Abstract
Purpose
To assess whether a new method of quantifying therapy-associated hemodynamic alterations may help to distinguish pseudoprogression from true progression in patients with high-grade glioma.
Patients and Methods
Patients with high-grade glioma received concurrent chemoradiotherapy. Relative cerebral blood volume (rCBV) and blood flow (rCBF) maps were acquired before chemoradiotherapy and at week 3 during treatment on a prospective institutional review board–approved study. Pseudoprogression was defined as imaging changes 1 to 3 months after chemoradiotherapy that mimic tumor progression but stabilized or improved without change in treatment or for which resection revealed radiation effects only. Clinical and conventional magnetic resonance (MR) parameters, including average percent change of rCBV and CBF, were evaluated as potential predictors of pseudoprogression. Parametric response map (PRM), an innovative, voxel-by-voxel method of image analysis, was also performed.
Results
Median radiation dose was 72 Gy (range, 60 to 78 Gy). Of 27 patients, stable disease/partial response was noted in 13 patients and apparent progression was noted in 14 patients. Adjuvant temozolomide was continued in all patients. Pseudoprogression occurred in six patients. Based on PRM analysis, a significantly reduced blood volume (PRMrCBV) at week 3 was noted in patients with progressive disease as compared with those with pseudoprogression (P < .01). In contrast, change in average percent rCBV or rCBF, MR tumor volume changes, age, extent of resection, and Radiation Therapy Oncology Group recursive partitioning analysis classification did not distinguish progression from pseudoprogression.
Conclusion
PRMrCBV at week 3 during chemoradiotherapy is a potential early imaging biomarker of response that may be helpful in distinguishing pseudoprogression from true progression in patients with high-grade glioma.
INTRODUCTION
Combination temozolomide and radiation significantly prolongs survival compared with radiation alone and has become standard treatment for glioblastoma multiforme (GBM).1,2 Response assessment in GBM is difficult as a result of the frequent occurrence of early imaging changes indistinguishable from tumor progression, termed pseudoprogression (PP).3–5 The majority of patients remain clinically stable. It is often unclear whether current therapy should be maintained or second-line therapy initiated. The incidence of PP after concurrent chemoradiation (cRT) is 15% to 30%.3–7 A potential mechanism of PP is that radiation-induced vascular changes may lead to focal transient increase in gadolinium enhancement.5 To date, no prospective studies have demonstrated that imaging biomarkers can distinguish true progression from PP.
Dynamic susceptibility contrast magnetic resonance imaging (DSC-MRI) provides a noninvasive means for quantifying tumor vascular properties.8,9 Temporal changes in blood volume and blood flow suggestive of tumor progression relate to increased tumor growth, necrosis, and angiogenesis. Increased contrast enhancement noted in pseudoprogression may result from transient radiation effects on the vasculature, leading to vasodilation, edema, and increased capillary permeability.8,10
Because accurate response assessment in malignant gliomas has significant clinical implications in patient management, we wished to develop a reliable method for distinguishing true progression from PP. Given significant tumor heterogeneity after therapy, we hypothesized that a voxel-based approach may be more sensitive than mean tumor average of relative cerebral blood volume (rCBV).11 Parametric response map (PRM) is a novel, voxel-wise analytic approach to quantify significant regional changes in tumor physiology after therapy. PRM was compared with standard whole-tumor statistical methods. We report on results using PRM applied to perfusion metrics obtained from DSC-MRI to establish a reliable method of distinguishing PP from true progression.
PATIENTS AND METHODS
Patient Characteristics
Twenty-seven patients with high-grade glioma (glioblastoma multiforme, n = 23; anaplastic astrocytoma, n = 4) received concurrent cRT. All patients participated in a prospective institutional review board (IRB)–approved MRI protocol. Patient characteristics are listed in Table 1. Mean age was 52 years. Stereotactic biopsy was performed in seven patients, subtotal tumor resection was performed in nine patients, and near-gross total resection (GTR) was performed in 11 patients. Eligibility criteria required at least 4 mL of enhancing tumor. Therefore, patients with GTR were excluded from study. Several patients were also enrolled onto consecutive cRT protocols. Concurrent chemotherapy included temozolomide 75 mg/m2/d followed by adjuvant temozolomide 150 to 200 mg/m2/d for 5 days of a 28-day cycle (n = 21), gemcitabine given as a radiosensitizer at 1,000 mg/m2 weekly over 2 to 4 weeks (n = 4), or immunotherapy, intramuscular polyinosinic-polysytidylic acid stabilized with polylysine and carboxymethylcellulose 20 μg/kg three times weekly (n = 2).
