients and Methods
Preclinical efficacy of the combination therapy was evaluated in PC3 xenograft mice. Sixty patients with progressive metastatic CRPC received intravenous docetaxel and bevacizumab plus oral thalidomide and prednisone. The primary end point was a prostate-specific antigen (PSA) decline of ≥ 50%. Secondary end points included time to progression, overall survival, and safety.
Results
In the mouse model, combination therapy of docetaxel, bevacizumab, and thalidomide inhibited tumor growth most effectively. In the clinical trial, 90% of patients receiving the combination therapy had PSA declines of ≥ 50%, and 88% achieved a PSA decline of ≥ 30% within the first 3 months of treatment. The median time to progression was 18.3 months, and the median overall survival was 28.2 months in this group with a Halabi-predicted survival of 14 months. While toxicities were manageable, all patients developed grade 3/4 neutropenia.
Conclusion
The addition of bevacizumab and thalidomide to docetaxel is a highly active combination with manageable toxicities. The estimated median survival is encouraging, given the generally poor prognosis of this patient population. These results suggest that definitive clinical trials combining antiangiogenic agent combinations with docetaxel are warranted to improve treatment outcomes for patients with metastatic CRPC.
INTRODUCTION
Metastatic castration-resistant prostate cancer (CRPC) is a leading cause of cancer death in men.1 The current recommended treatment of patients with CRPC is the chemotherapeutic agent docetaxel.2 Previous studies show a median overall survival (OS) of 19.2 months for patients receiving docetaxel and prednisone compared with 16.3 months for patients receiving mitoxantrone and prednisone.3 In an effort to prolong survival in this patient population, various antiangiogenic agents have been studied in combination with docetaxel.4–6
Angiogenesis plays an important role in the progression of prostate cancer and is inversely associated with survival rates.7–10 In a previous randomized phase II clinical trial of patients with CRPC we found that, compared with docetaxel alone, docetaxel plus thalidomide was associated with a higher prostate-specific antigen (PSA) response rate (51% v 37%) and improved OS (25.9 v 14.7 months; P = .040).6 Picus et al11 conducted a phase II study testing docetaxel in combination with bevacizumab and estramustine. The promising activity of this combination led to a phase III study comparing docetaxel and prednisone with docetaxel, bevacizumab, and prednisone. The antitumor activity in these phase II studies suggests a potential role for antiangiogenic therapy in combination with chemotherapy in the treatment of metastatic CRPC.
On the basis of these studies and the knowledge that a complex array of genes control tumor activity, it can be inferred that an optimal antiangiogenic approach will require a combination of different types of treatments.12–15 Since thalidomide and bevacizumab act through different mechanisms, it has been hypothesized that these two drugs would be excellent candidates for a treatment cocktail. Thalidomide appears to inhibit the action of basic fibroblast growth factors, endothelial cell proliferation, circulating endothelial cells, and expression of tumor necrosis factor α, while bevacizumab acts selectively by neutralizing vascular endothelial growth factor.16 Thus, we evaluated the combination of bevacizumab, thalidomide, and docetaxel first for safety in mouse models and then for efficacy in patients with metastatic CRPC as a phase II clinical trial.
PATIENTS AND METHODS
Xenograft Mouse Model
The National Cancer Institute (NCI) is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International and follows the Public Health Service (PHS) Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the Guide for the Care and Use of Laboratory Animals.17 The study protocol was approved by the NCI Animal Care and Use Committee.
Six-week-old, male, severe combined immunodeficiency mice were obtained from the NCI-Frederick Animal Production Area. PC3 cells, grown to 80% confluence, were washed with sterile phosphate-buffered saline buffer, and 1 million cells/100 μL were injected subcutaneously into the rear flank of each mouse. Mice were monitored and weighed three times per week. When tumors became palpable, mice were randomly assigned to eight groups (n = 5 to 8) and treated with single agents or with combinations of two or three agents. Mice receiving 100 μL of saline intraperitoneally (IP) 5 days/wk served as a vehicle control. Three groups of mice received a single agent alone: bevacizumab (5 mg/kg IP twice a week), docetaxel (10 mg/kg intravenously once a week), or thalidomide (100 mg/kg IP 5 days/wk). Three groups of mice were treated as follows at the same doses and schedules as mice receiving single agents: (1) docetaxel plus bevacizumab, (2) docetaxel plus thalidomide, or (3) docetaxel plus bevacizumab and thalidomide. Three weeks of treatment were followed by 1 week of follow-up, and treatment response was monitored by measuring tumor volume.
Patient Criteria
Eligible patients had castration-resistant metastatic adenocarcinoma of the prostate. Standard Prostate-Specific Antigen Working Group eligibility criteria were used.18 Patients must have had adequate organ functions (Appendix, online only). They were ineligible if they had grade ≥ 2 peripheral neuropathy, uncontrolled persistent systolic blood pressure ≥ 170 mmHg, diastolic blood pressure ≥ 100 mmHg, or were taking anticoagulant drugs. Patients were also excluded if they had brain and/or leptomeningeal metastases confirmed by imaging, or any other active malignancy within the past 2 years, excluding nonmelanoma skin cancer and superficial bladder carcinoma.
