Abstract
Effective treatment of chronic pain with morphine is limited by decreases in the drug’s analgesic action with chronic administration (antinociceptive tolerance). Because opioids are mainstays of pain management, restoring their efficacy has great clinical importance. We have recently reported that formation of peroxynitrite (ONOO−, PN) in the dorsal horn of the spinal cord plays a critical role in the development of morphine antinociceptive tolerance and have further documented that nitration and enzymatic inactivation of mitochondrial superoxide dismutase (MnSOD) at that site provides a source for this nitroxidative species. We now report for the first time that antinociceptive tolerance is also associated with the inactivation of MnSOD at supraspinal sites. Inactivation of MnSOD led to nitroxidative stress as evidenced by increased levels of products of oxidative DNA damage and activation of the nuclear factor poly (ADP-ribose) polymerase in whole brain homogenates. Co-administration of morphine with potent Mn porphyrin-based peroxynitrite scavengers, (MnTE-2-PyP5+ and MnTnHex-2-PyP5+) (1) restored the enzymatic activity of MnSOD, (2) attenuated PN derived nitroxidative stress, and (3) blocked the development of morphine induced antinociceptive tolerance. The more lipophilic analogue, MnTnHex-2-PyP5+ was able to cross the blood brain barrier at higher levels than its lipophylic counterpart MnTE-2-PyP5+ and was about 30 fold more efficacious. Collectively, these data suggest that peroxynitrite mediated enzymatic inactivation of supraspinal MnSOD provides a source of nitroxidative stress, which in turn contributes to central sensitization associated with the development of morphine antinociceptive tolerance. These results support our general contention that PN-targeted therapeutics may have potential as adjuncts to opiates in pain management.
Keywords: morphine, mitochondrial superoxide dismutase, superoxide, peroxynitrite, peroxynitrite decomposition catalysts, nitroxidative stress
Introduction
Opiate/narcotic analgesics, typified by morphine sulfate, are the most effective treatments for acute and chronic severe pain; but their clinical utility is often hampered by the development of analgesic tolerance as well as by de novo painful hypersensitivity to innocuous and noxious stimuli, phenomena observed in both animal and human studies (Arner et al., 1988, Mao et al., 1995). With respect to morphine in particular, tolerance necessitates escalating doses to achieve equivalent pain relief (Foley, 1995). This complex pathophysiological cycle contributes to decreased quality of life in the growing population of subjects with chronic pain because of oversedation, reduced physical activity, respiratory depression, constipation, potential for addiction, and other side-effects (Foley, 1995). Accordingly, there is major interest in new approaches to maintain opiate efficacy during repetitive dosing for chronic pain, without engendering tolerance or unacceptable side-effects. Our studies to date have shown that targeting peroxynitrite (ONOO−, PN) is an effective therapeutic strategy in blocking the development of antinociceptive tolerance (Salvemini, 2009, Salvemini and Neumann, 2009).
Spinal formation of PN, the reaction product between superoxide (O2·−) and nitric oxide (·NO) (Beckman et al., 1990), is a potent proinflammatory reactive nitroxidative species (Salvemini et al., 1998, Jagtap and Szabo, 2005). Since the rate of interaction between ·NO and O2·− to form PN is faster than the dismutation of O2·− by SOD, the most critical roles of O2·− and ·NO in pain and inflammation may be their formation of PN (Salvemini, 2009). Indeed, PN has been recently implicated in the development of thermal hyperalgesia associated with acute and chronic inflammation (Wang et al., 2004, Khattab, 2006, Bezerra et al., 2007, Ndengele et al., 2008), in response to spinal activation of the N-methyl-D-aspartate receptor (NMDAR) (Muscoli et al., 2004) and in the development of opiate induced antinociceptive tolerance (Muscoli et al., 2007, Batinic-Haberle et al., 2009b, Ndengele et al., 2009). Furthermore, the use of non-selective pharmacological probes (i.e. these agents react not only with O2·− but also with several nitroxidative species and derivatives thereov) (Muscoli et al., 2003) such as PBN [phenyl N-tert-butylnitrone (PBN)] and TEMPOL [4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl] has supported the role of nitroxidative stress (defined as stress caused by the presence of O2·−, ·NO and/or PN and related derivatives) in both neurogenic nociception (Gao et al., 2007, Lee et al., 2007, Schwartz et al., 2008, Schwartz et al., 2009), visceral pain (Wang et al., 2008) and neuropathic pain (Tal, 1996, Park et al., 2006, Gao et al., 2007, Siniscalco et al., 2007). Ultimately, selective PN decomposition catalysts should confirm the contribution of PN in such settings.
It is well documented that inactivation of manganese superoxide dismutase (MnSOD), the enzyme that normally keeps concentrations of O2·− under tight control (McCord and Fridovich, 1969) is a central source for O2·−-derived PN in many diseases driven by overt production of PN (MacMillan-Crow and Thompson, 1999, MacMillan-Crow et al., 2001). Such enzymatic inactivation results from nitration on Tyr-34 by PN in a Mn-catalysed process (MacMillan-Crow and Thompson, 1999). We have recently extended these findings to provide evidence that spinal inactivation of MnSOD is also a central source for the formation of PN in the development of morphine antinociceptive tolerance (Muscoli et al., 2007, Ndengele et al., 2009). Under these circumstances, increased spinal PN contributes to antinociceptive tolerance through three well defined biochemical pathways within the dorsal horn of the spinal cord: (1) post-translational nitration of proteins involved in glutamate homeostasis such as glutamate transporters and glutamine synthase (2) neuroimmune activation and (3) neuronal apoptosis (Muscoli et al., 2007, Batinic-Haberle et al., 2009b). Whether PN-mediated nitroxidative stress at supraspinal sites contributes to the development of morphine antinociceptive tolerance is not known. In this study we show that the development of antinociceptive tolerance following chronic administration of morphine was associated with supraspinal inactivation of MnSOD which provided a source for PN and PN-mediated nitroxidative stress. The PN decomposition catalysts MnTE-2-PyP5+ and MnTnHex-2-PyP5+ (Batinic-Haberle, 2002) potently blocked MnSOD nitration at supraspinal sites and the development of antinociceptive tolerance with MnTnHex-2-PyP5+ being more potent than MnTE-2-PyP5+. These results suggest that in addition to the documented spinal role of PN in tolerance (Muscoli et al., 2007, Batinic-Haberle et al., 2009a, Ndengele et al., 2009), supraspinal PN has an important role as well.
Methods
Mn porphyrins
MnTE-2-PyP5+ and MnTnHex-2-PyP5+ were synthesized and characterized as previously described (Batinic-Haberle, 2002).
Thin-layer chromatography
Thin-layer chromatography of the purified compounds was performed on plastic-backed silica gel TLC plates with 1:1:8 KNO3-saturated H2O: H2O: acetonitrile mixture as mobile phase. Typically, 1 µL of ~1 mM samples were applied at ~ 1cm of the strip border and the solvent front was allowed to run ~12 cm. The absolute Rf values and their ratio thereoff are sensitive to the degree of the saturation of vapor phase in the TLC chamber and may differ slightly from one to another experiment. Thus, the internal standardization of TLC is required for comparison purposes [Kos et al Free Radic Biol Med 2009]. For the purpose of this study and given high levels of ascorbate in brain Mn(III) porphyrins and ascorbate-reduced Mn(II) porphyrins (MnTE-2-PyP4+ and MnTnHex-2-PyP4+) were applied on silica plates. Mn(II) porphyrins are less stable and may lose Mn to some extent. Consequently in solution there may be some amount of metal-free porphyrins that bear same 4+ total charge and may have similar adsorption on the silica as reduced Mn(II) porphyrins. Thus we applied on TLC plates metal-free porphyrins, H2TE-2-PyP4+ and H2TnHex-2-PyP4+ as controls also. The thin-layer chromatographic behavior/lipophilicity of Mn porphyrins was described by retention factor, Rf which presents the ratio of Mn porphyrin path and solvent path. Based on Rf values, the POW values were then calculated (see Results).