Table 1.
Patient Characteristics
| Characteristic | Total | SD | PD | PP | P |
|---|---|---|---|---|---|
| Total no. of patients | 27 | 13 | 8 | 6 | |
| Age, years | 1 : 0.761 | ||||
| Mean | 51.8 | 53.6 | 51.1 | 48.8 | 2 : 0.561 |
| SEM | 3.1 | 4.1 | 6.9 | 6.8 | 3 : 0.817 |
| Pathology | 1 : 0.075 | ||||
| Grade 3 | 4 | 3 | 0 | 1 | 2 : 0.746 |
| Grade 4 | 23 | 10 | 8 | 5 | 3 : 0.180 |
| KPS | 1 : 0.590 | ||||
| < 70 | 4 | 2 | 2 | 0 | 2 : 0.202 |
| 70+ | 23 | 11 | 6 | 6 | 3 : 0.115 |
| Location | 1 : 0.380 | ||||
| Frontal/temporal | 16 | 9 | 4 | 3 | 2 : 0.423 |
| Other | 11 | 4 | 4 | 3 | 3 : 0.990 |
| RPA | |||||
| 1 | 2 | 2 | 0 | 0 | 1 : 0.689 |
| 2 | 0 | 0 | 0 | 0 | 2 : 0.305 |
| 3 | 7 | 2 | 2 | 3 | 3 : 0.606 |
| 4 | 6 | 3 | 2 | 1 | |
| 5 | 9 | 4 | 3 | 2 | |
| 6 | 3 | 2 | 1 | 0 | |
| Initial tumor volume, cm3 | 1 : 0.338 | ||||
| Mean | 36.4 | 36 | 46.9 | 23.1 | 2 : 0.230 |
| SEM | 4.6 | 6.6 | 8 | 7.8 | 3 : 0.065 |
| Surgery | |||||
| Biopsy | 7 | 0 | 5 | 2 | 1 : 0.002† |
| Subtotal | 9 | 7 | 1 | 1 | 2 : 0.045† |
| Near GTR | 11 | 6 | 2 | 3 | 3 : 0.537 |
| Radiation therapy dose, Gy | 1 : 0.009* | ||||
| Mean | 71.8 | 69.5 | 76 | 70.5 | 2 : 0.820 |
| SEM | 1.5 | 2 | 1.5 | 3.9 | 3 : 0.188 |
NOTE. Numerical identifiers refer to individual comparisons (1: SD v PD, 2: SD v PP, and 3: PP v PD).
Abbreviations: SD, stable disease; PD, progressive disease; PP, pseudoprogression; KPS, Karnofsky performance score; RPA, Recursive Partitioning Analysis; GTR, gross total resection.
Two-sided unpaired student's t test.
Likelihood ratio test.
Clinical parameters, including age, grade, Karnofsky performance status (KPS), Radiation Therapy Oncology Group (RTOG) Recursive Partitioning Analysis (RPA) classification,12 tumor volume, and extent of resection were also evaluated.
Radiation Therapy
Radiotherapy (RT) was delivered using highly conformal radiation techniques. Postoperative MRI scans were coregistered to the treatment planning computed tomography. Twelve patients received standard 60-Gy RT. For these 12 patients, the initial RT treatment volume (abnormal magnetic resonance fluid-attenuated inversion recovery signal plus a 2-cm margin) received 46 Gy. A boost volume (contrast-enhancing lesion plus a 2.5-cm margin) received a further 14 Gy. Fifteen patients with GBM were treated in an IRB-approved study of radiation dose escalation with concurrent temozolomide (75 mg/m2 daily) delivered in 30 fractions over 6 weeks with intensity-modulated radiation. For these patients, gross target volumes (GTV) were defined as the residual gross tumor or postoperative resection cavity, based on the contrast-enhanced T1-weighted images. Intensity-modulated radiation plans were generated to deliver 60 Gy to the GTV plus a 2-cm margin and a higher dose (range, 66 to 78 Gy) to the GTV plus a 5-mm margin. For all 27 patients, the median RT dose was 72 Gy with concurrent chemotherapy.
