Abstract
PUMA (p53 upregulated modulator of apoptosis) is a Bcl-2 homology 3 (BH3)-only Bcl-2 family member and a critical mediator of p53-dependent and -independent apoptosis induced by a wide variety of stimuli, including genotoxic stress, deregulated oncogene expression, toxins, altered redox status, growth factor/cytokine withdrawal and infection. It serves as a proximal signaling molecule whose expression is regulated by transcription factors in response to these stimuli. PUMA transduces death signals primarily to the mitochondria, where it acts indirectly on the Bcl-2 family members Bax and/or Bak by relieving the inhibition imposed by antiapoptotic members. It directly binds and antagonizes all known antiapoptotic Bcl-2 family members to induce mitochondrial dysfunction and caspase activation. PUMA ablation or inhibition leads to apoptosis deficiency underlying increased risks for cancer development and therapeutic resistance. Although elevated PUMA expression elicits profound chemo- and radio-sensitization in cancer cells, inhibition of PUMA expression may be useful for curbing excessive cell death associated with tissue injury and degenerative diseases. Therefore, PUMA is a general sensor of cell death stimuli and a promising drug target for cancer therapy and tissue damage.
Keywords: PUMA, BH3 domain, Bcl-2 family, p53, apoptosis
Introduction
The Bcl-2 family proteins are evolutionarily conserved key regulators of apoptosis. PUMA (p53 upregulated modulator of apoptosis) is one of the most potent killers among the Bcl-2 homology 3 (BH3)-only subgroup of Bcl-2 family members. There are more than 300 articles published on PUMA, the majority of which describe its involvement in stress-induced apoptosis regulated by the tumor suppressor p53. Until now the activity of PUMA seems to be exclusively controlled by transcription, whereas other BH3-only proteins are often activated through multiple mechanisms including posttranslational modifications. In response to genotoxic stress such as DNA damage, PUMA is transactivated by p53. Along with another BH3-only protein, Noxa, which in most cases has a minor function, PUMA accounts for virtually all of the proapoptotic activity of p53. PUMA is also activated by other transcription factors to initiate p53-independent apoptotic responses to nongenotoxic stimuli, including growth factor/cytokine deprivation, endoplasmic reticulum (ER) stress and ischemia/reperfusion. In contrast to its prominent function in p53-dependent apoptosis, the function of PUMA in p53-independent apoptosis remains to be fully appreciated.
Similar to other BH3-only proteins, PUMA serves as a proximal signaling molecule that transduces death signals to the mitochondria where it acts through multidomain Bcl-2 family members to induce mitochondrial dysfunction and caspase activation. PUMA primarily acts to indirectly activate Bax and/or Bak by relieving the inhibition of these proteins by antiapoptotic Bcl-2 family members, including Bcl-2, Bcl-XL, Mcl-1, Bcl-w and A1. It has also been suggested that PUMA can trigger apoptosis by directly activating Bax, or through cytoplasmic p53 in some cells. With such versatile functions, it is perhaps not surprising that PUMA has been implicated in many pathological and physiological processes including cancer, tissue injury, neurodegenerative diseases, immune response and bacterial or viral infection. PUMA may well be an excellent therapeutic target, as activating PUMA inhibits tumor growth by restoring apoptosis in cancer cells, whereas inhibiting PUMA curbs excessive apoptosis associated with tissue injury and neurodegeneration.
Discovery
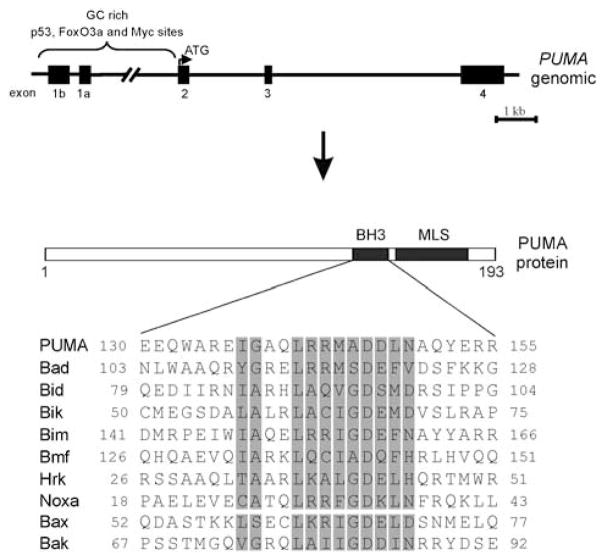
PUMA was independently cloned by three groups in 2001. Two groups identified PUMA as a transcriptional target of p53 through global gene expression profiling (Nakano and Vousden, 2001; Yu et al., 2001), whereas another group identified PUMA as an interacting partner of Bcl-2 (hence named as Bcl-2-binding component 3 or BBC3) through yeast two-hybrid screening (Han et al., 2001). PUMA is highly conserved between human and mouse, with over 90% sequence identity at both the DNA and protein levels (Yu et al., 2001), and is found in six other vertebrate genomes. The genomic structure of PUMA is also very similar in human and mouse, with the human locus mapped to 19q31 (Yu et al., 2001), and the mouse locus residing at 7A2. Five exons are identified in the PUMA locus, with the first two exons (1a or 1b) being noncoding (Figure 1). In addition to the two major transcripts, PUMA-α and PUMA-β, which both encode proteins containing the BH3 domain, extensive alternative splicing can result in multiple PUMA transcript species (Nakano and Vousden, 2001; Yu et al., 2001). The functions of these splice variants, some of which lack the BH3 domain, are currently unclear. The genomic region including the PUMA promoter, exon 1a and intron 1 contains a high percentage of guanine and cytosine nucleotides, suggesting a propensity of forming secondary structures that inhibit transcription, which may account for low basal expression levels in unstressed cells (Yu et al., 2001; Ming et al., 2008). PUMA is conserved and fulfills similar functions in zebrafish (Sidi et al., 2008). Although no PUMA homologue has been identified in lower eukaryotes, the BH3-only protein EGL-1 may be its functional counterpart in Caenorhabditis elegans (Horvitz, 1999).
Figure 1.
PUMA gene and protein structure. The human PUMA gene contains three coding exons (exons 2–4) and two noncoding exons (exons 1a and b), all of which (except for exon 1b) are conserved in mouse. The binding sites of several transcription factors such as p53, c-Myc and FoxO3a are found in the regulatory regions of both human and mouse genes. The PUMA protein has two functional domains, the BH3 domain and a C-terminal mitochondria-localization signal (MLS), which is not well defined. Alignment of the BH3 domain of PUMA with those of other proapoptotic Bcl-2 family members is shown with the conserved residues shaded in gray.
Protein structure
Initial characterization of PUMA revealed that the protein belongs to the BH3-only subgroup of Bcl-2 family proteins, which share sequence similarity only within the BH3 domain (Figure 1). The BH3 domain of PUMA is required for its interactions with Bcl-2-like proteins, such as Bcl-2 and Bcl-XL (Nakano and Vousden, 2001; Yu et al., 2001). Structural analysis indicates that the BH3 domain of PUMA forms an amphipathic α-helical structure that directly binds to antiapoptotic Bcl-2 family proteins (Day et al., 2008). The C-terminal portion of PUMA contains a hydrophobic domain that directs its mitochondrial localization (Figure 1; Yu et al., 2003b). Both the BH3 domain and mitochondrial localization are essential for the ability of PUMA to induce apoptosis or suppress cell growth (Yu et al., 2003b; Yee and Vousden, 2008). To date, no posttranslational modification on PUMA has been reported.
Regulation by transcription
PUMA is normally expressed at a very low level (Yu et al., 2001), but is rapidly induced in response to a wide range of stresses in human and mouse cells of virtually every tissue origin that has been examined. To date, only transcriptional induction of PUMA has been reported. Bioinformatics analyses reveal potential binding sites for multiple transcription factors in the promoter, exon 1 and intron 1 regions of the PUMA gene. Several of these sites are conserved in the human and mouse genomes, such as those for p53, c-Myc and forkhead box O3A (FoxO3a). These transcription factors can activate reporters containing their respective sites in the PUMA gene, and are recruited to these sites following stresses in vivo.
Among the transcription factors that activate PUMA, the function of p53 is best understood. Within hours of DNA damage, p53 is recruited to the two p53-responsive elements in the PUMA promoter (Kaeser and Iggo, 2002; Wang et al., 2007a). Gene targeting studies have indicated that both p53 and the p53 binding sites in the PUMA promoter are indispensable for PUMA induction by DNA damage (Yu et al., 2001; Wang et al., 2007a). The binding of p53 to the PUMA promoter facilitates modifications of the core histones such as acetylation of histones H3 and H4, which leads to opening of the chromatin structure and transcriptional activation (Kaeser et al., 2004; Wang et al., 2007a). In isolated cases, the p65 or p52 subunit of nuclear factor-κB can facilitate p53-dependent PUMA induction through p53-dependent recruitment to the PUMA promoter following some forms of DNA damage (Fujioka et al., 2004; Schumm et al., 2006).
In addition to p53, a number of other transcription factors are implicated in PUMA induction. The p53 homologue p73 can regulate PUMA expression independent of p53 by binding to the same p53-responsive elements in the PUMA promoter in response to a variety of stimuli (Melino et al., 2004; Matallanas et al., 2007; Ming et al., 2008). The forkhead family member FoxO3a mediates PUMA induction by cytokine/growth factor withdrawal (You et al., 2006a). C/EBP homologous protein (CHOP) and E2F1 are involved in PUMA induction following ER stress (Futami et al., 2005; Li et al., 2006; Zou et al., 2009). The oncoproteins E2F1 and c-Myc can induce PUMA through their respective binding sites in the PUMA promoter (Fernandez et al., 2003; Hershko and Ginsberg, 2004). Moreover, general transcription factors, including C/EBPβ, CREB, c-Jun and Sp1 have been implicated in PUMA induction, some of which may do so by cooperating with p53 or p73 (Qiao et al., 2003; Hayakawa et al., 2004; Koutsodontis et al., 2005; Ming et al., 2008). On the other hand, PUMA transcription is subject to negative regulation by transcriptional repressors, including Slug (Wu et al., 2005), some alternative splice products of p73 (ΔNp73 and p73α) (Melino et al., 2004; Nyman et al., 2005) or p63 (ΔNp63) (Rocco et al., 2006) and microRNA (Choy et al., 2008).