Induction of morphine-induced antinociceptive tolerance in mice
The development of morphine induced tolerance was performed as described (Muscoli et al., 2007, Batinic-Haberle et al., 2009b, Ndengele et al., 2009). Male CD-1 mice (24–30g, Charles River) were housed and cared for in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Saint Louis University Health Science Center and in accordance with the National Institute of Health Guidelines on Laboratory Animal and the University of Messina, in compliance with Italian regulations on protection of animals used for experimental and other scientific purposes (D.M. 116192) as well as with the EEC regulations (O.J. of E.C. L 358/1 12/18/1986). Mice were housed 4–5 per cage, maintained under identical conditions of temperature (21±1°C), humidity (60±5%) with a 12-hr light-dark cycle, and allowed food ad libitum. Nociceptive/pain thresholds were determined by measuring latencies (in seconds, s) of mice placed in a transparent glass cylinder on a hot plate (Ugo Basile, Italy) maintained at 52°C. Responses indicative of nociception included intermittent lifting and/or licking of the hindpaws or escape behavior. Determination of antinociception/pain relief effects was assessed between 7:00 and 10:00 AM. All injections were given by intraperitoneal (ip) or subcutaneous (sc) means in a 0.1 ml volume at approximately 7 AM and 4 PM. Drug or vehicle (saline) was given before each dose of morphine. Hot plate latencies were taken in mice from all groups on day five before (baseline latency) and 40 min after an acute dose of morphine (3 mg/kg) or its vehicle (saline) (response latency). Baseline values from all groups as measured on day five before injection of the acute dose of morphine or its vehicle, similarly ranged between 6–8 s. Results are expressed as percent maximal possible antinociceptive effect (% MPE) calculated as follows: (response latency − baseline latency)/(cut off latency − baseline latency) × 100 (Muscoli et al., 2007). A cut-off latency of 20 s was employed to prevent tissue damage. Twelve mice per group were used and all experiments were conducted with the experimenters blinded to treatment conditions. Unless specified, all drugs were purchased from Sigma. The following experimental groups were used.
Naïve group (N)
Mice were injected twice a day with an ip injection of saline and a sc injection of saline. On day five, mice received an ip injection of saline followed fifteen minutes later by a sc injection of saline.
Vehicle group (V)
Mice were injected twice a day with an ip injection of saline and a sc injection of saline. On day five, mice received an ip injection of saline followed fifteen minutes later by a sc injection of acute morphine eliciting near-to-maximal antinociception (3 mg/kg) at 40 minutes after injection.
Tolerant group (T)
Mice were injected twice a day with an ip injection of saline and sc injection of morphine (20 mg/kg/day). On day five, mice received an ip injection of saline followed fifteen minutes later by a sc injection of acute morphine (3 mg/kg). Hot plate latencies were taken 40 minutes after morphine injection.
Tolerant + drugs group
Mice were injected twice a day with an ip injection of varying doses of MnTE-2-PyP5+ (0.3–3 mg/kg/day) or MnTnHex-2-PyP5+ (0.01–0.1 mg/kg/day) followed by a sc injection of morphine (20 mg/kg/day). On day five, mice received an ip injection of MnTE-2-PyP5+ (1.5 mg/kg/day) or MnTnHex-2-PyP5+ (0.05 mg/kg/day) followed fifteen minutes later by the sc injection of acute morphine (3 mg/kg). Hot plate latencies were taken 40 minutes after morphine injection.
After the behavioral tests on day five, whole brains were removed and tissues processed for Western blot and biochemical analysis.
Western Blot
Whole brain lysates (n = 3 per group) were prepared as previously described (Wang et al., 2004). Briefly, whole frozen brain (395–500 mg) were pulverized in liquid nitrogen-chilled mortar and pestle prior to homogenization in 1.2 – 1.5 mL of lysis buffer [20 mM Tris-Cl (pH 7.4), 150 mM NaCl, 16.2 mM CHAPS, 12.5 mM EGTA, 1% protease cocktail (Sigma, St. Louis MO) (final concentration: 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF), 15 µM pepstatinA, 14 µM E-64, 40 µM bestatin, 20 µM leupeptin, and 850 nM aprotinin), 10% glycerol]. The homogenates were sonicated for 10 min on ice in an ultrasonic bath (VWR, Buffalo Grove IL), incubated an additional 10 min on ice, and clarified by centrifugation for 10 min at 12500g, 4°C. The supernatants were frozen at −80°C and the total protein content of an aliquot was measured by bicinchoninic acid assay (Pierce, Rockford IL). The total lysates (40 µg) were denatured with an equal volume of loading buffer (2X: 125 mM Tris-Cl (pH 6.8), 15 % glycerol, 3% SDS, 1.1 mM β-mercaptoethanol, and 0.075% bromophenol blue) and heat denatured for 5 min at 95°C. The total lysates were resolved by SDS-PAGE (170 V, ~55 min) with a 4–20% Tris-glycine minigel (Lonza, Basile Switzerland) and electrophoretically transferred (100V, 1.5 h) to 0.2 µm nitrocellulose membrane (Whatman, Fordham Park NJ) in Towbin transfer buffer. Additional transfers of total lysates serially diluted (2-fold) were prepared. The relative levels of total MnSOD were determined by enhanced chemiluminescence (Wang et al., 2004). Each membrane was blocked for 2 hr at RT (10% low-fat milk/PBS-T: 1X PBS, 0.05% Tween-20, and 0.1% thimerasol) and incubated for 1 hr at RT with a rabbit anti-MnSOD monoclonal antibody (Millipore) diluted 1:1000 in 5% low-fat milk/PBS-T. The membrane was washed 5 times in PBS-T (15 min and 4 × 5 min), then incubated with a goat anti-rabbit IgG-HRP antibody diluted 1:10000 in 5% low-fat milk/PBS-T. After 5 washes in PBS-T, the bands were visualized with Supersignal® West Femto chemiluminscence substrate (Pierce) and captured on film (GE Healthcare, Piscataway NJ). The membranes were subsequently washed twice with PBS-T for 10 min and equal loading was similarly verified with a mouse anti-β-actin monoclonal antibody (Sigma, St. Louis MO) diluted 1:10000 and goat anti-mouse IgG-HRP antibody diluted 1:8000 in 5% low-fat milk/PBS-T. The lack of species cross-reactivity of HRP-conjugated antibodies to the anti-MnSOD and anti-β-actin was also confirmed. The densities of the MnSOD and β-actin bands were quantified using ImageQuant 5.2 (GE Healthcare) and expressed as relative MnSOD:β-actin densities.