Assessment of Response
Standard MacDonald criteria were used to determine response to chemoradiation.13 The definition of PP was defined as (1) an initial assessment of progression using standard MacDonald criteria 1 to 3 months after completion of treatment, and (2) either subsequent MRIs showed stability or improvement for at least 2 months without alteration of corticosteroid dose or a change in therapy or a subsequent resection showed RT effects without evidence of viable tumor.
MRI Parameters
Research MRI scans was performed prior, week 1, and week 3 of cRT. Corticosteroid doses before scans were documented for all patients. Clinical MRI scans were obtained at 1 month post-treatment and every 2 to 3 months thereafter. MRI was performed either on a 1.5T MRI system (General Electric Medical Systems, Milwaukee, WI; n = 20) or a 3T Philips Achieva system (Philips Medical Systems, Best, the Netherlands).7
To monitor for treatment-induced alterations of tumor microvasculature, DSC-MRI was acquired using a gradient-echo T2-weighted echo-planar imaging pulse sequence (TR = 1.5 to 2 seconds, TE = 50 to 60 ms, field of view 220 × 220 mm2, matrix 128 × 128, flip angle 60°, and 4- to 6-mm thickness, 14 to 20 slices and 0 mm gap). Images were obtained before and after a bolus intravenous injection of gadolinium-diethylenetriamine pentaacetic acid using a dose of 0.05 to 0.1 mmol/kg using a power injector at a rate of 2 mL/sec. Cerebral blood volume (CBV) and cerebral blood flow (CBF) maps were generated from the subsequent DSC T2-weighted images as described previously.11 To assess changes in tumor blood volume and flow during cRT as well as between patients, CBV and CBF maps were normalized to values within white matter regions contralateral to the GTV to obtain relative CBV (rCBV) and CBF (rCBF) maps.
Image Analysis
Relative CBV and rCBF maps were coregistered to gadolinium-enhanced T1-weighted images acquired pretreatment using automated mutual information and simplex optimization module.14 After coregistration, GTV was contoured by a neuroradiologist or radiation oncologist, defined on the post contrast-enhanced T1-weighted images.
PRM is a voxel-wise imaging method of analysis applied to perfusion maps to quantify early hemodynamic alterations after treatment. PRMrCBV was determined by calculating the difference between serial rCBV maps (ΔrCBV = rCBVintratreatment – rCBVpretreatment) for each voxel within the GTV pretreatment and week 3.11 Changes in tumor volume may occur between scans, and therefore only voxels present in both volumes were analyzed. Voxels yielding ΔrCBV greater than a predetermined threshold set to 1.2 (details described below), were designated red (ie, ΔrCBV > 1.2). Blue voxels represent volumes whose rCBV decreased by more than 1.2 (ie, ΔrCBV < −1.2), and green voxels represent voxels within the tumor that were unchanged within the predetermined threshold. In summary, the volumes analyzed included increasing PRMrCBV+, decreasing PRMrCBV−, and unchanged, PRMrCBV0. Thresholds designating a significant change in rCBV within a voxel was calculated as previously described.11 Briefly, a volume of interest within the contralateral brain containing normal gray and white matter was used to acquire a range of rCBV values from registered baseline and week 1 cRT images. The resulting voxels have two serial rCBV values that have undergone no anatomic or physiological changes after treatment initiation. Variation in rCBV within these voxels represents error associated with data acquisition, processing, and alignment. To determine the degree of error, voxels of rCBV from seven randomly selected individuals were pooled and linear least squares regression analysis was performed. The 95% CI was determined from the fit of baseline and week 1 cRT rCBV values to be 1.2. A similar procedure was applied to obtain PRM rCBF maps (95% CI of 2.1). As previously reported, thresholds determined from week 1 data were found to generate consistent results when applied to the PRM analyses at week 3 after cRT.11
A standard statistical approach was used to calculate percent difference in mean rCBV over the tumor (%ΔrCBV = 100 × [rCBVintratreatment − rCBVpretreatment] × rCBVpretreatment−1). These results were compared with PRMrCBV results. A similar procedure was performed for rCBF.