Role in p53-dependent apoptosis induced by genotoxic stress
The tumor suppressor p53 eliminates damaged or stressed cells through induction of apoptosis (Vogelstein and Kinzler, 2004). PUMA is induced by DNA damage resulting from a variety of genotoxic agents, such as single- and double-strand breakers, inter- and intramolecular cross-linkers, nucleotide analogues and topoisomerase inhibitors, many of which are conventional chemotherapeutic drugs. The induction of PUMA by these agents is strictly p53 dependent, and is abolished in p53-deficient human cancer cells (Yu et al., 2001, 2006). p53-dependent PUMA induction also occurs in response to other classes of agents, such as neurotoxins (Gomez-Lazaro et al., 2005; Wong et al., 2005), proteasome inhibitors (Yu et al., 2003a), microtubule poisons (Giannakakou et al., 2002) and transcription inhibitors (Kalousek et al., 2007), likely owing to low levels of DNA damage elicited by these agents. PUMA is essential for p53-dependent apoptosis in a number of cell culture systems. PUMA-knockout HCT116 colon cancer cells are highly resistant to apoptosis induced by p53 overexpression or the DNA-damaging agents adriamycin, 5-fluorouracil (5-FU), cisplatin, oxaliplatin, etoposide, camptothecin, UV (ultraviolet) irradiation and γ-irradiation (Yu et al., 2003b; Chipuk et al., 2005; Ding et al., 2007; Wang et al., 2007a; Tsuruya et al., 2008). Additional examples of p53-dependent and PUMA-mediated apoptosis include neuronal cell death induced by 6-hydroxydopamine (Biswas et al., 2005), and cancer cell death induced by the proteasome inhibitors MG-132, bortezomib and epoxomicin (Concannon et al., 2007; Ding et al., 2007), and by the monoflavonoid wogonin (Lee et al., 2008).
γ-Irradiation induces p53-dependent apoptosis in vivo in mouse thymocytes and intestinal crypts (Clarke et al., 1993; Lowe et al., 1993). PUMA is induced in a p53-dependent fashion by γ-irradiation in a variety of tissues and cell compartments, including but not limited to mouse embryonic fibroblasts (MEFs), neurons, thymocytes, hematopoietic system, spleen and intestinal crypts. PUMA-knockout mice are highly protected from apoptosis induced by γ-irradiation in all of these tissues except for primary MEFs, which enter cell-cycle arrest following γ-irradiation (Jeffers et al., 2003; Villunger et al., 2003; Akhtar et al., 2006; Wyttenbach and Tolkovsky, 2006; Qiu et al., 2008). However, transformed MEFs are also susceptible to DNA-damage-induced and PUMA-dependent apoptosis (Villunger et al., 2003). These striking apoptotic deficiencies observed in PUMA-knockout mice resemble those found in p53-knockout mice. Importantly, recent studies demonstrated that p53-dependent PUMA induction is the major mechanism leading to γ-irradiation-induced apoptosis in the intestinal and hematopoietic stem/progenitor cells, and contributes significantly to the resulting tissue injury (Wu et al., 2005; Qiu et al., 2008). p53-dependent PUMA induction is also responsible for γ-irradiation-induced apoptosis in zebrafish embryos (Sidi et al., 2008). PUMA is likely to be required for p53-dependent apoptosis induced by other types of DNA damage in vivo. Among over a dozen p53 downstream targets implicated in regulating apoptosis (Yu and Zhang, 2005), only deficiency in PUMA results in such striking phenotypes, leaving no doubt that it is an essential mediator of p53-dependent apoptosis in vivo.
Under most conditions, PUMA accounts for a majority, if not all, of the proapoptotic activity of p53 in response to DNA damage. However, depending on cell types, mouse strains, apoptotic stimuli and status of other genes, another BH3-only protein, Noxa, can complement the function of PUMA in p53-dependent apoptosis (Villunger et al., 2003). Noxa has a minor function in apoptosis induced by genotoxic drugs or γ-irradiation in MEFs and thymocytes (Michalak et al., 2008). The dominant function of PUMA might be explained by differential regulation of PUMA and Noxa by p53. The induction of PUMA by genotoxic stimuli is strictly p53 dependent, whereas that of Noxa is not (Yu et al., 2001). The p53 binding sites in the PUMA promoter are among the few with the highest affinity for p53 following genotoxic stress (Kaeser and Iggo, 2002). Furthermore, PUMA was more potent than Noxa in apoptosis induction when overexpressed (Yu et al., 2001; Cregan et al., 2004). However, Noxa seems to have a prominent function in p53-dependent apoptosis induced by UV irradiation in oncogene-transformed MEFs and keratinocytes (Naik et al., 2007), and in NIH3T3 cells (Shibue et al., 2006).
In response to DNA damage, p53 also induces genes that regulate cell-cycle arrest such as the cyclindependent kinase (CDK) inhibitor p21, which is often antiapoptotic. Preferential activation of proapoptotic genes such as PUMA and/or suppression of p21 is found in cells that are poised to die (Seoane et al., 2002; Iyer et al., 2004; Sykes et al., 2006; Zhang et al., 2006; Patel et al., 2008). On the other hand, selective induction of cell-cycle regulators or suppression of PUMA occurs in cells that are resistant to DNA-damage-induced apoptosis (Wu et al., 2005; Jackson and Pereira-Smith, 2006). Moreover, radiosensitivity of several mouse tissues is correlated with the expression of PUMA, but inversely with that of p21 (Fei et al., 2002). Therefore, the choice between cell-cycle arrest and apoptosis can determine cell fate through differential regulation of p53 targets.
Functions in apoptosis induced by other stimuli
Oncogenic stress
PUMA-mediated apoptosis functions as a safeguard mechanism against neoplastic transformation. The cellular proto-oncoprotein c-Myc is a potent transcription factor that can induce p53-dependent apoptosis (Hermeking and Eick, 1994). Large-scale chromatin immunoprecipitation (ChIP) analysis identified several high-affinity c-Myc-binding E boxes in the PUMA promoter (Fernandez et al., 2003). c-Myc activates PUMA expression and facilitates PUMA-mediated apoptosis in colon cancer cells (Seoane et al., 2002). Expression of Eμ-Myc activates PUMA and induces PUMA-dependent apoptosis in lymphoma (Jeffers et al., 2003; Maclean et al., 2003). PUMA was also identified as a potential transcriptional target of the proto-oncoprotein c-Myb in human mammary cells (Rushton et al., 2003). Oncogenic E2F signaling, a common abnormality in cancer due to defects in the RB pathway, has been linked to p53 activation and apoptosis (Stanelle and Putzer, 2006). E2F1 expression activates PUMA and several other BH3-only proteins through the E2F sites in their promoters (Hershko and Ginsberg, 2004). Apoptosis induced by E2F1 is impaired in PUMA-knockout HCT116 cells (Hao et al., 2007), and in PUMA-knockdown SAOS-2 osteosarcoma cells (Hershko and Ginsberg, 2004). On the other hand, oncoproteins such as ΔNp63 and ΔNp73 can inhibit apoptosis by suppressing PUMA (Simoes-Wust et al., 2005; Rocco et al., 2006; Klanrit et al., 2008).
Several studies suggest that p53-dependent and PUMA-mediated apoptosis preferentially eliminates cells with chromosomal instability or compromised cell-cycle checkpoints, which are characteristics of transformed cells. For example, p53 overexpression or genotoxic agents normally induce cell-cycle arrest in HCT116 colon cancer cells, but trigger PUMA-dependent apoptosis in p21-deficient HCT116 cells (Yu et al., 2003b). Apoptosis was detected mostly in the polyploid or M-phase p21-deficient cells, and could be inhibited by other CDK inhibitors including p16 and p27 (Le et al., 2005). Interestingly, tetraploidy, which can lead to aneuploidy, triggered a higher rate of spontaneous apoptosis that is p53 and PUMA dependent (Castedo et al., 2006).
Growth factor/cytokine withdrawal and kinase inhibition
The expression of PUMA is kept in check in healthy cells by survival signals, including growth factors and cytokines (Han et al., 2001; Zou et al., 2009). Blocking these signals through growth factor or cytokine withdrawal leads to p53-independent transcriptional activation of PUMA by FoxO3a or p73 (Han et al., 2001; You et al., 2006a; Ming et al., 2008). The function of PUMA in p53-independent apoptosis under such conditions has been extensively studied in hematopoietic cells. Myeloid cells isolated from PUMA-knockout mice are resistant to apoptosis induced by withdrawal of interleukin (IL)-3 and IL-6 (Jeffers et al., 2003; Ekert et al., 2006). In mast cells, PUMA deficiency blocked apoptosis induced by cytokine withdrawal (Ekoff et al., 2007). PUMA knockdown also suppressed serum starvation-induced apoptosis in leukemia cells (Ming et al., 2008).
PUMA is induced by various kinase inhibitors targeting signaling pathways downstream of growth factors, conditions that can promote apoptosis in cancer cells. For example, the pan-kinase inhibitor staurosporine induces PUMA-dependent apoptosis in MEF and colon cancer cells (Villunger et al., 2003; Wang et al., 2007a). The mitogen-activated protein/extracellular signal-regulated kinase kinase (MEK) inhibitor U0126 or small-interfering RNA (siRNA) against MEK1 induces PUMA-dependent apoptosis in melanoma cells (Wang et al., 2007b). Other kinase inhibitors shown to activate PUMA in cancer cells include phosphoinositide-3 kinase inhibitors wortmannin and LY294002 (Han et al., 2001; Ming et al., 2008), the human epidermal growth factor receptor/vascular endothelial growth factor receptor inhibitor BMS-690514 (de La Motte Rouge et al., 2007) and the farnesyltransferase inhibitor BMS-214662 (Gomez-Benito et al., 2005).
ER stress
Apoptosis can occur as a result of ER stress. PUMA was identified as an ER stress-responsive gene in global gene expression profiling studies (Reimertz et al., 2003; Ward et al., 2004), and in an siRNA library screening of genes that inhibit ER stress-induced apoptosis (Futami et al., 2005). PUMA is induced by ER stress in multiple cell types (Luo et al., 2005; Nickson et al., 2007; Jiang et al., 2008). The induction of PUMA in response to ER stress is p53 independent in most cases, and mediated, in part, by the transcription factors CHOP (Li et al., 2006), E2F1 (Futami et al., 2005) and TRB3 (Zou et al., 2009). It is likely that other transcription factors also contribute to PUMA induction by ER stress.
PUMA mediates ER stress-induced apoptosis in a variety of cell types. Apoptosis induced by the ER poisons tunicamycin and thapsigargin is inhibited in PUMA-knockout HCT116 colon cancer cells (Reimertz et al., 2003; Luo et al., 2005), PUMA-knockdown cardiomyocytes (Nickson et al., 2007) and neuronal cells (Zou et al., 2009), and PUMA-knockout MEF cells (Li et al., 2006). At the same time, other BH3-only proteins such as Bim and Noxa are also critical in ER stress-induced apoptosis in other cell types (Li et al., 2006; Puthalakath et al., 2007). Deficiency in PUMA or Bim protects motor neurons from ER stress-induced apoptosis and delays motor neuron loss in amyotrophic lateral sclerosis mice (Hetz et al., 2007; Kieran et al., 2007), suggesting their overlapping functions in neurodegeneration.
