Abstract
Male breast cancer is a rare disease, accounting for less than 1% of all breast cancer diagnoses worldwide. Most data on male breast cancer comes from small single-institution studies, and because of the paucity of data, the optimal treatment for male breast cancer is not known. This article summarizes a multidisciplinary international meeting on male breast cancer, sponsored by the National Institutes of Health Office of Rare Diseases and the National Cancer Institute Divisions of Cancer Epidemiology and Genetics and Cancer Treatment and Diagnosis. The meeting included representatives from the fields of epidemiology, genetics, pathology and molecular biology, health services research, and clinical oncology and the advocacy community, with a comprehensive review of the data. Presentations focused on highlighting differences and similarities between breast cancer in males and females. To enhance our understanding of male breast cancer, international consortia are necessary. Therefore, the Breast International Group and North American Breast Cancer Group have joined efforts to develop an International Male Breast Cancer Program and to pool epidemiologic data, clinical information, and tumor specimens. This international collaboration will also facilitate the future planning of clinical trials that can address essential questions in the treatment of male breast cancer.
INTRODUCTION
Male breast cancer is a rare disease, accounting for less than 1% of all breast cancer diagnoses worldwide. It is estimated that 1,910 men will be diagnosed with breast cancer in 2009 in the United States alone.1 Almost all data on male breast cancer come from small single-institution studies, and because of the paucity of data, the optimal treatment for male breast cancer is not known. This article summarizes a multidisciplinary meeting on male breast cancer, sponsored by the National Institutes of Health (NIH) Office of Rare Diseases and the National Cancer Institute Divisions of Cancer Epidemiology and Genetics and Cancer Treatment and Diagnosis and held in Bethesda, Maryland on September 4, 2008. The meeting included representatives from North America and several European countries in the fields of epidemiology, genetics, pathology and molecular biology, health services research, and clinical oncology and from the advocacy community. The main goals of this multidisciplinary project were to build on the joint efforts of the Breast International Group (BIG) and North American Breast Cancer Group (NABCG), to form a community of international experts in male breast cancer research to forge fruitful and meaningful collaborations, and to develop a research program agenda by identifying the most important research question(s) that we need answered to enable the science to move forward.
The meeting presentations focused on highlighting differences and similarities between breast cancer in males and females. International data on male breast cancer epidemiology, biology, and treatment were reviewed, and the history of clinical trial efforts for male breast cancer was presented. A particular focus was to consider when it is appropriate to extrapolate data from female disease to the treatment of male breast cancer and, conversely, when specific trials are needed to establish optimal therapy for male breast cancer. The need for retrospective and prospective data on treatment and outcomes, biomarker studies, and the development of a tissue repository was stressed.
EPIDEMIOLOGY
Male breast cancer accounts for less than 1% of all cancers in men and less than 1% of breast cancers. The Surveillance, Epidemiology, and End Results (SEER) registry contains a total of 5,494 cases of male breast cancer and 835,000 cases of female breast cancer diagnosed from 1973 through 2005 (Anderson WF, submitted for publication). Eleven percent of breast cancers in men were in situ disease. The median age at diagnosis among registry patients was slightly older in men than women (67 v 61 years). Male breast cancer incidence rates were slightly higher among black than white men. Advanced stage-related tumor characteristics (tumor size > 2.0 cm and positive axillary lymph nodes) were more common in men than women; mean tumor size was 2.4 cm (SE = 0.03 cm) among men and 2.2 cm (SE = 0.003 cm) among women. In contrast, advanced biology-related variables (hormone receptor–negative expression and high tumor grade) were more common among women than men. For example, 23% and 7.6% of breast cancers were estrogen receptor (ER)–negative among women and men, respectively. The age-standardized incidence of male breast cancer increased slightly from 1975 to 2000 (approximately 1% to 4% per year) and seemed to plateau or decrease slightly from 2000 to 2005.
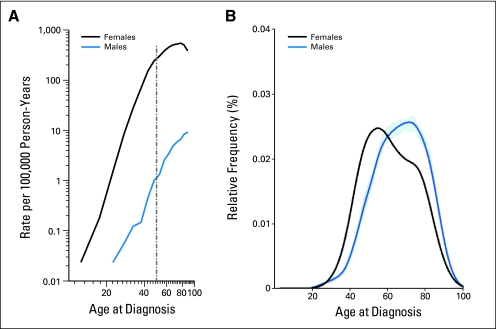
Age-specific incidence rate curves capture information about both time trends in incidence and age at diagnosis. In the United States, from 1973 to 2005, age-specific incidence rates for male breast cancer increase linearly and steadily with age (Fig 1). In contrast, the age-specific rates for female breast cancer increase rapidly until age 50 years and then continue to increase at a slower rate for older women.2 This phenomenon (in women), often referred to as Clemmesen's hook, has been attributed to a bimodal distribution with one peak of early-onset disease and a second peak with a later age at onset. The bimodal distribution is not seen for male breast cancer; rather, a single peak is present at approximately 75 years of age. Although the absolute rates of male breast cancer vary internationally, age-specific patterns are similar worldwide.
Fig 1.
Comparison of age at diagnosis for male and female breast cancer: Surveillance, Epidemiology, and End Results registry, 1973 to 2005. (A) Age-specific incidence rates. (B) Age distribution at diagnosis.
In the Association of Nordic Cancer Registries database (NORDCAN), age-standardized male breast cancer incidence from 1970 to 2000 seems quite stable at approximately 0.4%, with perhaps a slight increase during the latter part from 1990 onward, although these trends should be interpreted with caution because of small numbers. As expected, male breast cancer was more common in older men and very uncommon in younger men. For all the Nordic countries together, there seems to be a relatively stable age-standardized incidence of male breast cancer.3
RISK FACTORS AND GENETICS
Approximately 15% to 20% of men with breast cancer report a family history of breast or ovarian cancer. It is estimated that approximately 10% of men with breast cancer have a genetic predisposition, and BRCA2 is the most clearly associated gene mutation.4–7 BRCA1 mutation is also associated,8 and associations have also been suggested for PTEN, P53, and CHEK2.9–11 Klinefelter's syndrome (XXY) has been described in the literature as occurring in 3% to 7.5% of men with breast cancer.12,13
Among male BRCA2 mutation carriers, the estimated lifetime risk of breast cancer is 5% to 10% compared with a general population risk of 0.1%.14 The association between male breast cancer and BRCA1 mutations is not as strong as for BRCA2 mutations.8,15,16 The lifetime risk of male breast cancer with BRCA1 mutations is approximately 1% to 5%.8,16,17 In 2002, Frank et al18 reported on the Myriad Genetics database, which contained more than 10,000 individuals who had undergone clinical testing for BRCA1/2 over a 3-year period. At that time, there were only 76 male breast cancers in their database, and 21 had detectable mutations; approximately one third of the mutations were BRCA1 mutations.