Statistical Analysis
PRM and percentage change in the mean histogram for rCBV and rCBF at week 1 and 3 after cRT were determined for each clinical outcome group (stable disease [SD], progressive disease [PD], and PP). A one-sample Kolmogorov-Smirnov test was performed to ensure the assumption of normality was not violated in the entire data, for example, the P value of the Kolmogorov-Smirnov test for PRMrCBV for PP was nonsignificant (P = .664). Thus there is a lack of evidence to reject the assumption of normality.
The primary analysis of this article was performed to assess differences in response measures between groups were assessed using analysis of variance adjusted for multiple comparisons (Bonferronni posthoc test). These results were considered statistically significant at the two-sided 5% comparison-wise significance level, P < .0083. We performed a stepwise multinomial logistic regression with three outcomes: SD, PD, and PP. Four variables of interest (PRMrCBV−, PRMrCBF−, percent change in rCBV, and percent change in rCBF) were included in the stepwise procedure.
A secondary analysis was performed to assess clinical and MRI parameters, including age, tumor grade, KPS, resection versus biopsy, RT dose, pretreatment tumor volume, and RTOG RPA classification. These parameters were tested using likelihood ratio for categoric variables and t test (not adjusted for multiple comparisons) for continuous variables. All statistical computations were performed with a statistical software package (SPSS, Chicago, IL). Results were considered statistically significant at the two-sided 5% comparison-wise significance level (P < .05).
RESULTS
Between September 2003 and September 2007, 27 patients were enrolled on an IRB-approved MRI study and received concurrent chemoradiation. Post-treatment MRIs were performed 1 month after completion of chemoradiation and were analyzed for response using the Macdonald criteria. Partial response (PR) and SD was noted in 15 patients, and 12 patients were noted to be worsening. A significant clinical deterioration, defined as either KPS decreased by at least 30 points or the KPS level decreased to less than 60, was noted in five of the 12 patients. In addition, two patients met the criteria of PD based on MacDonald criteria at 3 months. Thus a total of 14 patients had progression in the first 3 months, and 13 patients did not. Adjuvant temozolomide was continued at 1 month in all patients, and MRIs were obtained clinically every 2 to 3 months.
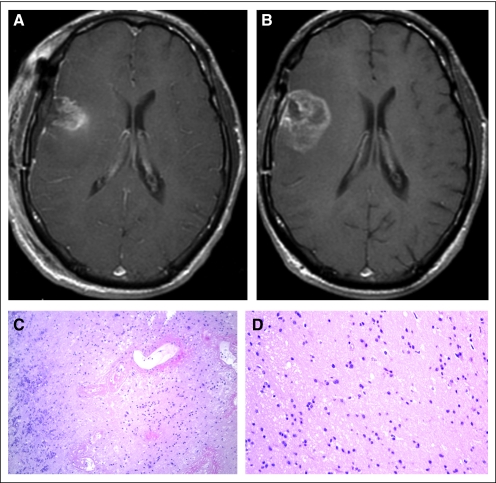
Review of clinical MRIs showed stability or improvement for at least 2 months without change in corticosteroids or treatment in three patients, and three additional patients had a subsequent resection that showed RT effects only with no tumor. A total of six of the 14 patients initially identified with radiographic progression were found to have PP, whereas PD was noted in eight patients. An example of a patient defined as having PP is shown in Figure 1. After treatment with concurrent temozolomide and 75 Gy RT, subsequent MRI showed progression based on Macdonald criteria. The patient underwent surgical resection revealed consistent with gliosis and radiation necrosis without evidence of viable tumor (Fig 1C).
Fig 1.
Representative patient with glioblastoma multiforme treated with concurrent temozolomide and radiation. T1 postgadolinium magnetic resonance imaging at (A) baseline and (B) 3 months after treatment showed a significant increase in contrast-enhancing lesion. At resection, pathology was notable for (C) fibrinoid radiation necrosis involving blood vessel wall and (D) predominantly gliotic brain parenchyma with no viable neoplasm, consistent with pseudoprogression.
We then determined whether any of the pretreatment clinical characteristics, including age, histology, KPS, tumor location, and RTOG RPA classification, were predictors of response (SD, PD, and PP). None of the clinical or conventional MRI parameters was a significant predictor of PP (Table 1).
