Abstract
The peripheral vestibular system is faced by a sensory ambiguity, where primary otolith afferents respond identically to translational (inertial) accelerations and changes in head orientation relative to gravity. Under certain conditions, this sensory ambiguity can be resolved using extra-otolith cues, including semicircular canal signals. Here we review and summarize how neurons in the vestibular nuclei, rostral fastigial nuclei, cerebellar nodulus/uvula, and thalamus respond during combinations of tilt and translation. We focus primarily on cerebellar cortex responses, as nodulus/uvula Purkinje cells reliably encode translation rather than net gravito-inertial acceleration. In contrast, neurons in the vestibular and rostral fastigial nuclei, as well as the ventral lateral and ventral posterior nuclei of the thalamus represent a continuum, with some encoding translation and some net gravito-inertial acceleration. This review also outlines how Purkinje cells use semicircular canal signals to solve the ambiguity problem and how this solution fails at low frequencies. We conclude by attempting to bridge the gap between the proposed roles of nodulus/uvula in tilt/translation discrimination and velocity storage.
Keywords: cerebellum, vestibular, Purkinje cell, vermis, linear acceleration, rotation, translation, velocity storage
Introduction
Like other sensory systems, the peripheral otolith sensors face a serious ambiguity: primary otolith afferents respond identically to an inertial motion stimulus (e.g., during lateral or forward translation) and a change in head orientation relative to gravity (e.g., roll or pitch tilt).1-3 At least under certain conditions (see below), this ambiguity can be resolved in the brain using extra-otolith signals that arise from either the semicircular canals4,5 or the visual system.6,7 Computational models have been proposed to explain how an angular position estimate arising from the semicircular canals can be used to estimate head orientation relative to gravity. The latter can then be subtracted from net gravito-inertial acceleration, the signal coded by primary otolith afferents, to extract translational motion information.4,8-16 Here we summarize how different subcortical areas might relate to this sensory ambiguity and its resolution.
Primary vestibular afferents project primarily to the vestibular nuclei and the cerebellar nodulus (vermal lobule X) and ventral uvula (vermal lobules IXc,d; collectively referred to here as NU).17-22 What is the role of the NU in vestibular processing? Lesions of the NU primarily affect velocity-storage properties: NU lesions change the time constant of the vestibulo-ocular reflex (VOR) and destroy the ability of velocity storage to align with gravity and to generate a bias velocity during offvertical axis rotations.23-27 Similarly, electrical stimulation of the nodulus shortens the VOR time constant, whereas electrical stimulation of the uvula induces nystagmus without changing the VOR time constant.28 Yet, since the functional significance of velocity storage remains obscure, these lesion/stimulation experiments do not by themselves shed light on the functional role of the NU for everyday spatial orientation.
Here we summarize the response properties of simple spike responses from NU Purkinje cells.29,30 We compare these properties with those in the vestibular nuclei (VN), rostral fastigial nuclei (FN), and thalamus (TH).1,31-34 We show that, among these areas, only the NU exhibits population responses with all neurons selectively responding to inertial motion (translation), while ignoring head re-orientations relative to gravity.29 In contrast, VN, FN, and TH neurons represent a mixed continuum, with some cells selectively encoding translation and some net gravito-inertial acceleration.1,31,33,34 We also outline how this tilt/translation ambiguity is solved by examining the characteristics of the canal signal used by Purkinje cells to cancel gravitational responses; such canal signals must be and indeed are spatially transformed and temporally integrated such that they encode head attitude relative to gravity.1,31,33 However, this ability of NU neurons to segregate translation from tilt is only limited to frequencies above 0.1 Hz; at lower frequencies, the otolith/canal matching necessary to cancel gravitational responses is no longer accurate, thus resulting in perceptual misinterpretation of all linear accelerations as tilt.35-37 We conclude by explaining how velocity storage can be identified as the temporal integrator needed to integrate spatially transformed canal signals into head altitude (orientation) in space.
Responses of NU Purkinje Cells during Combinations of Tilt and Translation
Whether single neurons respond selectively to translation or net gravito-inertial acceleration can be evaluated using a four-component experimental protocol consisting of combinations of 0.5 Hz sinusoidal tilt and translation stimuli.1,4 What is critical in these stimuli is that the peak amplitude of the sinusoidal tilt is adjusted to produce a 0.5 Hz horizontal plane gravitational acceleration component that is the same (0.2 G) as that during translation. The tilt and translation motions are then delivered either in isolation (Fig. 1A, B) or together (Fig. 1C, D). The relative phase of the two sinusoidal movements for combination stimuli can differ. Whenever a head tilt to the right occurs simultaneously with translation to the left, gravitational and inertial accelerations are oppositely directed and, since they are appropriately matched in amplitude, cancel out, and neither component is transduced to the brain (Fig. 1C; Tilt − Translation). In contrast, whenever a head tilt to the right occurs simultaneously with translation to the right, the two accelerations sum, resulting in net linear acceleration that is double that for each movement alone (Fig. 1D; “Tilt + Translation”).
Figure 1.