CHEK2 1100delC increases the risk of both male and female breast cancer, particularly among individuals with a family history and a CHEK2 mutation.19,20 It was initially postulated that CHEK2 may be responsible for 1% of female breast cancer and up to 9% of male breast cancer, although these initial estimates were not based on data from unselected populations. CHEK2 mutations are seen at a low frequency in certain populations, including the US population. The example of CHEK2 can also be applied to recent findings in genome-wide association studies, which have already identified a number of single nucleotide polymorphisms associated with female breast cancer. Although the relative risks associated with these low-penetrance genes are small, they likely cause a substantial fraction of both hereditary and sporadic breast cancer in men and women. Further information is needed regarding interactions with other genes and environmental factors. There are likely to be single nucleotide polymorphisms associated specifically with male breast cancer, but because of small numbers, there is limited power to look at this association, and the clinical value of this information at this time is questionable.
Other well-described risk factors for breast cancer in men include age, race, and radiation exposure. White men have an incidence of 1.1 per 100,000, and black men have an incidence of 1.8 per 100,000.21 At all ages, black men have a higher incidence than white men; in contrast, black women have a lower incidence of breast cancer than white women, except for at young ages (age < 40 years). Black men also tend to have poorer prognostic features, such as advanced-stage disease, larger tumor sizes, more nodal involvement, and higher tumor grade, compared with their white counterparts. A cohort of atomic bomb survivors showed a male breast cancer rate of 1.8 per 100,000 person-years.22
Support for associations with other risk factors, derived mainly from case-control studies, is less conclusive. These other risk factors include hormonal factors, previous breast cancer, and environmental exposures.23 A recent meta analysis23 showed an odds ratio of 2.7 for the association between previous breast disease and male breast cancer. Gynecomastia has been described as a risk factor, although it is unclear whether gynecomastia is a risk factor for male breast cancer or whether the risk factors for male breast cancer are the same as those for gynecomastia. Exposure to electromagnetic fields has been postulated to contribute to the risk of male breast cancer, although the data are inconclusive.23,24
Data from case-control and cohort studies also suggest that increased estradiol levels are associated with male breast cancer. Cirrhosis of the liver, obesity, and exogenous estrogen result in increased circulating estrogen levels and, therefore, may contribute to an increased risk of male breast cancer.23,25,26 There are also possible associations with testicular abnormalities, which are thought to be a result of low testosterone levels and alteration of the ratio of androgens to estrogens.
Recently published data from the NIH American Association of Retired Persons Diet and Health Study Cohort27 confirm an association between family history and male breast cancer. These data also suggest that obesity and osteoporotic fracture are risk factors. The study included 324,000 male study participants, in whom the incidence of subsequent male breast cancer could be assessed. Between 1995 and 2003, 121 male breast cancers were identified, nine of which were in situ and 107 of which were invasive (five patients had missing stage information). The results confirmed that a family history of breast cancer is an important risk factor. The correlation was stronger for patients who reported a sister alone compared with a mother alone; the small number of patients who reported both a mother and a sister with breast cancer had a 10-fold increased risk for male breast cancer. In this study, obesity was also a significant risk factor. Men with a body mass index of ≥ 30 had an 80% increased risk compared with men with a body mass index of less than 25. Conversely, men who were physically active, especially in adolescence, were at a reduced risk, although this was not statistically significant. The study also looked at the relationship of alcohol consumption and cigarette smoking and found a slight increased risk of male breast cancer associated with these factors, but there was no dose-response relationship, probably because of small numbers. An additional interesting finding was that a bone fracture after the age of 45 years was a significant risk factor for male breast cancer, with a relative risk of 2.20.
Men with breast cancer should be considered for genetic counseling and testing, and an adequate family history should be obtained. Current National Comprehensive Cancer Network guidelines for men with BRCA1/2 mutations recommend that providers teach and encourage breast self-examination and perform twice-yearly clinical breast examinations. In addition, consideration should be given to baseline mammogram and annual mammography in men with gynecomastia or glandular breast density on the baseline study. Men are advised to follow population screening guidelines for prostate cancer, and there is an ongoing trial looking at prospective prostate cancer screening for BRCA1 and BRCA2 mutation carriers.28
Future studies with larger cohorts are needed to more completely describe the risk factors for male breast cancer. There is also a need for studies that address how biomarkers may relate to male breast cancer, particularly with regard to endogenous hormones, and how these relate to some of the identified risk factors such as obesity, physical activity, and bone fractures. Ongoing work in genome-wide association studies will likely provide further information regarding the genetic basis of male breast cancer.
PROGNOSIS AND SURVIVAL
Overall, mortality from male breast cancer improved over the time period from 1975 to 2005. Studies examining survival for male breast cancer are quite small compared with population-based studies in female breast cancer. Most studies report overall survival, although a few more recent studies also look at disease-free survival. Five-year survival rates for male breast cancer range from 36% to 66%.29–38 The broad range of survival is likely related to a mix of different stages of disease and time frames of diagnosis, reflecting different treatment guidelines (dates range from the 1930s to the 2000s). In general, men are frequently diagnosed with more advanced-stage cancer than women, particularly in regions where women are routinely screened with mammography.
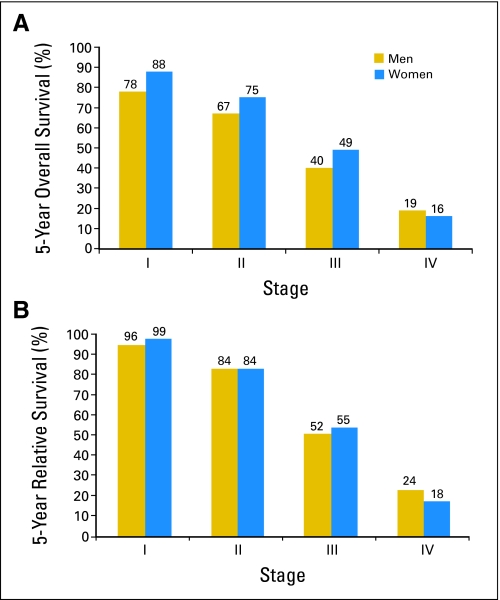
The largest published registry series to examine survival comes from the National Cancer Database,39 which included 4,755 men diagnosed with breast cancer between 1985 and 1994. Observed survival was highest for in situ disease (overall survival = 82%; disease-free survival = 97%) and decreased with successive stages. In that study, 36.9%, 41.9%, 9.6%, and 4.5% of men were diagnosed with stage I, II, III, and IV disease, respectively. In situ disease accounted for 7.1% of breast cancer diagnoses among men. Relative survival, which takes into account age and life expectancy, was higher than observed survival. This likely reflects the influence of greater intercurrent illness as a result of an older mean age at diagnosis. Data from several studies show that approximately 40% of men with breast cancer will die from something other than their cancer. In the SEER database,40 raw data suggest that men have poorer survival than women for stage I to III disease. However, the relative survival rates, which adjust for older age at diagnosis and poorer life expectancy in men than women, are quite similar for men and women (Fig 2).
Fig 2.
(A) Overall and (B) relative survival for male and female breast cancer: Surveillance, Epidemiology, and End Results registry, 1973 to 1998 (N = 2,537).
