Abstract
Background
The primary form of treatment for obesity today is behavioral therapy. Self-monitoring diet and physical activity plays an important role in interventions targeting behavior and weight change. The SMART weight loss trial examined the impact of replacing the standard paper record used for self-monitoring with a personal digital assistant (PDA). This paper describes the design, methods, intervention, and baseline sample characteristics of the SMART trial.
Methods
The SMART trial used a 3-group design to determine the effects of different modes of self-monitoring on short- and long-term weight loss and on adherence to self-monitoring in a 24-month intervention. Participants were randomized to one of three conditions (1) use of a standard paper record (PR); (2) use of a PDA with dietary and physical activity software (PDA); or (3), use of a PDA with the same software plus a customized feedback program (PDA + FB).
Results
We screened 704 individuals and randomized 210. There were statistically but not clinically significant differences among the three cohorts in age, education, HDL cholesterol, blood glucose and systolic blood pressure. At 24 months, retention rate for the first of three cohorts was 90%.
Conclusions
To the best of our knowledge, the SMART trial is the first large study to compare different methods of self-monitoring in a behavioral weight loss intervention and to compare the use of PDAs to conventional paper records. This study has the potential to reveal significant details about self-monitoring patterns and whether technology can improve adherence to this vital intervention component.
Keywords: Obesity, Weight loss, Adherence, Technology, Personal digital assistant, Behavioral treatment, Randomized clinical trial
1. Introduction
Obesity prevalence continues to increase at an alarming rate [1,2]. In 1999, 18.9% of adults in the US were affected; currently it is 34% [3,4]. This rise in the number of Americans afflicted by a chronic condition that is an independent risk factor for coronary heart disease (CHD) makes it one of our most pressing health challenges. Improving weight loss treatment and maintenance can improve several of the obesity-related risk factors for CHD (e.g., insulin resistance, type 2 diabetes) and help reduce the prevalence of the second most preventable cause of death today [5–7].
At present the primary form of treatment for obesity is behavioral therapy, an approach that combines instruction about nutrition and exercise with behavioral strategies that facilitate behavior change [8,9]. According to Jeffery, the greatest achievement in behavioral treatment is increased initial weight loss; however, long-term weight loss lags far behind [10]. Several studies have attempted to improve long-term weight loss maintenance by studying an array of strategies, e.g., increasing treatment intensity and or duration, enhancing motivation, modifying the dietary and exercise prescriptions, using the Internet for delivery, and teaching behavioral skills specific to maintenance [7,8,11–19].
Self-monitoring has been described as the “cornerstone” of behavioral treatment [20,21]. Early cross-sectional studies found that more consistent self-monitoring correlated significantly with weekly weight loss [21–23]. More recent studies have confirmed these earlier findings [24,25]. To date, no large clinical trials have focused on comparing the standard paper record to the use of a personal digital assistant (PDA) with and without tailored, daily feedback in response to the reported behavior.
Self-regulation provides the conceptual basis for the self-management intervention. Kanfer has described self-regulation as a process having three distinct stages: self-monitoring, self-evaluation, and self-reinforcement, and suggests that changing habits requires developed self-regulatory skills [26–28]. The behavioral strategy of self-monitoring is central to this process, and includes deliberate attention to some aspect of an individual’s behavior and recording some details of that behavior. In order to change behaviors, individuals need to pay adequate attention to their own actions, as well as to the conditions under which they occur and their immediate and long-term effects [29]. Therefore, successful self-regulation depends in part on the fidelity, consistency and temporal proximity of self-monitoring [29].
We based this intervention on data obtained from in-depth interviews focused on the experience of self-monitoring, which revealed that the process can be tedious, time-consuming and at times burdensome [30]. Thus, we sought an approach that might reduce the effort and time required to self-monitor in a weight loss study. The rationale for selecting a PDA diary in comparison to a paper record (PR) was that it might be easier if the participant did not need to look up the fat and calorie content of each food since it is in the PDA food database. Also, the PDA continually calculated the subtotals so participants always knew where their energy and fat intake were in relation to the daily goals, which assisted with the self-evaluation component of the self-regulation model. The screen showing the current intake in relation to the daily goals was another method of providing feedback to the participants. The participants in the paper record group needed to look up each food item, record the calorie and fat content and manually calculate the subtotal throughout the day. The PDA with feedback group received an additional message reminding them to record or if they had recorded, a message that was responsive to what they had entered. While the other two groups received feedback a week later from the notes provided by the interventionist in their review of the diaries, we hypothesized that providing feedback in real time would provide encouragement to the participant to record and reinforcement for their efforts to change their dietary or physical activity habits.
The purpose of this article is to describe the design, methods, intervention and baseline sample characteristics of the SMART (Self-Monitoring And Recording using Technology) weight loss trial, a randomized clinical trial of a behavioral intervention for weight loss and maintenance. We are testing a chronic disease treatment model that includes prolonged (24 months) supervision of self-management. The primary aim of the SMART trial is to determine if self-monitoring of eating and physical activity behaviors using a PDA, with or without a subject-tailored feedback intervention, is superior to using a PR for promoting and maintaining weight loss. Second, the study is designed to determine whether using the PDA with tailored feedback (PDA+FB) is superior to using the PDA without feedback. The secondary aim is to compare the effect of treatment group assignment (PR vs. PDA vs. PDA+FB) on the risk factors for coronary heart disease (lipids and glucose).
2. Materials and methods
The SMART trial is a single-center, 24-month clinical trial of adults seeking treatment for weight loss. The study uses a 3-group design to determine the effects of different modes of self-monitoring on short- and long-term weight loss and on adherence to self-monitoring. Participants are randomized to one of three conditions: (1) PPR, (2) PDA with dietary and physical activity software, and (3) PDA+FB with the same software plus a customized feedback program.
This study was approved by the University of Pittsburgh Institutional Review Board. All participants provided informed consent.
2.1. Eligibility
The eligibility criteria are listed in Table 1. Participants needed to be overweight or obese but otherwise in good health, and participants needed to be reasonably certain that they would be able to attend the treatment sessions and assessments throughout the two-year study. Eligibility was assessed several times throughout the screening process as described below.
Table 1.
Inclusion and exclusion criteria for the SMART trial.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
2.2. Recruitment, screening and enrollment
Participants were recruited from the general population in the Pittsburgh area in three annual cohorts (2006, 2007, and 2008). Several recruitment strategies were used to increase the representativeness of the sample. Participants from the principal investigator’s previous clinical trial, PREFER, were asked to refer friends and family to the SMART trial. We also maintained a weight research registry of individuals who expressed an interest in receiving information about future studies. Participants in the registry were sent a letter or e-mail describing the study. Those who were sent a letter were given an extra study flyer to share with a friend. The university and adjacent medical center both have voice mail announcement systems that were also used to recruit participants. Flyers were placed throughout the university, medical center and community. Mailing lists were purchased for recruitment of the second and third cohorts. An advertisement was placed on City of Pittsburgh buses for recruitment of the third cohort.