Altered redox status
Stimuli leading to altered redox status, including hypoxia, anoxia, oxidative stress and generation of reactive oxygen species (ROS), can upregulate PUMA expression in vitro and in vivo. PUMA and p53 are induced by hypoxia or anoxia in transformed baby mouse kidney cells (Nelson et al., 2004), by ROS or anoxia in cardiomyocytes (Li et al., 2008), by oxidative stress in neuronal cells (Steckley et al., 2007) and by ROS in colon cancer cells (Macip et al., 2003). Phenotypically, PUMA-knockout HCT116 cells are deficient in hypoxia-induced and p53-dependent apoptosis (Yu et al., 2003b). PUMA-deficient neurons are remarkably resistant to apoptosis induced by multiple oxidative stress inducers (Steckley et al., 2007). ROS induction impacts on p53-dependent and PUMA-mediated apoptosis in colon cancer cells (Macip et al., 2003). Although ROS is generated during PUMA-mediated apoptosis as a result of mitochondrial damage (Liu et al., 2005), induction of PUMA by ROS may provide a feed-forward mechanism for signal amplification in the execution of apoptosis. Multiple transcription factors including p53 may be involved in PUMA induction by ROS.
Ischemia/reperfusion
PUMA has been implicated in apoptosis induced by ischemia/reperfusion, a primary cause of tissue injury in neurological disorders, heart attack and gastrointestinal disorders (Webster, 2006). Ischemia/reperfusion induces p53-independent PUMA expression and apoptosis in mouse small intestine (Wu et al., 2007). Similar findings have been described in cultured cardiomyocytes and an ex vivo ischemic heart model (Toth et al., 2006), and in isolated neurons (Reimertz et al., 2003; Ward et al., 2004). At least two mechanisms might be responsible for triggering PUMA-dependent apoptosis in response to ischemia/reperfusion, including ROS and ER stress (Nickson et al., 2007; Wu et al., 2007). Immune response may also significantly contribute to apoptosis induction in the later phase of tissue injury.
Immune modulation
PUMA is an important regulator of apoptosis in the immune system. It complements the function of Bim in controlling T-cell apoptosis to terminate an immune response (Bauer et al., 2006; Fischer et al., 2008). Combined losses of PUMA and Bim result in significant changes in cell numbers in various hematopoietic compartments (Erlacher et al., 2006). Apoptosis induced by glucocorticoids, which has been implicated in the regulation of the immune response, is PUMA dependent in several cell types. PUMA is necessary for glucocorticoid-induced and p53-independent apoptosis in cultured thymocytes (Jeffers et al., 2003; Villunger et al., 2003), and in cerebellar neural progenitor cells (Noguchi et al., 2008). Apoptosis induced in vivo by the glucocorticoid dexamethasone is delayed and reduced in PUMA-deficient thymocytes and mature lymphocytes (Erlacher et al., 2005, 2006). Other immune modulators such as interferons can stimulate PUMA expression through p53 or Jak1 (Takaoka et al., 2003; Gomez-Benito et al., 2007). The salmonella flagellin protein induces a classical proinflammatory gene expression profile including PUMA, which is similarly activated by the cytokine tumor necrosis factor-α (Zeng et al., 2003).
Infection
PUMA expression is also modulated in response to viral or bacterial infection. p53-dependent PUMA induction appears to be an important component of AIDS-associated pathology (Castedo et al., 2005). Upregulation of p53 and PUMA was detected in peripheral blood mononuclear cells and lymph nodes (Castedo et al., 2005), circulating CD4+ lymphocytes, dying syncytia and neurons of HIV carriers (Nardacci et al., 2005), and in HIV-1-infected primary lymphoblasts (Perfettini et al., 2004). The HIV envelope (Env) protein appears to be responsible for activating PUMA through p53 to induce PUMA-dependent apoptosis (Perfettini et al., 2004). Helicobacter pylori infection leads to p73-mediated PUMA induction in gastric epithelial cells (Wei et al., 2008). Degradation or downregulation of PUMA has been reported to inhibit apoptosis in cells infected with Chlamydia (Fischer et al., 2004; Dong et al., 2005; Ying et al., 2005) or Epstein–Barr virus (Choy et al., 2008).
Other inducers
Table 1 summarizes the major stimuli that induce PUMA-dependent apoptosis, along with the related transcription factors and model systems. In addition to these stimuli, PUMA is necessary for apoptosis induced by kinase activators such as phorbol ester (Villunger et al., 2003; Ming et al., 2008), and during skeletal myoblast differentiation (Shaltouki et al., 2007). A variety of other anticancer agents and toxins can induce PUMA expression and apoptosis in human cancer cells. Examples include chemopreventive agents celecoxib (Ishihara et al., 2007; Liu et al., 2008), genistein (Tategu et al., 2008) and green tea polyphenol (Wang et al., 2008b), arsenic trioxide (Morales et al., 2008), the BH3 mimetic Gossypol (Meng et al., 2008) and the synthetic retinoid analogue 4-hydroxybenzylretinone (Anding et al., 2007). Induction of PUMA under these conditions is mostly p53 independent, but its function remains largely undetermined.
Table 1.
Stimuli and transcription factors that induce PUMA-dependent apoptosis
| Stimuli | Transcription modulators | In vitro system | In vivo system | References |
|---|---|---|---|---|
| Genotoxic drugs | p53, p73, c-Myc, E2F1 | Cancer, neuronal and renal cells | Neuron | Wong et al., 2005; Akhtar et al., 2006; Wyttenbach and Tolkovsky, 2006; Wang et al., 2007a; Tsuruya et al., 2008 |
| Proteasome inhibitors | p53 | Cancer cells | Concannon et al., 2007; Ding et al., 2007 | |
| γ- and UV irradiation | p53 | Cancer, fibroblast and hematopoietic cells | Hematopoietic system, small intestine and neuron | Jeffers et al., 2003; Villunger et al., 2003; Chipuk et al., 2005; Wu et al., 2005; Wang et al., 2007a, b; Qiu et al., 2008; Sidi et al., 2008 |
| Oncogenes | c-Myc, Eμ-Myc, E2F1 | Cancer and hematopoietic cells | Jeffers et al., 2003; Hershko and Ginsberg, 2004; Hao et al., 2007 | |
| Polyploidy | p53 | Cancer cells | Le et al., 2005; Castedo et al., 2006 | |
| Viral infection | p53, miRNA | Cancer cells | Perfettini et al., 2004; Choy et al., 2008 | |
| Hypoxia and oxidative stress | p53 | Cancer and neuronal cells | Yu et al., 2003b; Steckley et al., 2007 | |
| ER stress | CHOP, E2F1, p53 | Cancer, fibroblast, cardiomyocyte and neuronal cells | Neuron | Reimertz et al., 2003; Futami et al., 2005; Luo et al., 2005; Li et al., 2006; Kieran et al., 2007; Nickson et al., 2007; Zou et al., 2009 |
| Kinase inhibition | p53-independent | Cancer cells | Wang et al., 2007a, b | |
| Cytokine, growth factor and nutrient deprivation | FoxO3a, p73 | Hematopoietic and cancer cells | Jeffers et al., 2003; Ekert et al., 2006; Ekoff et al., 2007; Ming et al., 2008 | |
| Ischemia/reperfusion | p53-independent | Small intestine and heart | Toth et al., 2006; Wu et al., 2007 | |
| Termination of immune response | Unknown | T cells | Bauer et al., 2006; Chang et al., 2007; Cumont et al., 2007; Fischer et al., 2008 | |
| Glucocorticoids | p53-independent | Hematopoietic and neuronal cells | Hematopoietic system | Jeffers et al., 2003; Villunger et al., 2003; Erlacher et al., 2005; Noguchi et al., 2008 |
| Homeostasis | Unknown | Hematopoietic system | Erlacher et al., 2006 |
Abbreviations: CHOP, C/EBP homologous protein; FoxO3a, forkhead box O3A.
Mechanisms of PUMA-induced apoptosis
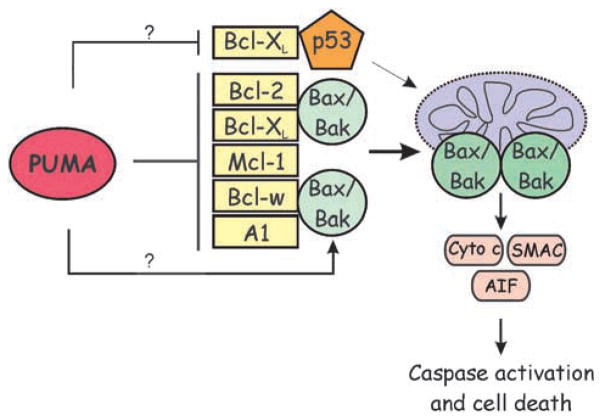
The proapoptotic activity of PUMA requires its interactions with other Bcl-2 family members and mitochondria localization (Nakano and Vousden, 2001; Yu et al., 2001). Biochemical studies indicate that PUMA induces apoptosis by activating the multidomain proapoptotic protein Bax and/or Bak through its interaction with antiapoptotic Bcl-2 family members, thereby triggering mitochondrial dysfunction and caspase activation. Some reports suggest that PUMA can also directly activate Bax/Bak or cytoplasmic p53 to induce mitochondrial dysfunction (Figure 2).
Figure 2.
A model for PUMA-mediated apoptosis. PUMA activates Bax/Bak indirectly by relieving the inhibition of all five antiapoptotic Bcl-2 family proteins to promote mitochondria dysfunction and release of the mitochondrial apoptogenic proteins cytochrome c (Cyto c), SMAC and apoptosis-inducing factor (AIF), which lead to caspase activation and cell death. Under some conditions, PUMA might directly activate Bax/Bak, or cytoplasmic p53 (by displacing it from Bcl-XL) to promote cell death through the mitochondria.
Functions through multidomain Bcl-2 family members
Similar to apoptosis induced by other BH3-only proteins, PUMA-induced apoptosis requires Bax and/or Bak. BAX-knockout HCT116 colon cancer cells are completely resistant to apoptosis induced by PUMA overexpression, or several stimuli that induce PUMA-dependent apoptosis (Yu et al., 2003b). PUMA over-expression leads to conformational change, multimerization and mitochondrial translocation of Bax (Yu et al., 2003b). In vivo PUMA-dependent apoptosis induced by γ-irradiation or ischemia/reperfusion also involves Bax multimerization and mitochondrial translocation (Wu et al., 2007; Qiu et al., 2008). These changes in Bax appear to be critical for PUMA-induced apoptosis (Ming et al., 2006). Although Bax is undoubtedly necessary, the function of Bak in PUMA-induced apoptosis cannot be ruled out (Wu et al., 2007; Qiu et al., 2008).
