Abstract
OBJECTIVES
To demonstrate that human smooth muscle cells derived from neurogenic bladders produce more collagen in vitro than smooth muscle cells derived from normal bladders, and that epigenetic therapy may normalize this increased collagen production.
METHODS
Human smooth muscle cells from normal (n = 3) and neurogenic bladders (n = 3) were cultured in normal culture media and at different concentrations of the histone deacetylase inhibitors trichostatin A, valproic acid, and the DNA methylation inhibitor 5-azacytidine (5-aza). Collagen type I and III gene expression was measured using real-time quantitative reverse transcription-polymerase chain reaction after varying doses of drug exposure. Cell viability was measured using trypan blue.
RESULTS
The smooth muscle cells from neurogenic bladders released significantly more collagen than the normal bladder cells (mean 4.1 vs 1.8 μg/mL in control media) when grown in normal conditions. Treatment with trichostatin A at 50 ng/mL decreased the collagen level in cells from neurogenic bladders to almost normal levels (2.1 μg/mL). In addition, valproic acid treatment decreased collagen types I and III gene expression relative to controls, with maximal effect at 300 mg/mL. These treatments had little effect on cell viability.
CONCLUSIONS
Histone deacetylase inhibitors decreased collagen production of smooth muscle cells from neurogenic bladders in vitro. These agents may be a means of effectively preventing bladder fibrosis in patients with this condition.
In children with myelodysplasia, a primary concern is the maintenance of low bladder storage pressure. Elevated intravesical pressure caused by a poorly compliant bladder in patients with spinal dysraphism is associated with damage to the upper urinary tract, and if not appropriately treated, renal failure.1 Many treatment modalities have been developed to prevent or treat this disease process. One example is the use of chronic oral antimuscarinic therapy from an early age, coupled with clean intermittent catheterization.2 Intravesical instillation of oxybutynin and other agents also has been attempted.3,4 However, these therapies vary in efficacy and are associated with a number of side effects. Thus, development of novel treatment options is desirable.
Neuropathic bladders have an increased ratio of type-III to type-I collagen, with an absolute increase in the amount of type-III collagen.5 This increased collagen content is at least in part produced by detrusor smooth muscle cells and is thought to be a major cause of decreased bladder compliance.6 However, it is not clear how elevated collagen composition affects total intravesical pressure in the setting of a neuropathic, spastic bladder.
To study the role of collagen composition and production in neuropathic bladder tissue, we performed genomic analyses of cells from normal bladders and from the bladders of myelomeningocele patients.7 When comparing the differential gene expression of neurogenic bladder smooth muscle to normal smooth muscle, we identified changes in the expression of 837 genes (520 were upregulated and 317 downregulated). Clustering this dataset using the Gene Ontology Cellular Component demonstrated that the predominant cellular location of gene upregulation was in the extracellular matrix, specifically the collagen and microfibrillar genes.7 Thus, smooth muscle cells in neurogenic bladders may have genomic differences that could promote excessive collagen production. Controlling the regulation of collagen production in bladder tissue may delay disease progression and enhance bladder compliance.
Epigenetic therapies (agents that induce changes in gene expression but do not change the DNA itself) such as histone deacetylase (HDAC) inhibitors have demonstrated antifibrogenic effects in several disease models through the hyperacetylation of histones H3 and H4.8,9 Acetylated histone proteins increase the accessibility of DNA to the transcriptional machinery. HDAC deacetylates the histone proteins and inhibits gene transcription. HDAC inhibitors, therefore, may function by restoring the expression of specific silenced genes. These agents reduce fibrosis in diseases affecting other organs, such as pulmonary and liver fibrosis.9,10
Epigenetic therapy may also be directed at the level of DNA. DNA is methylated by adding a methyl group to the 5′ position of cytosine residues in the cytosine-phosphoguanine (CpG) dinucleotide. Methylation at this site silences gene transcription, while demethylation promotes gene expression. Hypermethylation and gene suppression may lead to disease processes such as cancer and fibrosis. In these instances, DNA methylation inhibitors can restore normal gene function, and in some cases treat fibrosis in organs such as the liver.9
The goals of this pilot study were to evaluate collagen production in normal and neuropathic bladder smooth muscle cells in culture, and to attempt to modulate this collagen production using the HDAC inhibitors trichostatin A (TSA) and valproic acid (VA), and the DNA methylation-inhibitor 5-azacytidine (5-aza). This study was designed to provide proof-of-principle for the hypothesis that HDAC inhibitors would alter collagen production in tissues from neurogenic bladders, and to be the basis for larger and more definitive studies in the future.