The NORDCAN database showed an overall downward trend in age-standardized mortality over time for both women and men. Looking at total mortality broken down by (Nordic) country, the highest male breast cancer mortality rate was in Denmark, followed by Norway, Sweden, and Finland. Comparing breast cancer–specific survival in men and women in Sweden, it seemed that although in the first 5 years they were equivalent, after 5 years, women did statistically significantly better than men; however, these data should be interpreted with caution because of small numbers (777 men compared with approximately 130,000 women). For all the Nordic countries together, there was perhaps a slight downward trend in mortality for men.3
Race seems to be a prognostic factor in male breast cancer, particularly in the case of distant disease.41 Other prognostic factors are similar to what is seen in women with breast cancer. A 2004 analysis by Giordano et al,40 which included information on approximately 2,500 patients from the SEER database, identified age, tumor size, stage, and lymph node status as important in determining prognosis. This analysis did not show a correlation between hormone receptor status and prognosis, but only 5-year estimates were stratified by hormone receptor status because of lack of information in the older SEER data. Data on human epidermal growth factor receptor 2 (HER2) in male breast cancer is sparse; therefore, it is difficult to draw any conclusions about the effect of HER2 status on prognosis.
Men with a diagnosis of breast cancer are at increased risk of a second primary malignancy. In a SEER database review that included 4,873 men with breast cancer diagnosed between 1973 and 2003, there was a 1.9% incidence of second primary male breast cancer.42 In this study, 21% of men with breast cancer developed a second nonbreast malignancy; the most commonly reported second malignancies were prostate, colon, and genitourinary cancers.
HISTOPATHOLOGY AND MOLECULAR BIOLOGY
The diagnosis of male breast cancer is generally made on cytology or core biopsy. One study describing 19 years of experience with cytology in male breast cancer43 found that more than two thirds of patients had satisfactory specimens. Male breast tumors tend to be in the areolar region. They are usually identified by palpation and generally have associated mammographic and/or sonographic findings. Mammographically, microcalcifications are less commonly seen in lesions in men than in women.
In a recent review of male breast cancer,44 the majority of tumors were invasive ductal carcinoma (85% to 95%), followed by ductal carcinoma in situ (5% to 10%). Invasive papillary carcinoma is more common in males than in females, accounting for approximately 2% to 4% of breast cancers in men compared with up to 1% in women.
Information on the molecular biology of male breast cancer must be inferred from multiple, small, usually single-center studies using immunohistochemistry because currently there are no actual data on molecular subtypes in male breast cancer. The available literature covers almost 60 studies with varying numbers of patients (up to approximately 200 patients in the largest series) over more than two decades. However, the majority of these studies are small (average of 34 to 39 patients per study) institutional series. Clearly, the combination of data from such diverse series, with different methodologies and cut points for positivity for markers such as ER, progesterone receptor (PgR), and HER2, means that such data must be interpreted with extreme caution. However, the existing data cover just over 1,500 patients and may indicate some future research priorities. Overall, data on conventional markers do not seem markedly dissimilar to the data seen in female breast cancers. Table 1 summarizes the existing literature on receptor status of male breast cancer31,45–48 and compares the data with those seen in a large representative data set of women from a large contemporary phase III trial.49 Proportionately, male breast cancer seems to be more hormone receptor positive (ER and PgR) than female breast cancers, in line with the SEER data cited earlier. Paradoxically, HER2 positivity also seems higher in male breast cancer than in female breast cancer, but this contrasts with data collected from the SEER database. These data may suggest a real biologic difference in these diseases or simply highlight methodologic problems in the pooled analysis. Although the current data suggest that male breast cancer seems to most closely resemble postmenopausal female breast cancer, more robust data are needed to confirm this observation. One of the key requirements for the future management of male breast cancer is a clear understanding of the molecular pathology based on robust analysis of large populations.
Table 1.
Comparison of Existing Literature on Receptor Status in Male and Female Breast Cancer
| Receptor | Male Breast Cancer |
Female Breast Cancer |
Male-to-Female Ratio | Mean No. of Male Breast Cancer Patients on Study | ||||
|---|---|---|---|---|---|---|---|---|
| Total No. of Patients | Receptor-Positive Patients |
Total No. of Patients | Receptor-Positive Patients |
|||||
| No. | % | No. | % | |||||
| ER | 1,548 | 1,269 | 82 | 3,755 | 2,597 | 69 | 1.19 | 34 |
| PgR | 1,287 | 968 | 75 | 2,049 | 1,141 | 56 | 1.35 | 35 |
| HER2 | 741 | 250 | 34 | 3,755 | 942 | 25 | 1.34 | 39 |
Abbreviations: ER, estrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
The inconsistencies between studies can be illustrated by the discrepancies between a large American series,46 where hormone receptor status was similar to that seen in women (81% of tumors expressed ER, and 74% expressed PgR), and the European Institute of Oncology (IEO) experience,45 where ER was expressed in 100% of male breast tumors and PR was expressed in 96%.
The data on HER2 expression in male breast cancer are similarly inconsistent. In one series by Bloom et al,50 only one (1.7%) of 58 men with breast cancer had HER2 overexpression by immunohistochemistry, and none showed HER2 amplification by fluorescence in situ hybridization. In the IEO series,45 15% of male breast tumors were HER2 positive.
Data on other biomarkers of interest are even more sparse and variable. The current data tend to focus on whether or not a certain marker is expressed, rather than quantifying the level or degree of expression. Breast cancers in men express p53, but results range from 3.7% to 20.8% of patients with positive expression.47,48,51 In one small study focusing on cytokeratin (CK) expression, 32 male breast tumors were examined for expression of CK5/6, CK14, and CK17 (associated with basal phenotype) and CK18 and CK19 (associated with luminal subtype), along with ER and HER2 expression.52 Four tumors displayed a basal phenotype pattern of CK expression; the remaining 28 tumors had a luminal-like expression pattern. Interestingly, one tumor example was described in the report in which the basal CKs and ER were all highly expressed. CK profiles suggest that we can differentiate luminal from basal tumors in male breast cancer, as is the case for female breast cancer.
An abstract presented at the 45th Annual Meeting of the American Society of Clinical Oncology in 2009 by Shak et al53 compared quantitative gene expression by sex in tumor specimens submitted for Oncotype DX (Genomic Health, Redwood City, CA) recurrence score testing. The analysis included 347 male and 82,434 female tumor specimens. The distribution of recurrence scores in men and women was similar; the proportions of tumors with low, intermediate, and high recurrence scores were 53.6%, 35.2%, and 11.2%, respectively, in men and 53.4%, 36.2%, and 10.3%, respectively, in women. Notably, the level of quantitative ER expression increased with patient age in women but was essentially stable in men.53 Overall, current data suggest more similarities than dissimilarities between male and female breast cancers; however, additional research on molecular characteristics of male breast cancer is crucial.