Measurement of MnSOD and Cu,ZnSOD activities
Whole brain tissue was homogenized in a Polytron homogenizer in 4 – 5 ml of 10 mM phosphate buffered saline, pH 7.2; then sonicated twice on ice for 20 s. The sonicated samples were subsequently centrifuged at 1,100 g for 10–15 min and SOD activity was measured in the supernatants (Wang et al., 2004). In brief, a competitive inhibition assay was performed using xanthine-xanthine oxidase-generated superoxide to reduce nitroblue tetrazolium (NBT) to blue tetrazolium salt. The reaction was performed in 50 mM carbonate buffer, pH 10.1, containing 0.1 mM EDTA, 25 µM nitroblue tetrazolium, 0.1 mM xanthine and 2 nM xanthine oxidase (Boehringer, Germany). The rate of NTB reduction was monitored spectrophotometrically (Perkin Elmer, Lambda 5 Spectrophotometer, Milan, Italy) at 560 nm. The amount of protein required to inhibit the rate of NBT reduction by 50% was defined as one unit of enzyme activity. Cu,ZnSOD activity was inhibited by performing the assay in the presence of 2 mM NaCN after preincubation for 30 min. Enzymatic activity was expressed in mU per µg of protein as previously described (Wang et al., 2004).
Determination of 8-hydroxy-2'-deoxyguanosine (8-OHdG)
The isolation of DNA was performed as described previously by our group (Masini et al., 2005). In brief, whole brain tissue was homogenized in 4 – 5 ml of 10 mM PBS, pH 7.4, sonicated on ice 20 s twice, then 1 ml of 10 mM Tris-HCl buffer, pH 8, containing 10 mM EDTA, 10 mM NaCl, and 0.5% sodium dodecyl sulphate was added. The lysates were incubated for 1 h at 37°C with 20 µg/ml RNase I (Sigma-Aldrich, Milan, Italy) and then overnight at 37 °C under argon in the presence of 100 µg/ml proteinase K (Sigma-Aldrich). The DNA was extracted with chloroform/isoamyl alcohol (10/2 v/v), precipitated from the aqueous phase with 0.2 volumes of 10 M ammonium acetate, solubilized in 200 µl of 20 mM acetate buffer, pH 5.3, and denatured at 90°C for 3 min. The extract was then supplemented with 10 IU of P1 nuclease (Sigma-Aldrich) in 10 µl and incubated for 1 h at 37 °C with 5 IU of alkaline phosphatase (Sigma-Aldrich) in 0.4 M phosphate buffer, pH 8.8. All of the procedures were performed in the dark under argon. The mixture was filtered by an Amicon Micropure-EZ filter (Millipore Corporation, Billerica, MA), and 50 µl of each sample was used for 8-hydroxy-2'-deoxyguanosine (8-OHdG) determination using a Bioxytech enzyme immunoassay kit (Oxis, Portland, OR), per manufacturer’s protocol. The values are expressed as nanogram of 8-OHdG per milligram of protein (Bradford, 1976).
Measurement of PARP Activity
PARP-1 activity was measured as described previously (Suzuki et al., 2004). Whole brain tissue was gently homogenized at 4 °C, in 4 – 5 mL of 50 mM Tris HCI, pH 8, containing 0.1% tergitol-type NP-40 (NP-40), 200 mM KCI, 2 mM MgCl2, 50 µM ZnCl2, 2 mM dithiothreitol (DTT) and protease inhibitors: 1 mM phenylmethylsulfonyl fluoride (PMSF), 5 µl/ml leupeptin, and antipain. Samples were then centrifuged and 10 µl of each supernatant were incubated for 5 min at 25 °C with 2 µl of [3H]NAD+ (specific activity 25 Ci/mmol, G.E., Health Care, U.K.) in 50 mM Tris HCI, pH 8, containing 20 mM MgCl2, 1 mM DTT, and 20 µM NAD+ in the absence or presence of activated calf thymus DNA, in the final volume of 100 µl. The reaction was stopped by the addition of 5% trichloroacetic acid. Samples were filtered and the radioactivity in the acid-insoluble fraction was counted by a Beckman LS1801 liquid scintillation spectrometer. PARP activity estimated without activated DNA in the mixture was assigned as "endogenous" activity. Activity estimated in the presence of activated DNA in the assay mixture was assigned as "total" activity of PARP. Ratio between endogenous and total activity was considered as the measure of PARP activity in the tissue. The values are expressed as pmol of NAD+ per µg of protein/h (Bradford, 1976).
Statistics
For paired group analysis Students t-test were performed. For paired multiple groups, analysis of variance followed by Student-Newman-Keuls test were employed to analyze the data. Results are expressed as mean±s.e.m for n animals. A statistical significant difference was defined as a P value <0.05.
Results
Lipophilicity of Mn porphyrins
The thin-layer chromatographic behavior of reduced Mn(II) porphyrins, MnTE-2-PyP4+ and MnTnHEx-2-PyP4+, as described by retention factor Rf is given in Table 1. It is obvious that reduced porphyrins are significantly more lipophilic than their oxidized Mn(III) analogues. In brain due to the high level of ascorbate and their easy reducibility with ascorbate (Ferrer-Sueta et al Chem Res Toxicol 1999, Batinic-Haberle et al, Free Radic Biol Med 2004), Mn porphyrins will at least in part exist in reduced state which would further enhance their accumulation. Utilizing POW vs Rf relationship (Kos et al., 2009) as given by equation, log POW= 12.18 × Rf −7.43, we calculated the partition of both porphyrins between n-octanol and water, POW. POW is a more common indicator of drug lipophilicity. Both Rf and POW values are listed in Table 1.
Table 1.
The catalytic rate constants for O2·− dismutation (kcat in M−1s−1 at 25°C) and ONOO− reduction (kred in M−1 s−1 at 37°C); lipophilicity expressed in terms of partition between n-octanol and water, POW for oxidized, MnIIIPs and reduced MnIIPs; thermodynamic property, metal-centered reduction potential for the redox couple MnIIIP/MnIIP, E1/2 in mV vs NHE; and blood to brain ratio for ethyl, MnTE-2-PyP5+ and hexyl, MnTnHex-2-PyP5+ analogues.
| MnP | log kcat (O2·−) | log kred(ONOO−) | E1/2 | POW(MnIIIP) | POW(MnIIP) | Rf Blood : brain ratio | |
|---|---|---|---|---|---|---|---|
| MnTE-2-PyP5+ | 7.76a | 7.53b(>7)c | +228a | −6.89d | −4.69e | 0.23 | 100f |
| MnTnHex-2-PyP5+ | 7.48a | 7.11b | +314a | −2.76d | −1.64e | 0.48 | 8f |
value in parenthesis relates to MnII(Ferrer-Sueta et al., 2006);
Data calculated based on Rf values for metal-center reduced Mn(II) porphyrins (by ascorbate) on TLC silica plates in KNO3 - saturated H2O : H2O : acetonitrile = 1:1:8 and POW vs Rf relationships, log POW= 12.18 × Rf −7.43, from ref (Kos et al., 2009); Rf, a thin-layer chromatographic retention factor is a ratio of MnP path over solvent path, POW partition coefficient between n-octanol and water;
Spasojevic et al, unpublished.