It is controversial whether direct interactions of BH3-only proteins with Bax/Bak are critical for their ability to induce apoptosis. In one model, a limited subgroup of BH3-only proteins is suggested to induce apoptosis by activating Bax or Bak through direct interactions (Letai et al., 2002). A hierarchical regulation was thus proposed in which these proteins, including Bid, Bim and perhaps PUMA, are necessary for Bax activation and apoptosis induced by other BH3-only proteins (Kim et al., 2006). Although an interaction between the BH3 domain of PUMA and the first α-helical region (Halpha1) of Bax has been detected in a cell-free system (Cartron et al., 2004), many studies performed using intact cells or knockout animals argue against direct activation of Bax by PUMA. No interaction between PUMA and Bax could be detected following co-transfection into cancer or immortalized cells (Ming et al., 2006). Inconsistent results were obtained from experiments where the BH3 peptides of PUMA were used to induce Bax multimerization or cytochrome c release from isolated mitochondria (Kuwana et al., 2005; Kim et al., 2006; Deng et al., 2007). Even in cells where PUMA and Bax were found to interact, the C-terminal hydrophobic domain of PUMA that mediates this interaction was not required for apoptosis (Yee and Vousden, 2008). Furthermore, Bax and Bak mutants without a discernable interaction with BH3-only proteins are fully capable in apoptosis induction (Willis et al., 2007). Apoptosis still occurs in Bim/Bid double knockout cells with knockdown of PUMA (Willis et al., 2007). Collectively, these studies strongly suggest that BH3-only proteins do not rely on direct interactions with Bax/Bak to induce apoptosis.
On the other hand, PUMA is able to bind to all antiapoptotic Bcl-2 family members with high affinity. In 911 human embryonic kidney cells, PUMA co-precipitated with a large fraction of Bcl-2 or Bcl-XL (Yu et al., 2001). The interactions between PUMA and antiapoptotic proteins are solely dependent on the BH3 domain, as deletion or mutations in the BH3 domain completely abrogated these interactions (Yu et al., 2001), and the BH3 peptide of PUMA is fully capable of binding to antiapoptotic proteins (Chen et al., 2005; Kuwana et al., 2005). Much of the current data support a model in which the interactions of PUMA with antiapoptotic proteins cause displacement of Bax and Bak, resulting in activation of the proapoptotic activities of these proteins (Figure 2). PUMA overexpression has been shown to dissociate Bax from Bcl-XL, and PUMA deficiency blocked apoptosis and dissociation of Bax/Bcl-XL complexes following DNA damage (Ming et al., 2006). In cell-free binding assays, the BH3 peptide of PUMA suppressed the inhibitory effects of antiapoptotic proteins on Bax without directly binding to Bax (Kuwana et al., 2005). Furthermore, the cell-killing activities of BH3 domains correlate remarkably well with their binding affinities for antiapoptotic proteins (van Delft et al., 2006).
The notion that PUMA is extremely potent in inducing apoptosis in multiple cell types can be explained by its ability to promiscuously interact with different antiapoptotic Bcl-2 family proteins. The PUMA BH3 peptide can bind to all five antiapoptotic Bcl-2 family members, including Bcl-2, Bcl-XL, Mcl-1, Bcl-w and A1 (Chen et al., 2005). In contrast, BH3 peptides derived from the BH3 domains of most other proapoptotic proteins, such as those from Bad and Noxa, exhibited selective binding to antiapoptotic proteins. The molecular basis of the specificities remains poorly understood. Structural studies provided some insight into how BH3 domains bind to the antiapoptotic proteins Mcl-1 and A1 (Day et al., 2008; Smits et al., 2008). These studies suggest that the conserved residues of the BH3 domains (Figure 1) are responsible for optimal binding to antiapoptotic proteins, which does not seem to explain the disparities in binding affinity among different BH3 domains. This important issue may be better addressed by combining structural information and studies using molecular approaches such as systematic mutagenesis and domain swapping.
Binding of PUMA with several other proteins besides the multidomain Bcl-2 family proteins was reported to modulate its proapoptotic activity. A p53 transcriptional target and antiapoptotic protein called apoptosis repressor with caspase recruitment domain (ARC) was recently shown to interact with PUMA, and displace PUMA from Bcl-2 (Li et al., 2008). A small chaperone protein p23 binds to PUMA in healthy cells, and prolonged ER stress disrupts this interaction and promotes PUMA-dependent apoptosis (Rao et al., 2006). The functions of these and other potential PUMA-interacting proteins remain to be investigated.
Induction of mitochondrial dysfunction
Upon binding to antiapoptotic proteins and activating Bax/Bak, PUMA-induced apoptosis proceeds through a typical mitochondrial pathway, which is characterized by mitochondrial membrane permeabilization and depolarization, release of mitochondrial apoptogenic proteins including cytochrome c, SMAC and apoptosis-inducing factor (AIF), and activation of caspases (Figure 2; Yu et al., 2003b). Production of ROS, which is indicative of mitochondrial dysfunction, is associated with PUMA-mediated apoptosis (Macip et al., 2003; Liu et al., 2005). A SMAC-regulated mitochondrial feedback mechanism was recently identified in the execution of PUMA-induced apoptosis. SMAC deficiency suppressed PUMA-induced apoptosis and the release of cytochrome c and AIF (Yu et al., 2007). It is possible that SMAC is involved in regulating caspase-mediated mitochondrial events as described in other studies (Lakhani et al., 2006).
Several recent reports suggest that PUMA acts on cytoplasmic p53 to promote mitochondrial dysfunction (Figure 2). PUMA can displace cytoplasmic p53 from Bcl-XL following UV irradiation in colon cancer cells, which leads to mitochondrial membrane permeabilization (Chipuk et al., 2005). This intriguing model is supported by studies showing that pharmacological inhibitors of cytoplasmic p53 (Strom et al., 2006), but not similar inhibitors that suppress p53-mediated PUMA induction (Steele et al., 2008), can block DNA-damage-induced apoptosis. However, it is not understood why the structurally related inhibitors used in these studies exhibited such different activities. Furthermore, FoxO3a was shown to promote p53 cytoplasmic accumulation in addition to PUMA transcription, leading to apoptosis induction (You et al., 2006b). Despite the evidence supporting a role for PUMA in disrupting p53/Bcl-XL complexes, in most systems PUMA-mediated apoptosis is not dependent on p53. PUMA induces apoptosis in numerous cancer cell lines irrespective of their p53 status (Ito et al., 2005; Yu et al., 2006; Sun et al., 2007), as well as in p53-knockout mouse lymphocytes (Callus et al., 2008). Knockout of p53 binding sites in the PUMA promoter abolishes PUMA induction by p53 and genotoxic agents, and suppresses apoptosis induced by p53 and genotoxic agents to the same extent as PUMA targeting (Wang et al., 2007a). Moreover, apoptotic events induced by cytoplasmic p53, such as Bax and Bak oligomerization and mitochondrial membrane disruption, are unaffected in PUMA-knockout cells (Wolff et al., 2008), indicating that the apoptotic function of cytoplasmic p53 is PUMA independent in most cell types.
Function of PUMA in cancer
It is increasingly apparent that apoptosis acts as a barrier against oncogenesis. Deregulated apoptosis contributes to tumor formation, progression and impaired responsiveness to anticancer therapies (Johnstone et al., 2002; Adams and Cory, 2007). Several lines of evidence suggest that the function of PUMA is compromised in cancer cells. First, more than half of human tumors contain p53 mutations (Vogelstein and Kinzler, 2004), which abrogate the induction of PUMA by irradiation and many chemotherapeutic drugs (Yu and Zhang, 2005). Second, frequent overexpression of antiapoptotic Bcl-2 family proteins and other antiapoptotic oncoproteins in tumors antagonizes PUMA-induced apoptosis (Adams and Cory, 2007). Third, PUMA expression was found to be reduced in malignant cutaneous melanoma, and PUMA expression appears to be an independent predictor of poor prognosis in patients (Karst et al., 2005). In addition, approximately 40% of primary human Burkitt’s lymphomas do not express detectable levels of PUMA, which is attributable, in part, to DNA methylation (Garrison et al., 2008). However, PUMA does not appear to be a direct target of genetic inactivation in human cancer (Hoque et al., 2003; Kim et al., 2007; Yoo et al., 2007).
Several animal studies suggest a role for PUMA in tumor suppression, despite the fact that PUMA-deficient mice do not show increased risk for spontaneous malignancies (Jeffers et al., 2003; Villunger et al., 2003). Suppression of PUMA by short-hairpin RNAs enhanced Eμ-Myc-induced lymphoma by blocking p53-dependent apoptosis (Hemann et al., 2004). Loss of PUMA in Bim-deficient mice exacerbated hyperplasia of lymphatic organs, and promoted spontaneous malignancies (Erlacher et al., 2006). In a hypoxia-induced tumor model, loss of PUMA- and Bax/Bak-dependent apoptosis contributes to chromosomal instability and enhanced tumorigenesis (Nelson et al., 2004). Whether PUMA deficiency facilitates tumorigenesis in other tumor models is currently under investigation. Nonetheless, existing data already indicate that PUMA suppresses tumorigenesis, perhaps through both p53-dependent and -independent mechanisms.
Roles and considerations in cancer therapy
Evidence of PUMA induction by therapeutic agents in patients has just begun to emerge. For example, analysis of tissue biopsies from breast cancer patients showed that PUMA mRNA was induced within 6 h of chemotherapy (Middelburg et al., 2005). Increased expression of PUMA and Bim is associated with better prognosis in patients receiving 5-FU-based therapy in stage II and III colon cancer, and is an independent prognostic marker for overall and disease-free survival (Sinicrope et al., 2008). PUMA was induced by dexamethasone in leukemia cells isolated from patients that were sensitive to the treatment, but not from those that were resistant (Xu et al., 2006).