TREATMENT
The most common surgical procedure for male breast cancer is modified radical mastectomy. Literature from male breast cancer case series suggests that modified radical mastectomy is used in approximately 70% of patients, followed by radical mastectomy (8% to 30%), total mastectomy (5% to 14%), and lumpectomy with or without radiation (1% to 13%).54 It is important to note that in these series, T4 disease represented 20% to 25% of cancers, which likely affected the choice of surgical procedure. Radical mastectomy was more commonly used in older case series, likely reflecting both practice patterns and later stage at diagnosis of patients in the older series. Male breast anatomy may also contribute to the increased rate of radical mastectomy.
Axillary nodal involvement is the strongest predictor of both local recurrence and metastatic risk and is present in approximately 50% of men with breast cancer.30–32,40,41,55,56 In approximately 40% of patients with nodal involvement, there are more than three nodes involved.30,31,40,54 Sentinel lymph node (SLN) sampling has been examined in numerous small series and seems to be feasible and accurate.
The predictive value of SLN analysis in male breast cancer was investigated by the IEO in 32 patients with cN0 disease subjected to total mastectomy and dissection of the SLN and nonsentinel axillary lymph nodes.57 The SLN was identified by lymphoscintigraphy with colloid particles of human serum albumin labeled with technetium-99m. The SLN was analyzed intraoperatively in frozen sections cut at 50-μm intervals. Lymphoscintigraphy successfully identified all of the SLNs, and the mean number of SLNs was 1.5 (range, one to three SLNs). In six patients, SLNs (18.75%) were metastatic (four macrometastases and two micrometastases). In four of these six patients, the SLN was the only lymph node with metastatic deposits, and two patients had additional positive axillary lymph nodes. The Memorial Sloan-Kettering Cancer Center recently reviewed their experience with SLN sampling in male breast cancer.58 Of 78 male patients reviewed, SLN sampling was successful in 76 patients, yielding a failure rate (3%) that was identical to that seen in concurrently evaluated women with breast cancer. Forty-nine percent of male breast cancer patients had a positive SLN compared with 31% of women. At a median follow-up of 28 months, no axillary recurrences were noted. Similar data have been reported in other, smaller studies, suggesting that SLN analysis is a reliable tool in male breast cancer patients, sparing unnecessary axillary lymph node dissection in a significant fraction (35% in the IEO series) of the patients.
There are few studies describing the use of postmastectomy radiation in male breast cancer, and all are small retrospective analyses. In these studies, between 3% and 100% of patients received radiation therapy, and local recurrence rates ranged from 3% to 29%.59 A recent review suggested that indications for postmastectomy radiation should follow recommendations used for treatment of disease in women, keeping in mind that axillary nodal involvement is a strong predictor of local recurrence risk.54 In addition, retroareolar location of tumor and muscle invasion should be considered as further indications for locoregional radiation treatment.
The mainstay of systemic therapy for hormone receptor–positive male breast cancer is hormonal therapy. Tamoxifen is the most extensively studied. Other hormonal therapies include luteinizing hormone–releasing hormone (LHRH) agonists, orchidectomy, estrogens, and progestins. Case studies have described the use of aromatase inhibitors with or without concurrent LHRH agonist for treatment of male breast cancer, but there are no data supporting this approach.
In a recently reported French case series that included 489 men with breast cancer diagnosed between 1990 and 2005, 72% of patients received hormonal therapy; the majority of these patients (85%) were treated with tamoxifen.60 An additional report outlining patterns of care for male breast cancer in the United States using the SEER database between 2003 and 2004 will be published shortly. In a case series from The University of Texas M.D. Anderson Cancer Center,61 which observed 135 patients for a median follow-up time of 14 years, there was a clear benefit in terms of both recurrence and overall survival with hormonal therapy. A majority of patients in this study received tamoxifen (92%); the remainder received either an LHRH agonist or megestrol acetate. Other studies have also suggested a benefit for tamoxifen in the treatment of male breast cancer.32,34,62
Use of adjuvant chemotherapy in male breast cancer is associated with younger age, higher tumor grade, and axillary nodal involvement. In the French series, 6% of patients received chemotherapy alone, and 28% received the combination of chemotherapy and hormonal treatment.60 Of patients receiving chemotherapy, 73% were treated with an anthracycline-based regimen. In the M.D. Anderson Cancer Center report,61 25% of patients received chemotherapy alone, and 37% of patients received chemotherapy and hormonal therapy. The use of chemotherapy was associated with a nonsignificantly lower risk of death (hazard ratio = 0.78). A second study looking at 20-year survival after chemotherapy with cyclophosphamide, fluorouracil, and methotrexate in male breast cancer suggested a benefit for patients with lymph node involvement.63 Because chemotherapy benefits in general are more apparent in endocrine-nonresponsive breast cancer, in high-risk groups, and in younger patients, it is likely more difficult to detect a chemotherapy benefit in male breast cancer, where the majority of tumors are ER positive and where patients are older and have multiple comorbidities.
One study has examined the effect of sociodemographic and treatment factors on outcomes in male breast cancer.41 This study included 510 men with breast cancer (456 white and 34 black patients) from the SEER registry (1992 to 2002) who were over the age of 65 and covered by Medicare Parts A and B. Two thirds of the men with breast cancer were referred to a medical oncologist, and approximately a quarter of these patients received adjuvant chemotherapy. The strongest predictors of who would receive chemotherapy included age (younger men were more likely to receive chemotherapy) and stage of disease. Hormone receptor positivity and comorbidities were inversely related to chemotherapy use. Of note, although not statistically significant, black men were approximately 50% less likely to be referred to a medical oncologist and to receive chemotherapy. Approximately 50% of the sample population had died after a median follow-up time of 5 years; a slightly higher proportion of blacks died in the follow-up period. Among whites, only approximately 25% of the deaths were reported to be breast cancer related, whereas approximately 60% of the deaths among blacks were defined as breast cancer–related deaths. In a multivariate analysis adjusted for sociodemographics, known prognostic factors, and treatment received, there was approximately a three-fold increased risk of breast cancer–specific mortality for black men compared with their white counterparts. However, because only 34 black men with breast cancer were included in this series, these data should be interpreted with caution.
To date, in the United States, there has only been one prospective clinical trial in male breast cancer, fielded by the Southwest Oncology Group. The hypothesis was that the efficacy of aromatase inhibitors as single-agent therapy for male breast cancer should not be extrapolated from the data on their use in women. The study was a single-arm, phase II trial with a goal of 56 patients with static or recurring disease who were ER positive. Patients were to receive the combination of anastrozole (daily) and goserelin acetate (monthly injections). The primary end point was progression-free survival. The study planned to examine serial serum endocrine levels and to examine the genomics of drug metabolism with anastrozole in men. The study closed after less than 2 years with no patients accrued. The challenges faced were mainly logistic; sites were reluctant to activate a study (only 26 Southwest Oncology Group sites activated the study) and undergo the administrative costs for such a rare disease.