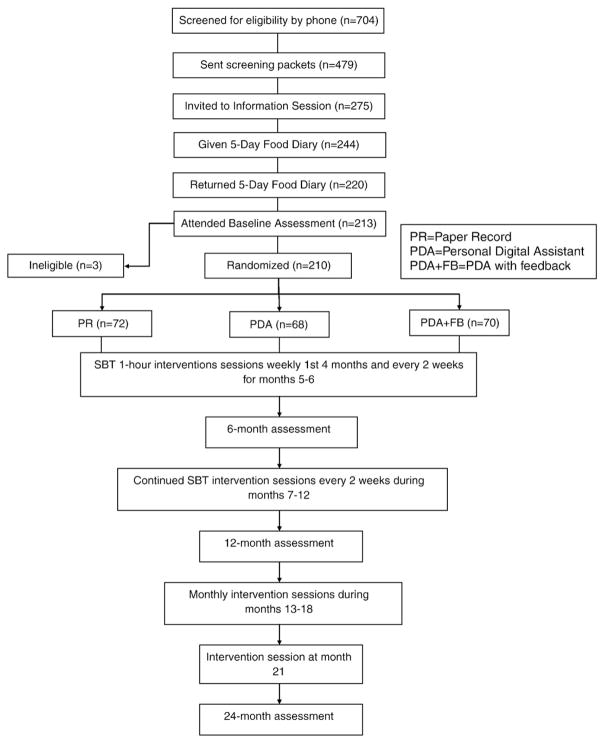
Participants were evaluated through a multi-stage screening process. Refer to Fig. 1 for the number of participants at each stage. The first stage of screening occurred over the telephone. Individuals calling in response to advertisements were asked a series of screening questions to determine basic eligibility. If an individual remained eligible he/she was sent a packet of three questionnaires to collect general demographic information, determine if the individual had a binge eating disorder and assess health history including medication use and alcohol consumption. If the individual scored above 36 on the Binge Eating Scale, which assesses disordered eating habits, the person was excluded from participation and given information on treatment sources [31]. Individuals who remained eligible after the review of their screening questionnaires were invited to an orientation session. At this session, which lasted approximately 1 h, the participants were measured to verify their self reported weight and height, the study was explained to them in detail, they had the opportunity to ask questions, and they were given a 5-day food diary. The diary served two purposes, 1) to provide participants a time to try self-monitoring and decide if they wanted to commit to a two-year study of self-monitoring and 2) to screen out individuals who would not be able to self-monitor or who demonstrated that they would not complete the diaries as instructed. A baseline assessment was scheduled for each participant who returned a completed diary.
Fig. 1.
SMART trial flowchart.
2.3. Baseline assessment
The baseline assessment included completing 2 unannounced 24-h dietary recalls (1 work day and 1 leisure day), a battery of questionnaires and a visit to the Clinical Translational Research Center (CTRC). The questionnaires were given or sent to the participants in advance of their assessment appointment and they were asked to bring the completed materials to the appointment. At the appointment, staff immediately reviewed all questionnaires for incompleteness and asked the participant for clarification if needed.
After completing a 12-h overnight fast, participants came to the CTRC for anthropometric (height, weight, body composition, and waist circumference) and blood pressure measurements, and had their blood drawn for lipid and glucose measurements. Participants with elevated glucose levels (>125 mg/DL) were not eligible to continue in the study. All other individuals who completed the assessment were eligible for randomization.
2.4. Randomization
Once we confirmed that all participants for a given cohort were eligible, we proceeded with randomization. Individuals were stratified by gender and ethnicity (White vs. non-White) to ensure balance across the three treatment groups.
2.5. Measurements
Table 2 shows the data collection schedule. Participants were asked to complete assessments that were similar to the baseline assessment every 6 months. Due to budgetary constraints lipid and glucose assays were conducted only at the baseline, 12-month and 24-month assessments. Participants were compensated for their time at the 6-, 12-, 18-, and 24-month assessments.
Table 2.
Schedule of data collection.
| Screening a | Baseline | 4 weeks | 3-mo | 6-mo | 12-mo | 18-mo | 24-mo | |
|---|---|---|---|---|---|---|---|---|
| Questionnaires | ||||||||
| Basic eligibility data (ex. age, BMI, availabile for 2 years) | X (1) | |||||||
| Sociodemographic data | X (2) | |||||||
| Health history | X (2) | |||||||
| Binge Eating Scale | X (2) | |||||||
| Barriers to Healthy Eating | X | X | X | X | X | |||
| Medical Outcomes Study – Short Form-36 | X | X | X | X | X | |||
| Cholesterol-lowering Diet Self-Efficacy – Short Form | X | X | X | X | X | |||
| Physical Activity Questionnaire | X | X | X | X | X | |||
| Perceived Stress Scale | X | X | X | X | X | |||
| Problem solving | X | X | X | X | X | |||
| Life events | X | X | X | X | X | |||
| Modifiable Activity Questionnaire | X | X | X | X | X | |||
| Experiences associated with following a low-fat diet | X | X | X | X | X | |||
| Medication update | X | X | X | X | ||||
| Symptom and Injury Survey | X | |||||||
| General Attitudes Toward Computers Scale | X | X | X | |||||
| Computer Thoughts Survey | X | X | X | |||||
| Using PDA (PDA groups) | X | X | ||||||
| Using The DietMate Pro Program (PDA Groups) | X | X | ||||||
| Labs | ||||||||
| Cholesterol – Total (mg/dL) | X | X | X | |||||
| HDL (mg/dL) | X | X | X | |||||
| LDL (mg/dL) | X | X | X | |||||
| VLDL (mg/dL) | X | X | X | |||||
| Trigycerides (mg/dL) | X | X | X | |||||
| Glucose (mg/dL) | X | X | X | |||||
| Measurements | ||||||||
| Waist (cm) | X | X | X | X | X | |||
| Weight (lbs) | X (3) | X | X | X | X | X | ||
| Height (in) | X (3) | X | X | |||||
| Blood pressure (mm Hg) | X | X | X | X | X | |||
| 24 hour dietary recall | X | X | X | X | X | |||
| Adherence to treatment Protocol | ||||||||
| Attendance | ||||||||
| Self-monitoring diaries | Collected at each treatment session: weekly for months 1–4, bi-weekly for months 5–12, monthly for months 13–18, month 21. | |||||||
| Calories and fat grams consumed, physical activity minutes | ||||||||
Screening occurred in several phases. The initial screening (1) was conducted via the telephone. The second screening (2) was conducted via mailed questionnaires. The third (3) screening occurred at the information session. The final screening was at the baseline assessment (glucose levels were checked).
2.5.1. Biological and physical measures
Venipuncture for the collection of serum for the lipid profile (total cholesterol, high density lipoprotein, low density lipoprotein, very low density lipoprotein and triglycerides) and glucose was performed by a trained phlebotomist with the subject sitting upright following a 12-h fast and a brief resting period. A wall-mounted stadiometer was used to measure height and a Tanita scale and body fat analyzer (Tanita Corporation of America, Inc., Illinois, USA) were used for weight and body compositions, which were assessed via bioelectrical impedance analysis. BMI was computed using the following formula: BMI=weight (kg)/height (meters2). Waist circumference was measured at least twice with a Gulick II measuring tape. When two measurements were obtained that were within 2 cm of each other, the average of the two measurements was used. Blood pressure was measured following the American Heart Association standard guidelines with the subject in a sitting position after at least a 5-min rest period, using an automatic blood pressure monitor.