Study Design
This was an open-label, single-arm, phase II study of bevacizumab and thalidomide plus docetaxel and prednisone in patients with progressive metastatic CRPC. The NCI's institutional review board approved the study, and all patients provided signed consent. The primary objective was to evaluate whether this combination regimen resulted in substantial antitumor activity. The trial followed a two-stage Min-Max design19 to rule out an undesirably low PSA response rate of 65% (P0 = .65) and to target an 80% PSA response rate (P1 = .80). The 65% response rate was based on the reported activity of the combination of bevacizumab with docetaxel and estramustine in the same disease setting, which showed 65% of patients experiencing a decrease in PSA of ≥ 50%.11 Secondary objectives included evaluation of progression-free survival (PFS), OS, pharmacokinetics of the study agents, circulating apoptotic endothelial cells (CAECs) before and after treatment, and safety.
Treatment Plan and Dose Modifications
Patients received docetaxel (Taxotere, Aventis Pharmaceuticals, Bridgewater, NJ) intravenously at 75 mg/m2 over 60 minutes and bevacizumab (Avastin, Genentech, South San Francisco, CA) at 15 mg/kg over 30 minutes on day 1 of each 21-day cycle, plus oral thalidomide (Celgene, Warren, NJ) at 200 mg/d and prednisone at 10 mg/d. Patients also received enoxaparin at 1 mg/kg/d subcutaneously beginning on day 1 to prevent thrombosis6,20 and received pegfilgrastim, zoledronic acid, and ongoing androgen deprivation therapy as indicated.
Patients were evaluated every 3 weeks, including PSA levels. Staging radiographic studies, including computed tomography scans of the chest, abdomen, and pelvis as well as technetium-99m (99mTc) bone scintigraphy, were performed at baseline, after the second cycle, and every three cycles thereafter.
Docetaxel was withheld for grade 4 hematologic toxicity or grade ≥ 3 nonhematologic toxicity at time of treatment. For grade 3 or 4 neutropenia, docetaxel was continued at the regular dose with the addition of pegfilgrastim administered 24 hours after docetaxel infusion. If patients receiving pegfilgrastim experienced grade 4 or febrile neutropenia, docetaxel was decreased by 25%. Thalidomide was withheld for any grade 3 or 4 nonhematologic toxicity related to thalidomide or for grade 2 peripheral neuropathy. Thalidomide was resumed at 50% when toxicities resolved to grade 1 or baseline, unless grade ≥ 2 peripheral neuropathy recurred, in which case thalidomide was discontinued. For patients with grade 2 thalidomide-related toxicities, thalidomide was reduced 50 to 100 mg at a time to alleviate adverse effects, and then slowly escalated to maximum-tolerated dose.
Patients with thalidomide-related toxicities were allowed to continue docetaxel and bevacizumab, whereas no dose reduction was allowed for bevacizumab. Bevacizumab was withheld if urine dipstick protein was ≥ 2 g, was resumed when 24-hour protein was < 2g, and was discontinued for grade 4 hypertension, grade ≥ 2 thrombosis, a nonhealing wound, or persistent (≥ 3 weeks) grade 3 or 4 adverse events. Patients withdrawn from bevacizumab were allowed to continue docetaxel and thalidomide unless thrombosis developed, in which case thalidomide and bevacizumab were simultaneously discontinued.
Evaluation of Response and Toxicity and Pharmacokinetic Analysis
Tumor response was based on the Prostate-Specific Antigen Working Group criteria and standard Response Evaluation Criteria in Solid Tumors (RECIST) for measurable disease.18,21 Adverse events were assessed according to the NCI Common Toxicity Criteria, version 3.
Initially, disease progression was determined by PSA criteria or tumor progression was determined by RECIST, new lesion on bone scan, or symptomatic deterioration. After 22 patients were enrolled, the protocol was amended to eliminate the use of PSA criteria alone for study discontinuation, because changes in overall clinical status were not well reflected by these criteria. Before this amendment, 12 patients had been removed from the study on the basis of PSA progression. For those 12 patients, the disease progression was based on Prostate-Specific Antigen Working Group I criteria (including PSA-only progression). Thereafter, patients continued treatment until there was evidence of clinical progression or radiographic progression.
Plasma bevacizumab concentrations were measured using a modified enzyme-linked immunosorbent assay, as previously described,22,23 and the CAEC analysis was performed using flow cytometry (LSRII, Becton Dickinson, Franklin Lakes, NJ; Appendix).
Statistical Analysis
For the primary end point, percentage of change in PSA from baseline to nadir was calculated and reported in a waterfall plot. Proportions of patients with PSA declines of ≥ 50% at any point, ≥ 75% at any point, and ≥ 30% within the first 3 months of treatment were tabulated.
For key secondary objectives, PFS was defined from on-study date to date of first observation of disease progression or last follow-up. OS was defined from on-study date to date of death from any cause. Patients remaining on-study or alive at time of analyses were censored at date of last follow-up. The probability of PFS and OS was estimated using the Kaplan-Meier method.