Inactivation of brain mitochondrial superoxide dismutase (MnSOD) as a source of PN in the development of antinociceptive tolerance
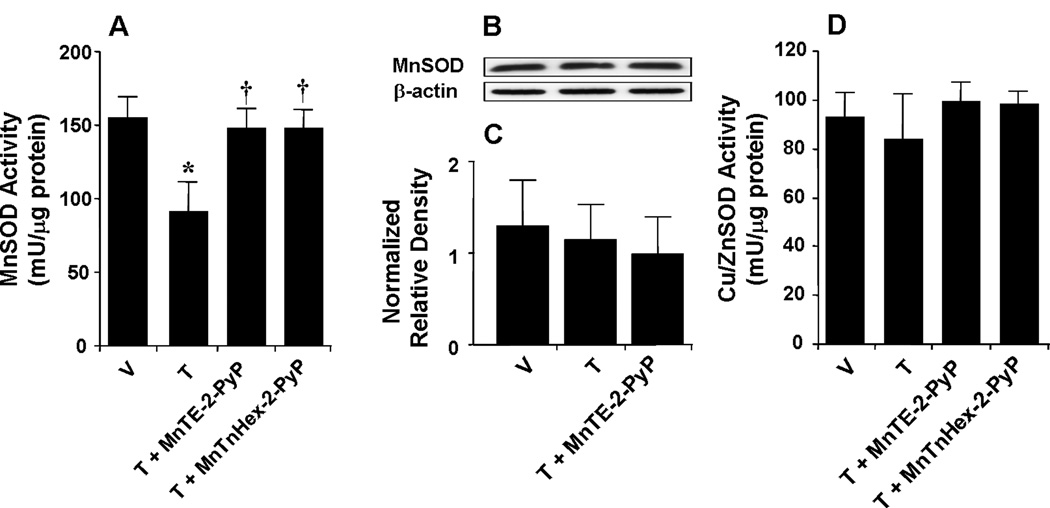
When compared to mice treated over 4 days with saline, chronic administration of morphine over the same time course led to the inactivation of MnSOD in whole brain homogenates as evidenced by loss of the catalytic activity of this enzyme to dismute superoxide measured spectrophotometrically on day 5 (n=12) (Fig. 2A). Chronic administration of morphine did not change the total amount of MnSOD protein in brain homogenates as measured by Western blotting analysis (Fig. 2B, C). The enzymatic activity of cytosolic SOD (Cu,ZnSOD) was not affected (n=12) (Fig. 2D). Inactivation of brain MnSOD was associated with the development of antinociceptive tolerance (Fig. 3A, B). When compared to animals receiving an equivalent injection of its vehicle (Naïve group), acute injection of morphine (3 mg/kg) in animals that received saline over four days (Vehicle group) produced a significant and near-to-maximal antinociceptive response [percent maximal possible antinociceptive effect (%MPE) ranging between 90–95%] (Fig. 3 A, B). On the other hand, when compared to the antinociceptive response to acute morphine in animals that received saline over four days, repeated administration of morphine over the same time course (Tolerant group) led to the development of antinociceptive tolerance as evidenced by a significant loss of its antinociceptive response (Fig. 3A, B). Baseline latencies in groups treated with saline or morphine over four days before acute administration of morphine on day five similarly ranged between 6–8 seconds (n=12).
Fig. 2.
When compared to the vehicle group (V) repeated administration of morphine (tolerant group, T) led to significant functional enzymatic inactivation of MnSOD (A) but not Cu,ZnSOD (D) as evidenced by loss of its catalytic activity to dismute superoxide as measured spectrophotometrically. Co-administration of morphine with MnTE-2-PyP5+ (3 mg/kg/day, n=12) or MnTnHex-2-PyP5+ (0.1 mg/kg/day, n=12) restored the enzymatic activity of MnSOD. When compared to the vehicle group, repeated administration of morphine did not change the total amount of MnSOD in whole brain tissue homogenates as measured by Western blotting analysis. Gels shown in B are representative from gels obtained in 3 animals. A composite of the densitometry data resulting from these experiments is shown in C. *P<0.001 for Morphine alone vs Vehicle; †P<0.001 for Morphine+drug-treated vs Morphine alone.
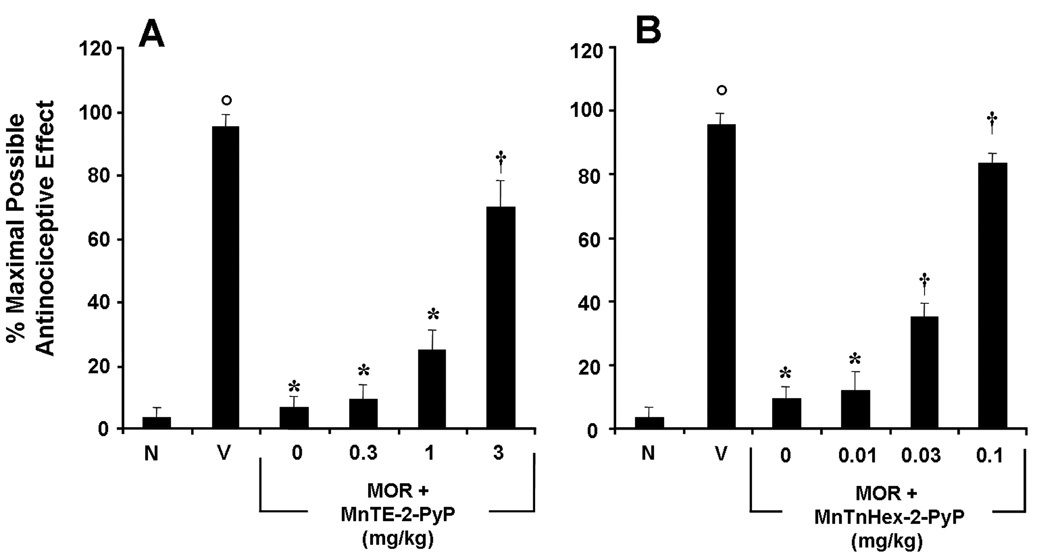
Fig. 3.
Inhibition of morphine antinociceptive tolerance with MnTE-2-PyP5+ or MnTnHex-2-PyP5+. On day five, acute injection of morphine (3 mg/kg) in animals that received saline over four days produced a significant antinociceptive response when compared to responses observed in animals that received an equivalent volume of saline (A, B, Vehicle, V vs Naïve, N). On the other hand, a significant loss to the antinociceptive effect from the acute injection of morphine was observed in animals that received repeated administration of morphine over four days (tolerant group) (A, B). Co-administration of morphine over four days with varying doses of MnTE-2-PyP5+ (0.3–3 mg/kg/day, n=12; A) or MnTnHex-2-PyP5+ (0.01–0.1 mg/kg/day, n=12; B) inhibited the development of tolerance in a dose-dependent manner. Results are expressed as mean ± s.e.m. for six animals. °P<0.001 for Vehicle, V vs Naïve, N; *P<0.001 for Morphine alone vs Vehicle; †P<0.001 for Morphine + drug treated vs Morphine alone.
Co-administration of morphine with varying doses of the PN scavengers MnTE-2-PyP5+ (0.3–3 mg/kg/day) or MnTnHex-2-PyP5+ (0.01–0.1 mg/kg/day) prevented the supraspinal inactivation of MnSOD and restored its ability to enzymatically dismute superoxide (n=12) (Fig. 2A) and blocked in a dose dependent fashion morphine antinociceptive tolerance [0.3–3 mg/kg/day for MnTE-2-PyP5+ and 0.01–0.1 mg/kg/day for MnTnHex-2-PyP5+, n=12] (Fig. 3A, B). We have previously demonstrated that MnTE-2-PyP5+ or MnTnHex-2-PyP5+ alone do not increase baseline latencies (Batinic-Haberle et al., 2009b).