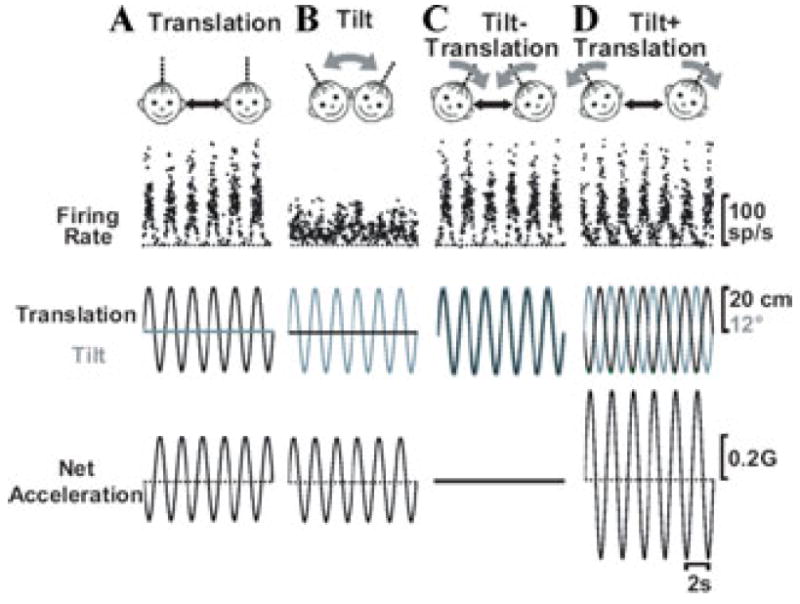
Instantaneous firing rate of a typical Purkinje cell during (A) Translation, (B) Tilt, (C) Tilt − Translation, and (D) Tilt + Translation (0.5 Hz). The translation/tilt stimuli were matched in amplitude to elicit an identical net acceleration in the horizontal plane. Straight black and curved gray arrows denote translation and tilt axes of stimulation, respectively.
Responses from a typical NU Purkinje cell are shown on the top traces of Figure 1. The stimuli (translation and tilt position) are shown in the middle traces. In addition, to facilitate interpretation of cell responses during tilt, translation, and their combinations, net linear acceleration (the stimulus encoded by otolith afferents)1,38,39 has also been plotted in the bottom traces of Figure 1. In contrast to otolith afferents, NU Purkinje cell responses are large during translation but small during tilt, despite identical net linear acceleration stimuli. Similarly, responses during Tilt + Translation and Tilt − Translation are similar to those during Translation (Fig. 1C, D versus Fig. 1A). Thus, during 0.5 Hz motion, NU Purkinje cells selectively encode translation, but ignore changes in orientation of the head relative to gravity.
These responses have been quantified using a partial correlation analysis in which the responses of each cell to all four stimuli (Translation, Tilt, Tilt − Translation, and Tilt + Translation) are simultaneously fitted with “afferent-like” and ”translation-coding” models.1,31 When the variances of these partial correlation coefficients are normalized (using Fisher’s r-to-z transform), comparison between the two models can be simple and visually convenient: data points falling on the upper-left quadrant (defined by the dashed lines corresponding to the 0.01 level of significance) illustrate cells that were significantly better fit with the translation-coding model. In contrast, data points falling on the lower-right quadrant correspond to cells that were significantly better fit with the afferent-like model. Indeed, data from a few otolith afferents fall, as expected, in the lower-right quadrant (Fig. 2A, open triangles; data from Angelaki et al.1). In contrast, data from all NU Purkinje cells fell in the upper-left quadrant (Fig. 2A, black circles; data from Yakusheva et al.29).
Figure 2.
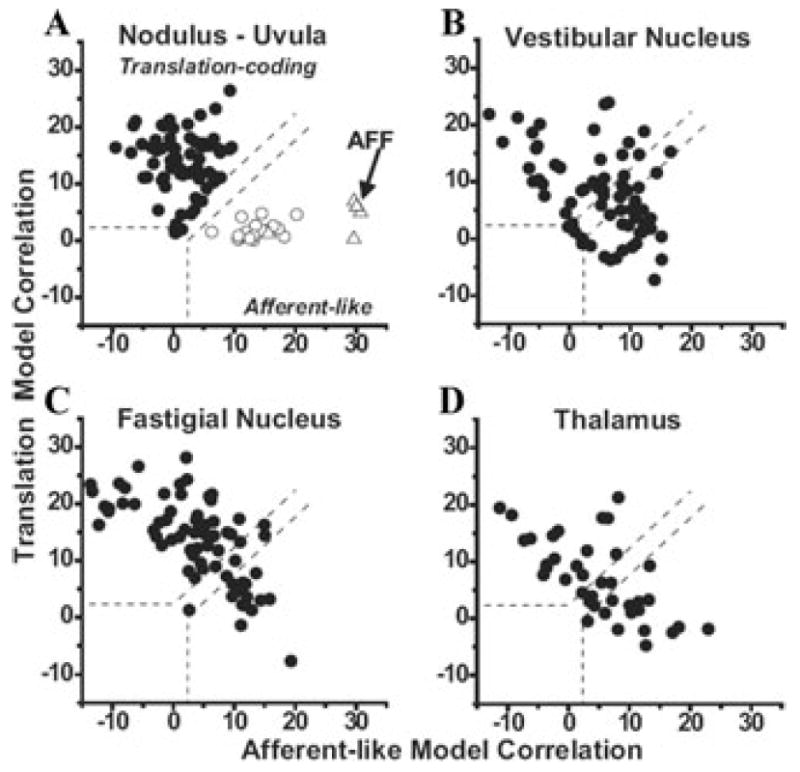
Scatter plots of partial correlation coefficients for fits of each cell responses with “translation-coding” and “afferent-like” models. (A) Data from NU Purkinje cells in labyrinthine-intact animals (filled circles) are compared with those in canal-plugged animals (open circles; both are replotted with permission from Yakusheva et al.29) and primary otolith afferents (AFF, open triangles; replotted with permission from Angelaki et al.1). (B) Data from vestibular nuclei neurons (replotted with permission from Angelaki et al.1). (C) Data from rostral fastigial nuclei neurons (replotted with permission from Angelaki et al.1). (D) Data from the ventral lateral and ventral posterior lateral nuclei of the thalamus (replotted with permission from Meng et al.34, © Journal of Neuroscience). The superimposed dashed lines divide the plots into three regions: an upper/left area corresponding to cell responses that were significantly better fit (P < 0.01) by the translation-coding model; a lower/right area that includes neurons that were significantly better fit by the afferent-like model; and an in-between area that would include cells that were not significantly better fit by either model. Data shown for the cell’s best-responding translation direction.