RESULTS
Efficacy Study in Mice
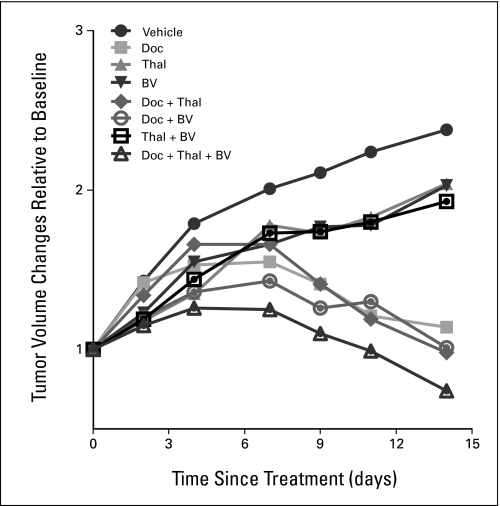
Treatment with thalidomide or bevacizumab alone inhibited tumor growth by 16% in PC3 xenograft mice compared with vehicle-treated mice (Fig 1). Combining these two agents did not further enhance antitumor activity. Docetaxel alone reduced tumor volume approximately 52% after 2 weeks of treatment. Bevacizumab plus docetaxel led to an earlier onset of tumor reduction than thalidomide plus docetaxel, although tumor shrinkage was similar for both treatment groups after 2 weeks. Mice receiving docetaxel with dual antiangiogenic therapy showed the greatest decrease in tumor volume (71%).
Fig 1.
Efficacy of therapeutic agents in PC3 xenograft mouse models following vehicle (solid circle), docetaxel (solid square), thalidomide (solid triangle), or bevacizumab (inverted solid triangle) alone; a dual combination of docetaxel and thalidomide (solid diamond), docetaxel and bevacizumab (open circle), thalidomide and bevacizumab (open square); and a combination of docetaxel, thalidomide, and bevacizumab (open triangle). Values are means from five to eight mice. Doc, docetaxel; Thal, thalidomide; BV, bevacizumab.
Clinical Study
The characteristics of the 60 patients enrolled in the first- and second-stage cohorts are summarized in Table 1. Many patients had unfavorable prognostic factors, as evidenced by involvement of soft tissue and visceral diseases, a high Gleason score at diagnosis, and short prestudy PSA doubling time. Two of the 60 patients had no detectable PSA activity. Based on the Halabi nomogram,24 the estimated median survival for this patient population was approximately 14 months.
Table 1.
Demographics and Disease Characteristics of Enrolled Patients (n = 60)
| Patient Demographics and Clinical Characteristics | No. | % |
|---|---|---|
| Age, years | ||
| Median | 66 | |
| Range | 44-79 | |
| Race | ||
| White | 49 | 82 |
| African American | 9 | 15 |
| Hispanic | 2 | 3 |
| Gleason score at diagnosis | ||
| Median | 8 | |
| Range | 5-10 | |
| ≥ 8 | 39 | 65 |
| ≤ 7 | 20 | 33 |
| Unclassified | 1 | 2 |
| Prior treatment | ||
| Prostatectomy | 56 | 93 |
| Radiotherapy | 6 | 10 |
| Neoadjuvant/adjuvant therapy | 6 | 10 |
| Secondary hormonal therapy | 57 | 95 |
| On-study PSA, ng/mL | ||
| Median | 99 | |
| Range | 0.9-4,399.0* | |
| Prestudy PSA doubling time, months | ||
| Median | 1.6 | |
| Range | 0.3-18.2 | |
| Metastases | ||
| Bone only | 23 | 38 |
| Soft tissue only | 6 | 10 |
| Bone and soft tissue | 31 | 52 |
| Visceral involvement | 8 | 13 |
| Patients with measurable disease according to RECIST | 33 | 55 |
| Hemoglobin, g/dL | ||
| Median | 12.7 | |
| Range | 8.3-14.3 | |
| Lactate dehydrogenase, U/L | ||
| Median | 191 | |
| Range | 112-1,280 | |
| Alkaline phosphatase, U/L | ||
| Median | 107 | |
| Range | 45-720 | |
| ECOG performance status | ||
| 0 | 8 | 13 |
| 1 | 48 | 80 |
| 2 | 4 | 7 |
| Pain at baseline | 29 | 48 |
| Predicted survival, months† | ||
| Median | 14 | |
| Range | < 6-27 | |
Abbreviations: PSA, prostate-specific antigen; RECIST, Response Evaluation Criteria in Solid Tumors; ECOG, Eastern Cooperative Oncology Group.
One patient had no detectable PSA activity.
Based on the Halabi survival nomogram.24
At the time data were evaluated, six patients remained on study. Of the remaining 54 patients, 41 had disease progression, seven withdrew voluntarily, five discontinued for toxicity, and one died of accidental aspiration. A total of 1,278 treatment cycles were administered, with a median of 20 cycles (range, 2 to 55). During treatment, 41 patients (68%) required dose modifications of thalidomide, including 33 (55%) with dose reductions and eight patients (13%) who discontinued thalidomide; 16 patients had a 25% dose reduction in docetaxel and seven had bevacizumab discontinued for toxicity. All patients were eligible for safety and efficacy analyses.