In a series of cell culture and xenograft studies, elevated PUMA expression, either alone or in combination with chemotherapy or irradiation, induced profound toxicity to cancer cells. A variety of cancer cells have been analysed, including those from the lung (Yu et al., 2006), head and neck (Sun et al., 2007), esophagus (Wang et al., 2006), drug-resistant choriocarcinoma (Chen et al., 2007), melanoma (Karst et al., 2006), malignant glioma (Ito et al., 2005), gastric glands (Dvory-Sobol et al., 2007), breast (Wang et al., 2008a) and prostate (Giladi et al., 2007). Driving PUMA expression by promoters that are selectively active in cancer cells, such as those from hTERT (Ito et al., 2005), β-catenin/Tcf-4 (Dvory-Sobol et al., 2007; Giladi et al., 2007) and survivin (Wang et al., 2008a), yields minimal toxicity to normal tissues. It is interesting to note that PUMA adenovirus appears to be more efficacious in apoptosis induction and chemosensitization than p53 adenovirus (Wang et al., 2006; Yu et al., 2006; Sun et al., 2007), suggesting that PUMA-based gene therapy may be particularly beneficial to patients whose tumors harbor cellular or viral proteins that inactivate p53. Furthermore, BH3 mimetics, including ABT-737 and several other small molecule inhibitors of Bcl-2 family proteins, have begun to show promise as novel anticancer agents in preclinical studies (Adams and Cory, 2007; Zhang et al., 2007). A BH3 mimetic of PUMA or Bim that can bind to all antiapoptotic proteins might exhibit even better anticancer activity, especially when used in combination with standard chemotherapeutic agents, irradiation and newer generation targeted therapies such as selective kinase inhibitors.
Radiation and chemotherapy can cause severe side effects in rapidly proliferating tissues, such as bone marrow, hair follicles and the gastrointestinal tract, which limit the dose that can be used to treat patients. Preventing these therapy-induced side effects can be extremely beneficial to cancer patients. PUMA is an important mediator of γ-irradiation-induced injury in the hematopoietic and intestinal systems (Wu et al., 2005; Qiu et al., 2008). Therefore, delivery of PUMA inhibitors to these normal tissues would be expected to alleviate the side effects of chemo and radiation therapies. On the basis of data from mice, inhibiting PUMA expression should pose minimal risk for cancer development, and may be more desirable than inhibitors of p53 for manipulating adverse toxicities.
Conclusion
PUMA is induced by a wide range of apoptotic stimuli through both p53-dependent and -independent mechanisms. It is central in mitochondria-mediated cell death by interacting with all known antiapoptotic Bcl-2 family members. A better understanding of PUMA regulation and its interactions with other Bcl-2 family proteins will not only reveal missing mechanistic links in apoptotic signaling pathways that lead to mitochondrial dysfunction, but will also provide tools and insights to identify PUMA activators, mimetics or inhibitors for treating cancer, as well as diseases associated with excessive apoptosis. A major challenge in cancer therapy will be to determine the differences in the signaling pathways between cancer and normal tissues that will allow selective killing of cancer cells by manipulation of PUMA-mediated apoptosis.
Acknowledgments
We thank Dr Daniel E Johnson, Dr Crissy P Dudgeon and Mr Joshua Jamison for helpful discussion and critical reading. Work in authors’ laboratories was supported by the NIH grants CA106348, CA121105, the American Cancer Society grant RSG-07-156-01-CNE, the American Lung Association (LZ), the NIH grant CA129829, P50CA097190 (Head and Neck Cancer SPORE Career Development Award), U19-A1068021 (University of Pittsburgh Center for Medical Countermeasures Against Radiation Developmental Research Program) and those from the Hillman Foundation, Alliance for Cancer Gene Therapy (ACGT) and Flight Attendant Medical Research Institute (FAMRI) (JY).
Footnotes
Conflict of interest
L Zhang acted as a consultant/an advisor for the Gerson Lehrman Group (New York, NY, USA) in April 2009. Together, J Yu and L Zhang hold two patents and have had several invention disclosures filed by the John Hopkins University (Baltimore, MD, USA) on the applications of SAGE technology and isogenic cell lines deficient in PUMA, Bax or SMAC. Both the authors are entitled to a share of the royalties for these inventions.
References
- Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar RS, Geng Y, Klocke BJ, Latham CB, Villunger A, Michalak EM, et al. BH3-only proapoptotic Bcl-2 family members Noxa and Puma mediate neural precursor cell death. J Neurosci. 2006;26:7257–7264. doi: 10.1523/JNEUROSCI.0196-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anding AL, Chapman JS, Barnett DW, Curley RW, Jr, Clagett-Dame M. The unhydrolyzable fenretinide analogue 4-hydroxybenzylretinone induces the proapoptotic genes GADD153 (CHOP) and Bcl-2-binding component 3 (PUMA) and apoptosis that is caspase-dependent and independent of the retinoic acid receptor. Cancer Res. 2007;67:6270–6277. doi: 10.1158/0008-5472.CAN-07-0727. [DOI] [PubMed] [Google Scholar]
- Bauer A, Villunger A, Labi V, Fischer SF, Strasser A, Wagner H, et al. The NF-kappaB regulator Bcl-3 and the BH3-only proteins Bim and Puma control the death of activated T cells. Proc Natl Acad Sci USA. 2006;103:10979–10984. doi: 10.1073/pnas.0603625103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SC, Ryu E, Park C, Malagelada C, Greene LA. Puma and p53 play required roles in death evoked in a cellular model of Parkinson disease. Neurochem Res. 2005;30:839–845. doi: 10.1007/s11064-005-6877-5. [DOI] [PubMed] [Google Scholar]
- Callus BA, Ekert PG, Heraud JE, Jabbour AM, Kotevski A, Vince JE, et al. Cytoplasmic p53 is not required for PUMA-induced apoptosis. Cell Death Differ. 2008;15:213–215. doi: 10.1038/sj.cdd.4402245. author reply 215–216. [DOI] [PubMed] [Google Scholar]
- Cartron PF, Gallenne T, Bougras G, Gautier F, Manero F, Vusio P, et al. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol Cell. 2004;16:807–818. doi: 10.1016/j.molcel.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Castedo M, Coquelle A, Vivet S, Vitale I, Kauffmann A, Dessen P, et al. Apoptosis regulation in tetraploid cancer cells. EMBO J. 2006;25:2584–2595. doi: 10.1038/sj.emboj.7601127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castedo M, Perfettini JL, Piacentini M, Kroemer G. p53-A pro-apoptotic signal transducer involved in AIDS. Biochem Biophys Res Commun. 2005;331:701–706. doi: 10.1016/j.bbrc.2005.03.188. [DOI] [PubMed] [Google Scholar]
- Chang KC, Unsinger J, Davis CG, Schwulst SJ, Muenzer JT, Strasser A, et al. Multiple triggers of cell death in sepsis: death receptor and mitochondrial-mediated apoptosis. FASEB J. 2007;21:708–719. doi: 10.1096/fj.06-6805com. [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Chen Y, Qian H, Wang H, Zhang X, Fu M, Liang X, et al. Ad-PUMA sensitizes drug-resistant choriocarcinoma cells to chemotherapeutic agents. Gynecol Oncol. 2007;107:505–512. doi: 10.1016/j.ygyno.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Bouchier-Hayes L, Kuwana T, Newmeyer DD, Green DR. PUMA couples the nuclear and cytoplasmic proapoptotic function of p53. Science. 2005;309:1732–1735. doi: 10.1126/science.1114297. [DOI] [PubMed] [Google Scholar]
- Choy EY, Siu K, Kok K, Lung RW, Tsang CM, To K, et al. An Epstein–Barr virus-encoded microRNA targets PUMA to promote host cell survival. J Exp Med. 2008;205:2551–2560. doi: 10.1084/jem.20072581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper ML, et al. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993;362:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Concannon CG, Koehler BF, Reimertz C, Murphy BM, Bonner C, Thurow N, et al. Apoptosis induced by proteasome inhibition in cancer cells: predominant role of the p53/PUMA pathway. Oncogene. 2007;26:1681–1692. doi: 10.1038/sj.onc.1209974. [DOI] [PubMed] [Google Scholar]
- Cregan SP, Arbour NA, Maclaurin JG, Callaghan SM, Fortin A, Cheung EC, et al. p53 activation domain 1 is essential for PUMA upregulation and p53-mediated neuronal cell death. J Neurosci. 2004;24:10003–10012. doi: 10.1523/JNEUROSCI.2114-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumont MC, Monceaux V, Viollet L, Lay S, Parker R, Hurtrel B, et al. TGF-beta in intestinal lymphoid organs contributes to the death of armed effector CD8 T cells and is associated with the absence of virus containment in rhesus macaques infected with the simian immunodeficiency virus. Cell Death Differ. 2007;14:1747–1758. doi: 10.1038/sj.cdd.4402192. [DOI] [PubMed] [Google Scholar]
- Day CL, Smits C, Fan FC, Lee EF, Fairlie WD, Hinds MG. Structure of the BH3 domains from the p53-inducible BH3-only proteins Noxa and Puma in complex with Mcl-1. J Mol Biol. 2008;380:958–971. doi: 10.1016/j.jmb.2008.05.071. [DOI] [PubMed] [Google Scholar]
- de La Motte Rouge T, Galluzzi L, Olaussen KA, Zermati Y, Tasdemir E, Robert T, et al. A novel epidermal growth factor receptor inhibitor promotes apoptosis in non-small cell lung cancer cells resistant to erlotinib. Cancer Res. 2007;67:6253–6262. doi: 10.1158/0008-5472.CAN-07-0538. [DOI] [PubMed] [Google Scholar]
- Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Ding WX, Ni HM, Chen X, Yu J, Zhang L, Yin XM. A coordinated action of Bax, PUMA, and p53 promotes MG132-induced mitochondria activation and apoptosis in colon cancer cells. Mol Cancer Ther. 2007;6:1062–1069. doi: 10.1158/1535-7163.MCT-06-0541. [DOI] [PubMed] [Google Scholar]
- Dong F, Pirbhai M, Xiao Y, Zhong Y, Wu Y, Zhong G. Degradation of the proapoptotic proteins Bik, Puma, and Bim with Bcl-2 domain 3 homology in Chlamydia trachomatis-infected cells. Infect Immun. 2005;73:1861–1864. doi: 10.1128/IAI.73.3.1861-1864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvory-Sobol H, Sagiv E, Liberman E, Kazanov D, Arber N. Suppression of gastric cancer cell growth by targeting the beta-catenin/T-cell factor pathway. Cancer. 2007;109:188–197. doi: 10.1002/cncr.22416. [DOI] [PubMed] [Google Scholar]