Clearly, as shown with a representative example in Figure 1, NU Purkinje cells are significantly better fit with the translation-coding as compared with the afferent-like model. To verify that the ability to discriminate translation from tilt depends on signals arising from the semicircular canals, this experiment was repeated after all six semicircular canals were inactivated by plugging the canal lumen.29,33 After canal plugging, NU Purkinje cell data fell in the lower-right quadrant (illustrating significantly better fit with the afferent-like model; Fig. 2A, open circles; data from Yakusheva et al.29). These data clearly demonstrate that when canal signals are no longer available to estimate the component of linear acceleration due to changes in head orientation relative to gravity, NU Purkinje cells respond similarly to tilt and translation.
Responses of Vestibular Nuclei, Rostral Fastigial Nuclei, and Thalamus Neurons during Combinations of Tilt and Translation
NU Purkinje cells project to the vestibular nuclei and rostral fastigial nuclei.40-42 From there areas vestibular information can be relayed to the oculomotor nuclei, the spinal cord, and the thalamus. Figure 2B, C, and D summarize how neurons in these areas respond to combinations of tilt and translation. Notably, unlike the selective coding of translation by all NU Purkinje cells, a mixture of response properties is found in the main projection nuclei. In the rostral fastigial nucleus most cells selectively encode translation, although in the vestibular nuclei and thalamus approximately equal proportions of neurons have responses that fit better with either the translation-coding or afferent-like models.1,34 Although preliminary, similar recordings in cortical areas MSTd (dorsal medical superior temporal area) and PIVC (parieto-insular vestibular cortex) show that neurons in these areas are more similar to those in the NU and FN. Future studies need to investigate the flow of information processing, as well as the targets of VN, FN, and TH neurons that have translation-coding versus net acceleration, afferent-like responses.
Properties of Canal Contribution to NU Purkinje Cell Responses: How Is the Tilt/Translation Ambiguity Problem Solved?
The fact that semicircular canal signals are critical for the ability of central neurons and behavior to solve the tilt/translation ambiguity problem has now been verified by numerous behavioral and neurophysiological studies. Merfeld and colleagues5,43,44 showed the contribution of canal cues in generating a neural estimate of translation by exploring “erroneous” behavioral responses (eye movements and perception) at low frequencies. Parallel studies by Angelaki and colleagues4,8 showed that there is an extra-otolith contribution to the compensatory eye movements during mid-to-high translation (> 0.1 Hz).
But how is this ambiguity resolved? Specifically, how is semicircular canal information used to create a central estimate of gravity, which can then be subtracted from net gravito-inertial acceleration? Mathematical considerations of this computational problem have proposed that, for canal signals to be useful for this purpose, they must be processed relative to canal afferent information in two important aspects.29,31,45 First, they should contribute to the resolution of this ambiguity only during earth-horizontal, but not during earth-vertical axis rotation (otherwise any head rotation, e.g., yaw from upright, would be interpreted as a change in head orientation relative to gravity). Second, they should also be temporally integrated, thus reflecting a tilt position (i.e., head attitude), rather than an angular velocity, signal. The properties of the canal-driven component can be isolated during the Tilt − Translation stimulus (Fig. 1C), as it is only during this motion that the dynamic acceleration stimulus to the otolith receptors is zero and any modulation arises exclusively from the semicircular canal signals. Indeed, using Tilt − Translation responses, both of these predictions were verified by examining the properties of the canal-driven component in NU Purkinje cells.29 The fact that the signal canceling gravitational responses arise from temporal integration of angular velocity from the semicircular canals has also been previously shown based on reflexive eye movements.8
It is important to appreciate that NU Purkinje cells only modulate during canal activation that involves rotations that change head orientation relative to gravity; such as during pitch and roll in upright orientation, but not during pitch or roll in ear-down, supine orientation.29 Thus, Purkinje cells selectively encode only the earth-horizontal rotational component, ωEH (in contrast to canal afferents, which encode the total angular velocity, ω = ωEH + ωEV; that is, both earth-horizontal and earth-vertical axis rotations). In addition, this canal-driven, spatially transformed signal has been temporally integrated, thus coding head position relative to gravity (∫ ωEH), rather than rotational velocity as do semicircular canal afferents.1,46 This transformation ensures that: (1) a canal-driven gravitational estimate is only created during rotations that change head orientation relative to gravity, and (2) this estimate is a head-orientation signal, that is, a signal that is temporally appropriate to cancel gravitational acceleration. (Remember that, during small tilts relative to gravity, the horizontal gravitational acceleration stimulus activating otolith afferents is proportional to tilt angle.) Such canal-driven estimate of head attitude could then be subtracted from net linear acceleration provided by the otoliths and used to estimate inertial motion during navigation. Importantly, for this subtraction to work, the canal-driven signal unmasked with the Tilt − Translation stimulus is both temporally and spatially matched on a cell-by-cell basis with the cell’s response during translation (Fig. 3A–C).29 Notice that this matching is remarkable, given the large cell-to-cell variability of translation response gain, phase, and preferred direction in central vestibular neurons.29,30,32,47-50 This “matched” convergence is a necessary condition for a computational solution to the tilt/translation ambiguity problem.