MATERIAL AND METHODS
After obtaining Institutional Review Board approval and parental consent, bladder biopsies were obtained from 3 children with primary vesicoureteral reflux undergoing ureteral reimplantation and 3 children with myelomeningocele undergoing bladder augmentation. The patients with vesicoureteral reflux had radiographically and urodynamically normal bladders, with normal filling cystometry. The patients with myelomeningocele had functionally abnormal bladders according to their urodynamic evaluation, with elevated pressures on filling cystometry (detrusor pressure at maximum cystometric capacity > 40 cm H2O).
Bladder smooth muscle cells were isolated from the tissue biopsies and cultured according to previously described methods.11 Briefly, the muscle cells were processed by the tissue explant technique and cultured in complete medium (Dulbecco’s Modified Eagle’s Medium [Gibco BRL, Carlsbad, CA] supplemented with 10% fetal bovine serum). The cells were incubated in a humidified chamber containing 5% CO2 and maintained at 37°C. The cells were expanded to 50% confluence in 35-mm cell culture plates. The cells had a uniform appearance in culture and expressed the smooth muscle marker proteins alpha-actin and myosin.
All experiments were performed in triplicate using cells between passages 2 and 5. Cells were first exposed to different doses and combinations of HDAC inhibitors (TSA) and DNA methylation inhibitors (5-aza) to screen the ability of these agents to affect collagen production. Cells from each patients were placed in 6 different media conditions for 24 hours: control (complete media), complete media with TSA at concentrations of either 50 or 100 ng/mL, complete media with 5-aza (5μM), complete media with TSA (50 ng/mL) and 5-aza (5 μM), and complete media with TSA (100 ng/mL) and 5-aza (5 μM). After a 24-hour treatment, the cells were returned to normal complete media for 6 days and the media was refreshed every 3 days. After the 1-week incubation, 200 μL of media from each cell culture plate was analyzed for collagen protein content using a Sircol Collagen Assay (Biocolor, Ltd., Carrick-fergus, Northern Ireland), according to the manufacturer’s instructions. The amount of collagen was quantified using spectrophotometry. The absorbance of reference collagen standards at 3 different concentrations was measured at 540 nm to produce a calibration curve. The absorbance of the culture media was also evaluated at 540 nm and correlated to this curve to calculate collagen composition. The cells from each plate were also counted and ranged in number from 200 000 to 250 000 cells, with an average of 225 000 cells/plate.
The ability of a less potent, but clinically available HDAC inhibitor, VA, to modulate smooth muscle cell collagen production at varying doses was also measured. Cells from each of the patients were placed in 5 different conditions for 24 hours: control media and media containing varying concentrations of VA (100, 200, 300, and 600 mg/mL). Cell viability was determined by trypan blue dye exclusion using a hematocytometer. Cellular RNA was then extracted using the RNeasy Mini kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The RNA concentrations were measured using spectrophotometry, and approximately 1–2 μg RNA was used for cDNA synthesis. The mRNA was converted to cDNA via reverse transcription using a cDNA Cycle Kit for First-Strand cDNA Synthesis for polymerase chain reaction (PCR) Template Generation (reverse transcription-PCR) (Invitrogen, Carlsbad, CA) per the manufacturer’s instructions.
Real-time PCR was performed to evaluate collagen gene expression, using the TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA) as described by the manufacturer. The reactions were performed in a 96-well optical Reaction Plate (Applied Biosystems). The primers included collagen type Ia and IIIa probes from TaqMan Gene Expression Assays (Applied Biosystems). Every reaction contained 8 μL of distilled RNase-free H2O, 10 μL TaqMan Universal PCR Master Mix, 1-μL primer, and 1 μL cDNA. The housekeeping gene GAPDH was used as an internal control to confirm the successful extraction of RNA, conversion to cDNA, and PCR reactions. The reactions were run on the ABI PRISM 7700 Sequence Detection System (Applied Biosystems). The amplification program consisted of incubation at 50°C for 2 minutes and denaturation at 95°C for 10 minutes, followed by 45 cycles of 95°C for 15 seconds, 60°C for 1 minute. The real-time PCR results were normalized based on GAPDH mRNA expression and relative gene expression levels were calculated using the 2(-delta delta C(T)) method.12 Analysis of gene expression data using the 2(-delta delta C(T)) formula has appeared in the literature,12 and it is a proven method to calculate relative changes in gene expression determined from real-time quantitative PCR experiments.