- Ekert PG, Jabbour AM, Manoharan A, Heraud JE, Yu J, Pakusch M, et al. Cell death provoked by loss of interleukin-3 signaling is independent of Bad, Bim, and PI3 kinase, but depends in part on Puma. Blood. 2006;108:1461–1468. doi: 10.1182/blood-2006-03-014209. [DOI] [PubMed] [Google Scholar]
- Ekoff M, Kaufmann T, Engstrom M, Motoyama N, Villunger A, Jonsson JI, et al. The BH3-only protein Puma plays an essential role in cytokine deprivation induced apoptosis of mast cells. Blood. 2007;110:3209–3217. doi: 10.1182/blood-2007-02-073957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlacher M, Labi V, Manzl C, Bock G, Tzankov A, Hacker G, et al. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med. 2006;203:2939–2951. doi: 10.1084/jem.20061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlacher M, Michalak EM, Kelly PN, Labi V, Niederegger H, Coultas L, et al. BH3-only proteins Puma and Bim are rate-limiting for gamma-radiation- and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood. 2005;106:4131–4138. doi: 10.1182/blood-2005-04-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei P, Bernhard EJ, El-Deiry WS. Tissue-specific induction of p53 targets in vivo. Cancer Res. 2002;62:7316–7327. [PubMed] [Google Scholar]
- Fernandez PC, Frank SR, Wang L, Schroeder M, Liu S, Greene J, et al. Genomic targets of the human c-Myc protein. Genes Dev. 2003;17:1115–1129. doi: 10.1101/gad.1067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer SF, Belz GT, Strasser A. BH3-only protein Puma contributes to death of antigen-specific T cells during shutdown of an immune response to acute viral infection. Proc Natl Acad Sci USA. 2008;105:3035–3040. doi: 10.1073/pnas.0706913105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer SF, Vier J, Kirschnek S, Klos A, Hess S, Ying S, et al. Chlamydia inhibit host cell apoptosis by degradation of proapoptotic BH3-only proteins. J Exp Med. 2004;200:905–916. doi: 10.1084/jem.20040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S, Schmidt C, Sclabas GM, Li Z, Pelicano H, Peng B, et al. Stabilization of p53 is a novel mechanism for proapoptotic function of NF-kappaB. J Biol Chem. 2004;279:27549–27559. doi: 10.1074/jbc.M313435200. [DOI] [PubMed] [Google Scholar]
- Futami T, Miyagishi M, Taira K. Identification of a network involved in thapsigargin-induced apoptosis using a library of small interfering RNA expression vectors. J Biol Chem. 2005;280:826–831. doi: 10.1074/jbc.M409948200. [DOI] [PubMed] [Google Scholar]
- Garrison SP, Jeffers JR, Yang C, Nilsson JA, Hall MA, Rehg JE, et al. Selection against PUMA gene expression in Myc-driven B-cell lymphomagenesis. Mol Cell Biol. 2008;28:5391–5402. doi: 10.1128/MCB.00907-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakakou P, Nakano M, Nicolaou KC, O’Brate A, Yu J, Blagosklonny MV, et al. Enhanced microtubule-dependent trafficking and p53 nuclear accumulation by suppression of microtubule dynamics. Proc Natl Acad Sci USA. 2002;99:10855–10860. doi: 10.1073/pnas.132275599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi N, Dvory-Sobol H, Sagiv E, Kazanov D, Liberman E, Arber N. Gene therapy approach in prostate cancer cells using an active Wnt signal. Biomed Pharmacother. 2007;61:527–530. doi: 10.1016/j.biopha.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Gomez-Benito M, Balsas P, Carvajal-Vergara X, Pandiella A, Anel A, Marzo I, et al. Mechanism of apoptosis induced by IFN-alpha in human myeloma cells: role of Jak1 and Bim and potentiation by rapamycin. Cell Signal. 2007;19:844–854. doi: 10.1016/j.cellsig.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Gomez-Benito M, Marzo I, Anel A, Naval J. Farnesyltransferase inhibitor BMS-214662 induces apoptosis in myeloma cells through PUMA up-regulation, Bax and Bak activation, and Mcl-1 elimination. Mol Pharmacol. 2005;67:1991–1998. doi: 10.1124/mol.104.007021. [DOI] [PubMed] [Google Scholar]
- Gomez-Lazaro M, Galindo MF, Fernandez-Gomez FJ, Prehn JH, Jordan J. Activation of p53 and the pro-apoptotic p53 target gene PUMA during depolarization-induced apoptosis of chromaffin cells. Exp Neurol. 2005;196:96–103. doi: 10.1016/j.expneurol.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Han J, Flemington C, Houghton AB, Gu Z, Zambetti GP, Lutz RJ, et al. Expression of bbc3, a pro-apoptotic BH3-only gene, is regulated by diverse cell death and survival signals. Proc Natl Acad Sci USA. 2001;98:11318–11323. doi: 10.1073/pnas.201208798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao H, Dong Y, Bowling MT, Gomez-Gutierrez JG, Zhou HS, McMasters KM. E2F-1 induces melanoma cell apoptosis via PUMA up-regulation and Bax translocation. BMC Cancer. 2007;7:24. doi: 10.1186/1471-2407-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa J, Mittal S, Wang Y, Korkmaz KS, Adamson E, English C, et al. Identification of promoters bound by c-Jun/ATF2 during rapid large-scale gene activation following genotoxic stress. Mol Cell. 2004;16:521–535. doi: 10.1016/j.molcel.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Hemann MT, Zilfou JT, Zhao Z, Burgess DJ, Hannon GJ, Lowe SW. Suppression of tumorigenesis by the p53 target PUMA. Proc Natl Acad Sci USA. 2004;101:9333–9338. doi: 10.1073/pnas.0403286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermeking H, Eick D. Mediation of c-Myc-induced apoptosis by p53. Science. 1994;265:2091–2093. doi: 10.1126/science.8091232. [DOI] [PubMed] [Google Scholar]
- Hershko T, Ginsberg D. Up-regulation of Bcl-2 homology 3 (BH3)-only proteins by E2F1 mediates apoptosis. J Biol Chem. 2004;279:8627–8634. doi: 10.1074/jbc.M312866200. [DOI] [PubMed] [Google Scholar]
- Hetz C, Thielen P, Fisher J, Pasinelli P, Brown RH, Korsmeyer S, et al. The proapoptotic BCL-2 family member BIM mediates motoneuron loss in a model of amyotrophic lateral sclerosis. Cell Death Differ. 2007;14:1386–1389. doi: 10.1038/sj.cdd.4402166. [DOI] [PubMed] [Google Scholar]
- Hoque MO, Begum S, Sommer M, Lee T, Trink B, Ratovitski E, et al. PUMA in head and neck cancer. Cancer Lett. 2003;199:75–81. doi: 10.1016/s0304-3835(03)00344-6. [DOI] [PubMed] [Google Scholar]
- Horvitz HR. Genetic control of programmed cell death in the nematode Caenorhabditis elegans. Cancer Res. 1999;59:1701s–1706s. [PubMed] [Google Scholar]
- Ishihara T, Hoshino T, Namba T, Tanaka K, Mizushima T. Involvement of up-regulation of PUMA in non-steroidal anti-inflammatory drug-induced apoptosis. Biochem Biophys Res Commun. 2007;356:711–717. doi: 10.1016/j.bbrc.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Ito H, Kanzawa T, Miyoshi T, Hirohata S, Kyo S, Iwamaru A, et al. Therapeutic efficacy of PUMA for malignant glioma cells regardless of p53 status. Hum Gene Ther. 2005;16:685–698. doi: 10.1089/hum.2005.16.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer NG, Chin SF, Ozdag H, Daigo Y, Hu DE, Cariati M, et al. p300 regulates p53-dependent apoptosis after DNA damage in colorectal cancer cells by modulation of PUMA/p21 levels. Proc Natl Acad Sci USA. 2004;101:7386–7391. doi: 10.1073/pnas.0401002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JG, Pereira-Smith OM. p53 is preferentially recruited to the promoters of growth arrest genes p21 and GADD45 during replicative senescence of normal human fibroblasts. Cancer Res. 2006;66:8356–8360. doi: 10.1158/0008-5472.CAN-06-1752. [DOI] [PubMed] [Google Scholar]
- Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- Jiang CC, Lucas K, Avery-Kiejda KA, Wade M, deBock CE, Thorne RF, et al. Up-regulation of Mcl-1 is critical for survival of human melanoma cells upon endoplasmic reticulum stress. Cancer Res. 2008;68:6708–6717. doi: 10.1158/0008-5472.CAN-08-0349. [DOI] [PubMed] [Google Scholar]
- Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Kaeser MD, Iggo RD. Chromatin immunoprecipitation analysis fails to support the latency model for regulation of p53 DNA binding activity in vivo. Proc Natl Acad Sci USA. 2002;99:95–100. doi: 10.1073/pnas.012283399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser MD, Pebernard S, Iggo RD. Regulation of p53 stability and function in HCT116 colon cancer cells. J Biol Chem. 2004;279:7598–7605. doi: 10.1074/jbc.M311732200. [DOI] [PubMed] [Google Scholar]
- Kalousek I, Brodska B, Otevrelova P, Roselova P. Actinomycin D upregulates proapoptotic protein Puma and downregulates Bcl-2 mRNA in normal peripheral blood lymphocytes. Anticancer Drugs. 2007;18:763–772. doi: 10.1097/CAD.0b013e3280adc905. [DOI] [PubMed] [Google Scholar]
- Karst AM, Dai DL, Cheng JQ, Li G. Role of p53 up-regulated modulator of apoptosis and phosphorylated Akt in melanoma cell growth, apoptosis, and patient survival. Cancer Res. 2006;66:9221–9226. doi: 10.1158/0008-5472.CAN-05-3633. [DOI] [PubMed] [Google Scholar]
- Karst AM, Dai DL, Martinka M, Li G. PUMA expression is significantly reduced in human cutaneous melanomas. Oncogene. 2005;24:1111–1116. doi: 10.1038/sj.onc.1208374. [DOI] [PubMed] [Google Scholar]
- Kieran D, Woods I, Villunger A, Strasser A, Prehn JH. Deletion of the BH3-only protein puma protects motoneurons from ER stress-induced apoptosis and delays motoneuron loss in ALS mice. Proc Natl Acad Sci USA. 2007;104:20606–20611. doi: 10.1073/pnas.0707906105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- Kim MR, Jeong EG, Chae B, Lee JW, Soung YH, Nam SW, et al. Pro-apoptotic PUMA and anti-apoptotic phospho-BAD are highly expressed in colorectal carcinomas. Dig Dis Sci. 2007;52:2751–2756. doi: 10.1007/s10620-007-9799-z. [DOI] [PubMed] [Google Scholar]