Figure 3.
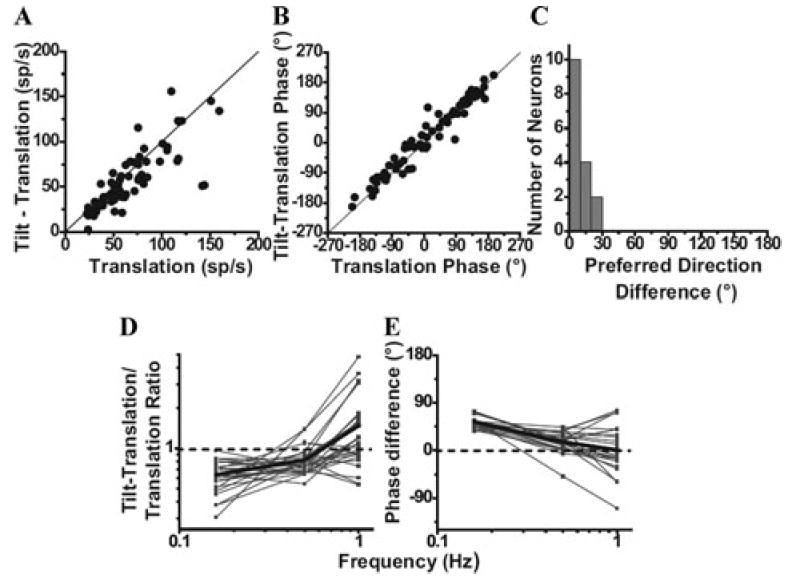
Spatio-temporal matching of canal-driven and otolith-driven signal contributions to NU Purkinje cell firing. (A), (B) Response amplitude and phase during the 0.5 Hz Tilt −Translation stimulus (canal-driven response) is plotted as a function of the respective amplitude and phase during Translation (otolith-driven response). (C) Distribution of the difference in preferred directions between the 0.5 Hz Tilt − “Translation and Translation responses. Data in A–C were replotted with permission from Yakusheva et al.29 (D), (E) Dependence of the relationship between otolith and canal-driven responses on frequency. (D) plots the ratio of peak response modulation during Tilt − Translation (canal-driven response) relative to that during Translation (otolith-driven response) as a function of frequency. (E) plots the phase difference between the response modulation during Tilt − Translation and Translation as a function of frequency. Thin lines and symbols illustrate data from single neurons, whereas thick lines indicate population averages. Data in D–E were replotted with permission from Yakusheva et al.,30 © Journal of Neuroscience.
Failure to Cancel Otolith-Driven Gravitational Responses at Low Frequencies
Notably, the tilt/translation ambiguity is not always correctly resolved; low-frequency linear accelerations in the absence of other, extra-vestibular cues are incorrectly interpreted as tilt even when generated by translational motion. This is because the semicircular canals do not provide a veridical estimate of angular velocity at low frequencies (<≈0.1 Hz) or when the head is statically tilted. Thus, the ability to discriminate between tilt and translation based solely on vestibular cues (e.g., during passive motion in darkness) deteriorates at low frequencies.9,10,16,51,52 In fact, it is typically at these low frequencies that perceptual illusions occur (“somatogravic/oculogravic illusion”).35-37 It is also during these low frequencies that extra-vestibular information (e.g., visual signals) is necessary to avoid these illusions. For example, visual cues can significantly influence our percept of head orientation relative to gravity.53,54 In addition, visual rotational cues contribute to estimation of inertial motion in an apparently similar fashion to canal-derived sensory signals.6,7
How NU Purkinje cells respond during low-frequency tilt has been recently investigated in macaques.30 Similar to previous observations in anesthetized rabbits55,56 and unlike the small response modulation during 0.5 Hz roll and pitch tilt (see Fig. 1B and Ref. 29), tilt modulation amplitude of NU Purkinje cells increases with decreasing frequency.30 Thus, as predicted, semicircular canal signals are no longer appropriate to cancel gravitational acceleration at low frequencies. The departure from ideal spatio-temoral matching is illustrated in Figure 3D, which plots the ratio of Tilt − Translation (canal-driven response) gain versus Translation (otolith-driven response) gain, and Figure 3E, which plots the corresponding phase difference. Ideal matching requires the ratio to be 1 and the phase difference to be 0 (dashed lines in Fig. 3D, E). However, because the dynamics of the otolith-driven and canal-driven response components are not temporally matched across all frequencies, both of these measures (ratio of Tilt − Translation versus Translation gain and corresponding phase difference) are significantly dependent on frequency.30 At 0.16 Hz, for example, canal-driven responses are smaller than otolith-driven responses (ratio less than 1, Fig. 3D) and lead by ~54° (Fig. 3E). Thus, simple spike responses from NU Purkinje cells might represent the neural substrate for both the resolution of the tilt/translation ambiguity problem and its failure at low frequencies.