RESULTS
The amount of soluble collagen produced by smooth muscle cells from neurogenic bladders after 1 week of growth was twice that produced by normal smooth muscle cells. Average amounts of collagen production for the control neurogenic and normal smooth muscle cells were 4.1 μg/mL (average of 4.0, 4.1, and 4.2 per patient sample), and 2.0 μg/mL (average of 1.8, 1.9, and 2.3 per patient sample), respectively. This difference was statistically significant (P <.001 via Student t test).
Treatment of the smooth muscle cells from neurogenic bladders with TSA (50 ng/mL) for 24 hours decreased the average amount of soluble collagen produced by over half (1.8 μg/mL; average of 1.5, 1.9, and 2 per patient sample). When compared with the collagen production in the control group using a paired t test this reduction was also statistically significant (P = .003). All other drug concentrations and combinations increased collagen production. TSA and 5-aza treatment of smooth muscle cells from normal bladders produced mixed results in which collagen production was sometimes increased, but never decreased.
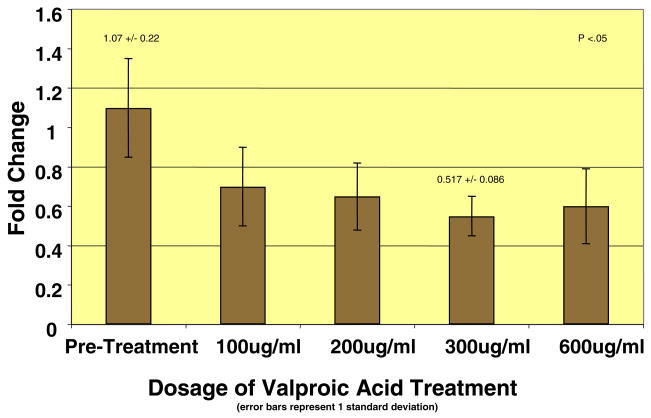
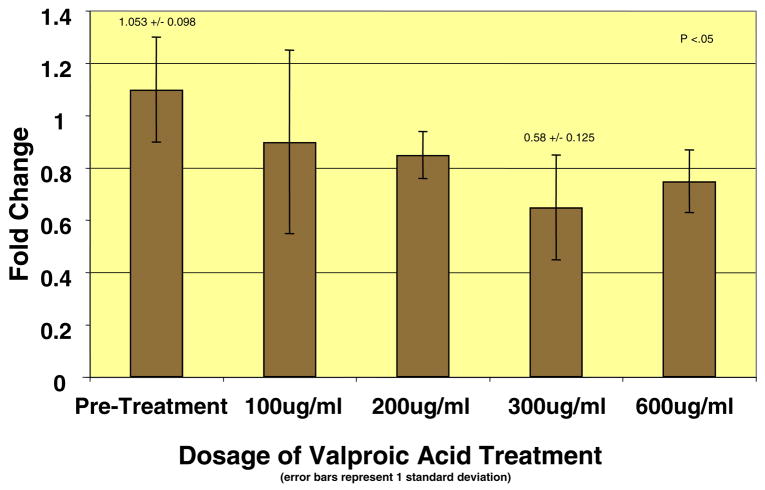
The results of the VA treatments also demonstrated decreases in collagen gene expression. All doses of VA (100, 200, 300, and 600 μg/mL) produced decreases in relative collagen type I and III mRNA expression in smooth muscle cells from neurogenic bladders, with a maximal effect at the 300 μg/mL dose (Figs. 1 and 2). At this dose, more than 50% decrease (P <.05) in the relative mRNA expression of both collagen type I and III mRNAs was observed. However, this experiment was not performed in normal bladder smooth muscle cells, as the first screening test did not demonstrate any effect of epigenetic therapies on collagen production in these cells. Cell viability studies using trypan blue exclusion confirmed that reduced collagen production with VA therapy was not due to cell death, as cell viability remained above well above 90% in all treatment groups except for the 600 μg/mL dose.
Figure 1.
Relative mRNA expression of collagen type I decreased at all doses, with maximal effect at 300 mg/mL.
Figure 2.
Relative mRNA expression of collagen type III decreased at all doses, with maximal effect at 300 mg/mL.