Response and Survival
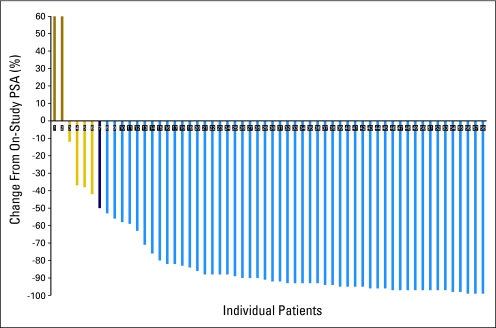
Of the 58 patients with PSA-active disease (Fig 2), 52 had PSA declines of ≥ 50% (89.6%; 95% CI, 78.8% to 96.1%), four had PSA declines of < 50%, two had rising PSA while on study, 44 (76%) had ≥ 75% PSA decline, and 51 (87.9%) achieved PSA declines of ≥ 30% in the first 3 months of treatment. Among the 33 patients with measurable disease, there were two confirmed complete responses and 19 confirmed partial responses, with an overall response rate of 64%. Eleven patients had stable disease as their best response and one had disease progression.
Fig 2.
Maximum percent change in prostate-specific antigen (PSA) from baseline in patients.
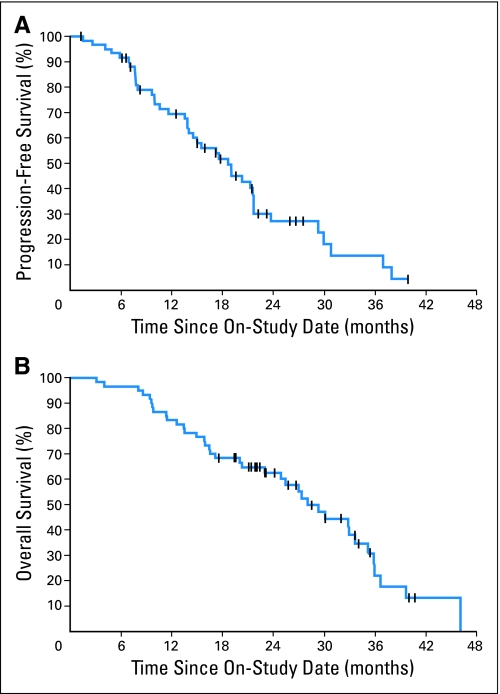
PFS was a secondary end point (Fig 3A). Estimated median time to progression was 18.3 months. This number includes the 12 patients who came off the study for PSA progression and the remaining patients who were required to have clinical or radiographic progression. Thirty-eight of the 60 patients (63%) died after a median potential follow-up of 34 months. The estimated OS curve is shown in Figure 3B. The median survival time from study entry was 28.2 months.
Fig 3.
Kaplan-Meier analysis of (A) progression-free survival and (B) overall survival for all patients on study.
Toxicity
The most common and clinically significant toxicities are summarized in Table 2. All of the patients developed grade 3/4 neutropenia, while only 20% had grade 3/4 anemia or thrombocytopenia. Grade 3/4 nonhematologic toxicities with an incidence of > 10% included hypertension and syncope. Significant grade 2 thalidomide-related adverse reactions included constipation (55%), fatigue (35%), peripheral neuropathy (13%, sensory in most cases), and depression (10%). In addition, grade 2 osteonecrosis of the jaw was observed in 18.3% of patients, which was considerably higher than the previously reported rate.25,26
Table 2.
Type and Number of Most Frequent and Important Treatment-Emergent Adverse Events
| Adverse Event | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|
| Constitutional | |||
| Fatigue | 21 | 2 | 0 |
| Weight loss | 5 | 0 | 0 |
| Hematologic | |||
| Neutropenia* | 0 | 39 | 21 |
| Lymphopenia | 15 | 19 | 1 |
| Anemia | 23 | 6 | 2 |
| Thrombocytopenia | 12 | 3 | 1 |
| Nonhematologic | |||
| Cardiovascular/pulmonary system | |||
| Epistaxis | 3 | 3 | 0 |
| Dysarthria/changes in voice | 2 | 0 | 0 |
| Dyspnea | 11 | 0 | 0 |
| Pleural effusion | 3 | 1 | 0 |
| Thrombosis | 0 | 2 | 2 |
| Hypertension | 8 | 7 | 0 |
| Aortic dissection | 0 | 0 | 1 |
| Myocardial infarction | 0 | 0 | 1 |
| Dermatology/skin system | |||
| Ulcer/cellulitis | 4 | 3 | 0 |
| Hand-foot reaction | 3 | 1 | 0 |
| Digestive/hepatic system | |||
| Taste alteration | 3 | 0 | 0 |
| Diarrhea | 4 | 2 | 0 |
| Constipation | 33 | 0 | 0 |
| Hypoalbuminemia | 26 | 4 | 0 |
| Increased ALT/AST | 5 | 3 | 0 |
| Increased bilirubin | 3 | 0 | 0 |
| GI hemorrhage | 2 | 2 | 0 |
| GI perforation | 0 | 2 | 0 |
| Rectal ulcer/fistula | 0 | 1 | 2 |
| Infection | |||
| Neutropenic fever | N/A | 5 | 0 |
| Musculoskeletal system | |||
| Osteonecrosis of the jaw | 10 | 1 | 0 |
| Nervous system | |||
| Depression | 6 | 0 | 0 |
| Syncope | N/A | 10 | 0 |
| Peripheral neuropathy | 8 | 0 | 0 |
| Ocular/visual system | |||
| Tearing or dryness | 4 | 1 | 0 |
| Renal/urinary system | |||
| Proteinuria | 3 | 1 | 0 |
| Nephrotic syndrome | 0 | 0 | 1 |
Abbreviation: N/A, not applicable.