Relationship of the Tilt/Translation Integrator and Velocity Storage
As summarized in the Introduction, both lesion and electrical stimulation studies have coupled the cerebellar nodulus and ventral uvula with velocity storage.25-28 However, there is absolutely no modulation of simple spike NU Purkinje cell during yaw rotations.29,30,56-59 At first glance, this complete lack of yaw modulation might thus appear at odds with the fact that NU lesions result in dramatic changes in the horizontal angular VOR (AVOR) time constant (velocity storage) during constant velocity rotations and destroy the capacity of velocity storage to align with gravity when subjects are suddenly tilted post-rotation.23,25
This seemingly bizarre finding can be easily understood if one considers two important findings. First, NU Purkinje cells only encode the earth-horizontal component of angular velocity, ωEH (see sections above). Second, NU Purkinje cells inhibit VOR pathways, through projections to NU-target neurons in the VN (Fig. 4). As a result, whenever an earth-horizontal rotation signal (ωEH, encoded by output of the NU) is subtracted from the net canal activation, what remains is the earth-vertical component of angular velocity (ωEV = ω − ωEH). Importantly, as Purkinje cells only modulate during low-frequency tilt, NU-target neurons in the VN (and thus AVOR pathways) should code for ω (the total canal activation) at high frequencies and ωEV at low frequencies. Indeed, only the low-frequency components of the AVOR have ωEV-like properties.60 Importantly, because the canal-driven responses in the NU are temporally integrated, AVOR (and likely NU-target neurons in the VN) encode earth-vertical canal signals with a longer time constant than canal afferents.30 Indeed, many VN neurons exhibit longer time constants, attributed to velocity storage.61 Taken together, in agreement with experimental findings, this framework predicts that NU lesions would destroy the computation of both ωEH and ωEV. Moreover, as this computation involves a temporal integration, NU lesions should also alter the low-frequency dynamics of the AVOR.
Figure 4.
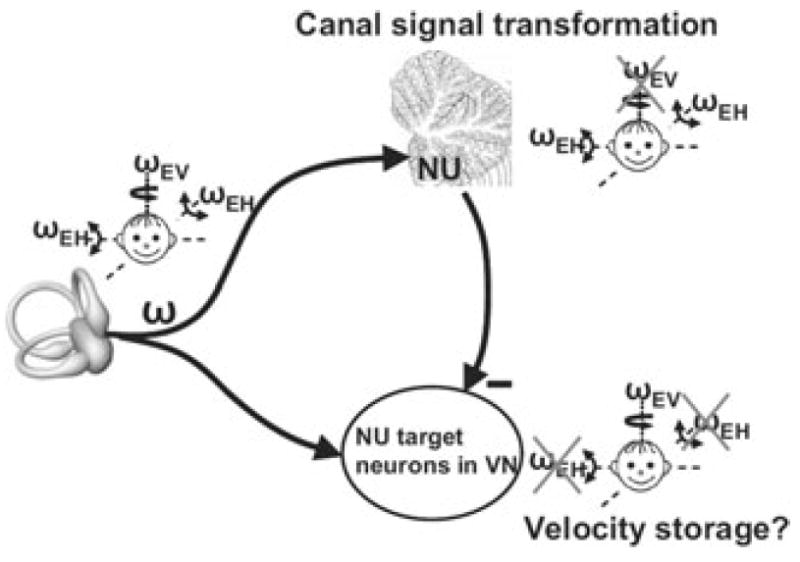
Schematic illustrating a hypothesis regarding the relationship between velocity storage and tilt/translation discrimination. Semicircular canal afferents carry head-referenced angular velocity (ω), but the NU encodes only the earth-horizontal component (ωEH). Thus, because of Purkinje cell inhibition, NU-target neurons in the VN should carry the earth-vertical component (ωEV = ω − ωEH). Importantly, because the NU canal-driven responses are temporally integrated, VN responses are predicted to encode earth-vertical canal signals with a longer time constant than canal afferents. Replotted with permission from Yakusheva et al.30 © Journal of Neuroscience.
Such hypothesized link between the tilt/translation integrator and velocity storage, pioneered by Green and Angelaki,8,45 gives a different functional meaning to velocity storage than previously assumed. The Green and Angelaki hypothesis suggests that the functional role of the velocity storage integrator is to temporally integrate canal-borne angular velocity signals, ωEH. Thus, typical of a true integrator of velocity, its function is to generate a dynamic estimate of head orientation relative to gravity, whereas the lengthening of the AVOR time constant might be simply an indirect inlfuence.8,45 Such proposed coupling between tilt/translation discrimination for the translational VOR (TVOR) and velocity storage for the AVOR is supported by parallel TVOR/AVOR differences between humans and monkeys. In particular, the Green and Angelaki hypothesis predicts that the more the TVOR compensates for translational (as opposed to net gravito-inertial) acceleration, the larger the contribution of this network to eye movements, and thus the stronger the gravity-dependent properties of velocity storage in the AVOR. Indeed, not only does monkey TVOR discriminate translation from tilt,4, but monkey velocity storage has a strong dependence on gravity.24,62-64 In contrast, human TVOR is not directly proportional to translation,9,10 and human responses show little or no shift in the axis of rotation after postrotatory tilt.44,65
Acknowledgments
The work was supported by NIH R01 EY12814.
Footnotes
Conference: Basic and Clinical Aspects of Vertigo and Dizziness (T. Brandt’s retirement), Kloster Seeon, Germany.
Conflicts of Interest The authors declare no conflicts of interest.