2.5.2. Measures of treatment adherence
Adherence to the dietary protocol was assessed through two 24-h dietary recalls, which were conducted using the Nutrition Data System for Research Software (NDSR). At the orientation sessions, individuals were given a booklet of measurement figures to keep near their telephone so they could use this for estimating portion size when they received the phone call for the dietary recall. They were also asked to complete a form that identifies which phone number (mobile, home or work) to use and the best times for the interviewer to reach them to conduct the recall. The two calls were made unannounced, one on a work day and one on a leisure day. The interviews were conducted using the Five-Step Automated Multiple-Pass Method [32,33] and the NDSR software to guide the food recall process. The NDSR is a comprehensive nutrient calculation software program that is maintained by the Nutrition Coordinating Center at the University of Minnesota and is updated annually to reflect marketplace changes and new analytic data. The NDSR database contains over 18,000 foods, 7000 brand names and an increasing number of ethnic foods. It also features optional dietary supplement data that we include with 24-h dietary recalls. These data provide total calories, fat grams and values for 156 nutrients with 9 food groups and 166 subgroups for our analysis. A member of the team who has a master’s degree in nutrition science has been trained at the University of Minnesota in the use of the NDSR program; she either conducts the recalls or supervises dietetic students whom she has trained.
The Modified Activity Questionnaire[34,35] assessed daily and leisure activities and provided a measure of adherence to the physical activity protocol. Participants also recorded the number of steps that were recorded on their pedometer in their diary.
Additional measures of adherence included attendance at the treatment sessions and recording of food and exercise behaviors either in the PRs or PDAs. PDAs were uploaded at each treatment session and the diary content printed to replicate the paper record for the interventionists’ review and to assess completion of the diary. The software in the PDA (Dietmate Pro©[36,37] and CalculFit©, PICS, Reston, VA) date- and time-stamped each entry in the PDA, permitting us to examine adherence to self-monitoring as well as temporal patterns of adherence to self-monitoring over the course of the 24-month study.
2.5.3. Measures of factors related to adherence
Questionnaires evaluated potential moderators and mediators of adherence such as barriers to healthy eating, self-efficacy, problem solving, stress and the effects of life events on weight. Additional questionnaires addressed the participants’ experiences with and acceptance of technology. These measures included Barriers to Healthy Eating, a 22-item questionnaire in which subjects used a scale of 1 (no problem) to 5 (very important problem) to rate various situations or conditions related to following the diet, e.g., feelings of deprivation and cost of the regimen [38]. A higher score indicates more barriers. Experiences Associated with Following a Low-Fat diet (ELF) is a 25-item scale that assessed experiences thought to be related to diet maintenance. The scale had a total of 6 factors: wellness, distaste (for fat), cost, inconvenience, deprivation, and family and asked about past experiences; therefore, it was administered at 3 months rather than at baseline. The response scale was 1 (strongly disagree) to 5 (strongly agree) with a higher score meaning a more positive experience and attitude related to following the low-fat diet [39]. The Medical Outcomes Study, Short Form-36 Questionnaire (SF-36), was being used to measure general health-related quality of life, how it was affected by weight reduction, and its impact on adherence to the lifestyle change. The scale provided two summary scores (Physical Component Score and Mental Component Score) with a range of 0 to 100 [40]. The General Attitudes Toward Computers Scale (GATCS-C) consisted of 20 items that used a 5-point Likert scale to measure attitudes toward technology [41]. We used this instrument because a person’s attitude toward technology could influence success in using the PDA; it could also change with use over time.
2.5.4. Measurement quality control
Data were collected at the CTRC by trained and certified staff using standardized procedures and questionnaires. Equipment was standardized and routinely calibrated. Questionnaires were checked for accuracy and completeness prior to the participant leaving the center. Each staff person who oversaw or completed an assessment procedure recorded his or her initials on a form that documented each assessment procedure. The form was reviewed and initialed by the participant at the end of the appointment.
2.6. Intervention sessions
Group meetings were held in the evening and lasted approximately 45 to 90 min. They were held weekly for the first 4 months, then every two weeks for the remainder of the first year. In the second year group meetings were held monthly for months 13–18 and then once in month 21, halfway between the 18- and 24-month assessments. Participants were weighed at the beginning of each group session in a private office. At the 39 group sessions they received nutritional and behavioral counseling and practical hands-on experiences to develop skills to implement a healthy lifestyle. A detailed listing of the central features and strategies of the intervention has been published previously [42]. In response to comments received during an evaluation of a previous study, every fourth session was a supervised exercise session where participants were able to experience a variety of activities such as yoga, indoor and outdoor walking, and strength training with supervision and receive feedback on performance provided by the lead interventionist, a master’s level prepared exercise physiologist.
Participants received a calorie goal based on their baseline weight and gender (women: 1200 kcal for <200 lb or 1500 kcal for ≥200 lb; men: 1500 kcal for <200 lb or 1800 kcal for ≥200 lb) and were asked to limit their fat intake to no more than 25% of their calories, e.g., 33 or 42 g per day for females.
2.7. Treatment assignment
All participants received the same standard intervention; the only difference was in the mode of self-monitoring that participants were asked to use. Participants in the PR group were asked to self-monitor using standard paper diaries. Participants in the PDA and PDA+ FB group were provided with Palm Tungsten E2™ PDAs and self-monitoring software (Dietmate Pro©[36,37] and CalculFit©, PICs, Reston, VA). Participants in the PDA+FB group had additional custom software loaded onto their PDAs that tracked their adherence to calorie, fat gram and exercise goals and provided a daily message related to goal attainment.
Participants in the PR group were provided with a reference book with nutrition information. The first cohort was given the T-factor book [43] and the second and third cohorts were given the Calorie King book [44,45]. These participants were trained in how to look up the nutritional information for their foods at the first group session, and they were given a pen and calculator set with the study logo.
Participants in the PDA and PDA+FB group were trained how to use a PDA and how to use the self-monitoring software during the first two group sessions. The training included the use of PowerPoint slides and the use of a PDA simulator projected on the screen so all participants could see the functions of the PDA software and work along with the instructor. Adequate mastery of the basic functions for self-monitoring was achieved in the first two sessions for most participants. One-on-one assistance was provided for participants who needed additional help.
2.8. Self-monitoring
Self-monitoring was the focus of this trial. Participants were asked to self-monitor their eating and exercise behaviors in a timely fashion throughout the 2-year study. The importance of timeliness in recording intake and subtotals of calories and fat consumed was stressed because it gives participants a chance to adjust their behavior during the day to avoid exceeding their calorie or fat gram goals. The PDA automatically calculated the subtotals after each food entry whereas the subtotals needed to be calculated for the PR. At each session, PR participants turned in their diaries and received new diaries to use until the next session. They also received the diaries turned in at the previous session that had been reviewed by and contained feedback from the interventionists. When the meeting frequency decreased to less than weekly, the diaries for the past two weeks were reviewed; however, if a person missed a session and then turned in four diaries, only the last two were reviewed and feedback was provided by the interventionists. The PDA and PDA+FB participants turned in their PDAs at the beginning of the session. During the session the self-monitoring data from the participants’ PDAs were uploaded to the PICS website and converted to a format that could be downloaded to the study’s Microsoft (MS) Access Adherence database. The interventionists then received printed reports that looked similar to the standard paper diaries for their review and comments. These annotated printouts were given to the participants at the next session with feedback. Multiple weeks and missed sessions were handled as described for the PR group.