COMMENT
Interventions to prevent or treat the development of high-pressure urine storage problems in children with myelodysplasia have been disappointing results, and preventive medical therapies have had limited efficacy and various side effects.13 Treatment options (eg, enterocystoplasty, neurostimulation devices) have been somewhat beneficial, but the ultimate goal remains to prevent surgery by maintaining a larger-volume, compliant bladder.14–17
Excessive collagen deposition is highly associated with decreased bladder compliance in neuropathic bladders. The increase in the collagen type III: I ratio, and the absolute increase in collagen III levels, has been well documented in histologic studies.5 These changes may also be due to the physiological obstruction induced by detrusor sphincter dysynergia, as they mirror those seen in obstructed bladders (ie, posterior urethral valves, prostatic hyperplasia).16 The exact effect of increased collagen deposition on bladder pressure is unknown, as many factors such as bladder wall composition and abnormal innervation affect total intravesical pressures. Several studies have shown that increased bladder collagen decreases bladder wall compliance.6
The smooth muscle cells of neuropathic bladders develop and grow in an abnormal environment.16 It is not known, however, whether the abnormal environment causes genetic changes in these cells that may lead to elevated collagen production, and whether these changes can be prevented. We have shown previously7 that specific genomic differences do exist among normal and neuropathic bladder smooth muscle cells, with a specific upregulation of extracellular matrix genes, such as collagen. The increased collagen production in neuropathic bladder smooth muscle cells documented in this study may also represent the results of epigenetic changes in these cells. Epigenetic modifications can involve DNA methylation, histone acetylation, and RNA interference. Such changes may explain why alterations in gene expression in cells from neuropathic bladders persist even in normal culture conditions.
TSA, an antifungal antibiotic that is a potent and specific inhibitor of HDAC in vivo and in vitro, reduces collagen production by inhibiting HDAC activity, primarily at histones H3 and H4.8,9 The results of the present study indicate that histone acetylation state may regulate collagen gene expression, and that the maintenance of acetylated histones may allow transcription of genes involved in inhibiting collagen synthesis in bladder smooth muscle cells. This inhibition could have clinical relevance in the case of neuropathic bladder. However, TSA is not approved for clinical use. In contrast, VA has been used for years as an antiepileptic agent for the treatment of both generalized and partial seizures in adults and children.18 Interestingly, it also has potent HDAC inhibitor activity, and because it is already clinically available, it could offer more hope as a therapy for bladder fibrosis.
There is some evidence regarding the mechanisms of action of TSA and VA in smooth muscle cells derived from neuropathic bladder tissue. Unraveling the chromosomal DNA of chromatin may participate in the control of gene expression. For example, tightly packed DNA is inaccessible to the transcriptional apparatus, while unwound DNA is easily accessible. One mechanism of these processes involves the acetylation and deacetylation of the histone proteins, which serve as “spools” around which the strands of DNA are wound. Acetylated histone proteins increase the accessibility of DNA to the transcriptional machinery for expression. Because HDACs deacetylate histone proteins and thus may inhibit gene transcription, HDAC inhibitors may restore the expression of silenced genes.19 The addition of methyl groups to the 5-position of cytosines after DNA synthesis alters the adherence of DNA-binding proteins, thus silencing these genes. These changes can be copied after DNA synthesis, resulting in heritable alterations in chromatin structure. The role of DNA methylation in tissue-specific gene expression is poorly understood, but it may act to increase specific gene expression.20
The finding that collagen production can be decreased in smooth muscle cells treated with 50 ng/mL of TSA suggests that this may reflect epigenetic changes such as histone acetylation/deacetylation. The varying results found with other doses of TSA within our study are not surprising, as the effects of these agents have been very dose-specific in other cases. Confirmation of the concept is further supported by the positive results also seen with VA. TSA and VA are 2 very different drugs, yet both are able to decrease collagen production in neuropathic bladder smooth muscle cells. This is likely due to the HDAC-inhibiting activity that they are known to share. Recently, an analogue of TSA (suberoylanilide hydroxamic acid) was approved for oral use in the treatment of T-cell lymphoma. Future studies could evaluate the ability of either of these orally available agents to prevent fibrosis in patients with neurogenic bladder.
LIMITATIONS
Our findings should be interpreted with caution. Because this study was intended as a pilot experiment, the number of subjects was deliberately small so that we could establish proof-of-principle for the underlying hypothesis. Furthermore, since we wanted to conduct a dose–response study, using fewer subjects made our initial experiments much more feasible. We plan future studies with a larger sample size and more detailed analyses of the tissues.
CONCLUSIONS
This exploratory pilot study in a small group of patients and controls is the first demonstration that epigenetic treatment with the HDAC inhibitors TSA and VA may reverse the abnormal collagen production of smooth muscle cells from neuropathic bladders. If replicated in larger future studies, this novel therapeutic approach may improve treatment options for patients with neurogenic bladder.
Acknowledgments
This study was supported by American Urological Association Foundation.