Initiation of prophylactic pegfilgrastim required for grade ≥ 3 neutropenia.
Severe adverse events possibly related to the use of bevacizumab included death (n = 1) due to myocardial infarction that was complicated by the development of aortic dissection, grade 4 aortic dissection (n = 1) that resolved after conservative management, grade 3 GI perforation amenable to repair (n = 2), grade 3/4 rectal fistula or ulcer (n = 3), grade 4 nephrotic syndrome (n = 1), grade 3/4 thrombosis (n = 4), and grade 3 bleeding (n = 5). Except for the patient with myocardial infarction, all of these patients recovered and the majority were able to remain on study with discontinuation of bevacizumab.
Pharmacokinetics
Bevacizumab pharmacokinetics were assessed in 57 of 60 patients for cycle 1 and 50 of 60 patients for cycle 2. On average, plasma concentrations remained above 52.84 μg/mL (range, 11.94 to 96.09 μg/mL) at 3 weeks after the first dose and above 90.57 μg/mL (range, 39.63 to 194.08 μg/mL) before the third dose. Area under the plasma concentration-time curve up to the last sampling (AUClast) was calculated based on plasma concentrations collected approximately 21 to 23 days after each dose. The mean AUClast from the first dose was 2,805 μg/mL/d, with a percent coefficient of variation of 20%. The median accumulation ratio after the second dose, calculated on the basis of AUClast, was 1.22 (range, 1.01 to 1.72). As with other immunoglobulin G antibodies,27,28 a low clearance and limited volume of distribution were seen in bevacizumab pharmacokinetics. The mean bevacizumab clearance (0.401 L/d) was slightly higher than previously reported values.29 This was not attributed to concomitant docetaxel and/or thalidomide, since studies have shown that bevacizumab disposition displays linearly over a wide range of doses, and chemotherapy has a minimal effect on bevacizumab pharmacokinetics.29,30
Changes in CAECs
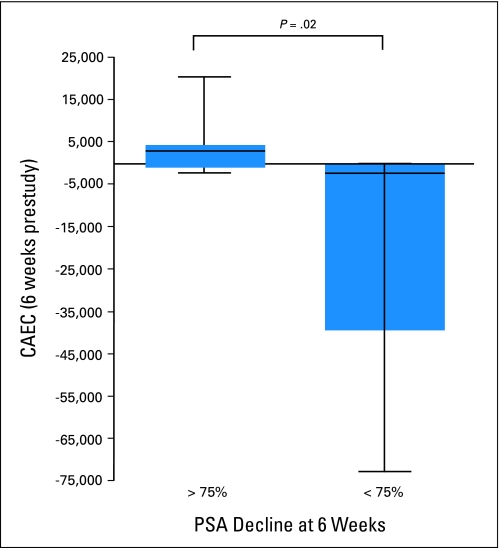
CAECs may serve as a marker for assessing a treatment's antiangiogenic activity. CAEC levels were measured at baseline and again at 6 weeks (after two cycles of treatment) in 17 study patients. CAEC levels were compared by using the Wilcoxon rank sum test, categorizing patients with < 75% PSA decline in one group and > 75% PSA decline in another group (Fig 4). Patients with ≥ 75% PSA decline had a significant increase in CAEC levels compared with those who had < 75% PSA decline (P = .02). There was also a strong inverse correlation between relative change in PSA over 6 weeks and the absolute difference in CAECs (r = −0.82; P < .001).
Fig 4.
Changes in circulating apoptotic endothelial cells (CAECs) at 6 weeks. PSA, prostate-specific antigen.
DISCUSSION
We evaluated the efficacy of an antiangiogenic therapy combining bevacizumab and thalidomide, plus a conventional regimen of docetaxel and prednisone, in both a preclinical model of prostate cancer and in patients with CRPC. PC3 xenograft experiments showed that this triple regimen was more effective in reducing tumor volumes throughout the treatment period compared with docetaxel alone or docetaxel plus either one of the antiangiogenic agents. Docetaxel alone elicited significant antitumor activity, but its effect was not seen until 5 days after initiation of treatment. However, adding an antiangiogenic agent such as bevacizumab or thalidomide to docetaxel induced inhibition of tumor growth as early as 1 day after initial treatment. Adding both bevacizumab and thalidomide to docetaxel therapy further potentiated this early onset of antitumor activity. These preclinical results provided the impetus for the clinical investigation in patients with CRPC.
In the phase II study of CRPC patients, the addition of both bevacizumab and thalidomide to docetaxel resulted in 90% of all patients having PSA declines of ≥ 50% and a 64% overall response rate in those with measurable disease. Bearing in mind the limitations of using PSA response to predict survival, the antitumor activity, as measured by PSA response, is compelling.