DISCUSSION AND PANEL RECOMMENDATIONS
Panel members agreed that based on current epidemiologic and histopathologic data, male breast cancer seems to resemble postmenopausal hormone receptor–positive disease in women. However, in view of the paucity of data, the rarity of the disease, and different hormonal milieu, male breast cancer should be considered and managed as a distinct entity. Although there seem to be racial and ethnic differences in both incidence and survival of male breast cancer, additional data are needed to confirm these observations. Discussants agreed that given the rarity of male breast cancer, large collaborative efforts are crucial to moving research forward. Two major consortial efforts were discussed, one led from the United States and one from the BIG and the European Organisation for Research and Treatment of Cancer. The National Cancer Institute and NIH Office of Rare Diseases are planning an epidemiologically focused male breast cancer meeting. It will bring together investigators from large cohort studies with banked blood and biomarker data to further examine risk factors for male breast cancer and to look at specific associations between biomarkers such as endogenous hormone levels and breast cancer risk in men.
The second planned research collaboration is an International Male Breast Cancer Program, coordinated by the European Organisation for Research and Treatment of Cancer under the BIG and NABCG networks. This program has three planned parts. The main objective of part 1, or the retrospective part, is to perform a meta-analysis of clinical data and a central pathology review of tumor specimens from patients with male breast cancer diagnosed at participating institutions over the last 20 years. This effort has the potential to overcome many of the difficulties seen in individual studies where biomarker data suffered from lack of harmonization in both definitions and techniques used. Proposed analyses using paraffin-embedded tumor material include quantitative ER and PgR protein levels, HER2 status, Ki67, androgen receptors, cyclin D1, p21, p27, intertumoral aromatase, and survivin. Gene expression profiling studies using frozen tumor material are planned to evaluate the presence and relative incidence of the breast cancer biologic subtypes (basal, luminal, and HER2), the prognostic value of the 70-gene profile, the wound signature, and the stromal signature, among others.
Part 2, or the prospective part, of the program consists of a prospective international registry of all patients with male breast cancer diagnosed at participating institutions for a period of 2 years. The registry would initially use a virtual tumor bank until funding is secured for central analysis of the biologic material collected (paraffin-embedded and frozen tumor samples and blood/serum). Additionally, data on demographics, risk factors, treatment, and outcome will be collected through a remote data capture system.
Data collected from the first two parts of the International Male Breast Cancer Program will enable us to determine the feasibility of a randomized clinical trial that could be launched as part 3 of the program. In view of the failure of previous attempted male breast cancer clinical trials to accrue patients, it is crucial to establish a fully committed global effort to successfully run such a trial.
To date, far more than 19 research groups have expressed interest in participating in the retrospective component of this effort, which would provide approximately 1,700 patients. It is estimated that paraffin blocks can be obtained in 75% to 80% of patients, with frozen material available for 25% to 30%. For the prospective part, more than 16 research groups have confirmed their interest, which should lead to accrual of approximately 100 patients per year.
Funding for such a non–drug-related, purely academic effort is an important hurdle. Thus far, grants have been obtained from the Breast Cancer Research Foundation for the retrospective part and from the European Breast Cancer Conference for the prospective registry. Efforts continue to be made to find the remaining funds needed for this major cooperative academic endeavor for male breast cancer research.
Panel members noted that efforts to understand the biology of male breast cancer, such as those described earlier, are essential to guiding therapy. Most data regarding treatment of male breast cancer is retrospective in nature and comes from small single-institution series; thus, the choice of treatment modalities is generally guided by extrapolation of data from female breast cancer. The established standard of care for male breast cancer is modified radical mastectomy followed by tamoxifen for endocrine-responsive positive disease, although other options are being explored. SLN biopsy seems to be feasible and accurate in men with small tumors and clinically negative axillae. Chemotherapy seems to benefit patients with endocrine-nonresponsive disease, large tumors, and/or node-positive disease.
Patterns of care studies suggest that aromatase inhibitors are being used in the community for treatment of hormone receptor–positive disease despite a lack of solid data supporting their use. Indeed, this is in contradiction to biologic hypotheses that aromatase inhibitors might increase circulating testosterone in men, leading to an increase in androgen available for conversion to estrogen.64 Aside from case reports, there are no clinical studies addressing the efficacy of aromatase inhibitors for male breast cancer. Biologic data suggest that if used in men, aromatase inhibitors should be combined with surgical or medical orchidectomy.64,65 The consensus among panel members was that although it would be reasonable to study aromatase inhibitors in male breast cancer, tamoxifen should remain the standard of care for adjuvant treatment of endocrine-responsive male breast cancer and that use of aromatase inhibitors for male breast cancer should be limited to the clinical trial setting until additional data on aromatase inhibitors in male breast cancer are available. To this end, a small study analyzing the pharmacokinetics and pharmacodynamics of aromatase inhibitors in men is being run as a joint effort between some members of the panel. For the treatment of metastatic tamoxifen-resistant male breast cancer, aromatase inhibitors may be considered but should only be administered in combination with surgical or medical orchidectomy (LHRH agonist).
Members felt that the development of treatment guidelines for male breast cancer would be beneficial. To our knowledge, the Arbeitsgemeinschaft Gynäkologische Onkologie is currently the only organization with published guidelines for male breast cancer, and the majority of data used to form these recommendations come from small case series and expert opinion. The panel stressed that male breast cancer should be considered a rare and unique disease, rather than being considered as analogous to postmenopausal female breast cancer. Treatment of male breast cancer should be driven by data collected from studies that include male participants. Furthermore, it was felt that an educational session at one of the large breast cancer meetings would be helpful in educating physicians on the current data regarding treatment options for male breast cancer.
Several discussion points regarding the prospect of clinical trials in male breast cancer were raised. First, it was felt that more etiologic data are necessary before embarking on clinical trials. Second, given the past experience with poor accrual, a large-scale international effort with a pre-existing infrastructure is strongly advised if a trial of this nature is to succeed; to this end, the International Male Breast Cancer Program is being developed through the BIG and NABCG networks. In addition, awareness at the community oncology level of such an effort is crucial. This could be facilitated through educational sessions at national meetings and potentially through raising awareness of clinical trial availability at the advocacy level.
Several trial design issues were also discussed. Because the majority of male breast tumors are endocrine responsive, the most logical first step would be to develop a trial on endocrine therapy options for these patients. One possibility would be to create a trial looking at tamoxifen versus an aromatase inhibitor with a safety, rather than an efficacy, end point. This would facilitate performing a smaller study and would provide important toxicity data on tamoxifen in men (for which there is scant information currently), as well as preliminary data on aromatase inhibitors. A second suggestion was the creation of a blanket orphan disease protocol that could be opened at multiple institutions and could facilitate gathering information on many of the rarer subsets of patients with breast cancer, including male breast cancer, inflammatory breast cancer, breast cancer in pregnant women, and breast cancer after treatment for Hodgkin's lymphoma. A third option would be to have a separate stratum for male breast cancer in large clinical trials in female breast cancer; this would allow for the collection of data on male breast cancer across studies that could be looked at in meta-analyses.
CONCLUSION
The panel concluded that much still needs to be learned about male breast cancer. It is clear that for this disease, and indeed for many rare diseases, the key to understanding is the pooling of data from a wide range of sources. International consortia are essential for moving forward in our understanding of male breast cancer. Current efforts at pooling epidemiologic data, clinical information, and tumor specimens will lead to a greater understanding of the etiology of this disease. Current international collaborations will also facilitate the future planning of successful clinical trials that can address essential questions in the treatment of male breast cancer. Education of both patients and providers is needed to increase awareness of male breast cancer, to guide evidence-based treatment, and to encourage enrollment onto future clinical and biologic studies aimed at optimizing treatment for this rare disease.