2.9. DietMatePro and Calcufit software
The DietMatePro© (DM Pro) software contains a USDA-based database of nutrient information for over 6000 food items. Moreover, the database contains nutritional information for foods from many national restaurant chains and participants have the option of adding unlisted foods to the database. Besides being able to add individual food items, participants also can add entire meals to the database. For example, if a participant always had a cup of coffee, a bowl of cereal with skim milk, and a medium banana for breakfast, those items could be entered as “Breakfast 1” then each morning the participant could simply enter “Breakfast 1” into the PDA instead of entering each individual food item.
The Calcufit software allowed participants to enter the intensity of and amount of time in aerobic exercise, the amount of weight and number of repetitions of strength training exercises, and details about stretching, as well as steps counted and water consumed. As with DietMatePro, the date and time of each entry were recorded, and no forward entry was allowed with either software.
The DM Pro developer created a custom desktop application that facilitated the transfer or upload of data from the PDA to the web site (hotsync) and server of DMPro and Calcufit. During uploading, this application copied all food and exercise records from the PDAs to the server hosted by the software developer. If there was a technical failure of a PDA and the data were lost, previous records could be brought back to the PDA from the web server during the upload process. Moreover, back-up software (BackupMan) was loaded onto the PDAs and set to back-up data to secure digital memory cards every night, further reducing the loss of data that would have occurred during a device failure.
2.10. Customized feedback program
A customized feedback program was designed for this study. The algorithm for the dietary goal feedback was written in four groups corresponding to time of day with five sections in each group corresponding to goal attainment. Table 3 displays the criteria for each group/section combination. The program randomly selected a time of day such that each participant received one dietary feedback message each day. This randomization was weighted to reduce the likelihood of the participants receiving a message at the same time on (the same part) on two consecutive days. The algorithm intentionally excluded some participants. We did not think that a feedback message was appropriate for participants who were overly restrictive in their eating. Presumably these participants were identified by the interventionists and were offered appropriate counseling. It was impossible to distinguish in an algorithm those participants who were engaging in restrictive eating from those who only partially recorded their intake, but we thought that the most prudent action was to allow the interventionists to explore these issues in person. We also counted participants as adherent if they slightly exceeded their goal (101% of calorie goal or 102% of fat gram goal). This rule took into account the imprecision of calorie and fat gram counting and avoided discouraging participants who simply appeared to exceed the goal due to rounding.
Table 3.
Feedback algorithm for calorie goals.
| 0) 10 AM–noon | 1) 1:00 PM–3:00 PM | 2) 5:00 PM–7:00 PM | 3) 8:00 PM–9:00 PM | |
|---|---|---|---|---|
| 1) No recordings | 0 calories, 0 fat ex.“Hope you can find some time to record your breakfast.” |
0 calories, 0 fat ex. “Self-monitoring can be a lot of work, but it will help you be successful.” |
0 calories, 0 fat ex. “Please take some time to record your meals today.” |
0 calories, 0 fat ex. “Recording your food intake is helpful. Hope you are still able to do it today.” |
| 2) At risk for exceeding or already exceeded calorie goal, fat OK | >40% of calorie goal, 20–40% of fat goal ex. “Good job making choices low in fat! Watch portion sizes to control calories.” |
>60% calorie goal, 40–60% of fat goal ex. “Nice job limiting fats; might want to limit sweets/candy this afternoon.” |
>95% calorie goal, 70–80% of fat goal ex. “Super job on staying within fat limits; watch high sugar foods this evening.” |
>101% calorie goal, 82–102% of fat goal ex. “You are making very nice progress. Watch the calories more closely.” |
| 3) Calories OK, at risk for exceeding or already exceeded fat gram goal | 20–40% of calorie goal, >40% of fat goal ex. “Your fat intake has been high so far. Try to eat more fruits and vegetables for rest of day.” |
40–60% of calorie goal, >60% of fat goal ex. “Go easy on high fat food tonight, big salad for dinner will help.” |
70–80% of calorie goal, >95% of fat goal ex. “Fat grams high, may want to add physical activity this evening.” |
80–101% of calorie goal, >102% of fat goal ex. “Very good changes are occurring, just watch the high fat foods — might wish to choose fresh fruit or veggies more often.” |
| 4) At risk for exceeding or already exceeded both goals | >40% of calorie goal, >40% of fat gram goal ex. “Must have been a tough morning. Do not get discouraged. Focus on meeting at least one of your dietary goals today.” |
>60% of calorie goal, >60% of fat gram goal ex. “Try a little harder to watch high fat, high calorie foods.” |
>95% of calorie goal, >95% of fat gram goal ex. “Do not get discouraged about not meeting dietary goals; you can still begin anew today.” |
>101% of calorie goal, 102% of fat gram goal ex. “Reducing portion sizes might help you meet your calorie and fat gram goals.” |
| 5) OK for both goals | 20–40% of calorie goal, 20–40% of fat gram goal ex. “Way to go! Congratulate yourself.” |
40–60% of calorie goal, 40–60% of fat gram goal ex. “Nice food choices; this will serve you well.” |
70–80% of calorie goal, 70–80% of fat gram goal ex. “Great job staying on target; reward yourself with a relaxing walk or movie.” |
80–101% of calorie goal, 82–102% of fat goal ex. “Super job on the eating! Do the same tomorrow.” |
The exercise algorithm was much simpler because measuring adherence to exercise is much more straightforward than is adherence to the dietary goals. Since exercise goals were given as weekly minutes to achieve, the weekly goal was divided by 7 to determine an average daily goal. The exercise messages were delivered once every other day since participants were not expected to exercise every day but were encouraged to exercise on most days. Thus, the average daily goal was multiplied by 2 and the participants’ exercise minutes for the previous 2 days were compared to the 2-day goal. Participants could receive a message between 3 and 5 PM or between 7 and 8 PM. Each group of messages contained three sections: 1) the participant did not record, 2) the participant met or was likely to meet the goal (minutes were equal to or greater than 80% of the goal), 3) the participant did not meet or was not likely to meet the goal (minutes were equal to or less than 50% of the goal).
The algorithm remained the same throughout the study, but the library of messages was changed after a year to reduce the risk of the participants becoming desensitized by repeatedly seeing the same messages throughout the two-year study.
2.11. Data and safety monitoring plan
Because this was a low risk study, a data and safety monitoring committee was not required; however, we had a detailed plan and a Data and Safety Monitoring Officer (DSMO). The primary safety concerns were gastrointestinal and musculoskeletal. A Symptom and Injury Survey was developed for the study and was administered at each assessment appointment. Biological measures were routinely collected and reviewed for safety monitoring, e.g., lipid profile, glucose and blood pressure. Results were reviewed by the PI and shared with the DSMO, a physician with extensive experience in weight management. If any of these parameters were elevated, the participant was advised to inform his or her physician. All participants were provided a written report of the biological and physical measure results from each assessment.
2.12. Data management
The study used a password protected centralized Oracle 9i database for storage of the outcome data, which were identified by subject identification number only. The outcome data were collected on forms designed to permit scanning using Teleform 6.1 software. Data were verified for accuracy after they were scanned into the database. Visual Basic 6.0 program was used to clean all scanned data by employing logic for data correction, range checks, skip and fill, coding missing values, and coding non-applicable questions. The progress of all participants was tracked throughout the study in a MS Access tracking program that permitted the staff to query the system and develop reports at any time. Process data (completion of weekly diary, achievement of dietary and physical activity goals, weights and attendance) were entered weekly in a MS Access database so retention and progress could be reviewed monthly. Status reports of screening, recruitment, and assessments were provided frequently. Only trained project staff were permitted to scan or clean the data and first had to provide a logon name and password.