Results of this study suggest that using bevacizumab and thalidomide in combination with docetaxel is more effective than a double combination employing either antiangiogenic agent with docetaxel. The single arm and small sample size of these trials clearly limit the significance of this conclusion; nevertheless, the patients in this study represent a population with a predicted median OS of 14 months. The current estimated median survival of 28.2 months after treatment almost doubles the predicted value. Furthermore, the estimated survival might increase with further follow-up, since there were only 38 deaths included in the analysis and most patients remaining alive are 18 to 36 months from their on-study dates. The Cancer and Leukemia Group B is conducting a phase III trial (CALGB-90401) assessing docetaxel with or without bevacizumab in patients with metastatic CRPC. Results of this CALGB trial validate the concept of adding an antiangiogenic agent with chemotherapy in prostate cancer. We are continuing to develop this treatment and to test the activity that lenalidomide may have over thalidomide in this regimen.
The addition of combined antiangiogenic therapy to docetaxel was generally well tolerated in most patients with expected and manageable toxicities observed. Serious adverse events such as thrombosis and other vascular complications were consistent with known adverse reactions to bevacizumab and may be related to a prolonged time on treatment (median 20 cycles, equivalent to 13.8 months). The single myocardial infarction 39 months after treatment and the episode of aortic dissection 18 months after treatment is consistent with increased risk of thromboembolic events with prolonged use of this drug. Additional positive effects of thalidomide and bevacizumab can be seen since none of the thrombotic events led to permanent disability or death despite prophylactic use of enoxaparin.
The exploratory translational study showed a strong inverse correlation between changes in CAECs and PSA level, suggesting that the drug combination may effectively inhibit tumor angiogenesis, and the antitumor activity of the combination may be caused by early changes in CAECs. This is consistent with observations from preclinical studies in prostate cancer models31 that suggest CAECs may serve as a biomarker for assessing antiangiogenic activity for metastatic CRPC.
Combined antiangiogenic therapy represents a new approach in the treatment of metastatic CRPC. The addition of both bevacizumab and thalidomide to docetaxel and prednisone resulted in unprecedented high response rates based on PSA changes and measurable disease, with expected and manageable toxicities. As with many combination regimens, increased toxicity (eg, GI bleeding/perforation, hypertension, syncope, and thrombosis) is noticed with this treatment, which may raise concerns regarding tolerability in the elderly CRPC patient population. However, the impressive response rate for this treatment warrants definitive evaluation. The high response rate was accompanied by an encouraging estimated median survival rate in this patient population. This warrants further studies of multiple antiangiogenic agents with different acting mechanisms combined with docetaxel.
Acknowledgment
We acknowledge Avi Retter, MD, David Draper, RN, and Marcia Mulquin, RN, for their assistance on the clinical trial and Stephen Pisle for his assistance on the animal study.
Appendix
Patient Criteria
Adequate organ functions were defined by the following laboratory values: leukocytes (≥ 3,000/μL), absolute neutrophil count (≥ 1,500/μL), platelets (≥ 100,000/μL), hemoglobin (≥ 8 g/L), total bilirubin (≤ 1.5 × institutional upper limit of normal [ULN]), AST/ALT (≤ 2.5 × ULN), creatinine (≤ 1.5 × ULN). Patients were eligible only if there was no history of myocardial infarction within the past 6 months, uncontrolled chronic heart failure, or uncontrolled angina pectoris.
To compare our outcomes to other trials in this population, we used the Prostate-Specific Antigen Working Group 1 eligibility criteria, which allowed patients to be enrolled with no detectable prostate-specific antigen (PSA) as long as they met other criteria for progressive disease. In the current study, while all other patients had quantifiable PSA values, only one patient had progressive disease by scan with no detectable PSA (< 0.2 ng/mL). Although he did not meet the primary end point, he met the secondary end point; thus, he was included.
Pharmacokinetic Measurement and Analysis
Blood samples were collected on day 1 of cycles 1 and 2. Samples were drawn at predose on day 1 of cycle 1, 5 minutes before the end of docetaxel infusion and at 15 and 30 minutes and 1, 2, 4, 8, and 24 hours after the end of docetaxel infusion. Plasma bevacizumab concentrations were measured using an enzyme-linked immunosorbent assay (ELISA) designed with modifications.25,26 Briefly, the surface of a flat-bottom Maxisorp 96-well microplate was coated with 200 μL of 1 μg/mL vascular endothelial growth factor 165 (R&D Systems, Minneapolis, MN) in 0.05 M carbonate-bicarbonate buffer (pH 9.6) and incubated overnight at 4°C. Nonspecific interaction was blocked with blocking buffer (SuperBlock; Pierce Biotechnology, Rockford, IL) by 2-hour incubation at room temperature. Standards, samples, and quality controls were transferred into the wells in duplicate and incubated for 2 hours. After washing any unbound substances using phosphate buffer containing 0.05% Tween 20, 200 μL of goat antibody specific for human immunoglobulin G (IgG) conjugated to horseradish peroxidase (1:30,000; Sigma-Aldrich, St. Louis, MO) was added to the wells. Following a wash to remove any unbound enzyme conjugate, 3,3′,5,5′-tetramethylbenzidine (TMB; Pierce Biotechnology, Rockford, IL) was added to the wells for color development. Color development was stopped by 2 N sulfuric acid, and the intensity of the color was read by SpectraMax plate reader (Molecular Devices, Sunnyvale, CA) at 405 nm. The lower limit of quantitation was 6.25 ng/mL, and mean accuracy and precision for quality controls were 96.2% to 104.8% and 0.25% to 9.43%, respectively.