References
- 1.Sidi AA, Dykstra DD, Gonzalez R. The value of urodynamic testing in the management of neonates with myelodysplasia: a prospective study. J Urol. 1986;135:90–93. doi: 10.1016/s0022-5347(17)45527-3. [DOI] [PubMed] [Google Scholar]
- 2.Webster GD, el-Mahrouky A, Stone AR, et al. The urological evaluation and management of patients with myelodysplasia. Br J Urol. 1986;58:261–265. doi: 10.1111/j.1464-410x.1986.tb09051.x. [DOI] [PubMed] [Google Scholar]
- 3.Edelstein RA, Bauer SB, Kelly MD, et al. The long-term urological response of neonates with myelodysplasia treated proactively with intermittent catheterization and anticholinergic therapy. J Urol. 1995;154:1500–1504. [PubMed] [Google Scholar]
- 4.Painter KA, Vates TS, Bukowski TP, et al. Long-term intravesical oxybutynin chloride therapy in children with myelodysplasia. J Urol. 1996;156:1459–1462. [PubMed] [Google Scholar]
- 5.Deveaud CM, Macarak EJ, Kucich U, et al. Molecular analysis of collagens in bladder fibrosis. J Urol. 1998;160:1518–1527. [PubMed] [Google Scholar]
- 6.Kaplan EP, Richier JC, Howard PS, et al. Type III collagen messenger RNA is modulated in non-compliant human bladder tissue. J Urol. 1997;157:2366–2369. [PubMed] [Google Scholar]
- 7.Hipp JA, Hipp JD, Yoo JJ, et al. Microarray analysis of bladder smooth muscle from patients with myelomeningocele. BJU Int. 2008;102:741–746. doi: 10.1111/j.1464-410X.2008.07606.x. [DOI] [PubMed] [Google Scholar]
- 8.Rombouts K, Niki T, Wielant A, et al. Trichostatin A, lead compound for development of antifibrogenic drugs. Acta Gastroenterol Belg. 2001;64:239–246. [PubMed] [Google Scholar]
- 9.Mann DA, Mann J. Epigenetic regulation of hepatic stellate cell activation. J Gastroenterol Hepatol. 2008;23(suppl 1):S108–S111. doi: 10.1111/j.1440-1746.2007.05295.x. [DOI] [PubMed] [Google Scholar]
- 10.Rishikof DC, Ricupero DA, Liu H, et al. Phenylbutyrate decreases type I collagen production in human lung fibroblasts. J Cell Biochem. 2004;91:740–748. doi: 10.1002/jcb.10742. [DOI] [PubMed] [Google Scholar]
- 11.Lai JY, Yoon CY, Yoo JJ, et al. Phenotypic and functional characterization of in vivo tissue engineered smooth muscle from normal and pathological bladders. J Urol. 2002;168(4 Pt 2):1853–1857. doi: 10.1097/01.ju.0000030040.76258.5a. [DOI] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;4:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Andersson KE. Antimuscarinics for treatment of overactive bladder. Lancet Neurol. 2004;3:46–53. doi: 10.1016/s1474-4422(03)00622-7. [DOI] [PubMed] [Google Scholar]
- 14.Gough DC. Enterocystoplasty. BJU Int. 2001;88:739–743. doi: 10.1046/j.1464-4096.2001.gough.2464.x. [DOI] [PubMed] [Google Scholar]
- 15.Jezernik S, Craggs M, Grill WM, et al. Electrical stimulation for the treatment of bladder dysfunction: current status and future possibilities. Neurol Res. 2002;24:41330. doi: 10.1179/016164102101200294. [DOI] [PubMed] [Google Scholar]
- 16.Madersbacher H. The various types of neurogenic bladder dysfunction: an update of current therapeutic concepts. Paraplegia. 1990;28:217–229. doi: 10.1038/sc.1990.28. [DOI] [PubMed] [Google Scholar]
- 17.Smith CP, Somogyi GT, Chancellor MB. Emerging role of botulinum toxin in the treatment of neurogenic and non-neurogenic voiding dysfunction. Curr Urol Rep. 2002;3:382–387. doi: 10.1007/s11934-002-0081-9. [DOI] [PubMed] [Google Scholar]
- 18.Loscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16:669–694. doi: 10.2165/00023210-200216100-00003. [DOI] [PubMed] [Google Scholar]
- 19.Villar-Garea A, Esteller M. Histone deacetylase inhibitors: understanding a new wave of anticancer agents. Int J Cancer. 2004;112:171–178. doi: 10.1002/ijc.20372. [DOI] [PubMed] [Google Scholar]
- 20.Ng HH, Bird A. DNA methylation and chromatin modification. Curr Opin Genet Dev. 1999;9:158–163. doi: 10.1016/s0959-437x(99)80024-0. [DOI] [PubMed] [Google Scholar]