2.13. Analysis plan
The primary outcome measure is weight change from baseline to 6 months and baseline to 24 months. The design of the study required data collection at baseline, 6, 12, 18, and 24 months. Therefore, missing data may present a problem in the data analyses. Analyses will follow the intention-to-treat model (ITT), that is, all randomized participants will be included in the analyses according to their original assignment. Individuals who withdraw or do not complete the final assessment will be included in the analysis by using the baseline weight as imputed values; in other words, no weight change will be assumed. The ITT approach will tend to bias the analyses in the direction of the null hypothesis of no difference among groups. We will examine the cohort effect in the analyses.
2.14. Sample size justification
The sample size for this study was determined considering 1) the effect sizes observed in the PI’s preliminary work for changes in the primary outcome of weight from baseline to follow-up (6, 12, and 18 months) across the estimated levels of self-monitoring (>25% to ≦ 50%, >50% to ≦75%, and > 75% to ≦100%); these effect sizes are expected to parallel the differences in weight loss across the treatment groups (PR, PDA, PDA+FB) with the level of significance adjusted to .01 to accommodate multiple testing; and 2) the expected rate of attrition during the 24-month study period. Taking these two factors into consideration we determined that a final sample size of 156 participants would be required to adequately test our hypotheses. We anticipated that we would need to enroll 198 individuals to retain 156 participants at 24 months.
3. Results
A total of 210 people were randomized to the SMART trial. No differences in sociodemographic or baseline measures were evident among the three treatment groups; thus, the results are not separated by treatment group. Table 4 shows the baseline characteristics of all individuals who completed the screening questionnaires. Few differences were found between the participants who were randomized and those who completed the second stage of screening but were ultimately not randomized. As is typical with weight loss trials, the randomized sample was predominately female and White. Most participants were employed full time and were fairly well educated; by BMI status they were obese. Many participants were overweight by early adulthood and most had tried to lose weight previously. Although participants could not be diabetic to be eligible for SMART, more than half had a family history of diabetes. Baseline measures for the participants are shown in Table 5. As expected, at baseline participants reported consuming a diet that exceeded the calorie and fat gram restrictions of the intervention. Statistically significant differences were found among cohorts for several of the baseline measures as displayed in Table 6.
Table 4.
Baseline sample description (randomized n=210, not randomized n=121).
| Characteristic | Randomized % (n) | Not Randomized % (n) | p |
|---|---|---|---|
| Gender (female) | 84.76 (178) | 88.99 (98) | .375 |
| Race (White) | 78.57 (165) | 64.46 (78) | .007 |
| Marital status | <.001 | ||
| Currently married | 68.57 (144) | 51.24 (62) | |
| Never married | 13.81 (29) | 30.58 (37) | |
| Formerly married (divorced or separated) | 17.62 (37) | 18.18 (22) | |
| Employment status | |||
| Employed full time | 82.86 (174) | 70.25 (85) | .007 |
| Gross household income | .005 | ||
| >$50,000 | 60.00 (123) | 43.97 (51) | |
| $30,000–$50,000 | 23.90 (49) | 25.86 (30) | |
| $10,000–$30,000 | 16.10 (33) | 30.17 (35) | |
| History of high cholesterol | 27.14 (57) | 27.27 (33) | .979 |
| History of high blood pressure | 29.05 (61) | 25.00 (30) | .429 |
| Family history of diabetes | 54.29 (114) | 52.09 (63) | .700 |
| History of being overweight | |||
| Pre-school period | 10.48 (22) | 6.72 (8) | .251 |
| Elementary school period | 23.81 (50) | 23.33 (28) | .922 |
| Junior high school period | 30.48 (64) | 35.00 (42) | .397 |
| High school period | 30.95 (65) | 35.00 (42) | .450 |
| Age 19–25 years | 37.80 (79) | 44.17 (53) | .257 |
| Age 26–35 years* | 64.39 (132) | 67.86 (76) | .535 |
| Age 36–45 years* | 88.54 (156) | 88.54 (85) | .921 |
| Age 46–55 years* | 100.00 (132) | 100.00 (60) | NA |
| Have intentionally lost 10–19 lb | |||
| Never | 8.13 (17) | 15.00 (18) | |
| 1–2 times | 44.50 (93) | 42.50 (51) | |
| 3–5 times | 24.40 (51) | 25.83 (31) | |
| 6–10 times | 13.88 (29) | 9.17 (11) | |
| 10+ times | 9.09 (19) | 7.50 (9) | |
| Have intentionally lost 20–49 lb | .618 | ||
| Never | 40.67 (85) | 41.18 (49) | |
| 1–2 times | 46.89 (98) | 42.86 (51) | |
| 3–5 times | 12.44 (26) | 15.97 (19) | |
| Median (IQR) | Median (IQR) | ||
| Age (years) | 49.00 (13.00) | 45.00 (13.00) | <.001 |
| Education (years) | 16.00 (5.00) | 14.00 (4.00) | .013 |
| Body mass index (kg/m2) | 33.09 (6.89) | 33.70 (7.47) | .226 |
Percentages and significance were calculated including only participants who reached the specified age range at or prior to baseline.
No significant differences were found among treatment groups at baseline.
IQR = interquartile range.
Table 5.
Descriptive statistics for the baseline measures (N=210).
| Measurement | M (SD) |
|---|---|
| Cholesterol (mg/dL) | 194.80 (35.39) |
| Low density lipoprotein (LDL) cholesterol (mg/dL) | |
| Females | 120.00 (31.57) |
| Males | 115.80 (27.63) |
| High density lipoprotein (HDL) cholesterol (mg/dL) | |
| Females | 56.97 (14.96) |
| Males | 44.88 (13.44) |
| Triglycerides (mg/dL) | |
| Females | 126.60 (80.68) |
| Males | 133.70 (79.70) |
| Glucose (mg/dL) | 88.70 (8.99) |
| Blood pressure (mm Hg) | |
| Systolic | 124.20 (15.06) |
| Diastolic | 75.99 (8.15) |
| Waist circumference (cm) | |
| Females | 103.40 (11.58) |
| Males | 117.90 (11.24) |
| Body Mass Index (kg/m2) | |
| Females | 33.86 (4.42) |
| Males | 34.88 (4.79) |
| Barriers to Healthy Eating | 61.32 (13.95) |
| Medical Outcomes Study – Short Form-36 | |
| Physical Component Score | 52.17 (7.25) |
| Mental Component Score | 47.95 (10.94) |
| Mean energy (kcal) | 2114.00 (724.70) |
| Mean % calories from fat | 33.77 ( 7.18) |
No significant differences were found between the treatment groups at baseline.
SD = Standard Deviation.
Table 6.