References
- 1.Angelaki DE, et al. Neurons compute internal models of the physical laws of motion. Nature. 2004;430:560–564. [Google Scholar]
- 2.Dickman JD, Angelaki DE, Correia MJ. Response properties of gerbil otolith afferents to small angle pitch and roll tilts. Brain Res. 1991;556:303–310. doi: 10.1016/0006-8993(91)90320-u. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez C, Goldberg JM, Abend WK. Response to static tilts of peripheral neurons innervating otolith organs of the squirrel monkey. J Neurophysiol. 1972;35:978–987. doi: 10.1152/jn.1972.35.6.978. [DOI] [PubMed] [Google Scholar]
- 4.Angelaki DE, et al. Computation of inertial motion: neural strategies to resolve ambiguous otolith information. J Neurosci. 1999;19:316–327. doi: 10.1523/JNEUROSCI.19-01-00316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merfeld DM, Zupan L, Peterka RJ. Humans use internal models to estimate gravity and linear acceleration. Nature. 1999;398:615–618. doi: 10.1038/19303. [DOI] [PubMed] [Google Scholar]
- 6.Zupan LH, Merfeld DM. Neural processing of gravito-inertial cues in humans. IV. Influence of visual rotational cues during roll optokinetic stimuli. J Neurophysiol. 2003;89:390–400. doi: 10.1152/jn.00513.2001. [DOI] [PubMed] [Google Scholar]
- 7.MacNeilage PR, et al. A Bayesian model of the disambiguation of gravitoinertial force by visual cues. Exp Brain Res. 2007;179:263–290. doi: 10.1007/s00221-006-0792-0. [DOI] [PubMed] [Google Scholar]
- 8.Green AM, Angelaki DE. Resolution of sensory ambiguities for gaze stabilization requires a second neural integrator. J Neurosci. 2003;23:9265–9275. doi: 10.1523/JNEUROSCI.23-28-09265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merfeld DM, et al. Vestibular perception and action employ qualitatively different mechanisms. I. Frequency response of VOR and perceptual responses during Translation and Tilt. J Neurophysiol. 2005;94:186–198. doi: 10.1152/jn.00904.2004. [DOI] [PubMed] [Google Scholar]
- 10.Merfeld DM, et al. Vestibular perception and action employ qualitatively different mechanisms. II. VOR and perceptual responses during combined Tilt and Translation. J Neurophysiol. 2005;94:199–205. doi: 10.1152/jn.00905.2004. [DOI] [PubMed] [Google Scholar]
- 11.Glasauer S, Merfeld DM. Modeling three-dimensional responses during complex motion stimulation. In: Fetter M, et al., editors. Three-dimension al kinematics of eye, head and limb movements. Harwood Academic; Amsterdam: 1997. pp. 387–398. [Google Scholar]
- 12.Merfeld DM. Modeling the vestibulo-ocular reflex of the squirrel monkey during eccentric rotation and roll tilt. Exp Brain Res. 1995;106:123–134. doi: 10.1007/BF00241362. [DOI] [PubMed] [Google Scholar]
- 13.Merfeld DM, Zupan LH. Neural processing of gravitoinertial cues in humans. III. Modeling tilt and translation responses. J Neurophysiol. 2002;87:819–833. doi: 10.1152/jn.00485.2001. [DOI] [PubMed] [Google Scholar]
- 14.Mergner T, Glasauer S. A simple model of vestibular canal-otolith signal fusion. Ann N Y Acad Sci. 1999;871:430–434. doi: 10.1111/j.1749-6632.1999.tb09211.x. [DOI] [PubMed] [Google Scholar]
- 15.Zupan LH, Merfeld DM, Darlot C. Using sensory weighting to model the influence of canal, otolith and visual cues on spatial orientation and eye movements. Biol Cybern. 2002;86:209–230. doi: 10.1007/s00422-001-0290-1. [DOI] [PubMed] [Google Scholar]
- 16.Glasauer S. Linear acceleration perception: frequency dependence of the hilltop illusion. Acta Otolaryngol Suppl. 1995;520(Pt 1):37–40. doi: 10.3109/00016489509125184. [DOI] [PubMed] [Google Scholar]
- 17.Carpenter MB, Stein BM, Peter P. Primary vestibulocerebellar fibers in the monkey: distribution of fibers arising from distinctive cell groups of the vestibular ganglia. Am J Anat. 1972;135:221–249. doi: 10.1002/aja.1001350209. [DOI] [PubMed] [Google Scholar]
- 18.Gerrits NM, et al. The primary vestibulocere-bellar projection in the rabbit: absence of primary afferents in the flocculus. Neurosci Lett. 1989;105:27–33. doi: 10.1016/0304-3940(89)90006-2. [DOI] [PubMed] [Google Scholar]
- 19.Barmack NH, et al. Vestibular primary afferent projection to the cerebellum of the rabbit. J Comp Neurol. 1993;327:521–534. doi: 10.1002/cne.903270405. [DOI] [PubMed] [Google Scholar]
- 20.Kevetter GA, Perachio AA. Distribution of vestibular afferents that innervate the sacculus and posterior canal in the gerbil. J Comp Neurol. 1986;254:410–424. doi: 10.1002/cne.902540312. [DOI] [PubMed] [Google Scholar]
- 21.Kevetter GA, et al. Central distribution of vestibular afferents that innervate the anterior or lateral semicircular canal in the mongolian gerbil. J Vestib Res. 2004;14:1–15. [PubMed] [Google Scholar]