The development of morphine-induced antinociceptive tolerance is associated with supraspinal oxidative DNA damage and PARP activation: inhibition by MnTE-2-PyP5+ or MnTnHex-2-PyP5+
It is now well established that inactivation of MnSOD increases superoxide levels thereby favoring PN formation and thus increased nitroxidative stress (Yamakura et al., 1998, MacMillan-Crow and Thompson, 1999, Macmillan-Crow and Cruthirds, 2001, MacMillan-Crow et al., 2001, Yamakura et al., 2001). Here we show that the development of morphine antinociceptive tolerance was associated with increased formation of nitroxidative stress markers as measured in whole brain homogenates. Thus and as can be seen in Fig. 4, when compared to mice treated over 4 days with saline, chronic administration of morphine over the same time course increased 8OHdG (n=12, Fig. 4A), a marker of oxidative DNA damage and increased the activation of PARP a nuclear enzyme known to be activated following oxidative DNA damage (Fig. 4B). Co-administration of morphine with MnTE-2-PyP5+ (3 mg/kg/day) or MnTnHex-2-PyP5+ (0.1 mg/kg/day) significantly reduced the increased production of 8-OHdG (n=12) and significantly decreased PARP activity (Fig. 4A, B).
Fig. 4.
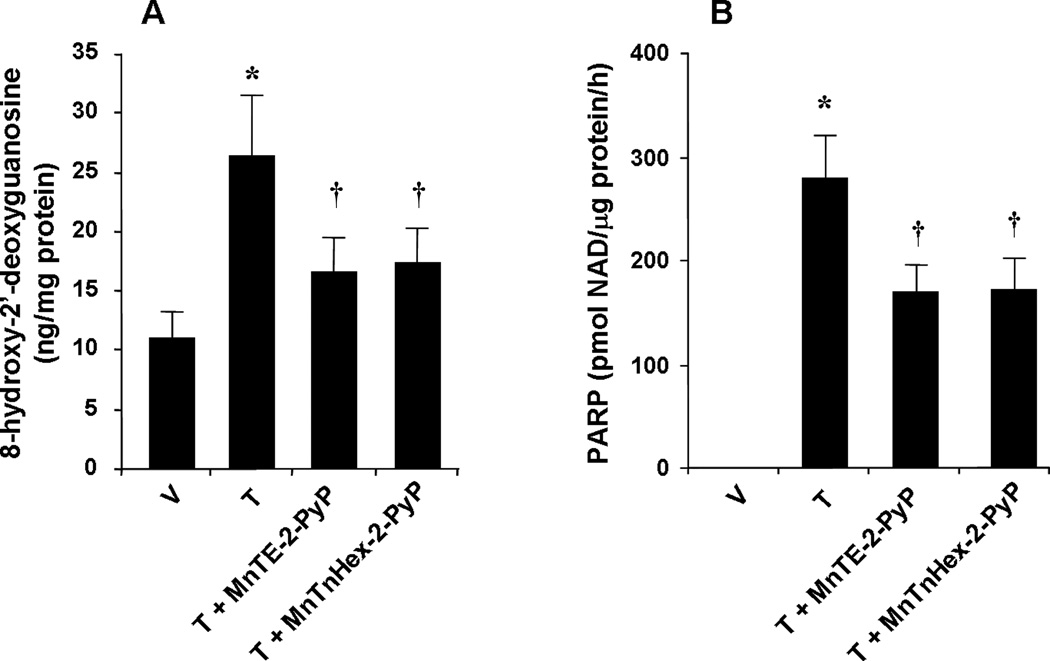
When compared to the vehicle group (V) repeated administration of morphine (tolerant group, T) led to significant increase in dorsal horn tissue levels of 8OHdG (A) and substantially activated PARP (B). These biochemical changes were significantly attenuated by co-administration of morphine over four days with MnTE-2-PyP5+ (3 mg/kg/day, n=12) or MnTnHex-2-PyP5+ (0.1 mg/kg/day, n=12) (A, B). Results are expressed as mean±s.e.m. for twelve animals. *P<0.001 for Morphine alone vs Vehicle; †P<0.001 for Morphine + drug-treated vs Morphine alone.
Discussion
Mitochondrial superoxide dismutase (MnSOD), the enzyme that normally keeps concentrations of superoxide under tight control (McCord and Fridovich, 1969) is an exquisite and sensitive target for PN-mediated nitration and enzymatic inactivation (MacMillan-Crow and Thompson, 1999). This process favors the accumulation of PN which in turn, nitrates and alters additional proteins and receptors, thereby perpetuating and extending the initial damage (Radi et al., 2002). Over the years a great body of experimental evidence has been gathered to link MnSOD inactivation as a source for PN and PN-driven nitroxidative stress to several disease states (Macmillan-Crow and Cruthirds, 2001). More recently, spinal inactivation of MnSOD has been linked to the development of peripheral and central sensitization associated with inflammation and in the development of opiate-induced antinociceptive tolerance (Muscoli et al., 2004, Wang et al., 2004, Muscoli et al., 2007, Schwartz et al., 2008, Batinic-Haberle et al., 2009b, Ndengele et al., 2009, Schwartz et al., 2009). The main objective of the study presented herein was to address whether in addition to its spinal role (Muscoli et al., 2007, Batinic-Haberle et al., 2009b, Ndengele et al., 2009), supraspinal inactivation of MnSOD, as a source of PN, contributes to the development of morphine antinociceptive tolerance. Our results clearly show that the development of antinociceptive tolerance was associated with enzymatic inactivation of MnSOD in whole brain homogenates which in turn contributed to PN formation and PN-mediated nitroxidative stress as evidenced by increased formation of 8-OHdG and increase in PARP activity well know markers of the presence of severe nitroxidative stress. Inhibition of PN with two PN scavengers (MnTE-2-PyP5+, MnTnHex-2-PyP5+) prevented MnSOD inactivation and attenuated antinociceptive tolerance. The more lipophilic MnTnHex-2-PyP5+ was found to be approximately 30-fold more potent a finding that can be explained at least in part by the fact that MnTnHex-2-PyP5+ has better organ uptake (i.e brain). The finding that the development of morphine-induced antinociceptive tolerance was not associated with enzymatic inactivation of the cytosolic isoform of SOD, namely Cu,ZnSOD, is consistent with previous studies that have shown that the interaction of Cu,ZnSOD with PN does not affect the catalytic activity of the protein (Smith et al., 1992). Although little is known about supraspinal locations and mechanisms of nitroxidative species in hyperalgesic states, the rostral ventromedial medulla (RVM) may provide a point of origin for further characterization of the roles of peroxynitrite during central sensitization.