Differences among enrollment cohorts.
| Measurement | Cohort 1 n=73 |
Cohort 2 n=63 |
Cohort 3 n=74 |
p* |
|---|---|---|---|---|
| Mean (SD) |
Mean (SD) |
Mean (SD) |
||
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| Age (years) | 46.03 (10.17) | 44.51 (9.21) | 49.53 (6.81) | .007 |
| 49.00 (18.00) | 46.00 (13.00) | 51.00 (31.00) | ||
| Education (years) | 16.03 (3.01) | 14.95 (2.89) | 15.86 (3.01) | .053 |
| 16.00 (4.00) | 14.00 (4.00) | 16.00 (15.00) | ||
| High density lipoprotein (HDL) cholesterol (mg/dL) | 59.32 (14.65) | 53.92 (14.88) | 52.03 (15.66) | .011 |
| 57.00 (22.00) | 52.00 (24.00) | 49.00 (27.00) | ||
| Glucose (mg/dL) | 89.01 (9.44) | 90.21 (7.96) | 87.11 (9.23) | .041 |
| 89.00 (12.00) | 90.00 (35.00) | 85.00 (11.00) | ||
| Blood pressure (mm Hg) | ||||
| Systolic | 120.45 (15.08) | 128.22 (13.96) | 124.53 (15.21) | .010 |
| 119.00 (21.00) | 129.00 (58.00) | 122.50 (88.00) | ||
| Diastolic | 76.00 (8.91) | 77.27 (7.34) | 74.89 (7.97) | .190 |
| 76.00 (10.50) | 76.00 (10.00) | 74.00 (10.00) | ||
| Barriers to healthy eating | 58.51 (12.85) | 64.95 (15.12) | 61.00 (13.44) | .050 |
| 60.00 (16.00) | 63.00 (20.00) | 62.00 (20.25) | ||
For all except HDL, non-parametric tests of significance are reported.
SD = standard deviation, IQR = interquartile range.
4. Issues encountered and how managed
4.1. PDA and software issues from the participants’ perspective
Participants provided anecdotal data on their acceptability of the PDA. Those who liked the PDA reported that it was portable and socially acceptable as they could record in any public setting without having to let others know that they were self-monitoring. It was also easier for them to check the nutrition information of their next meal and make necessary changes to stay within the dietary goals. Another added benefit was that they could use its other features, such as the calendar, memo, and downloading of books and music. However, participants who did not like the PDA reported frustration that it did not include more user-friendly software. They reported difficulty in searching the database for food items and recording portion size for commonly consumed food (i.e., ounce was not a portion option for poultry or deli meats). In addition, they were unable to modify or delete a custom food added to the database. Some participants indicated that the technical problems affected their desire to use the PDA to self-monitor. While some participants focused on the negative aspects of the software, others accepted the problems as being part of technology. These participants did not let the technical problems deter their self-monitoring with the PDA.
The interventionists provided support for all of the PDA issues and encouraged its continued use. There were a number of open discussions at the group meetings to allow the participants to discuss PDA problems and find out how other group members were resolving them. The interventionists met individually with the participants having difficulty until everyone felt confident in their ability to use the PDA.
4.2. DietMate Pro© and Calcufit© technical issues and lessons learned
We tested the software programs for the PDA (DM Pro and Calcufit) as well as the custom upload applications in a small pilot study prior to the start of the SMART trial. Nevertheless, technical problems started to appear at the very beginning of the study, which were mainly caused by the hotsync upload process and the large number of transfers. These problems occurred while using the added foods feature and sometimes the PDA would save incorrect nutritional information or the daily totals would display zero; it should be noted that these problems did not occur with all participants’ PDAs. Other problems included freezing of PDAs and getting fatal errors, which prevented participants from self-monitoring; again this did not affect all participants.
The SMART team served as the first-line for all troubleshooting and attended to the technical problems in the shortest time possible to prevent an interruption of participants’ self-monitoring. A research assistant managed basic problems and was assisted by the Data Manager when necessary. Any issue that was fixable without contacting the software developer was solved immediately and the PDA was returned to the participant. An important member of the team was the Data Manager who was also trained in information science and knowledgeable in technology and data transfer issues. This person communicated with the programmer when issues needed the developer’s assistance, which was done the same day via e-mail and/or through telephone contact. Log files, which were created on a local PC when the upload process was initiated, contained all food, exercise, and header information about possible errors. We sent these files to the developer along with a full description of the problems reported by the participants and the steps that we took in solving and replicating the issue. Upon resolving the problem, the developer sent patches that were applied to all PDAs. In cases that the PDAs needed to be kept for further analysis for more complex problems or hardware issues, participants were given a ‘loaner’ PDA with all of their information transferred to it from back-up memory cards, and dietary and exercise software were reinstalled.
Team work, communication, timely troubleshooting, and developing a trusting relationship between participants and members of the SMART team were crucial for success and continuation of the study. Based on our initial experience in the trial, we would recommend that future interventions that rely on newly developed technology do some initial testing after the pilot study on a large enough scale to invoke possible unpredicted errors. On a related matter, we were cautioned by colleagues about loss of PDAs and to anticipate at least a 10% loss rate; however, at this point in the trial, we have not experienced the loss of any PDAs including the first cohort that has already completed the study. We have experienced breakage of the PDAs but these were covered by the warranty contract.
5. Discussion
The SMART trial is a single-center randomized controlled trial designed to examine three different methods of self-monitoring tools in a lifestyle behavioral intervention intended to achieve weight reduction. To the best of our knowledge, it is the first large study to compare different methods of self-monitoring in a behavioral weight loss intervention and to compare the use of PDAs to conventional paper diaries. Other investigators have examined different approaches to self-monitoring. Helsel et al. conducted a 16-week correspondence behavioral weight loss program (N=42) and compared traditional paper diary vs. traditional paper diary with transition to abbreviated diary with checklists and found no difference between the two groups in weight loss; however, retention was less than 60% [46]. Another study compared paper diary use in one study to the use of a PDA in a subsequent study and also found no difference between the groups in weight loss [47]. Glanz et al. conducted a one-month pilot study using hand-held computers in the Diet Modification arm of the Women’s Health Initiative and found that participants (N=33) significantly increased self-monitoring and met their dietary goals more often compared to previous use of PR for self-monitoring [48]. While the latter study had positive results, it was short in duration and not focused on weight loss. Taken together, findings from these studies are equivocal regarding the use of a PDA to reduce subject burden for self-monitoring and improved long-term adherence and better weight loss compared with using a paper diary. Our study with its large sample, 2-year duration and use of three groups will permit us to answer this question, and moreover, reveal if providing tailored feedback in real time will improve adherence to self-monitoring.
We demonstrated in a previous study that participants often back-fill their diaries, e.g. filling in the diary days after the eating behavior occurred and thus needing to rely on recall [24]. We also showed that timeliness of recording one’s intake is related to weight loss [24,49]. Because the PDA software date-and-time-stamps each entry, we will be able to describe adherence to self-monitoring in the two PDA groups over the duration of the study and examine in a larger sample how time of recording is associated with weight loss.
Like most behavioral weight loss studies, our sample is predominantly female and White. However, the ethnic composition reflects the region’s demographics with 79% Whites. We made extra effort to recruit men, such as posting flyers and running phone announcements targeting men only and also posted flyers in women’s clinics appealing to their concern for an overweight spouse, sibling, or father. We had a higher representation of men in the early phases of screening; however, they subsequently did not continue through the full screening process. Even though the reported prevalence of obesity is increasing among men, [50] there does not seem to be an increase in their willingness to enroll in weight loss studies. Our retention rates, thus far, are excellent. We had 90% study completion of the first cohort at 24 months and 92% retention at 6 months for the third cohort. The second cohort had a lower retention rate of 76% at 18 months.