- 22.Newlands SD, et al. Central projections of the saccular and utricular nerves in macaques. J Comp Neurol. 2003;466:31–47. doi: 10.1002/cne.10876. [DOI] [PubMed] [Google Scholar]
- 23.Angelaki DE, Hess BJ. The cerebellar nodulus and ventral uvula control the torsional vestibulo-ocular reflex. J Neurophysiol. 1994;72:1443–1447. doi: 10.1152/jn.1994.72.3.1443. [DOI] [PubMed] [Google Scholar]
- 24.Angelaki DE, Hess BJ. Inertial representation of angular motion in the vestibular system of rhesus monkeys. I. Vestibuloocular reflex. J Neurophysiol. 1994;71:1222–1249. doi: 10.1152/jn.1994.71.3.1222. [DOI] [PubMed] [Google Scholar]
- 25.Angelaki DE, Hess BJ. Inertial representation of angular motion in the vestibular system of rhesus monkeys. II. Otolith-controlled transformation that depends on an intact cerebellar nodulus. J Neurophysiol. 1995;73:1729–1751. doi: 10.1152/jn.1995.73.5.1729. [DOI] [PubMed] [Google Scholar]
- 26.Angelaki DE, Hess BJ. Lesion of the nodulus and ventral uvula abolish steady-state off-vertical axis otolith response. J Neurophysiol. 1995;73:1716–1720. doi: 10.1152/jn.1995.73.4.1716. [DOI] [PubMed] [Google Scholar]
- 27.Wearne S, Raphan T, Cohen B. Control of spatial orientation of the angular vestibuloocular reflex by the nodulus and uvula. J Neurophysiol. 1998;79:2690–2715. doi: 10.1152/jn.1998.79.5.2690. [DOI] [PubMed] [Google Scholar]
- 28.Solomon D, Cohen B. Stimulation of the nodulus and uvula discharges velocity storage in the vestibulo-ocular reflex. Exp Brain Res. 1994;102:57–68. doi: 10.1007/BF00232438. [DOI] [PubMed] [Google Scholar]
- 29.Yakusheva TA, et al. Purkinje cells in posterior cerebellar vermis encode motion in an inertial reference frame. Neuron. 2007;54:973–985. doi: 10.1016/j.neuron.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Yakusheva T, Blazquez PM, Angelaki DE. Frequency-selective coding of translation and tilt in macaque cerebellar nodulus and uvula. J Neurosci. 2008;28:9997–10009. doi: 10.1523/JNEUROSCI.2232-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green AM, Shaikh AG, Angelaki DE. Sensory vestibular contributions to constructing internal models of self-motion. J Neural Eng. 2005;2:S164–S179. doi: 10.1088/1741-2560/2/3/S02. [DOI] [PubMed] [Google Scholar]
- 32.Shaikh AG, et al. Properties of cerebellar fastigial neurons during translation, rotation, and eye movements. J Neurophysiol. 2005;93:853–863. doi: 10.1152/jn.00879.2004. [DOI] [PubMed] [Google Scholar]
- 33.Shaikh AG, et al. Sensory convergence solves a motion ambiguity problem. Curr Biol. 2005;15:1657–1662. doi: 10.1016/j.cub.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Meng H, et al. Vestibular signals in primate thalamus: properties and origins. J Neurosci. 2007;27:13590–13602. doi: 10.1523/JNEUROSCI.3931-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graybiel A, Clark B. Duration of oculogyral illusion as a function of the interval between angular acceleration and deceleration; its significance in terms of dynamics of semicircular canals in man. J Appl Physiol. 1952;5:147–152. doi: 10.1152/jappl.1952.5.4.147. [DOI] [PubMed] [Google Scholar]
- 36.Clark B, Graybiel A. Contributing factors in the perception of the oculogravic illusion. Am J Psychol. 1963;76:18–27. [PubMed] [Google Scholar]
- 37.Clark B, Graybiel A. Factors contributing to the delay in the perception of the oculogravic illusion. Am J Psychol. 1966;79:377–388. [PubMed] [Google Scholar]
- 38.Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long-duration centrifugal force. J Neurophysiol. 1976;39:970–984. doi: 10.1152/jn.1976.39.5.970. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. III. Response dynamics. J Neurophysiol. 1976;39:996–1008. doi: 10.1152/jn.1976.39.5.996. [DOI] [PubMed] [Google Scholar]
- 40.Shojaku H, et al. Topographical distribution of Purkinje cells in the uvula and the nodulus projecting to the vestibular nuclei in cats. Brain Res. 1987;416:100–112. doi: 10.1016/0006-8993(87)91501-0. [DOI] [PubMed] [Google Scholar]
- 41.Voogd J, Gerrits NM, Ruigrok TJ. Organization of the vestibulocerebellum. Ann N Y Acad Sci. 1996;781:553–579. doi: 10.1111/j.1749-6632.1996.tb15728.x. [DOI] [PubMed] [Google Scholar]
- 42.Wylie DR, et al. Projections of individual Purkinje cells of identified zones in the ventral nodulus to the vestibular and cerebellar nuclei in the rabbit. J Comp Neurol. 1994;349:448–463. doi: 10.1002/cne.903490309. [DOI] [PubMed] [Google Scholar]
- 43.Merfeld DM, Zupan LH, Gifford CA. Neural processing of gravito-inertial cues in humans. II. Influence of the semicircular canals during eccentric rotation. J Neurophysiol. 2001;85:1648–1660. doi: 10.1152/jn.2001.85.4.1648. [DOI] [PubMed] [Google Scholar]