Glutamate neurotransmission, in particular that mediated via activation of NMDAR and glial cell activation in the RVM, contributes to central sensitization (see below). The µ-opioid receptors, NMDA receptors, and glial cell activation in the RVM are all potential sources for PN formation since each of these can release its precursors: O2·− and ·NO [see (Watkins et al., 2007, Salvemini and Neumann, 2009) for reviews]. Furthermore, PN contributes to NMDA-mediated central sensitization (Muscoli et al., 2004). Investigations using intracerebroventricular injections of non-selective NOS inhibitor (Moore et al., 1991, Kawabata et al., 1993, Salter et al., 1996, Zhao and Bhargava, 1996) or free radical scavengers (Kim et al., 2004, Lee et al., 2007) attenuate central sensitization in various animal models of hypersensitivity including opiate tolerance, implicating roles for nitroxidative species at supraspinal sites. More specifically, RVM microinjections of NOS inhibitors and NMDAR antagonists were all able to significantly reduce secondary hyperalgesia following peripheral insult (Coutinho et al., 1998, 2001). Conversely, RVM microinjections of ·NO donors or NMDA enhanced hyperalgesia (Urban et al., 1999, Guan et al., 2002). Collectively these results suggest that supraspinal formation of nitroxidative species formed upon activation of the NMDAR modulates central sensitization.
The homeostasis of extracellular glutamate, a primary endogenous ligand for the NMDAR, is tightly regulated by sodium-dependent high-affinity glutamate transporters (GTs) in the plasma membranes of both neurons and glial cells (Danbolt, 2001). Loss of the transport function of these proteins leads to increased glutamate levels in the synaptic cleft, overstimulation of NMDAR and neurotoxicity (Mennerick et al., 1999, Lievens et al., 2000). In contradistinction to the key role of GTs, glutamine synthetase (GS) plays a pivotal role in its intracellular metabolic fate by converting glutamate into nontoxic glutamine. Pertinent to the context of our studies, is that PN nitrates and inactivates the transport activity of GTs and the enzymatic activity of GS (Trotti et al., 1996, Minana et al., 1997, Trotti et al., 1999, Gorg et al., 2005). Furthermore, Zanelli and co-workers demonstrated that PN nitrates tyrosine residues on NMDA receptor subunits, an event associated with constant potentiation of synaptic currents, calcium influx, and ultimately increased NMDA-receptor excitability and neuronal excitotoxicity (Zanelli et al., 2000, Zanelli et al., 2002). We have recently shown that the development of opiate antinociceptive tolerance was associated with nitration of GTs and GS (Muscoli et al., 2007) and of NMDAR (Salvemini, unpublished observations) in dorsal horn tissues. Taken collectively and in an attempt to provide a mechanism whereby PN formation at supraspinal sites modulates opiate tolerance, it is possible that PN fosters tolerance via a pathway involving nitroxidative alterations of glutamate homeostasis.
Spinal neuroimmune activation contributes to opiate antinociceptive tolerance [(Watkins et al., 2007) (Salvemini and Neumann, 2009)for reviews] and inhibition of PN blocks spinal glial cell activation and cytokine formation during antinociceptive tolerance (Muscoli et al., 2007, Batinic-Haberle et al., 2009b, Ndengele et al., 2009, Salvemini and Neumann, 2009). Since neuroimmune activation in the RVM has been associated with central sensitization (Wei et al., 2008) we hypothesize that a second mechanism by which supraspinal PN fosters tolerance is neuroimmune activation.
In summary, we have shown for the first time that supraspinal inactivation of MnSOD and PN mediated nitroxidative stress plays a role in antinociceptive tolerance. We have further shown that potent peroxynitrite scavengers, MnTE-2-PyP5+ and MnTnHex-2-PyP5+ were able to prevent MnSOD inactivation and nitroxidative stress at that level thereby inhibiting morphine tolerance. Investigations to determine the region of and cellular sources of supraspinal PN will be important to determine neuroanatomical site of action during antinociceptive tolerance. The RVM appears to provide a plausible site of origin to begin these investigations.
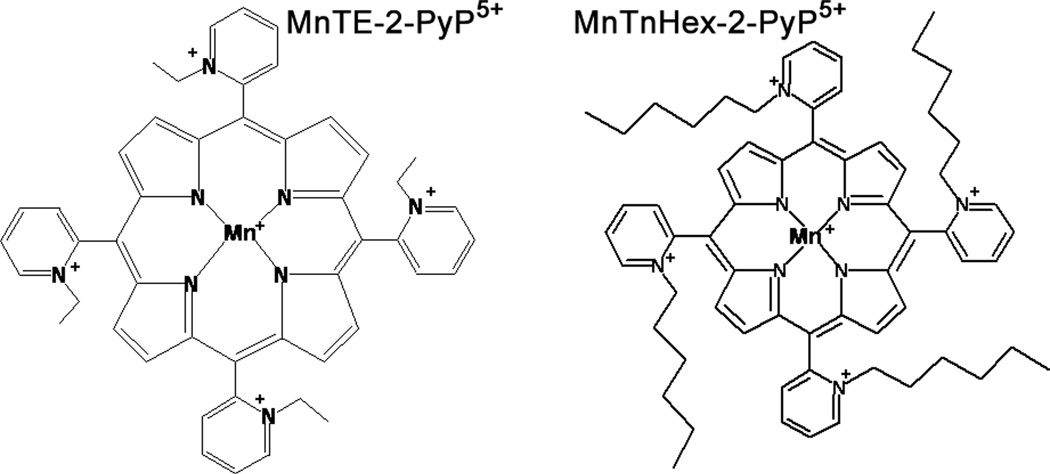
Fig. 1.
Structures of MnTE-2-PyP5+ and MnTnHex-2-PyP5+. Replacement of shorter ethyl with longer hexyl side chains increases lipophilicity by several orders of magnitude (see Table 1).
Acknowledgments
This work was supported by IRCCS Centro Neurolesi “Bonino-Pulejo” grant (SC), by 1RO1DA024074-01A1 (DS, IBH), R21DA023056-01A2 (DS), NIH U19AI67798-01 (IBH), William H. Coulter Translation Partners Grant Program (IBH), and NIH/NCI Duke Comprehensive Cancer Center Core Grant (5-P30-CA014236-33) (IS).