Individual bevacizumab concentration versus time profiles were analyzed using WinNonlin (version 5, Pharsight, Mountain View, CA) to obtain noncompartmental parameters, including the area under the plasma concentration-time curve up to the last sampling (AUClast) and AUC to infinity (AUCinf), total clearance (CL), and steady-state volume of distribution (Vss). The accumulation ratio of bevacizumab after the second dose was calculated using AUClast relative to cycle 1 for individual patients whose values were available for both cycle 1 and cycle 2.
Assessment of Circulating Apoptotic Endothelial Cells
Mononuclear cells were isolated from heparinized blood by Ficoll gradient centrifugation. Cells were washed with phosphate-buffered saline (PBS), resuspended in freezing medium (10% dimethyl sulfoxide [DMSO] in fetal bovine serum), transferred to a cryotube, and stored at −80°C. For analysis of circulating apoptotic endothelial cells (CAECs), samples were thawed and washed with PBS, mixed with FcR blocking reagent (Miltenyi Biotec, Auburn, CA) and stained for 30 minutes on ice with anti-CD45-PerCP (2D1 clone; BD Biosciences, San Jose, CA), anti-CD31-FITC (WM59 clone; BD Biosciences), anti-CD133-APC (AC133 clone; Miltenyi Biotec), and anti-CD146-PE (P1H12 clone; Chemicon International, Billerica, MA). Cells were washed with 0.1% bovine serum albumin in PBS and resuspended in PBS containing 0.1% bovine serum albumin and 10 μg/mL of Hoechst 33258 (Invitrogen, Carlsbad, CA). CAEC analysis was performed using an LSRII (Becton Dickinson, Franklin Lakes, NJ) and at least 1 × 105 cells were acquired. Data were analyzed using FlowJo software. CAECs were defined as negative for the hematopoietic marker CD45, positive for the endothelial markers CD31 and CD146, and negative for the progenitor marker CD133. CAEC was defined as the CAEC subset positive for Hoechst 33258.
Footnotes
Supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Presented in part at the 44th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2008, Chicago, IL.
The results and discussions presented herein do not represent the views of these federal agencies, nor does use of the combined antiangiogenic therapy imply approval of the combination by these agencies.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00091364.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Yang-Min Ning, James L. Gulley, Seth M. Steinberg, Jane B. Trepel, William D. Figg, William L. Dahut
Financial support: Jane B. Trepel, William D. Figg, William L. Dahut
Provision of study materials or patients: Yang-Min Ning, James L. Gulley, Philip M. Arlen, John J. Wright, Howard L. Parnes, Ravi A. Madan, Elizabeth Jones, Clara C. Chen, William D. Figg, William L. Dahut
Collection and assembly of data: Yang-Min Ning, James L. Gulley, Philip M. Arlen, Sukyung Woo, Seth M. Steinberg, John J. Wright, Howard L. Parnes, Jane B. Trepel, Min-Jung Lee, Yeong Sang Kim, Haihao Sun, Ravi A. Madan, Lea Latham, Elizabeth Jones, Clara C. Chen, William D. Figg, William L. Dahut
Data analysis and interpretation: Yang-Min Ning, James L. Gulley, Philip M. Arlen, Sukyung Woo, Seth M. Steinberg, John J. Wright, Howard L. Parnes, Jane B. Trepel, Min-Jung Lee, Yeong Sang Kim, Haihao Sun, Ravi A. Madan, Lea Latham, Elizabeth Jones, Clara C. Chen, William D. Figg, William L. Dahut
Manuscript writing: Yang-Min Ning, James L. Gulley, Sukyung Woo, John J. Wright, Howard L. Parnes, Jane B. Trepel, Ravi A. Madan, Elizabeth Jones, William D. Figg, William L. Dahut
Final approval of manuscript: Yang-Min Ning, James L. Gulley, Philip M. Arlen, Sukyung Woo, Seth M. Steinberg, John J. Wright, Howard L. Parnes, Jane B. Trepel, Min-Jung Lee, Yeong Sang Kim, Haihao Sun, Ravi A. Madan, Lea Latham, Elizabeth Jones, Clara C. Chen, William D. Figg, William L. Dahut
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 3.Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: Updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 4.Petrylak D. Therapeutic options in androgen-independent prostate cancer: Building on docetaxel. BJU Int. 2005;96(suppl 2):41–46. doi: 10.1111/j.1464-410X.2005.05946.x. [DOI] [PubMed] [Google Scholar]
- 5.Di Lorenzo G, Figg WD, Fossa SD, et al. Combination of bevacizumab and docetaxel in docetaxel-pretreated hormone-refractory prostate cancer: A phase 2 study. Eur Urol. 2008;54:1089–1094. doi: 10.1016/j.eururo.2008.01.082. [DOI] [PubMed] [Google Scholar]
- 6.Dahut WL, Gulley JL, Arlen PM, et al. Randomized phase II trial of docetaxel plus thalidomide in androgen-independent prostate cancer. J Clin Oncol. 2004;22:2532–2539. doi: 10.1200/JCO.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 7.Cox MC, Permenter M, Figg WD. Angiogenesis and prostate cancer: Important laboratory and clinical findings. Curr Oncol Rep. 2005;7:215–219. doi: 10.1007/s11912-005-0076-z. [DOI] [PubMed] [Google Scholar]