Appendix
Meeting participants included the following: William Anderson (Planning Committee), Biostatistics Branch, Division of Cancer Epidemiology and Genetics (DCEG), National Cancer Institute, Bethesda, MD; John M.S. Bartlett, University of Edinburgh, Endocrine Cancer Group, Department of Pathology, Edinburgh Cancer Research Centre, Edinburgh, United Kingdom; Jonas Bergh, Karolinska Institutet and University Hospital, Stockholm, Sweden; Louise Brinton, Hormonal and Reproductive Epidemiology Branch, DCEG, National Cancer Institute, Bethesda, MD; Fatima Cardoso, Jules Bordet Institute, Breast International Group (BIG), Brussels, Belgium; Katherine Crew, Columbia University, New York, NY; Bruno Cutuli, Polyclinique de Courlancy, Reims, France; Neelima Denduluri, US Oncology, Arlington, VA; Susan Domchek, University of Pennsylvania, Philadelphia, PA; Karen Gelmon, National Cancer Institute of Canada Clinical Trials Group, BC Cancer Agency, Vancouver, British Columbia, Canada; Gretchen Gierach, Hormonal and Reproductive Epidemiology Branch, DCEG, National Cancer Institute, Bethesda, MD; Sharon Giordano, Department of Breast Medical Oncology, The University of Texas M.D. Anderson Cancer Center, Houston, TX; Julie Gralow, University of Washington, Seattle Cancer Care Alliance, Seattle, WA; Mark Greene, Clinical Genetics Branch, DCEG, National Cancer Institute, Bethesda, MD; Linda Harlan (Planning Committee), Health Services and Economics Branch, Applied Research Program, Division of Cancer Control and Population Sciences (DCCPS), National Cancer Institute, Bethesda, MD; Gabriel N. Hortobagyi, Department of Breast Medical Oncology, The University of Texas M.D. Anderson Cancer Center, Houston, TX; James Ingle, Mayo Clinic, Rochester, MN; Guy Jones, Male Breast Cancer Advocate; Marty Jones, Male Breast Cancer Advocate; Larissa Korde (Meeting Co-Chair), Clinical Genetics Branch, DCEG, Bethesda, MD; Jean Lynn, Coordinating Center for Clinical Trials, Office of the Director, National Cancer Institute, Bethesda, MD; Worta McCaskill-Stevens (Planning Committee), Division of Cancer Prevention, National Cancer Institute, Bethesda, MD; Zeina Nahleh, Wayne State University, Detroit, MI; Melanie Palomares, Division of Population Sciences, Department of Outcomes Research, Beckman Research Institute of the City of Hope, Duarte, CA; Giancarlo Pruneri, European Institute of Oncology, Milan, Italy; Anne Reiner, Memorial Sloan-Kettering Cancer Center, New York, NY; Stephen Taplin, Applied Cancer Screening Research Branch, Behavioral Research Program, DCCPS, National Cancer Institute, Bethesda, MD; Laura Van't Veer, Department of Pathology, Netherlands Cancer Institute, Amsterdam, the Netherlands; Larry Wickerham, National Surgical Adjuvant Breast and Bowel Project, Pittsburgh, PA; William Wood, Department of Surgery, Emory University, Atlanta, GA; Jo Anne Zujewski (Meeting Co-Chair), Clinical Investigations Branch, Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Bethesda, MD.
Footnotes
Written on behalf of the Multidisciplinary Male Breast Cancer Meeting Participants.
Supported by the Intramural Research Program of the National Institutes of Health (NIH) and National Cancer Institute (NCI). Funding for the NCI Multidisciplinary Male Breast Cancer Scientific Meeting was provided by the Office of Rare Diseases, NIH, and by the Division of Cancer Epidemiology and Genetics and Division of Cancer Treatment and Diagnosis, NCI.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Gabriel Hortobagyi, Novartis (U), Bristol-Myers Squibb (C) Stock Ownership: None Honoraria: None Research Funding: Gabriel Hortobagyi, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Larissa A. Korde, Jo Anne Zujewski, William F. Anderson, Worta McCaskill-Stevens, Gabriel Hortobagyi, Fatima Cardoso
Financial support: Larissa A. Korde, Jo Anne Zujewski, Fatima Cardoso
Administrative support: Leah Kamin
Collection and assembly of data: Larissa A. Korde, Sharon Giordano, Susan Domchek, William F. Anderson, Jonas Bergh
Data analysis and interpretation: Sharon Giordano, Susan Domchek, William F. Anderson, John M.S. Bartlett, Jonas Bergh, Bruno Cutuli
Manuscript writing: Larissa A. Korde, Leah Kamin, Sharon Giordano, Susan Domchek, William F. Anderson, John M.S. Bartlett, Karen Gelmon, Zeina Nahleh, Jonas Bergh, Bruno Cutuli, Giancarlo Pruneri, Worta McCaskill-Stevens, Julie Gralow, Gabriel Hortobagyi, Fatima Cardoso
Final approval of manuscript: Larissa A. Korde, Jo Anne Zujewski, Leah Kamin, Sharon Giordano, Susan Domchek, William F. Anderson, John M.S. Bartlett, Karen Gelmon, Zeina Nahleh, Jonas Bergh, Bruno Cutuli, Giancarlo Pruneri, Worta McCaskill-Stevens, Julie Gralow, Gabriel Hortobagyi, Fatima Cardoso
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Anderson WF, Chu KC, Chang S, et al. Comparison of age-specific incidence rate patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol Biomarkers Prev. 2004;13:1128–1135. [PubMed] [Google Scholar]
- 3.Engholm G, Ferlay J, Christensen N, et al. Copenhagen, Denmark: Danish Cancer Society; 2009. NORDCAN: Cancer Incidence, Mortality and Prevalence in the Nordic Countries, Version 3.4, Association of Nordic Cancer Registries. [Google Scholar]
- 4.Couch FJ, Farid LM, DeShano ML, et al. BRCA2 germline mutations in male breast cancer cases and breast cancer families. Nat Genet. 1996;13:123–125. doi: 10.1038/ng0596-123. [DOI] [PubMed] [Google Scholar]
- 5.Haraldsson K, Loman N, Zhang QX, et al. BRCA2 germ-line mutations are frequent in male breast cancer patients without a family history of the disease. Cancer Res. 1998;58:1367–1371. [PubMed] [Google Scholar]
- 6.Thorlacius S, Tryggvadottir L, Olafsdottir GH, et al. Linkage to BRCA2 region in hereditary male breast cancer. Lancet. 1995;346:544–545. doi: 10.1016/s0140-6736(95)91383-1. [DOI] [PubMed] [Google Scholar]
- 7.Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- 8.Brose MS, Rebbeck TR, Calzone KA, et al. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002;94:1365–1372. doi: 10.1093/jnci/94.18.1365. [DOI] [PubMed] [Google Scholar]