Abbreviations
- 8-OHdG
8-hydroxy-2'-deoxyguanosine
- %MPE
percent maximal possible antinociceptive effect
- Cu,ZnSOD
copper/zinc superoxide dismutase
- GS
glutamine synthase
- GT
glutamate transporters
- kcat and kred
catalytic and reduction rate constants
- MnSOD
manganese superoxide dismutase
- MnTE-2-PyP5+
Mn(III) 5,10,15,20-tetrakis(N-ethylpyridinium-2-yl)porphyrin
- MnTnHex-2-PyP5+
Mn(III) 5,10,15,20-tetrakis(N-n-hexylpyridinium-2-yl)porphyrin
- NMDA
N-methyl-D-aspartate
- NMDAR
N-methyl-D-aspartate receptor
- ·NO
nitric oxide
- O2·−
superoxide
- ONOO− and PN
peroxynitrite
- PARP
poly(ADP-ribose) polymerase; PMSF
- POW
n-octanol/water partitioning constant
- RVM
rostral ventromedial medulla
- SOD
superoxide dismutase
- E1/2
metal-centered reduction potential for Mn(III)P/Mn(II)P redox couple
- NHE
normal hydrogen electrode
- Rf thin-layer chromatographic retention factor
ration of Mn porphyrin path and solvent path on silica plates
- POW
partition coefficient between n-octanol and water
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- Arner S, Rawal N, Gustafsson LL. Clinical experience of long-term treatment with epidural and intrathecal opioids--a nationwide survey. Acta Anaesthesiol Scand. 1988;32:253–259. doi: 10.1111/j.1399-6576.1988.tb02725.x. [DOI] [PubMed] [Google Scholar]
- Batinic-Haberle I, Cuzzocrea S, Reboucas JS, Ferrer-Sueta G, Mazzon E, Di Paola R, Radi R, Spasojevic I, Benov L, Salvemini D. Pure MnTBAP selectively scavenges peroxynitrite over superoxide: Comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two models of oxidative stress injury, an SOD-specific Escherichia coli model and carrageenan-induced pleurisy. Free Radic Biol Med. 2009a;46:192–201. doi: 10.1016/j.freeradbiomed.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batinic-Haberle I, Ndengele MM, Cuzzocrea S, Reboucas JS, Spasojevic I, Salvemini D. Lipophilicity is a critical parameter that dominates the efficacy of metalloporphyrins in blocking the development of morphine antinociceptive tolerance through peroxynitrite-mediated pathways. Free Radic Biol Med. 2009b;46:212–219. doi: 10.1016/j.freeradbiomed.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batinic-Haberle ISI, Stevens RD, Hambright P, Fridovich I. Manganese(III) Meso Tetrakis Ortho N-alkylpyridylporphyrins. Synthesis, Characterization and Catalysis of O2.- Dismutation. J Chem Soc, Dalton Trans. 2002:2689–2696. [Google Scholar]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra MM, Brain SD, Girao VC, Greenacre S, Keeble J, Rocha FA. Neutrophils-derived peroxynitrite contributes to acute hyperalgesia and cell influx in zymosan arthritis. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:265–273. doi: 10.1007/s00210-006-0123-9. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, Urban MO, Gebhart GF. Role of glutamate receptors and nitric oxide in the rostral ventromedial medulla in visceral hyperalgesia. Pain. 1998;78:59–69. doi: 10.1016/S0304-3959(98)00137-7. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, Urban MO, Gebhart GF. The role of CNS NMDA receptors and nitric oxide in visceral hyperalgesia. European journal of pharmacology. 2001;429:319–325. doi: 10.1016/s0014-2999(01)01331-0. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Ferrer-Sueta G, Hannibal L, Batinic-Haberle I, Radi R. Reduction of manganese porphyrins by flavoenzymes and submitochondrial particles: a catalytic cycle for the reduction of peroxynitrite. Free Radic Biol Med. 2006;41:503–512. doi: 10.1016/j.freeradbiomed.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Ferrer-Sueta G, Vitturi D, Batinic-Haberle I, Fridovich I, Goldstein S, Czapski G, Radi R. Reactions of manganese porphyrins with peroxynitrite and carbonate radical anion. J Biol Chem. 2003;278:27432–27438. doi: 10.1074/jbc.M213302200. [DOI] [PubMed] [Google Scholar]
- Foley KM. Misconceptions and controversies regarding the use of opioids in cancer pain. Anticancer Drugs. 1995;6 Suppl 3:4–13. doi: 10.1097/00001813-199504003-00002. [DOI] [PubMed] [Google Scholar]
- Gao X, Kim HK, Chung JM, Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain. 2007;131:262–271. doi: 10.1016/j.pain.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorg B, Wettstein M, Metzger S, Schliess F, Haussinger D. Lipopolysaccharide-induced tyrosine nitration and inactivation of hepatic glutamine synthetase in the rat. Hepatology. 2005;41:1065–1073. doi: 10.1002/hep.20662. [DOI] [PubMed] [Google Scholar]
- Guan Y, Terayama R, Dubner R, Ren K. Plasticity in excitatory amino acid receptor-mediated descending pain modulation after inflammation. The Journal of pharmacology and experimental therapeutics. 2002;300:513–520. doi: 10.1124/jpet.300.2.513. [DOI] [PubMed] [Google Scholar]
- Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- Kawabata A, Umeda N, Takagi H. L-arginine exerts a dual role in nociceptive processing in the brain: involvement of the kyotorphin-Met-enkephalin pathway and NO-cyclic GMP pathway. British journal of pharmacology. 1993;109:73–79. doi: 10.1111/j.1476-5381.1993.tb13533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattab MM. TEMPOL, a membrane-permeable radical scavenger, attenuates peroxynitrite- and superoxide anion-enhanced carrageenan-induced paw edema and hyperalgesia: a key role for superoxide anion. European journal of pharmacology. 2006;548:167–173. doi: 10.1016/j.ejphar.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Kos I, Reboucas JS, DeFreitas-Silva G, Salvemini D, Vujaskovic Z, Dewhirst MW, Spasojevic I, Batinic-Haberle I. Lipophilicity of potent porphyrin-based antioxidants: comparison of ortho and meta isomers of Mn(III) N-alkylpyridylporphyrins. Free Radic Biol Med. 2009;47:72–78. doi: 10.1016/j.freeradbiomed.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain. 2007;133:9–17. doi: 10.1016/j.pain.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievens JC, Bernal F, Forni C, Mahy N, Kerkerian-Le Goff L. Characterization of striatal lesions produced by glutamate uptake alteration: cell death, reactive gliosis, and changes in GLT1 and GADD45 mRNA expression. Glia. 2000;29:222–232. doi: 10.1002/(sici)1098-1136(20000201)29:3<222::aid-glia4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Macmillan-Crow LA, Cruthirds DL. Invited review: manganese superoxide dismutase in disease. Free Radic Res. 2001;34:325–336. doi: 10.1080/10715760100300281. [DOI] [PubMed] [Google Scholar]
- MacMillan-Crow LA, Cruthirds DL, Ahki KM, Sanders PW, Thompson JA. Mitochondrial tyrosine nitration precedes chronic allograft nephropathy. Free Radic Biol Med. 2001;31:1603–1608. doi: 10.1016/s0891-5849(01)00750-x. [DOI] [PubMed] [Google Scholar]