There were 65 individuals who were eligible at the initial screening and invited to the Orientation Session but were not randomized. These individuals were significantly different from those who were randomized on race (more Whites were randomized), marital status, employment, income and education (those currently or formerly married, working full time, or who had a higher income or education were more likely to be randomized). This may have been due to several reasons, e.g., as they learned more about the involvement of the study, they were unwilling or unable to make that commitment; individuals who were never married or had less income may have thought that study participation would interfere with work or care of a family member. Since the study covers all expenses related to participation, the study did not create a financial burden for the individual; however, some may have still seen participation for two years as a burden. The difference in age for the two groups was four years (49 vs. 45) with the older person more likely to be randomized. This difference did not seem to be clinically significant but it may represent a time when an individual, particularly a female, may have more time to engage in this pursuit.
We also found some statistically significant differences in sociodemographic and physiological variables among the three cohorts at baseline. While some of these differences might be considered clinically significant, e.g., a systolic BP of 128 vs. 120, other differences such as the age range from 52 to 59 and the Barriers to Healthy Eating score range of 60 to 63 are likely not to have a significant effect. However, these differences in the cohorts at baseline will be examined fully in the later analysis of the outcomes.
The design used in this trial is the current state of the science for standard behavioral treatment for weight loss; however, the 2-year duration is longer than most studies [15,51–54]. We are using this approach to address the chronic disease model of obesity and to promote the ongoing contact that has been demonstrated so often to improve long-term adherence to behavior change [55–58]. For the same reason, we encourage participants to enroll in Weight Watchers at the end of the study so that they will have ongoing support and reinforcement of their new behaviors. To promote this, the study covers the cost of enrollment in the commercial weight loss program.
In summary, we have learned a great deal from this ongoing trial, especially about the potential threats to study integrity when utilizing state-of-the art technology. Even a long-term pilot study of technology did not uncover the technical issues that we had to confront in the early phase of the study. However, having the necessary expertise in the research team members and the cooperation of the software developer helped to overcome those obstacles in a timely manner. Study participants quickly learned the use of the software and PDA and some used it extensively and incorporated it into their daily life. e.g., one participant read several books on the PDA. We anticipate that study outcomes will reveal significant detail about self-monitoring patterns and whether the use of technology can improve adherence to this vital component of the intervention.
Acknowledgments
This study was supported by the National Institutes of Health grants #RO1-DK71817 and partial support for LE Burke by NIH K24 Award, NR010742. The conduct of the study was also supported by the Data Management Core of the Center for Research in Chronic Disorders NIH-NINR #P30-NR03924 and the General Clinical Research Center, NIH-NCRR-GCRC #5MO1-RR00056 and the Clinical Translational Research Center, NIH/NCRR/CTSA Grant UL1 RR024153 at the University of Pittsburgh. We gratefully acknowledge the participants in this study who so willingly gave of their time to complete the assessments.
References
- 1.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The continuing epidemic of obesity in the United States [Letters] JAMA. 2000 October 4;284(13):1650–1. doi: 10.1001/jama.284.13.1650. [DOI] [PubMed] [Google Scholar]
- 3.Wadden TA, Brownell KD, Foster GD. Obesity: responding to the global epidemic. J Consult Clin Psychol. 2002;70(3):510–25. doi: 10.1037//0022-006x.70.3.510. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, McDowell MA, Flegal KM. Obesity among adults in the United States — no Change since 2003–2004. National Center for Health Statistics; Hyattsville, MD: 2007. [Google Scholar]
- 5.Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, et al. Clinical implications of obesity with specific focus on cardiovascular disease. A statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2004;110:2952–67. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 6.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on obesity and heart disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 7.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009 February 26;360(9):859–73. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132:226–2238. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 9.Wing RR. Behavioral approaches to the treatment of obesity. In: Bray GA, Bourchard C, James WPT, editors. Handbook of obesity. New York: Marcel Dekker; 1998. pp. 855–77. [Google Scholar]
- 10.Jeffery RW, Drewnowski A, Epstein LH, Stunkard AJ, Wilson GT, Wing RR, et al. Long-term maintenance of weight loss: current status. Health Psychol. 2000;19(1 Suppl):5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- 11.Hollis JF, Gullion CM, Stevens VJ, Brantley PJ, Appel LJ, Ard KD, et al. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med. 2008;35:118–26. doi: 10.1016/j.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosler A, Varadarajulu D, Ronsani A, Fredrick B, Fisher B. Low-fat milk and high-fiber bread availability in food stores in urban and rural communities. J Public Health Manag Pract. 2006;12(6):556–62. doi: 10.1097/00124784-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Smith West D, DiLillo V, Burzac Z, Gore SA, Greene PG. Motivational interviewing improves weight loss in women with type 2 diabetes. Diabetes Care. 2007;30(5):1081–7. doi: 10.2337/dc06-1966. [DOI] [PubMed] [Google Scholar]
- 14.Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. [see comment] JAMA. 2008;299(10):1139–48. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 15.Tate DF, Jackvony EH, Wing RR. A randomized trial comparing human e-mail counseling, computer-automated tailored counseling, and no counseling in an Internet weight loss program. Arch Intern Med. 2006;166:1620–5. doi: 10.1001/archinte.166.15.1620. [DOI] [PubMed] [Google Scholar]
- 16.Tate DF, Jeffery RW, Sherwood NE, Wing RR. Long-term weight losses associated with prescription of higher physical activity goals. Are higher levels of physical activity protective against weight regain? Am J Clin Nutrition. 2007;85(4):954–9. doi: 10.1093/ajcn/85.4.954. [DOI] [PubMed] [Google Scholar]
- 17.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355(15):1563–71. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 18.Turk MW, Yang K, Hravnak M, Sereika SM, Ewing LJ, Burke LE, et al. Randomized clinical trials of weight loss maintenance: a review. J Cardiovasc Nurs. 2009;24(1):58–80. doi: 10.1097/01.JCN.0000317471.58048.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakicic JM, Marcus BH, Gallagher KI, Napolitano M, Lang W. Effect of exercise duration and intensity on weight loss in overweight, sedentary women: a randomized trial. JAMA. 2003;290(10):1323–30. doi: 10.1001/jama.290.10.1323. [DOI] [PubMed] [Google Scholar]