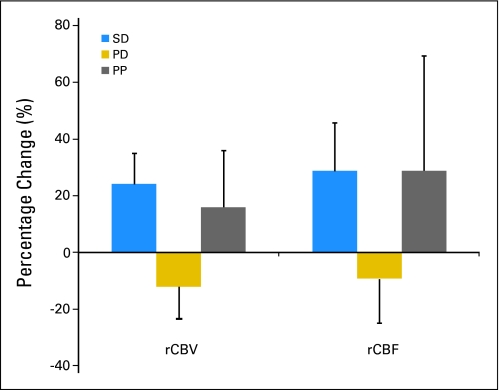
We analyzed standard imaging methods of analyzing hemodynamic alterations after chemoradiation such as percentage change in whole tumor average of rCBV as a predictor of response. No difference was noted between patients in the SD and PD groups. There was also no difference noted in patients with PP compared with those with progression. Analyses using percent change in rCBF also did not demonstrate any significant differences among the patient groups (Fig 2).
Fig 2.
Percent change of relative cerebral blood volume (rCBV) and blood flow (rCBF) 3 weeks after treatment initiation compared with pretreatment values is shown for the entire group (N = 27). Data are presented as mean ± SEM. Statistical significance was assessed at P < .0083 using an analysis of variance and Bonferonni posthoc test for multiple comparisons. SD, stable disease; PD, progressive disease; PP, pseudoprogression.
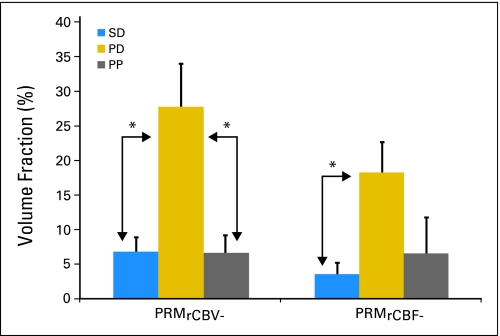
We hypothesized that PRM, a voxel-wise method of image analysis, would better predict clinical outcomes in patients with high-grade gliomas than standard imaging methods due to significant tumor heterogeneity. PRM analysis demonstrated a significant difference in PRMrCBV- in patients in the PP compared with the PD group (P = .001; Fig 3). A similar trend was observed in PRMrCBF− but was not found to be significant (P = .107).
Fig 3.
A significant difference in fractional tumor volume with decrease in relative cerebral blood volume (parametric response map [PRM]rCBV−) and relative cerebral blood flow (PRMrCBF−) week 3 chemoradiation versus baseline was noted in patients with pseudoprogression (PP) and progressive disease (PD). There was also a significant difference in PRMrCBV− between patients with stable disease (SD) and PD. Statistical significance was assessed at P < .0083.
We performed a multivariate analysis using a stepwise multinomial logistic regression. Based on Akaike's information criteria, only PRMrCBV− remained in the model as a significant predictor of outcome (P = .002; likelihood ratio test, χ2 = 12.405 on 2 df).
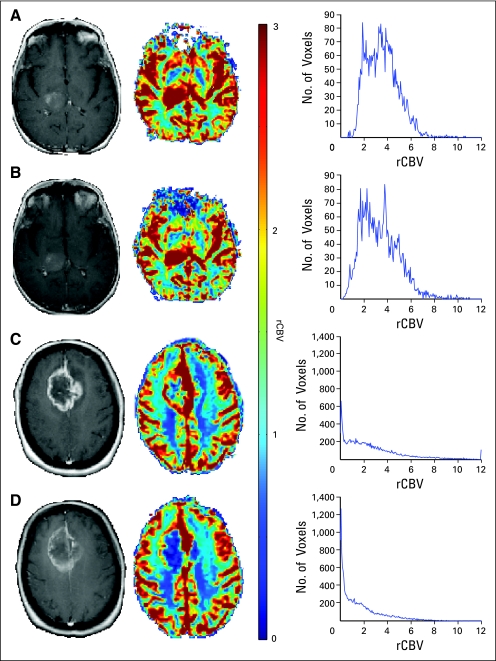
A representative patient with GBM with PP and PD analyzed using standard method analysis averaged over the entire tumor is shown in Figure 4. No changes in tumor volume were noted based on anatomic postgadolinium MRI at week 3 cRT compared with baseline. (Figs 4A through 4D) The corresponding rCBV map and histogram for the respective patients are shown (Figs 4C and 4D).
Fig 4.