- MacMillan-Crow LA, Thompson JA. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Archives of biochemistry and biophysics. 1999;366:82–88. doi: 10.1006/abbi.1999.1202. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62:259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- Masini E, Bani D, Vannacci A, Pierpaoli S, Mannaioni PF, Comhair SA, Xu W, Muscoli C, Erzurum SC, Salvemini D. Reduction of antigen-induced respiratory abnormalities and airway inflammation in sensitized guinea pigs by a superoxide dismutase mimetic. Free Radic Biol Med. 2005;39:520–531. doi: 10.1016/j.freeradbiomed.2005.04.006. [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- Mennerick S, Shen W, Xu W, Benz A, Tanaka K, Shimamoto K, Isenberg KE, Krause JE, Zorumski CF. Substrate turnover by transporters curtails synaptic glutamate transients. J Neurosci. 1999;19:9242–9251. doi: 10.1523/JNEUROSCI.19-21-09242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minana MD, Kosenko E, Marcaida G, Hermenegildo C, Montoliu C, Grisolia S, Felipo V. Modulation of glutamine synthesis in cultured astrocytes by nitric oxide. Cell Mol Neurobiol. 1997;17:433–445. doi: 10.1023/A:1026339428059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PK, Oluyomi AO, Babbedge RC, Wallace P, Hart SL. L-NG-nitro arginine methyl ester exhibits antinociceptive activity in the mouse. British journal of pharmacology. 1991;102:198–202. doi: 10.1111/j.1476-5381.1991.tb12153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscoli C, Cuzzocrea S, Ndengele MM, Mollace V, Porreca F, Fabrizi F, Esposito E, Masini E, Matuschak GM, Salvemini D. Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance in mice. J Clin Invest. 2007;117:3530–3539. doi: 10.1172/JCI32420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, Salvemini D. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. British journal of pharmacology. 2003;140:445–460. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscoli C, Mollace V, Wheatley J, Masini E, Ndengele M, Wang ZQ, Salvemini D. Superoxide-mediated nitration of spinal manganese superoxide dismutase: a novel pathway in N-methyl-D-aspartate-mediated hyperalgesia. Pain. 2004;111:96–103. doi: 10.1016/j.pain.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Ndengele MM, Cuzzocrea S, Esposito E, Mazzon E, Di Paola R, Matuschak GM, Salvemini D. Cyclooxygenases 1 and 2 contribute to peroxynitrite-mediated inflammatory pain hypersensitivity. Faseb J. 2008;22:3154–3164. doi: 10.1096/fj.08-108159. [DOI] [PubMed] [Google Scholar]
- Ndengele MM, Cuzzocrea S, Masini E, Vinci MC, Esposito E, Muscoli C, Petrusca DN, Mollace V, Mazzon E, Li D, Petrache I, Matuschak GM, Salvemini D. Spinal ceramide modulates the development of morphine antinociceptive tolerance via peroxynitrite-mediated nitroxidative stress and neuroimmune activation. The Journal of pharmacology and experimental therapeutics. 2009;329:64–75. doi: 10.1124/jpet.108.146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park ES, Gao X, Chung JM, Chung K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett. 2006;391:108–111. doi: 10.1016/j.neulet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Radi R, Cassina A, Hodara R. Nitric oxide and peroxynitrite interactions with mitochondria. Biol Chem. 2002;383:401–409. doi: 10.1515/BC.2002.044. [DOI] [PubMed] [Google Scholar]
- Salter M, Strijbos PJ, Neale S, Duffy C, Follenfant RL, Garthwaite J. The nitric oxide-cyclic GMP pathway is required for nociceptive signalling at specific loci within the somatosensory pathway. Neuroscience. 1996;73:649–655. doi: 10.1016/0306-4522(96)00060-7. [DOI] [PubMed] [Google Scholar]
- Salvemini D. Peroxynitrite and opiate antinociceptive tolerance: a painful reality. Archives of biochemistry and biophysics. 2009;484:238–244. doi: 10.1016/j.abb.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D, Jensen MP, Riley DP, Misko TP. Therapeutic manipulations of peroxynitrite. Drug News Perspect. 1998;11:204–214. [PubMed] [Google Scholar]
- Salvemini D, Neumann WL. Peroxynitrite: a strategic linchpin of opioid analgesic tolerance. Trends in pharmacological sciences. 2009;30:194–202. doi: 10.1016/j.tips.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Schwartz ES, Kim HY, Wang J, Lee I, Klann E, Chung JM, Chung K. Persistent pain is dependent on spinal mitochondrial antioxidant levels. J Neurosci. 2009;29:159–168. doi: 10.1523/JNEUROSCI.3792-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz ES, Lee I, Chung K, Chung JM. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain. 2008;138:514–524. doi: 10.1016/j.pain.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniscalco D, Fuccio C, Giordano C, Ferraraccio F, Palazzo E, Luongo L, Rossi F, Roth KA, Maione S, de Novellis V. Role of reactive oxygen species and spinal cord apoptotic genes in the development of neuropathic pain. Pharmacol Res. 2007;55:158–166. doi: 10.1016/j.phrs.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Smith CD, Carson M, van der Woerd M, Chen J, Ischiropoulos H, Beckman JS. Crystal structure of peroxynitrite-modified bovine Cu,Zn superoxide dismutase. Archives of biochemistry and biophysics. 1992;299:350–355. doi: 10.1016/0003-9861(92)90286-6. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Masini E, Mazzocca C, Cuzzocrea S, Ciampa A, Suzuki H, Bani D. Inhibition of poly(ADP-ribose) polymerase prevents allergen-induced asthma-like reaction in sensitized Guinea pigs. The Journal of pharmacology and experimental therapeutics. 2004;311:1241–1248. doi: 10.1124/jpet.104.072546. [DOI] [PubMed] [Google Scholar]
- Tal M. A novel antioxidant alleviates heat hyperalgesia in rats with an experimental painful peripheral neuropathy. Neuroreport. 1996;7:1382–1384. doi: 10.1097/00001756-199605310-00010. [DOI] [PubMed] [Google Scholar]
- Trotti D, Rolfs A, Danbolt NC, Brown RH, Jr, Hediger MA. SOD1 mutants linked to amyotrophic lateral sclerosis selectively inactivate a glial glutamate transporter. Nat Neurosci. 1999;2:848. doi: 10.1038/12227. [DOI] [PubMed] [Google Scholar]
- Trotti D, Rossi D, Gjesdal O, Levy LM, Racagni G, Danbolt NC, Volterra A. Peroxynitrite inhibits glutamate transporter subtypes. J Biol Chem. 1996;271:5976–5979. doi: 10.1074/jbc.271.11.5976. [DOI] [PubMed] [Google Scholar]
- Urban MO, Zahn PK, Gebhart GF. Descending facilitatory influences from the rostral medial medulla mediate secondary, but not primary hyperalgesia in the rat. Neuroscience. 1999;90:349–352. doi: 10.1016/s0306-4522(99)00002-0. [DOI] [PubMed] [Google Scholar]
- Wang J, Cochran V, Abdi S, Chung JM, Chung K, Kim HK. Phenyl N-t-butylnitrone, a reactive oxygen species scavenger, reduces zymosan-induced visceral pain in rats. Neurosci Lett. 2008;439:216–219. doi: 10.1016/j.neulet.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. The Journal of pharmacology and experimental therapeutics. 2004;309:869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the "bad guys": implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Guo W, Zou S, Ren K, Dubner R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J Neurosci. 2008;28:10482–10495. doi: 10.1523/JNEUROSCI.3593-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakura F, Matsumoto T, Fujimura T, Taka H, Murayama K, Imai T, Uchida K. Modification of a single tryptophan residue in human Cu,Zn-superoxide dismutase by peroxynitrite in the presence of bicarbonate. Biochimica et biophysica acta. 2001;1548:38–46. doi: 10.1016/s0167-4838(01)00212-6. [DOI] [PubMed] [Google Scholar]
- Yamakura F, Taka H, Fujimura T, Murayama K. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J Biol Chem. 1998;273:14085–14089. doi: 10.1074/jbc.273.23.14085. [DOI] [PubMed] [Google Scholar]
- Zanelli SA, Ashraf QM, Delivoria-Papadopoulos M, Mishra OP. Peroxynitrite-induced modification of the N-methyl-D-aspartate receptor in the cerebral cortex of the guinea pig fetus at term. Neurosci Lett. 2000;296:5–8. doi: 10.1016/s0304-3940(00)01608-6. [DOI] [PubMed] [Google Scholar]
- Zanelli SA, Ashraf QM, Mishra OP. Nitration is a mechanism of regulation of the NMDA receptor function during hypoxia. Neuroscience. 2002;112:869–877. doi: 10.1016/s0306-4522(02)00141-0. [DOI] [PubMed] [Google Scholar]
- Zhao GM, Bhargava HN. Nitric oxide synthase inhibition attenuates tolerance to morphine but not to [D-Ala2, Glu4] deltorphin II, a delta 2-opioid receptor agonist in mice. Peptides. 1996;17:619–623. doi: 10.1016/0196-9781(96)00073-3. [DOI] [PubMed] [Google Scholar]