- 8.Bok RA, Halabi S, Fei DT, et al. Vascular endothelial growth factor and basic fibroblast growth factor urine levels as predictors of outcome in hormone-refractory prostate cancer patients: A Cancer and Leukemia Group B study. Cancer Res. 2001;61:2533–2536. [PubMed] [Google Scholar]
- 9.Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321:419–424. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 10.Hudes GR, Greenberg R, Krigel RL, et al. Phase II study of estramustine and vinblastine, two microtubule inhibitors, in hormone-refractory prostate cancer. J Clin Oncol. 1992;10:1754–1761. doi: 10.1200/JCO.1992.10.11.1754. [DOI] [PubMed] [Google Scholar]
- 11.Picus J, Halabi S, Rini B, et al. The use of bevacizumab (B) with docetaxel (D) and estramustine (E) in hormone refractory prostate cancer (HRPC): Initial results of CALGB 90006. Proc Am Soc Clin Oncol. 2003;22:393. abstr 1578. [Google Scholar]
- 12.Kotoh T, Dhar DK, Masunaga R, et al. Antiangiogenic therapy of human esophageal cancers with thalidomide in nude mice. Surgery. 1999;125:536–544. [PubMed] [Google Scholar]
- 13.Fox WD, Higgins B, Maiese KM, et al. Antibody to vascular endothelial growth factor slows growth of an androgen-independent xenograft model of prostate cancer. Clin Cancer Res. 2002;8:3226–3231. [PubMed] [Google Scholar]
- 14.Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65:671–680. [PubMed] [Google Scholar]
- 15.Dorrell MI, Aguilar E, Scheppke L, et al. Combination angiostatic therapy completely inhibits ocular and tumor angiogenesis. Proc Natl Acad Sci U S A. 2007;104:967–972. doi: 10.1073/pnas.0607542104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrara N, Chen H, Davis-Smyth T, et al. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat Med. 1998;4:336–340. doi: 10.1038/nm0398-336. [DOI] [PubMed] [Google Scholar]
- 17.Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press; 1996. [PubMed] [Google Scholar]
- 18.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: Recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 19.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 20.Zangari M, Fink LM, Elice F, et al. Thrombotic events in patients with cancer receiving antiangiogenesis agents. J Clin Oncol. 2009;27:4865–4873. doi: 10.1200/JCO.2009.22.3875. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Yoeruek E, Ziemssen F, Henke-Fahle S, et al. Safety, penetration and efficacy of topically applied bevacizumab: Evaluation of eyedrops in corneal neovascularization after chemical burn. Acta Ophthalmol. 2008;86:322–328. doi: 10.1111/j.1600-0420.2007.01049.x. [DOI] [PubMed] [Google Scholar]
- 23.Bakri SJ, Snyder MR, Pulido JS, et al. Six-month stability of bevacizumab (Avastin) binding to vascular endothelial growth factor after withdrawal into a syringe and refrigeration or freezing. Retina. 2006;26:519–522. doi: 10.1097/01.iae.0000225354.92444.7a. [DOI] [PubMed] [Google Scholar]
- 24.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–1237. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 25.Aragon-Ching JB, Ning YM, Chen CC, et al. Higher incidence of osteonecrosis of the Jaw (ONJ) in patients with metastatic castration resistant prostate cancer treated with anti-angiogenic agents. Cancer Invest. 2009;27:221–226. doi: 10.1080/07357900802208608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: Bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–761. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 27.Bruno R, Washington CB, Lu JF, et al. Population pharmacokinetics of trastuzumab in patients with HER2+ metastatic breast cancer. Cancer Chemother Pharmacol. 2005;56:361–369. doi: 10.1007/s00280-005-1026-z. [DOI] [PubMed] [Google Scholar]
- 28.Trang J. Pharmacokinetics and metabolism of therapeutic and diagnostic antibodies. In: Ferraiolo B, Mohler M, Gloff C, editors. Protein Pharmacokinetics and Metabolism: Pharmaceutical Biotechnology. New York, NY: Plenum Press; 1992. pp. 223–270. [Google Scholar]
- 29.Lu JF, Bruno R, Eppler S, et al. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol. 2008;62:779–786. doi: 10.1007/s00280-007-0664-8. [DOI] [PubMed] [Google Scholar]
- 30.Gordon MS, Margolin K, Talpaz M, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19:843–850. doi: 10.1200/JCO.2001.19.3.843. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Raia V, Bertolini F, et al. Circulating endothelial cells as a therapeutic marker for thalidomide in combined therapy with chemotherapy drugs in a human prostate cancer model. BJU Int. 2008;101:884–888. doi: 10.1111/j.1464-410X.2007.07342.x. [DOI] [PubMed] [Google Scholar]