Gadolinium-enhanced images (column 1), relative cerebral blood volume (rCBV) maps with color scale (column 2), and rCBV histograms (column 3) are shown in a patient with pseudoprogression (A, B) and progression (C, D) at baseline and at week 3 of chemoradiation. Additional reduction in mean rCBV was observed in the patient with progressive disease after therapy.
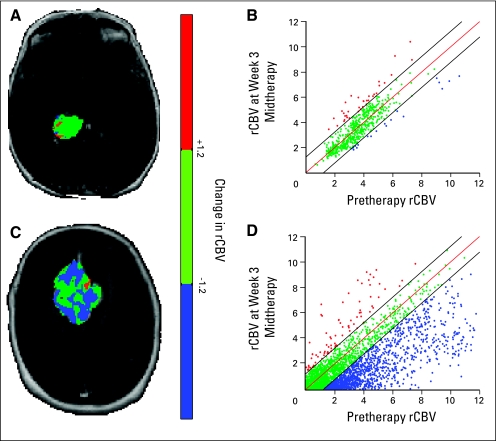
PRMrCBV color-coded overlay of the same patient with pseudoprogression (Figs 5A and 5B) is shown in contrast to a patient with PD (Figs 5A and 5B). A corresponding quantitative scatterplot shows the distribution of rCBV at baseline compared with week 3 cRT for the entire tumor volume region (Figs 5B and 5D).
Fig 5.
Parametric response map of relative cerebral blood volume (PRMrCBV) color-coded overlay of a patient with (A) pseudoprogression and (C) progressive disease. Voxels were designated red with significant increase in rCBV: blue for a significant decrease in rCBV, and green if they remained statistically unchanged. Corresponding quantitative scatter plot analysis showing the distribution of rCBV at baseline compared with week 3 chemoradiation is shown respectively (B, D).
We performed a detailed analysis of baseline mean rCBV and rCBF. There was no significant difference in baseline mean rCBV in patients with PD (3.1 ± 0.6) compared with those with PP (2 ± 0.4). There was also no difference in mean rCBV at week 3 cRT in patients with PD (2.8 ± 0.6) as compared with those with PP (2.3 ± 0.5; P < .8). These analyses using mean rCBF showed similar results.
However, a significant difference in baseline mean rCBV was noted in patients with SD (1.3 ± 0.1) compared with those with PD (3.1 ± 0.6; P = .002). Similar results were noted for mean rCBF at week 3 cRT (P = .02). Extent of resection differed between the SD and PD groups. Resection was more frequent in the SD group, whereas stereotactic biopsy was more frequent in the PD group. Pretreatment tumor volumes based on MRI were not significant. Conventional MRI no significant changes in tumor volume at week 3 cRT when compared with baseline.
DISCUSSION
Transient increases in contrast enhancement have been detected after chemoradiation (PP) that present difficulties in determining whether to continue current therapy or switch to second-line therapy.3–7 In high-grade gliomas, tumor vasculature is compromised because of rapid tumor growth and angiogenesis leading to a high density of immature and leaky vasculature in the contrast-enhancing tumor rim, whereas the tumor core is characterized by regression and low vessel density.8–10 In this study, temporal characterization of tumor vasculature and perfusion using a novel voxel-by-voxel method of analysis, PRM, was predictive of PP, whereas standard methods of analysis were not.
Substantial debate surrounds the possible causes of PP. One hypothesis is that treatment effects on the vasculature leads to transient vasodilation, increased vessel permeability, and local inflammation, with a resultant increase in contrast enhancement and edema that may mimic early tumor progression.3,4 The combination of chemotherapy and RT may increase the incidence of PP compared with that observed from RT alone, possibly due to the radiosensitizing effect of temozolomide on adjacent normal tissue.4–7
Multimodality metabolic MRI including magnetic resonance spectroscopy and positron emission tomography imaging may provide additional anatomic, biochemical, and molecular information to better assess therapeutic response and guide treatment.8,15,16 Initial studies have suggested functional MRI and positron emission tomography assessing neovascularity, tumor proliferation, and cellularity are potential imaging biomarkers of response to therapy in GBM.8,17–19 Increased enhancement has been used as a surrogate for measuring tumor progression as part of the standard MacDonald criteria in response assessment of GBM.13 It is clear that increased gadolinium enhancement due to a disrupted blood-brain barrier may be influenced by a number of factors, including acute changes immediately after surgery or RT, corticosteroids, radiation necrosis and effects to the vasculature, as well as technical issues of MRI equipment and technique of gadolinium administration.20,21 To date, no single imaging technique has been shown to adequately distinguish pseudoprogression from true progression.20,21
GBMs are heterogenous tumors. PRM analysis is a sensitive and voxel-wise analytic method to quantify regional changes in perfusion after therapy. PRMrCBV is derived for each voxel within the tumor and regions of increasing or decreasing rCBV values are measured separately. By comparison, mean changes in perfusion averaged throughout the tumor (increasing and decreasing regions cancel out) will lack sufficient sensitivity in predicting response and outcome in GBM. For this reason, PRM rCBV values may be more reliable in distinguishing PP from PD.