- Klanrit P, Flinterman MB, Odell EW, Melino G, Killick R, Norris JS, et al. Specific isoforms of p73 control the induction of cell death induced by the viral proteins, E1A or apoptin. Cell Cycle. 2008;7:205–215. doi: 10.4161/cc.7.2.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsodontis G, Vasilaki E, Chou WC, Papakosta P, Kardassis D. Physical and functional interactions between members of the tumour suppressor p53 and the Sp families of transcription factors: importance for the regulation of genes involved in cell-cycle arrest and apoptosis. Biochem J. 2005;389:443–455. doi: 10.1042/BJ20041980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Lakhani SA, Masud A, Kuida K, Porter GA, Jr, Booth CJ, Mehal WZ, et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–851. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le HV, Minn AJ, Massague J. Cyclindependent kinase inhibitors uncouple cell cycle progression from mitochondrial apoptotic functions in DNA-damaged cancer cells. J Biol Chem. 2005;280:32018–32025. doi: 10.1074/jbc.M504689200. [DOI] [PubMed] [Google Scholar]
- Lee DH, Kim C, Zhang L, Lee YJ. Role of p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer cells. Biochem Pharmacol. 2008;75:2020–2033. doi: 10.1016/j.bcp.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Li J, Lee B, Lee AS. Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J Biol Chem. 2006;281:7260–7270. doi: 10.1074/jbc.M509868200. [DOI] [PubMed] [Google Scholar]
- Li YZ, Lu DY, Tan WQ, Wang JX, Li PF. p53 initiates apoptosis by transcriptionally targeting the antiapoptotic protein ARC. Mol Cell Biol. 2008;28:564–574. doi: 10.1128/MCB.00738-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HF, Hsiao PW, Chao JI. Celecoxib induces p53-PUMA pathway for apoptosis in human colorectal cancer cells. Chem Biol Interact. 2008;176:48–57. doi: 10.1016/j.cbi.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Liu Z, Lu H, Shi H, Du Y, Yu J, Gu S, et al. PUMA overexpression induces reactive oxygen species generation and proteasome-mediated stathmin degradation in colorectal cancer cells. Cancer Res. 2005;65:1647–1654. doi: 10.1158/0008-5472.CAN-04-1754. [DOI] [PubMed] [Google Scholar]
- Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Luo X, He Q, Huang Y, Sheikh MS. Transcriptional upregulation of PUMA modulates endoplasmic reticulum calcium pool depletion-induced apoptosis via Bax activation. Cell Death Differ. 2005;12:1310–1318. doi: 10.1038/sj.cdd.4401659. [DOI] [PubMed] [Google Scholar]
- Macip S, Igarashi M, Berggren P, Yu J, Lee SW, Aaronson SA. Influence of induced reactive oxygen species in p53-mediated cell fate decisions. Mol Cell Biol. 2003;23:8576–8585. doi: 10.1128/MCB.23.23.8576-8585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean KH, Keller UB, Rodriguez-Galindo C, Nilsson JA, Cleveland JL. c-Myc augments gamma irradiation-induced apoptosis by suppressing Bcl-XL. Mol Cell Biol. 2003;23:7256–7270. doi: 10.1128/MCB.23.20.7256-7270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matallanas D, Romano D, Yee K, Meissl K, Kucerova L, Piazzolla D, et al. RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol Cell. 2007;27:962–975. doi: 10.1016/j.molcel.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melino G, Bernassola F, Ranalli M, Yee K, Zong WX, Corazzari M, et al. p73 Induces apoptosis via PUMA transactivation and Bax mitochondrial translocation. J Biol Chem. 2004;279:8076–8083. doi: 10.1074/jbc.M307469200. [DOI] [PubMed] [Google Scholar]
- Meng Y, Tang W, Dai Y, Wu X, Liu M, Ji Q, et al. Natural BH3 mimetic (−)-gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of Puma and Noxa. Mol Cancer Ther. 2008;7:2192–2202. doi: 10.1158/1535-7163.MCT-08-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak EM, Villunger A, Adams JM, Strasser A. In several cell types tumour suppressor p53 induces apoptosis largely via Puma but Noxa can contribute. Cell Death Differ. 2008;15:1019–1029. doi: 10.1038/cdd.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middelburg R, de Haas RR, Dekker H, Kerkhoven RM, Pohlmann PR, Fuentes-Alburo A, et al. Induction of p53 up-regulated modulator of apoptosis messenger RNA by chemotherapeutic treatment of locally advanced breast cancer. Clin Cancer Res. 2005;11:1863–1869. doi: 10.1158/1078-0432.CCR-04-1372. [DOI] [PubMed] [Google Scholar]
- Ming L, Sakaida T, Yue W, Jha A, Zhang L, Yu J. Sp1 and p73 activate PUMA following serum starvation. Carcinogenesis. 2008;29:1878–1884. doi: 10.1093/carcin/bgn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming L, Wang P, Bank A, Yu J, Zhang L. PUMA dissociates Bax and BclX(L) to induce apoptosis in colon cancer cells. J Biol Chem. 2006;281:16034–16042. doi: 10.1074/jbc.M513587200. [DOI] [PubMed] [Google Scholar]
- Morales AA, Gutman D, Lee KP, Boise LH. BH3-only proteins Noxa, Bmf, and Bim are necessary for arsenic trioxide-induced cell death in myeloma. Blood. 2008;111:5152–5162. doi: 10.1182/blood-2007-10-116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik E, Michalak EM, Villunger A, Adams JM, Strasser A. Ultraviolet radiation triggers apoptosis of fibroblasts and skin keratinocytes mainly via the BH3-only protein Noxa. J Cell Biol. 2007;176:415–424. doi: 10.1083/jcb.200608070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Nardacci R, Antinori A, Larocca LM, Arena V, Amendola A, Perfettini JL, et al. Characterization of cell death pathways in human immunodeficiency virus-associated encephalitis. Am J Pathol. 2005;167:695–704. doi: 10.1016/S0002-9440(10)62044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DA, Tan TT, Rabson AB, Anderson D, Degenhardt K, White E. Hypoxia and defective apoptosis drive genomic instability and tumorigenesis. Genes Dev. 2004;18:2095–2107. doi: 10.1101/gad.1204904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickson P, Toth A, Erhardt P. PUMA is critical for neonatal cardiomyocyte apoptosis induced by endoplasmic reticulum stress. Cardiovasc Res. 2007;73:48–56. doi: 10.1016/j.cardiores.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi KK, Walls KC, Wozniak DF, Olney JW, Roth KA, Farber NB. Acute neonatal glucocorticoid exposure produces selective and rapid cerebellar neural progenitor cell apoptotic death. Cell Death Differ. 2008;15:1582–1592. doi: 10.1038/cdd.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyman U, Sobczak-Pluta A, Vlachos P, Perlmann T, Zhivotovsky B, Joseph B. Full-length p73alpha represses drug-induced apoptosis in small cell lung carcinoma cells. J Biol Chem. 2005;280:34159–34169. doi: 10.1074/jbc.M500394200. [DOI] [PubMed] [Google Scholar]
- Patel S, George R, Autore F, Fraternali F, Ladbury JE, Nikolova PV. Molecular interactions of ASPP1 and ASPP2 with the p53 protein family and the apoptotic promoters PUMA and Bax. Nucleic Acids Res. 2008;36:5139–5151. doi: 10.1093/nar/gkn490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfettini JL, Roumier T, Castedo M, Larochette N, Boya P, Raynal B, et al. NF-kappaB and p53 are the dominant apoptosis-inducing transcription factors elicited by the HIV-1 envelope. J Exp Med. 2004;199:629–640. doi: 10.1084/jem.20031216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Qiao L, Han SI, Fang Y, Park JS, Gupta S, Gilfor D, et al. Bile acid regulation of C/EBPbeta, CREB, and c-Jun function, via the extracellular signal-regulated kinase and c-Jun NH2-terminal kinase pathways, modulates the apoptotic response of hepatocytes. Mol Cell Biol. 2003;23:3052–3066. doi: 10.1128/MCB.23.9.3052-3066.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W, Carson-Walter EB, Liu H, Epperly M, Greenberger JS, Zambetti GP, et al. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell. 2008;2:576–583. doi: 10.1016/j.stem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RV, Niazi K, Mollahan P, Mao X, Crippen D, Poksay KS, et al. Coupling endoplasmic reticulum stress to the cell-death program: a novel HSP90-independent role for the small chaperone protein p23. Cell Death Differ. 2006;13:415–425. doi: 10.1038/sj.cdd.4401761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimertz C, Kogel D, Rami A, Chittenden T, Prehn JH. Gene expression during ER stress-induced apoptosis in neurons: induction of the BH3-only protein Bbc3/PUMA and activation of the mitochondrial apoptosis pathway. J Cell Biol. 2003;162:587–597. doi: 10.1083/jcb.200305149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco JW, Leong CO, Kuperwasser N, DeYoung MP, Ellisen LW. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell. 2006;9:45–56. doi: 10.1016/j.ccr.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Rushton JJ, Davis LM, Lei W, Mo X, Leutz A, Ness SA. Distinct changes in gene expression induced by A-Myb, B-Myb and c-Myb proteins. Oncogene. 2003;22:308–313. doi: 10.1038/sj.onc.1206131. [DOI] [PubMed] [Google Scholar]
- Schumm K, Rocha S, Caamano J, Perkins ND. Regulation of p53 tumour suppressor target gene expression by the p52 NF-kappaB subunit. EMBO J. 2006;25:4820–4832. doi: 10.1038/sj.emboj.7601343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoane J, Le HV, Massague J. Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature. 2002;419:729–734. doi: 10.1038/nature01119. [DOI] [PubMed] [Google Scholar]
- Shaltouki A, Freer M, Mei Y, Weyman CM. Increased expression of the pro-apoptotic Bcl2 family member PUMA is required for mitochondrial release of cytochrome c and the apoptosis associated with skeletal myoblast differentiation. Apoptosis. 2007;12:2143–2154. doi: 10.1007/s10495-007-0135-z. [DOI] [PubMed] [Google Scholar]
- Shibue T, Suzuki S, Okamoto H, Yoshida H, Ohba Y, Takaoka A, et al. Differential contribution of Puma and Noxa in dual regulation of p53-mediated apoptotic pathways. EMBO J. 2006;25:4952–4962. doi: 10.1038/sj.emboj.7601359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidi S, Sanda T, Kennedy RD, Hagen AT, Jette CA, Hoffmans R, et al. Chk1 suppresses a caspase-2 apoptotic response to DNA damage that bypasses p53, Bcl-2, and caspase-3. Cell. 2008;133:864–877. doi: 10.1016/j.cell.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes-Wust AP, Sigrist B, Belyanskaya L, Hopkins Donaldson S, Stahel RA, Zangemeister-Wittke U. DeltaNp73 antisense activates PUMA and induces apoptosis in neuroblastoma cells. J Neurooncol. 2005;72:29–34. doi: 10.1007/s11060-004-3118-8. [DOI] [PubMed] [Google Scholar]