- 9.Anelli A, Anelli TF, Youngson B, et al. Mutations of the p53 gene in male breast cancer. Cancer. 1995;75:2233–2238. doi: 10.1002/1097-0142(19950501)75:9<2233::aid-cncr2820750907>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Fackenthal JD, Marsh DJ, Richardson AL, et al. Male breast cancer in Cowden syndrome patients with germline PTEN mutations. J Med Genet. 2001;38:159–164. doi: 10.1136/jmg.38.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meijers-Heijboer H, van den Ouweland A, Klijn J, et al. Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet. 2002;31:55–59. doi: 10.1038/ng879. [DOI] [PubMed] [Google Scholar]
- 12.Evans DB, Crichlow RW. Carcinoma of the male breast and Klinefelter's syndrome: Is there an association? CA Cancer J Clin. 1987;37:246–251. doi: 10.3322/canjclin.37.4.246. [DOI] [PubMed] [Google Scholar]
- 13.Hultborn R, Hanson C, Kopf I, et al. Prevalence of Klinefelter's syndrome in male breast cancer patients. Anticancer Res. 1997;17:4293–4297. [PubMed] [Google Scholar]
- 14.Cancer risks in BRCA2 mutation carriers. The Breast Cancer Linkage Consortium. J Natl Cancer Inst. 1999;91:1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 15.Chodick G, Struewing JP, Ron E, et al. Similar prevalence of founder BRCA1 and BRCA2 mutations among Ashkenazi and non-Ashkenazi men with breast cancer: Evidence from 261 cases in Israel, 1976-1999. Eur J Med Genet. 2008;51:141–147. doi: 10.1016/j.ejmg.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tai YC, Domchek S, Parmigiani G, et al. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:1811–1814. doi: 10.1093/jnci/djm203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson D, Easton DF. Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94:1358–1365. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 18.Frank TS, Deffenbaugh AM, Reid JE, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: Analysis of 10,000 individuals. J Clin Oncol. 2002;20:1480–1490. doi: 10.1200/JCO.2002.20.6.1480. [DOI] [PubMed] [Google Scholar]
- 19.CHEK2 Breast Cancer Case-Control Consortium. CHEK2*1100delC and susceptibility to breast cancer: A collaborative analysis involving 10,860 breast cancer cases and 9,065 controls from 10 studies. Am J Hum Genet. 2004;74:1175–1182. doi: 10.1086/421251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vahteristo P, Bartkova J, Eerola H, et al. A CHEK2 genetic variant contributing to a substantial fraction of familial breast cancer. Am J Hum Genet. 2002;71:432–438. doi: 10.1086/341943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson WF, Althuis MD, Brinton LA, et al. Is male breast cancer similar or different than female breast cancer? Breast Cancer Res Treat. 2004;83:77–86. doi: 10.1023/B:BREA.0000010701.08825.2d. [DOI] [PubMed] [Google Scholar]
- 22.Ron E, Ikeda T, Preston DL, et al. Male breast cancer incidence among atomic bomb survivors. J Natl Cancer Inst. 2005;97:603–605. doi: 10.1093/jnci/dji097. [DOI] [PubMed] [Google Scholar]
- 23.Sasco AJ, Lowenfels AB, Pasker-de Jong P. Review article: Epidemiology of male breast cancer—A meta-analysis of published case-control studies and discussion of selected aetiological factors. Int J Cancer. 1993;53:538–549. doi: 10.1002/ijc.2910530403. [DOI] [PubMed] [Google Scholar]
- 24.Demers PA, Thomas DB, Rosenblatt KA, et al. Occupational exposure to electromagnetic fields and breast cancer in men. Am J Epidemiol. 1991;134:340–347. doi: 10.1093/oxfordjournals.aje.a116095. [DOI] [PubMed] [Google Scholar]
- 25.Hsing AW, McLaughlin JK, Cocco P, et al. Risk factors for male breast cancer (United States) Cancer Causes Control. 1998;9:269–275. doi: 10.1023/a:1008869003012. [DOI] [PubMed] [Google Scholar]
- 26.Sorensen HT, Friis S, Olsen JH, et al. Risk of liver and other types of cancer in patients with cirrhosis: A nationwide cohort study in Denmark. Hepatology. 1998;28:921–925. doi: 10.1002/hep.510280404. [DOI] [PubMed] [Google Scholar]
- 27.Brinton LA, Richesson DA, Gierach GL, et al. Prospective evaluation of risk factors for male breast cancer. J Natl Cancer Inst. 2008;100:1477–1481. doi: 10.1093/jnci/djn329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.IMPACT Study. Homepage. http://www.impact-study.co.uk/public/home.
- 29.Adami HO, Holmberg L, Malker B, et al. Long-term survival in 406 males with breast cancer. Br J Cancer. 1985;52:99–103. doi: 10.1038/bjc.1985.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cutuli B, Lacroze M, Dilhuydy JM, et al. Male breast cancer: Results of the treatments and prognostic factors in 397 cases. Eur J Cancer. 1995;31A:1960–1964. doi: 10.1016/0959-8049(95)00366-5. [DOI] [PubMed] [Google Scholar]
- 31.Donegan WL, Redlich PN, Lang PJ, et al. Carcinoma of the breast in males: A multiinstitutional survey. Cancer. 1998;83:498–509. doi: 10.1002/(sici)1097-0142(19980801)83:3<498::aid-cncr19>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 32.Goss PE, Reid C, Pintilie M, et al. Male breast carcinoma: A review of 229 patients who presented to the Princess Margaret Hospital during 40 years: 1955-1996. Cancer. 1999;85:629–639. doi: 10.1002/(sici)1097-0142(19990201)85:3<629::aid-cncr13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 33.Norris HJ, Taylor HB. Carcinoma of the male breast. Cancer. 1969;23:1428–1435. doi: 10.1002/1097-0142(196906)23:6<1428::aid-cncr2820230626>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro G. Male breast carcinoma: A review of 301 cases from the Christie Hospital & Holt Radium Institute, Manchester. Br J Cancer. 1985;51:115–119. doi: 10.1038/bjc.1985.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribeiro GG. Carcinoma of the male breast: A review of 200 cases. Br J Surg. 1977;64:381–383. doi: 10.1002/bjs.1800640602. [DOI] [PubMed] [Google Scholar]
- 36.Scheike O. Male breast cancer: 6. Factors influencing prognosis. Br J Cancer. 1974;30:261–271. doi: 10.1038/bjc.1974.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stierer M, Rosen H, Weitensfelder W, et al. Male breast cancer: Austrian experience. World J Surg. 1995;19:687–692. doi: 10.1007/BF00295904. [DOI] [PubMed] [Google Scholar]