- 44.Zupan LH, Peterka RJ, Merfeld DM. Neural processing of gravito-inertial cues in humans. I. Influence of the semicircular canals following postrotatory tilt. J Neurophysiol. 2000;84:2001–2015. doi: 10.1152/jn.2000.84.4.2001. [DOI] [PubMed] [Google Scholar]
- 45.Green AM, Angelaki DE. An integrative neural network for detecting inertial motion and head orientation. J Neurophysiol. 2004;92:905–925. doi: 10.1152/jn.01234.2003. [DOI] [PubMed] [Google Scholar]
- 46.Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol. 1971;34:661–675. doi: 10.1152/jn.1971.34.4.661. [DOI] [PubMed] [Google Scholar]
- 47.Angelaki DE, Dickman JD. Spatiotemporal processing of linear acceleration: primary afferent and central vestibular neuron responses. J Neurophysiol. 2000;84:2113–2132. doi: 10.1152/jn.2000.84.4.2113. [DOI] [PubMed] [Google Scholar]
- 48.Dickman JD, Angelaki DE. Vestibular convergence patterns in vestibular nuclei neurons of alert primates. J Neurophysiol. 2002;88:3518–3533. doi: 10.1152/jn.00518.2002. [DOI] [PubMed] [Google Scholar]
- 49.Musallam S, Tomlinson RD. Asymmetric integration recorded from vestibular-only cells in response to position transients. J Neurophysiol. 2002;88:2104–2113. doi: 10.1152/jn.2002.88.4.2104. [DOI] [PubMed] [Google Scholar]
- 50.Zhou W, et al. Responses of monkey vestibular-only neurons to translation and angular rotation. J Neurophysiol. 2006;96:2915–2930. doi: 10.1152/jn.00013.2006. [DOI] [PubMed] [Google Scholar]
- 51.Kaptein RG, Van Gisbergen JA. Canal and otolith contributions to visual orientation constancy during sinusoidal roll rotation. J Neurophysiol. 2006;95:1936–1948. doi: 10.1152/jn.00856.2005. [DOI] [PubMed] [Google Scholar]
- 52.Seidman SH, Telford L, Paige GD. Tilt perception during dynamic linear acceleration. Exp Brain Res. 1998;119:307–314. doi: 10.1007/s002210050346. [DOI] [PubMed] [Google Scholar]
- 53.Dichgans J, et al. Moving visual scenes influence the apparent direction of gravity. Science. 1972;178:1217–1219. doi: 10.1126/science.178.4066.1217. [DOI] [PubMed] [Google Scholar]
- 54.Howard IP, Hu G. Visually induced reorientation illusions. Perception. 2001;30:583–600. doi: 10.1068/p3106. [DOI] [PubMed] [Google Scholar]
- 55.Barmack NH, Shojaku H. Vestibular and visual climbing fiber signals evoked in the uvula-nodulus of the rabbit cerebellum by natural stimulation. J Neurophysiol. 1995;74:2573–2589. doi: 10.1152/jn.1995.74.6.2573. [DOI] [PubMed] [Google Scholar]
- 56.Fushiki H, Barmack NH. Topography and reciprocal activity of cerebellar Purkinje cells in the uvula-nodulus modulated by vestibular stimulation. J Neurophysiol. 1997;78:3083–3094. doi: 10.1152/jn.1997.78.6.3083. [DOI] [PubMed] [Google Scholar]
- 57.Barmack NH, Yakhnitsa V. Vestibularly evoked climbing-fiber responses modulate simple spikes in rabbit cerebellar Purkinje neurons. Ann N Y Acad Sci. 2002;978:237–254. doi: 10.1111/j.1749-6632.2002.tb07571.x. [DOI] [PubMed] [Google Scholar]
- 58.Barmack NH, Yakhnitsa V. Cerebellar climbing fibers modulate simple spikes in Purkinje cells. J Neurosci. 2003;23:7904–7916. doi: 10.1523/JNEUROSCI.23-21-07904.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yakhnitsa V, Barmack NH. Antiphasic Purkinje cell responses in mouse uvula-nodulus are sensitive to static roll-tilt and topographically organized. Neuroscience. 2006;143:615–626. doi: 10.1016/j.neuroscience.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 60.Angelaki DE, Hess BJ, Suzuki J. Differential processing of semicircular canal signals in the vestibulo-ocular reflex. J Neurosci. 1995;15:7201–7216. doi: 10.1523/JNEUROSCI.15-11-07201.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reisine H, Raphan T. Unit activity in the vestibular nuclei of monkeys during off-vertical axis rotation. Ann N Y Acad Sci. 1992;656:954–956. doi: 10.1111/j.1749-6632.1992.tb25305.x. [DOI] [PubMed] [Google Scholar]
- 62.Merfeld DM, et al. A multidimensional model of the effect of gravity on the spatial orientation of the monkey. J Vestib Res. 1993;3:141–161. [PubMed] [Google Scholar]
- 63.Merfeld DM, Young LR. The vestibuloocular reflex of the squirrel monkey during eccentric rotation and roll tilt. Exp Brain Res. 1995;106:111–122. doi: 10.1007/BF00241361. [DOI] [PubMed] [Google Scholar]
- 64.Wearne S, Raphan T, Cohen B. Effects of tilt of the gravito-inertial acceleration vector on the angular vestibuloocular reflex during centrifugation. J Neurophysiol. 1999;81:2175–2190. doi: 10.1152/jn.1999.81.5.2175. [DOI] [PubMed] [Google Scholar]
- 65.Fetter M, et al. The influence of head reorientation on the axis of eye rotation and the vestibular time constant during postrotatory nystagmus. Ann N Y Acad Sci. 1992;656:838–840. doi: 10.1111/j.1749-6632.1992.tb25269.x. [DOI] [PubMed] [Google Scholar]