- Sinicrope FA, Rego RL, Okumura K, Foster NR, O’Connell MJ, Sargent DJ, et al. Prognostic impact of bim, puma, and noxa expression in human colon carcinomas. Clin Cancer Res. 2008;14:5810–5818. doi: 10.1158/1078-0432.CCR-07-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits C, Czabotar PE, Hinds MG, Day CL. Structural plasticity underpins promiscuous binding of the prosurvival protein A1. Structure. 2008;16:818–829. doi: 10.1016/j.str.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Stanelle J, Putzer BM. E2F1-induced apoptosis: turning killers into therapeutics. Trends Mol Med. 2006;12:177–185. doi: 10.1016/j.molmed.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Steckley D, Karajgikar M, Dale LB, Fuerth B, Swan P, Drummond-Main C, et al. Puma is a dominant regulator of oxidative stress induced Bax activation and neuronal apoptosis. J Neurosci. 2007;27:12989–12999. doi: 10.1523/JNEUROSCI.3400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele AJ, Prentice AG, Hoffbrand AV, Yogashangary BC, Hart SM, Nacheva EP, et al. p53-mediated apoptosis of CLL cells: evidence for a transcription-independent mechanism. Blood. 2008;112:3827–3834. doi: 10.1182/blood-2008-05-156380. [DOI] [PubMed] [Google Scholar]
- Strom E, Sathe S, Komarov PG, Chernova OB, Pavlovska I, Shyshynova I, et al. Small-molecule inhibitor of p53 binding to mitochondria protects mice from gamma radiation. Nat Chem Biol. 2006;2:474–479. doi: 10.1038/nchembio809. [DOI] [PubMed] [Google Scholar]
- Sun Q, Sakaida T, Yue W, Gollin SM, Yu J. Chemosensitization of head and neck cancer cells by PUMA. Mol Cancer Ther. 2007;6:3180–3188. doi: 10.1158/1535-7163.MCT-07-0265. [DOI] [PubMed] [Google Scholar]
- Sykes SM, Mellert HS, Holbert MA, Li K, Marmorstein R, Lane WS, et al. Acetylation of the p53 DNA-binding domain regulates apoptosis induction. Mol Cell. 2006;24:841–851. doi: 10.1016/j.molcel.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoka A, Hayakawa S, Yanai H, Stoiber D, Negishi H, Kikuchi H, et al. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424:516–523. doi: 10.1038/nature01850. [DOI] [PubMed] [Google Scholar]
- Tategu M, Arauchi T, Tanaka R, Nakagawa H, Yoshida K. Puma is a novel target of soy isoflavone genistein but is dispensable for genistein-induced cell fate determination. Mol Nutr Food Res. 2008;52:439–446. doi: 10.1002/mnfr.200700218. [DOI] [PubMed] [Google Scholar]
- Toth A, Jeffers JR, Nickson P, Min JY, Morgan JP, Zambetti GP, et al. Targeted deletion of Puma attenuates cardiomyocyte death and improves cardiac function during ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2006;291:H52–H60. doi: 10.1152/ajpheart.01046.2005. [DOI] [PubMed] [Google Scholar]
- Tsuruya K, Yotsueda H, Ikeda H, Taniguchi M, Masutani K, Hayashida H, et al. Involvement of p53-transactivated Puma in cisplatin-induced renal tubular cell death. Life Sci. 2008;83:550–556. doi: 10.1016/j.lfs.2008.08.002. [DOI] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- Wang H, Qian H, Yu J, Zhang X, Zhang L, Fu M, et al. Administration of PUMA adenovirus increases the sensitivity of esophageal cancer cells to anticancer drugs. Cancer Biol Ther. 2006;5:380–385. doi: 10.4161/cbt.5.4.2477. [DOI] [PubMed] [Google Scholar]
- Wang P, Yu J, Zhang L. The nuclear function of p53 is required for PUMA-mediated apoptosis induced by DNA damage. Proc Natl Acad Sci USA. 2007a;104:4054–4059. doi: 10.1073/pnas.0700020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Wang X, Li B, Lin F, Dong K, Gao P, et al. Tumor-specific adenovirus-mediated PUMA gene transfer using the survivin promoter enhances radiosensitivity of breast cancer cells in vitro and in vivo. Breast Cancer Res Treat. 2008a doi: 10.1007/s10549-008-0163-6. 2008; e-pub ahead of print 13 September 2008. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang R, Hao MW, Dong K, Wei SH, Lin F, et al. The BH3-only protein PUMA is involved in green tea polyphenol-induced apoptosis in colorectal cancer cell lines. Cancer Biol Ther. 2008b;7:902–908. doi: 10.4161/cbt.7.6.5911. [DOI] [PubMed] [Google Scholar]
- Wang YF, Jiang CC, Kiejda KA, Gillespie S, Zhang XD, Hersey P. Apoptosis induction in human melanoma cells by inhibition of MEK is caspase-independent and mediated by the Bcl-2 family members PUMA, Bim, and Mcl-1. Clin Cancer Res. 2007b;13:4934–4942. doi: 10.1158/1078-0432.CCR-07-0665. [DOI] [PubMed] [Google Scholar]
- Ward MW, Kogel D, Prehn JH. Neuronal apoptosis: BH3-only proteins the real killers? J Bioenerg Biomembr. 2004;36:295–298. doi: 10.1023/B:JOBB.0000041756.23918.11. [DOI] [PubMed] [Google Scholar]
- Webster KA. Puma joins the battery of BH3-only proteins that promote death and infarction during myocardial ischemia. Am J Physiol Heart Circ Physiol. 2006;291:H20–H22. doi: 10.1152/ajpheart.00111.2006. [DOI] [PubMed] [Google Scholar]
- Wei J, O’Brien D, Vilgelm A, Piazuelo MB, Correa P, Washington MK, et al. Interaction of Helicobacter pylori with gastric epithelial cells is mediated by the p53 protein family. Gastroenterology. 2008;134:1412–1423. doi: 10.1053/j.gastro.2008.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- Wolff S, Erster S, Palacios G, Moll UM. p53’s mitochondrial translocation and MOMP action is independent of Puma and Bax and severely disrupts mitochondrial membrane integrity. Cell Res. 2008;18:733–744. doi: 10.1038/cr.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HK, Fricker M, Wyttenbach A, Villunger A, Michalak EM, Strasser A, et al. Mutually exclusive subsets of BH3-only proteins are activated by the p53 and c-Jun N-terminal kinase/c-Jun signaling pathways during cortical neuron apoptosis induced by arsenite. Mol Cell Biol. 2005;25:8732–8747. doi: 10.1128/MCB.25.19.8732-8747.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Qiu W, Wang P, Yu H, Cheng T, Zambetti GP, et al. p53 independent induction of PUMA mediates intestinal apoptosis in response to ischaemia-reperfusion. Gut. 2007;56:645–654. doi: 10.1136/gut.2006.101683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WS, Heinrichs S, Xu D, Garrison SP, Zambetti GP, Adams JM, et al. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell. 2005;123:641–653. doi: 10.1016/j.cell.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Wyttenbach A, Tolkovsky AM. The BH3-only protein Puma is both necessary and sufficient for neuronal apoptosis induced by DNA damage in sympathetic neurons. J Neurochem. 2006;96:1213–1226. doi: 10.1111/j.1471-4159.2005.03676.x. [DOI] [PubMed] [Google Scholar]
- Xu B, Wang BJ, Li AM, Lock R. Effect of glucocorticoid on the expression of Puma in acute lymphoblastic leukemia. Zhongguo Dang Dai Er Ke Za Zhi. 2006;8:151–154. [PubMed] [Google Scholar]
- Yee KS, Vousden KH. Contribution of membrane localization to the apoptotic activity of PUMA. Apoptosis. 2008;13:87–95. doi: 10.1007/s10495-007-0140-2. [DOI] [PubMed] [Google Scholar]
- Ying S, Seiffert BM, Hacker G, Fischer SF. Broad degradation of proapoptotic proteins with the conserved Bcl-2 homology domain 3 during infection with Chlamydia trachomatis. Infect Immun. 2005;73:1399–1403. doi: 10.1128/IAI.73.3.1399-1403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo NJ, Lee JW, Jeong EG, Lee SH. Immunohisto-chemical analysis of pro-apoptotic PUMA protein and mutational analysis of PUMA gene in gastric carcinomas. Dig Liver Dis. 2007;39:222–227. doi: 10.1016/j.dld.2006.11.006. [DOI] [PubMed] [Google Scholar]
- You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, et al. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006a;203:1657–1663. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H, Yamamoto K, Mak TW. Regulation of transactivation-independent proapoptotic activity of p53 by FOXO3a. Proc Natl Acad Sci USA. 2006b;103:9051–9056. doi: 10.1073/pnas.0600889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Tiwari S, Steiner P, Zhang L. Differential apoptotic response to the proteasome inhibitor Bortezomib [VELCADE, PS-341] in Bax-deficient and p21-deficient colon cancer cells. Cancer Biol Ther. 2003a;2:694–699. [PubMed] [Google Scholar]
- Yu J, Wang P, Ming L, Wood MA, Zhang L. SMAC/Diablo mediates the proapoptotic function of PUMA by regulating PUMA-induced mitochondrial events. Oncogene. 2007;26:4189–4198. doi: 10.1038/sj.onc.1210196. [DOI] [PubMed] [Google Scholar]
- Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci USA. 2003b;100:1931–1936. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Yue W, Wu B, Zhang L. PUMA sensitizes lung cancer cells to chemotherapeutic agents and irradiation. Clin Cancer Res. 2006;12:2928–2936. doi: 10.1158/1078-0432.CCR-05-2429. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang L. The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun. 2005;331:851–858. doi: 10.1016/j.bbrc.2005.03.189. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- Zeng H, Carlson AQ, Guo Y, Yu Y, Collier-Hyams LS, Madara JL, et al. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J Immunol. 2003;171:3668–3674. doi: 10.4049/jimmunol.171.7.3668. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zaugg K, Mak TW, Elledge SJ. A role for the deubiquitinating enzyme USP28 in control of the DNA-damage response. Cell. 2006;126:529–542. doi: 10.1016/j.cell.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ming L, Yu J. BH3 mimetics to improve cancer therapy; mechanisms and examples. Drug Resist Updat. 2007;10:207–217. doi: 10.1016/j.drup.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou CG, Cao XZ, Zhao YS, Gao SY, Li SD, Liu XY, et al. The molecular mechanism of endoplasmic reticulum stress-induced apoptosis in PC-12 neuronal cells: the protective effect of insulin-like growth factor-1. Endocrinology. 2008;150:277–285. doi: 10.1210/en.2008-0794. [DOI] [PubMed] [Google Scholar]