- 20.Wadden TA. The treatment of obesity. In: Stunkard AJ, Wadden TA, editors. Obesity: theory and therapy. New York: Raven Press; 1993. pp. 197–217. [Google Scholar]
- 21.Baker RC, Kirschenbaum DS. Self-monitoring may be necessary for successful weight control. Behav Ther. 1993;24:377–94. [Google Scholar]
- 22.Baker RC, Kirschenbaum DS. Weight control during the holidays: Highly consistent self-monitoring as a potentially useful coping mechanism. Health Psychol. 1998;17(4):367–70. doi: 10.1037//0278-6133.17.4.367. [DOI] [PubMed] [Google Scholar]
- 23.Sperduto WA, Thompson HS, O’Brien RM. The effect of target behavior monitoring on weight loss and completion rate in a behavior modification program for weight reduction. Addict Behav. 1986;11:337–40. doi: 10.1016/0306-4603(86)90060-2. [DOI] [PubMed] [Google Scholar]
- 24.Burke LE, Sereika SM, Music E, Warziski M, Styn MA, Stone AA. Using instrumented paper diaries to document self-monitoring patterns in weight loss. Contemporary Clinical Trials. 2008;29(2):182–93. doi: 10.1016/j.cct.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353(20):2111–20. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 26.Kanfer FH. Self-monitoring: methodological limitations and clinical applications. J Consult Clin Psychol. 1970;35:148–52. [Google Scholar]
- 27.Kanfer FH. The maintenance of behavior by self-generated stimuli and reinforcement. In: Jacobs A, Sachs LB, editors. The psychology of private events. New York: Academic Press; 1971. [Google Scholar]
- 28.Kanfer FH. Self-management methods. In: Kanfer EF, Goldstein AP, editors. Helping people change: a textbook of methods. 2. Elmsford, NY: Pergamon Press; 1980. [Google Scholar]
- 29.Bandura A. Health promotion from the perspective of social cognitive theory. Psychol Health. 1998;13:623–49. [Google Scholar]
- 30.Burke LE, Swigart V, Derro N, Warziski Turk M, Ewing L. Experiences of self-monitoring: narratives of success and struggle during treatment for obesity. Qual Health Res. 2009;19:815–28. doi: 10.1177/1049732309335395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav. 1982;7:47–55. doi: 10.1016/0306-4603(82)90024-7. [DOI] [PubMed] [Google Scholar]
- 32.Blanton CA, Moshfegh AJ, Baer DJ, Kretsch MJ. The USDA automated multiple-pass method accurately estimates group total energy and nutrient intake. J Nutr. 2006;136(10):2594–9. doi: 10.1093/jn/136.10.2594. [DOI] [PubMed] [Google Scholar]
- 33.Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. 2003;77 (5):1171–8. doi: 10.1093/ajcn/77.5.1171. [DOI] [PubMed] [Google Scholar]
- 34.Kriska AM, Caspersen CJ. Introduction to a collection of physical activity questionnaires. Med Sci Sports Exerc. 1997;29:S5–9. [PubMed] [Google Scholar]
- 35.Kriska AM, Knowler W, LaPorte RE, Drash AL, Wing RR, Blair SN, et al. Development of a questionnaire to examine the relationship of physical activity and diabetes in the Pima Indians. Diabetes Care. 1990;13:404–11. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 36.Beasley J, Riley WT, Jean-Mary J. Accuracy of a PDA-based dietary assessment program. Nutrition. 2005;21(6):672–7. doi: 10.1016/j.nut.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Beasley JM, Riley WT, Davis A, Singh J. Evaluation of a PDA-based dietary assessment and intervention program: a randomized controlled trial. J Am Coll Nutr. 2008;27(2):280–6. doi: 10.1080/07315724.2008.10719701. [DOI] [PubMed] [Google Scholar]
- 38.Burke LE, Kim Y, Music E. The barriers to healthy eating scale: psychometric report. Ann Behav Med. 2004;27(Supp):S101. [Google Scholar]
- 39.Urban N, White E, Anderson GL, Curry S, Kristal AR, Shattuck AL, et al. Correlates of maintenance of a low-fat diet among women in the women’s health trial. Prev Med. 1992;21:279–91. doi: 10.1016/0091-7435(92)90027-f. [DOI] [PubMed] [Google Scholar]
- 40.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Rosen LD, Sears DC, Weil MM. Computerphobia. Behav Res Meth Instrum Comput. 1987;19:167–79. [Google Scholar]
- 42.Burke LE, Choo J, Music E, Warziski M, Styn MA, Kim Y, et al. PREFER study: a randomized clinical trial testing treatment preference and two dietary options in behavioral weight management — rationale, design and baseline characteristics. Contemp Clin Trials. 2006;27(1):34–48. doi: 10.1016/j.cct.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Pope J, Katahn M. The T-factor fat gram counter, revised and updated edition. New York: W.W. Norton & Co.; 2006. [Google Scholar]
- 44.Borushek A. The calorie king calorie, fat and carbohydrate counter. Costa Mesa, CA: Family Health Publications; 2007. [Google Scholar]
- 45.Borushek A. The calorie king calorie, fat and carbohydrate counter. Costa Mesa, CA: Family Health Publications; 2008. [Google Scholar]
- 46.Helsel DL, Jakicic JM, Otto AD. Comparison of techniques for self-monitoring eating and exercise behaviors on weight loss in a correspondence-based intervention. J Am Diet Assoc. 2007;107(10):1807–10. doi: 10.1016/j.jada.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 47.Yon BA, Johnson RK, Harvey-Berino J, Gold BC, Howard AB. Personal digital assistants are comparable to traditional diaries for dietary self-monitoring during a weight loss program. J Behav Med. 2007;30(2):165–75. doi: 10.1007/s10865-006-9092-1. [DOI] [PubMed] [Google Scholar]
- 48.Glanz K, Murphy S, Moylan J, Evensen D, Curb JD. Improving dietary self-monitoring and adherence with hand-held computers: a pilot study. Am J Health Promot. 2006;20(3):165–70. doi: 10.4278/0890-1171-20.3.165. [DOI] [PubMed] [Google Scholar]
- 49.Burke LE, Sereika S, Choo J, Warziski M, Music E, Styn MA, et al. Ancillary study to the PREFER trial: a descriptive study of participants’ patterns of self-monitoring: rationale, design and preliminary experiences. Contemp Clin Trials. 2006;27(1):23–33. doi: 10.1016/j.cct.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Ogden C, Carroll M, Curtin L, McDowell M, Tabak C, Flegal K. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 51.Jakicic JM, Winters C, Lang W, Wing RR. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women: a randomized trial. JAMA. 1999;282 (16):1554–60. doi: 10.1001/jama.282.16.1554. [DOI] [PubMed] [Google Scholar]
- 52.Tate DF, Wing RR, Winett RA. Using Internet technology to deliver a behavioral weight loss program. JAMA. 2001;285(9):1172–7. doi: 10.1001/jama.285.9.1172. [DOI] [PubMed] [Google Scholar]
- 53.Wadden T, Berkowitz R, Womble L, Sarwer D, Phelan S, Cato R, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353(20):2111–20. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 54.Wing RR, Jeffery RW. Benefits of recruiting participants with friends and increasing social support for weight loss and maintenance. J Consult Clin Psychol. 1999;67(1):132–8. doi: 10.1037//0022-006x.67.1.132. [DOI] [PubMed] [Google Scholar]
- 55.Burke LE, Dunbar-Jacob JM, Hill MN. Compliance with cardiovascular disease prevention strategies: a review of the research. Ann Behav Med. 1997;19(3):239–63. doi: 10.1007/BF02892289. [DOI] [PubMed] [Google Scholar]
- 56.Osterberg L, Blaschke TF. Adherence to medication. N Engl J Med. 2005;353(5):487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 57.van Dulmen S, Sluijs E, van Dijk L, de Ridder D, Heerdink R, Bensing J. Patient adherence to medical treatment: a review of reviews. BMC Health Serv Res. 2007;7:55. doi: 10.1186/1472-6963-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weingarten SR, Henning JM, Badamgarav E, Knight K, Hasselblad V, Gano A, Jr, et al. Interventions used in disease management programmes for patients with chronic illness-which ones work? Meta-analysis of published reports. BMJ. 2002;325:925–8. doi: 10.1136/bmj.325.7370.925. [DOI] [PMC free article] [PubMed] [Google Scholar]