- 38.van Geel AN, van Slooten EA, Mavrunac M, et al. A retrospective study of male breast cancer in Holland. Br J Surg. 1985;72:724–727. doi: 10.1002/bjs.1800720918. [DOI] [PubMed] [Google Scholar]
- 39.Scott-Conner CE, Jochimsen PR, Menck HR, et al. An analysis of male and female breast cancer treatment and survival among demographically identical pairs of patients. Surgery. 1999;126:775–780. [PubMed] [Google Scholar]
- 40.Giordano SH, Cohen DS, Buzdar AU, et al. Breast carcinoma in men: A population-based study. Cancer. 2004;101:51–57. doi: 10.1002/cncr.20312. [DOI] [PubMed] [Google Scholar]
- 41.Crew KD, Neugut AI, Wang X, et al. Racial disparities in treatment and survival of male breast cancer. J Clin Oncol. 2007;25:1089–1098. doi: 10.1200/JCO.2006.09.1710. [DOI] [PubMed] [Google Scholar]
- 42.Wernberg JA, Yap J, Murekeyisoni C, et al. Multiple primary tumors in men with breast cancer diagnoses: A SEER database review. J Surg Oncol. 2009;99:16–19. doi: 10.1002/jso.21153. [DOI] [PubMed] [Google Scholar]
- 43.Joshi A, Kapila K, Verma K. Fine needle aspiration cytology in the management of male breast masses: Nineteen years of experience. Acta Cytol. 1999;43:334–338. doi: 10.1159/000331077. [DOI] [PubMed] [Google Scholar]
- 44.Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet. 2006;367:595–604. doi: 10.1016/S0140-6736(06)68226-3. [DOI] [PubMed] [Google Scholar]
- 45.Curigliano G, Colleoni M, Renne G, et al. Recognizing features that are dissimilar in male and female breast cancer: Expression of p21Waf1 and p27Kip1 using an immunohistochemical assay. Ann Oncol. 2002;13:895–902. doi: 10.1093/annonc/mdf166. [DOI] [PubMed] [Google Scholar]
- 46.Giordano SH, Buzdar AU, Hortobagyi GN. Breast cancer in men. Ann Intern Med. 2002;137:678–687. doi: 10.7326/0003-4819-137-8-200210150-00013. [DOI] [PubMed] [Google Scholar]
- 47.Rayson D, Erlichman C, Suman VJ, et al. Molecular markers in male breast carcinoma. Cancer. 1998;83:1947–1955. doi: 10.1002/(sici)1097-0142(19981101)83:9<1947::aid-cncr10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 48.Weber-Chappuis K, Bieri-Burger S, Hurlimann J. Comparison of prognostic markers detected by immunohistochemistry in male and female breast carcinomas. Eur J Cancer. 1996;32A:1686–1692. doi: 10.1016/0959-8049(96)00154-2. [DOI] [PubMed] [Google Scholar]
- 49.Ellis P, Barrett-Lee P, Johnson L, et al. Sequential docetaxel as adjuvant chemotherapy for early breast cancer (TACT): An open-label, phase III, randomised controlled trial. Lancet. 2009;373:1681–1692. doi: 10.1016/S0140-6736(09)60740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bloom KJ, Govil H, Gattuso P, et al. Status of HER-2 in male and female breast carcinoma. Am J Surg. 2001;182:389–392. doi: 10.1016/s0002-9610(01)00733-4. [DOI] [PubMed] [Google Scholar]
- 51.Wang-Rodriguez J, Cross K, Gallagher S, et al. Male breast carcinoma: Correlation of ER, PR, Ki-67, Her2-Neu, and p53 with treatment and survival, a study of 65 cases. Mod Pathol. 2002;15:853–861. doi: 10.1097/01.MP.0000022251.61944.1D. [DOI] [PubMed] [Google Scholar]
- 52.Ciocca V, Bombonati A, Gatalica Z, et al. Cytokeratin profiles of male breast cancers. Histopathology. 2006;49:365–370. doi: 10.1111/j.1365-2559.2006.02519.x. [DOI] [PubMed] [Google Scholar]
- 53.Shak S, Palmer G, Baehner FL, et al. Molecular characterization of male breast cancer by standard quantitative RT-PCR analysis: First large genomic study of 347 male breast cancers compared to 82,434 female breast cancers. J Clin Oncol. 2009;27(suppl 15S):18s. abstr 549. [Google Scholar]
- 54.Cutuli B. Strategies in treating male breast cancer. Expert Opin Pharmacother. 2007;8:193–202. doi: 10.1517/14656566.8.2.193. [DOI] [PubMed] [Google Scholar]
- 55.Nahleh ZA, Srikantiah R, Safa M, et al. Male breast cancer in the veterans affairs population: A comparative analysis. Cancer. 2007;109:1471–1477. doi: 10.1002/cncr.22589. [DOI] [PubMed] [Google Scholar]
- 56.Salvadori B, Saccozzi R, Manzari A, et al. Prognosis of breast cancer in males: An analysis of 170 cases. Eur J Cancer. 1994;30A:930–935. doi: 10.1016/0959-8049(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 57.Gentilini O, Chagas E, Zurrida S, et al. Sentinel lymph node biopsy in male patients with early breast cancer. Oncologist. 2007;12:512–515. doi: 10.1634/theoncologist.12-5-512. [DOI] [PubMed] [Google Scholar]
- 58.Flynn LW, Park J, Patil SM, et al. Sentinel lymph node biopsy is successful and accurate in male breast carcinoma. J Am Coll Surg. 2008;206:616–621. doi: 10.1016/j.jamcollsurg.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 59.Chakravarthy A, Kim CR. Post-mastectomy radiation in male breast cancer. Radiother Oncol. 2002;65:99–103. doi: 10.1016/s0167-8140(02)00210-4. [DOI] [PubMed] [Google Scholar]
- 60.Cutuli B, Le-Nir CC, Serin D, et al. Male breast cancer: Evolution of treatment and prognostic factors—Analysis of 489 cases. Crit Rev Oncol Hematol. doi: 10.1016/j.critrevonc.2009.04.002. epub ahead of print on May 11, 2009. [DOI] [PubMed] [Google Scholar]
- 61.Giordano SH, Perkins GH, Broglio K, et al. Adjuvant systemic therapy for male breast carcinoma. Cancer. 2005;104:2359–2364. doi: 10.1002/cncr.21526. [DOI] [PubMed] [Google Scholar]
- 62.Ribeiro G, Swindell R. Adjuvant tamoxifen for male breast cancer (MBC) Br J Cancer. 1992;65:252–254. doi: 10.1038/bjc.1992.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walshe JM, Berman AW, Vatas U, et al. A prospective study of adjuvant CMF in males with node positive breast cancer: 20-year follow-up. Breast Cancer Res Treat. 2007;103:177–183. doi: 10.1007/s10549-006-9363-0. [DOI] [PubMed] [Google Scholar]
- 64.Czene K, Bergqvist J, Hall P, et al. How to treat male breast cancer. Breast. 2007;16(suppl 2):S147–S154. doi: 10.1016/j.breast.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 65.Giordano SH, Hortobagyi GN. Leuprolide acetate plus aromatase inhibition for male breast cancer. J Clin Oncol. 2006;24:e42–e43. doi: 10.1200/JCO.2006.07.2397. [DOI] [PubMed] [Google Scholar]