Several studies have now described the incidence of PP. Chamberlain et al22 reported on 51 patients treated at two different institutions who received concurrent temozolomide and RT. Twenty-six patients (51%) were noted to have tumor progression within 6 months of completion of therapy, and all patients were considered to have tumor progression. Fifteen patients underwent reresection, and seven patients were noted to have necrosis without any evidence of tumor. Median time to treatment-related necrosis was 3 months (range, 2 to 6 months). Early radiation necrosis within 6 months from completion of concomitant therapy was noted in 15% of patients. One potential explanation for the increased rate of PP was due to the increase in treatment intensity from addition of temozolomide resulting in early treatment-related radiation necrosis. Although the median radiation dose used in the present study was higher than the standard 60 Gy, our rates of radiographic progression and pathologically proven PP are similar to what was observed in the Chamberlain et al study.22
Brandes et al6 reported an incidence of PP of 31% in 208 patients treated according to the European Organisation for the Research and Treatment of Cancer/National Cancer Institute of Canada protocol. Approximately 50% of patients had sufficient tissue for 06-methylguanine-DNA methyltransferase (MGMT) analysis. A higher incidence of PP was observed in patients with methylated MGMT promoter (58%) as compared with those with the unmethylated promoter (16%). The median time to radiographic evidence of tumor progression was 2 months (range, 0.1 to 10 months) after completion of treatment, but only a third of cases also exhibited clinical deterioration. This suggests that methylated MGMT promoter is not only associated with improved overall survival, but also increased incidence of PP in GBM.6 Whether this is a reflection of clinically relevant radiosensitization in part to the increased efficacy noted or increased sensitivity to the normal tissue is unclear.
In summary, PRM applied to physiologic MRI maps is a potentially important biomarker in determining PP from PD in patients with high-grade glioma receiving concurrent chemoradiation. Preliminary findings suggest PRM analysis of perfusion MRI provides important information regarding assessment of response when standard imaging methods of analysis were not. Recent US Food and Drug Administration approval of bevacizumab in recurrent glioma and other promising new agents make it of increasing importance to know early whether a treatment is working so that patients can achieve the best responses and avoid the toxicity of ineffective agents.
Footnotes
Supported by National Institutes of Health Grants No. 3PO1CA 59827, P01CA85878, and P01CA87634.
Presented in part at the Annual Meeting of the American Society for Therapeutic Radiation and Oncology, September 21-25, 2008, Boston, MA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Alnawaz Rehemtulla, ImBio (U); Brian D. Ross, ImBio (U) Consultant or Advisory Role: None Stock Ownership: Alnawaz Rehemtulla, ImBio; Brian D. Ross, ImBio Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Christina Tsien, Craig J. Galbán, Thomas L. Chenevert, Alnawaz Rehemtulla, Brian D. Ross
Provision of study materials or patients: Christina Tsien, Larry Junck, Theodore Lawrence
Collection and assembly of data: Christina Tsien, Craig J. Galbán, Thomas L. Chenevert, Daniel A. Hamstra, Pia C. Sundgren, Charles R. Meyer, Brian D. Ross
Data analysis and interpretation: Christina Tsien, Craig J. Galbán, Thomas L. Chenevert, Timothy D. Johnson, Pia C. Sundgren, Charles R. Meyer, Brian D. Ross
Manuscript writing: Christina Tsien, Craig J. Galbán, Thomas L. Chenevert, Timothy D. Johnson, Daniel A. Hamstra, Larry Junck, Theodore Lawrence, Brian D. Ross
Final approval of manuscript: Christina Tsien, Craig J. Galbán, Thomas L. Chenevert, Timothy D. Johnson, Daniel A. Hamstra, Pia C. Sundgren, Larry Junck, Charles R. Meyer, Alnawaz Rehemtulla, Theodore Lawrence, Brian D. Ross
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.de Wit MC, de Bruin HG, Eijkenboom W, et al. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63:535–537. doi: 10.1212/01.wnl.0000133398.11870.9a. [DOI] [PubMed] [Google Scholar]
- 4.Brandsma D, Stalphers L, Taal W, et al. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 5.Taal W, Brandsma D, de Bruin H, et al. Incidence of early pseudoprogression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113:405–410. doi: 10.1002/cncr.23562. [DOI] [PubMed] [Google Scholar]
- 6.Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192–2197. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 7.Gerstner ER, McNamara MB, Norden AD, et al. Effect of adding temozolomide to radiation therapy on the incidence of pseudoprogression. J Neurooncol. 2009;94:97–101. doi: 10.1007/s11060-009-9809-4. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y, Sundgren PC, Tsien CI, et al. Physiologic and metabolic magnetic resonance imaging in gliomas. J Clin Oncol. 2006;24:1228–1235. doi: 10.1200/JCO.2005.04.7233. [DOI] [PubMed] [Google Scholar]
- 9.Cao Y, Tsien CI, Nagesh V, et al. Clinical investigation survival prediction in high-grade gliomas by MRI perfusion before and during early stage of RT. Int J Radiat Oncol Biol Phys. 2006;64:876–885. doi: 10.1016/j.ijrobp.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Jensen RL. Brain tumor hypoxia: Tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a therapeutic target. J Neurooncol. 2009;92:317–335. doi: 10.1007/s11060-009-9827-2. [DOI] [PubMed] [Google Scholar]
- 11.Galbán CJ, Chenevert TL, Meyer CR. The parametric response map: An imaging biomarker for early cancer treatment outcome. Nat Med. 2009;15:572–576. doi: 10.1038/nm.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curran WJ, Scott C, Horton J, et al. Recursive portioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 13.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 14.Meyer CR, Boes JL, Kim B, et al. Demonstration of accuracy and clinical versatility of mutual information for automatic multimodality image fusion using affine and thin-plate spline warped geometric deformations. Med Image Anal. 1997;1:195–206. doi: 10.1016/s1361-8415(97)85010-4. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Delaloye S, Silverman DH, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: A pilot study. J Clin Oncol. 2007;25:4714–4721. doi: 10.1200/JCO.2006.10.5825. [DOI] [PubMed] [Google Scholar]
- 16.Chan AA, Lau A, Pirzkall A, et al. Proton magnetic resonance spectroscopy imaging in the evaluation of patients undergoing gamma knife surgery for grade IV glioma. J Neurosurg. 2004;101:467–475. doi: 10.3171/jns.2004.101.3.0467. [DOI] [PubMed] [Google Scholar]
- 17.Law M, Young RJ, Babb JS, et al. Gliomas: Predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2008;247:490–498. doi: 10.1148/radiol.2472070898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aronen HJ, Pardo FS, Kennedy DN, et al. High microvascular blood volume is associated with high glucose uptake and tumor angiogenesis in human gliomas. Clin Cancer Res. 2000;6:2189–2200. [PubMed] [Google Scholar]
- 19.Provenzale JM, Wang GR, Brenner T, et al. Comparison of permeability in high-grade and low-grade brain tumors using dynamic susceptibility contrast MR imaging. AJR Am J Roentgenol. 2002;178:711–716. doi: 10.2214/ajr.178.3.1780711. [DOI] [PubMed] [Google Scholar]
- 20.Clarke JL, Chang S. Pseudoprogression and pseudoresponse: Challenges in brain tumor imaging. Curr Neurol Neurosci Rep. 2009;9:241–246. doi: 10.1007/s11910-009-0035-4. [DOI] [PubMed] [Google Scholar]
- 21.Sorensen AG, Batchelor T, Wen Patrick Y. Response criteria for glioma. Nat Clin Pract Oncol. 2008;5:634–644. doi: 10.1038/ncponc1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chamberlain M, Glantz M, Chalmers L, et al. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82:81–83. doi: 10.1007/s11060-006-9241-y. [DOI] [PubMed] [Google Scholar]